Features of Profiles of Biologically Active Compounds of Primary and Secondary Metabolism of Lines from VIR Flax Genetic Collection, Contrasting in Size and Color of Seeds
Abstract
:1. Introduction
2. Results and Discussion
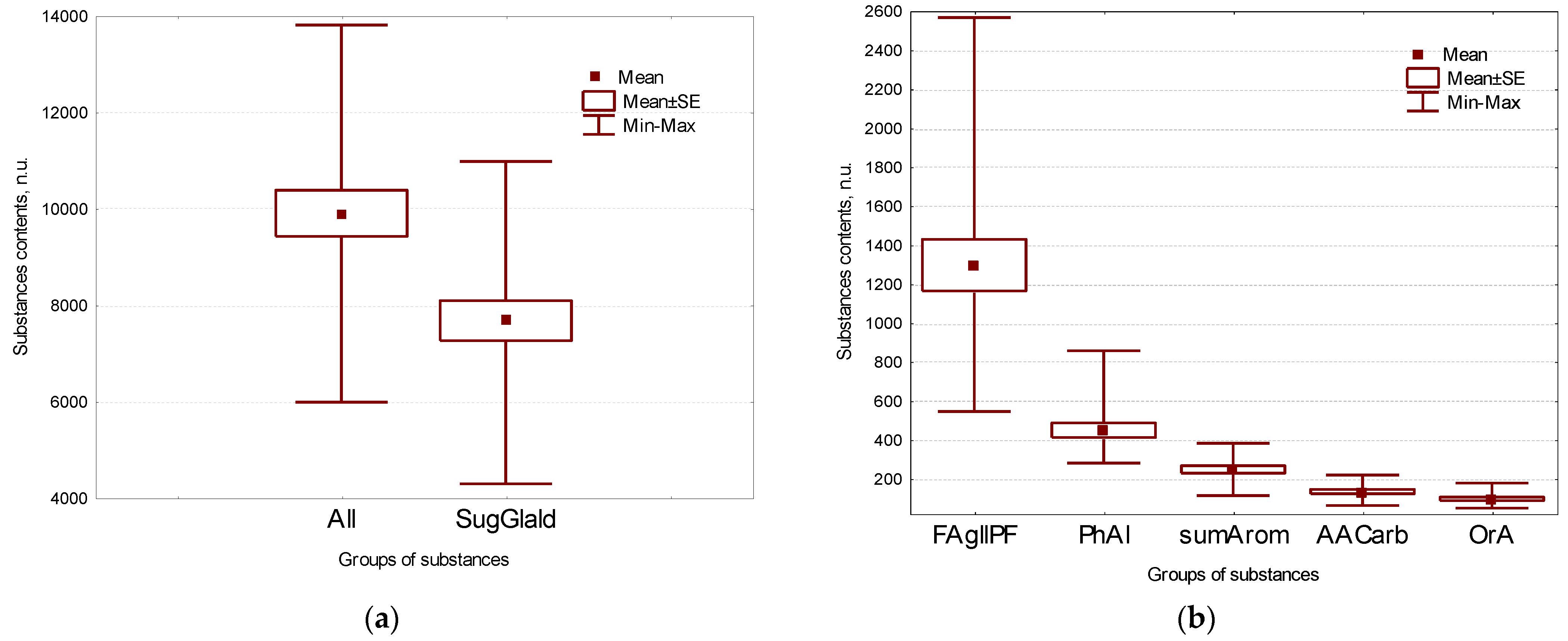
2.1. Main Groups of Substances in Flax Seeds
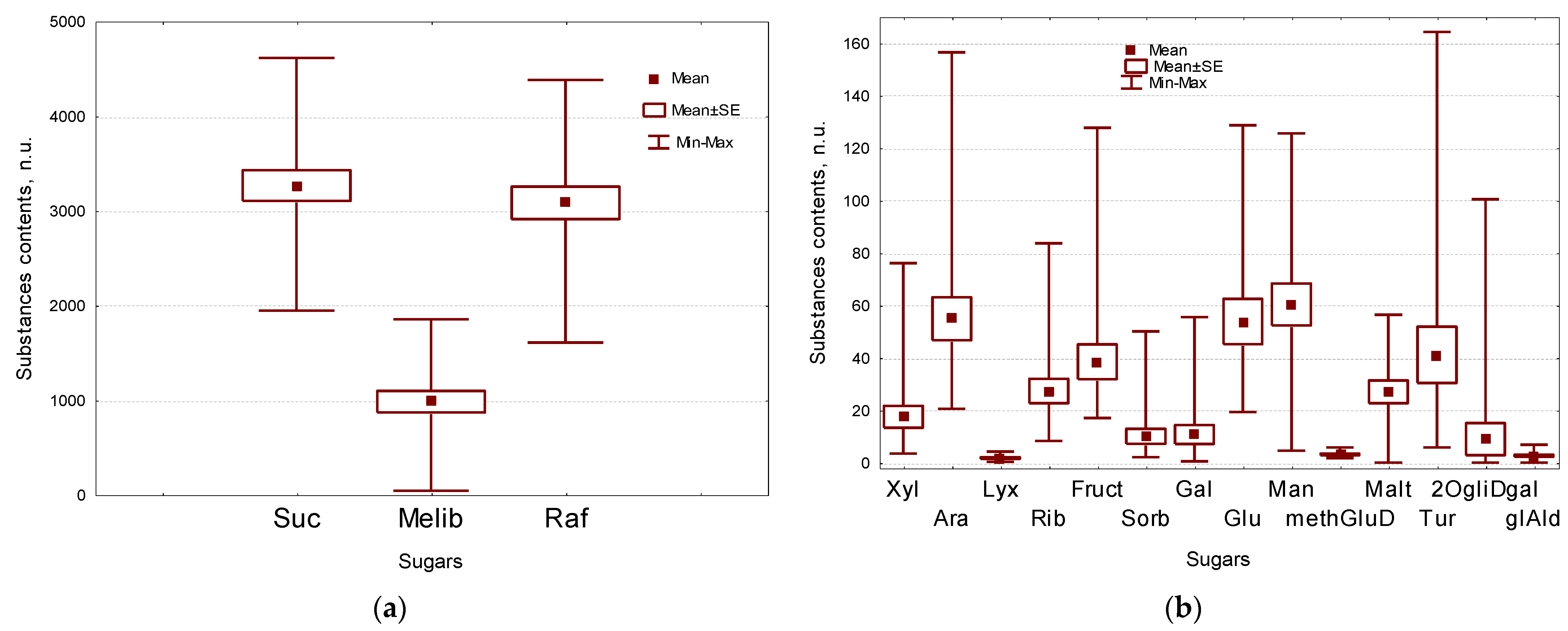
2.2. Sugars Composition of Flax Seeds
2.3. Fatty Acids, Acylglycerols and Paraffinscomposition of Flax Seeds
2.4. Polyatomic Alcohols Composition of Flax Seeds
2.5. Secondary Metabolites Composition of Flax Seeds
2.6. Amino Acids and Other Nitrogen-Containing Compounds Composition of Flax Seeds
2.7. Organic Acids Composition of Flax Seeds
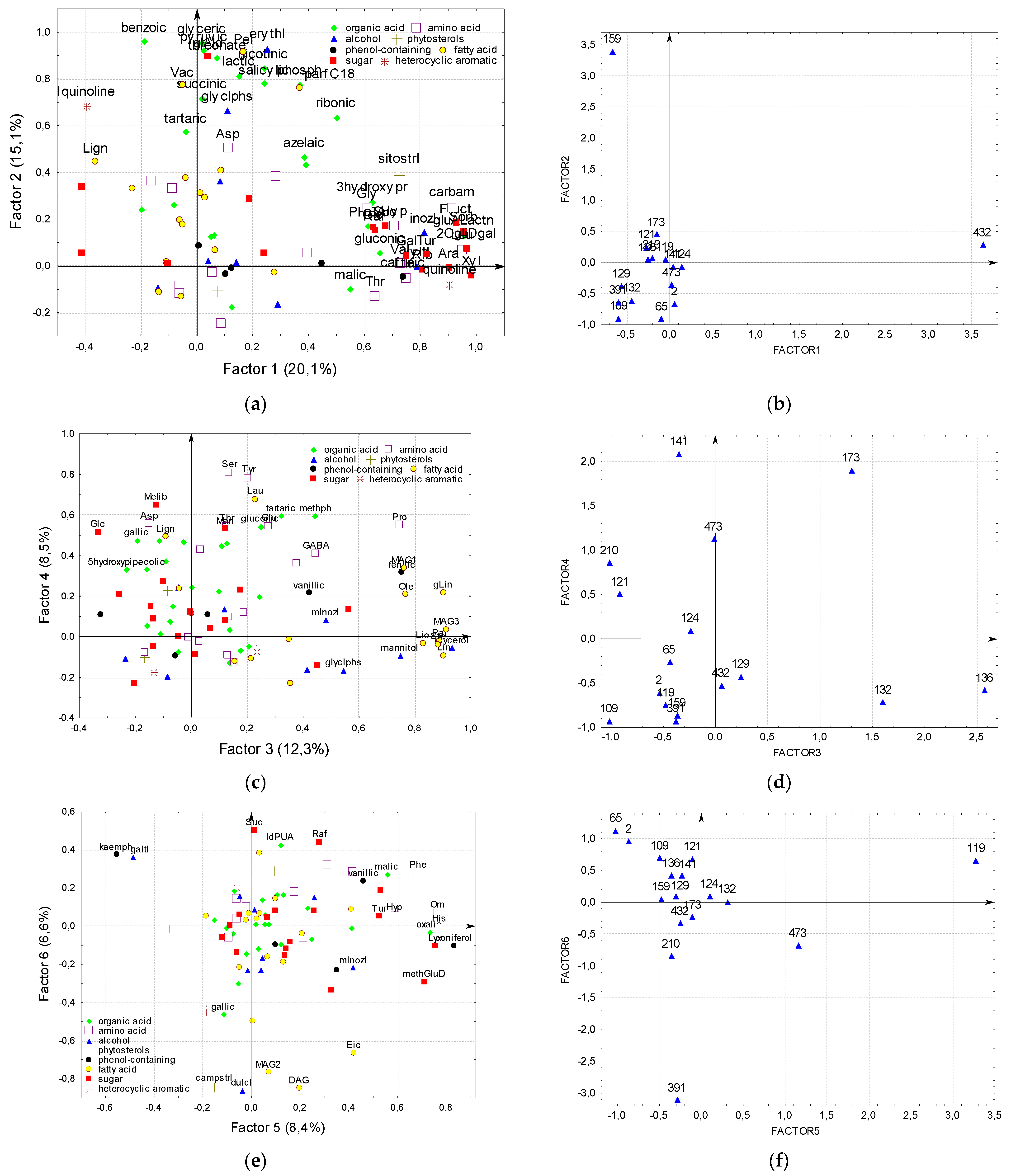
2.8. Factor Analysis of Flax Seeds Chamical Composition
2.9. Association of Plants Morphological Features with the Chemical Composition of Flax Seeds
3. Materials and Methods
3.1. Material
3.2. Chemicals and Reagents
3.3. Samples Preparation and Metabolomic Analysis
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oliver, S.G.; Winson, M.K.; Kell, D.B.; Baganz, F. Systematic functional analysis of the yeast genome. Trends Biotechnol. 1998, 16, 373–378. [Google Scholar] [CrossRef]
- Smolikova, G.N.; Shavarda, A.L.; Alekseichuk, I.V.; Chantseva, V.V.; Medvedev, S.S. Metabolic approach to assessing the varietal specificity of seeds of Brassica napus L. Russ. J. Genet. Appl. Res. 2015, 19, 121–127. (In Russian) [Google Scholar]
- Puzanskiy, R.K.; Yemelyanov, V.V.; Gavrilenko, T.A.; Shishova, M.F. The perspectives of metabolomics studies of potato plants. Vavilov J. Genet. Breed. 2017, 21, 112–123. [Google Scholar] [CrossRef]
- Loskutov, I.G.; Shelenga, T.V.; Konarev, A.V.; Vargach, Y.A.; Porokhovinova, E.A.; Blinova, E.V.; Gnutikov, A.A.; Rodionov, A.V. Biochemical approach to structuring the variety diversity of the naked and covered forms of cultural oats (Avena sativa L.). Ecol. Genet. 2020, 1, 27–41. [Google Scholar] [CrossRef]
- Puzanskiy, R.K.; Yemelyanov, V.V.; Shishova, M.F. Metabolomics as a modern approach for the investigation of potato plant adaptation to biotic and abiotic stress factors (review). Agrobiology 2018, 53, 15–28. [Google Scholar]
- Rangani, J.; Panda, A.; Parida, A.K. Metabolomic study reveals key metabolic adjustments in the xerohalophyte Salvadora persica L. during adaptation to water deficit and subsequent recovery conditions. Plant Physiol. Biochem. 2020, 150, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Golovkin, B.N.; Rudenskaya, R.N.; Trofimova, I.A.; Schroeter, A.I. Biologically Active Substances of Plant Origin; Nauka: Moscow, Russia, 2001; Volume 1, 369p. (In Russian) [Google Scholar]
- Campos-Vega, R.; Oomah, B.D. Chemistry and classification of phytochemicals. In Handbook of Plant Food Phytochemicals: Sources, Stability and Extraction; John Willey & Sons: NewYork, NY, USA, 2013; pp. 7–48. [Google Scholar]
- Loskutov, I.G.; Shelenga, T.V.; Konarev, A.V.; Shavarda, A.L.; Blinova, E.V.; Dzubenko, N.I. The metabolomic approach to the comparative analysis of wild and cultivated species of oats (Avena L.). Russ. J. Genet. Appl. Res. 2017, 7, 501–508. [Google Scholar] [CrossRef]
- Solovyeva, A.E.; Shelenga, T.V.; Shavarda, A.L.; Burlyaeva, M.O. Comparative analysis of wild and cultivated Lathyrus L. spp. according to their primary and secondary metabolite contents. Vavilov J. Genet. Breed. 2019, 23, 667–674. [Google Scholar] [CrossRef] [Green Version]
- Teplyakova, S.B.; Shavarda, A.L.; Shelenga, T.V.; Dzyubenko, E.A.; Potokina, E.K. A simple and efficient method to extract polar metabolites from guar leaves (Cyamopsis tetragonoloba (L.) Taub.) for GC-MS metabolome analysis. Vavilov J. Genet. Breed. 2019, 23, 49–54. [Google Scholar] [CrossRef]
- Perchuk, I.; Shelenga, T.; Gurkina, M.; Miroshnichenko, E.; Burlyaeva, M. Composition of Primary and Secondary Metabolite Compounds in Seeds and Pods of Asparagus Bean (Vigna unguiculata (L.) Walp.) from China. Molecules 2020, 25, 3778. [Google Scholar] [CrossRef]
- Loskutov, I.G.; Shelenga, T.V.; Konarev, A.V.; Horeva, V.I.; Shavarda, A.L.; Blinova, E.V.; Gnutikov, A.A. Biochemical aspects of interrelations between fungi and plants in the case study of Fusarium head blight in oats. Agric. Biol. 2019, 54, 575–588. [Google Scholar]
- Shelenga, T.V.; Malyshev, L.L.; Kerv Yu, A.; Diubenko, T.V.; Konarev, A.V.; Horeva, V.I.; Belousova, M.K.; Koleva, M.A.; Chikida, N.N. Metabolomic approach to search for fungal resistant forms of Aegilops tauschii Coss. from the VIR collection. Vavilov J. Genet. Breed. 2020, 24, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D. Flaxseed By-products. In Food Wastes and By-Products; Campos-Vega, R., Oomah, B.D., Vergara, H., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 267–289. [Google Scholar]
- Oomah, B.D. Flaxseed as a functional food source. J. Sci. Food Agric. 2001, 81, 889–894. [Google Scholar] [CrossRef]
- Morris, D.H. Flax—A Health and Nutrition Primer Flax Council of Canada. 2007. Available online: https://flaxcouncil.ca/resources/nutrition/technical-nutrition-information/flax-a-health-and-nutrition-primer (accessed on 15 October 2021).
- Soni, R.; Katoch, M.; Kumar, A.; Verma, P. Flaxseed—Composition and its health benefits. Res. Environ. Life Sci. 2016, 9, 310–316. [Google Scholar]
- Sharma, M.; Dadhwal, K.; Gat, Y.; Kumar, V.; Panghal, A.; Prasad, R.; Kaur, S.; Gat, P. A review on newer techniques in extraction of oleaginous flaxseed constituents. OCL 2019, 26, 14. [Google Scholar] [CrossRef]
- Podobey, A.I. Flax cake replenishes the range of protein supplements for animals and poultry. Oils Fats 2019, 11–12, 25–27. [Google Scholar]
- Tolkachev, O.N.; Zhuchenko, A.A. Biologically active substances of flax: Medicinal and nutritional properties (a review). Pharm. Chem. J. 2000, 34, 360–367. [Google Scholar] [CrossRef]
- Zubtsov, V.A.; Osipova, L.L.; Antipova, N.V. A new competitive product of flax growing—Flax flour. Achiev. Sci. Technol. Agro Ind. Complex 2007, 6, 56. [Google Scholar]
- Singh, K.; Mridula, D.; Rehal, J.; Barnwal, P. Flaxseed: A potential source of food, feed and fiber. Food Sci. Nutr. 2011, 51, 210–222. [Google Scholar] [CrossRef]
- Oomah, B.D.; Mazza, G. Flaxseed proteins—A review. Food Chem. 1993, 48, 109–114. [Google Scholar] [CrossRef]
- Touré, A.; Xueming, X. Flaxseed lignans: Source, biosynthesis, metabolism, antioxidant activity, bio-active components, and health benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, A.; Paynel, F.; Rihouey, C.; Porokhovinova, E.; Brutch, N.; Morvan, C. Variability of seed traits and properties of soluble mucilages in lines of the flax genetic collection of Vavilov Institute. Plant Physiol. Biochem. 2014, 80, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Thambugala, D.; Duguid, S.; Loewen, E.; Rowland, G.; Booker, H.; You, F.M.; Cloutier, S. Genetic variation of six desaturase genes in flax and their impact on fatty acid composition. Theor. Appl. Genet. 2013, 126, 2627–2641. [Google Scholar] [CrossRef] [Green Version]
- Brutch, N.B.; Porokhoviniva, E.A.; Shelenga, T.V. Innovative possibilities of oil flax breeding orientated at the different oil composition. Dostizheniya Nauki Tekhniki APK 2016, 30, 5–8. (In Russian) [Google Scholar]
- Bhatty, R.S.; Cherdkiatgumchai, P. Compositional analysis of laboratory-prepared and commercial samples of linseed meal and of hull isolated from flax. JAOCS 1990, 67, 79–83. [Google Scholar] [CrossRef]
- Khan, M.L.; Sharif, M.; Sarwar, M.; Ameen, S.M. Chemical composition of different varieties of linseed. Pak. Vet. J. 2010, 30, 79–82. [Google Scholar]
- Mazza, G.; Oomah, B.D. Flaxseed, dietary fiber, cyanogens. In Flaxseed in Human Nutrition; Cunnane, S.C., Thompson, L.U., Eds.; AOCS Press: Champaign, IL, USA, 1995; pp. 56–81. [Google Scholar]
- Ramsay, A.; Fliniaux, O.; Fang, J.; Molinie, R.; Roscher, A.; Grand, E.; Guillot, X.; Kovensky, J.; Fliniaux, M.A.; Schneider, B.; et al. Development of an NMR metabolomics-based tool for selection of flaxseed varieties. Metabolomics 2014, 10, 1258–1267. [Google Scholar] [CrossRef]
- Kasote, D.M. Flaxseed phenolics as natural antioxidants. Int. Food Res. J. 2013, 20, 27–34. [Google Scholar]
- Herchi, W.; Sawalha, S.; Arráez-Román, D.; Boukhchina, S.; Segura-Carretero, A.; Kallel, H.; Fernández-Gutierrez, A. Determination of phenolic and other polar compounds in flaxseed oil using liquid chromatography coupled with time-of-flight mass spectrometry. Food Chem. 2011, 126, 332–338. [Google Scholar] [CrossRef]
- Anisimova, N.V.; Vakula, S.I.; Leontiev, V.N.; Titok, V.V. Assessment of phytin and inorganic phosphate content in seeds of oilseed flax varieties Trudy BSTU. Series IV. Chem. Technol. Org. Subst. Biotechnol. 2010, 13, 262–265. (In Russian) [Google Scholar]
- Smirnov, V.G. Importance of genetic collections for fundamental research and breeding programs. In Identified Plant Genepool and Breeding; Rigin, B.V., Gaevskaya, E.I., Eds.; VIR: St. Petersburg, Russia, 2005; pp. 783–806. [Google Scholar]
- Mueller, K.; Eisner, P.; Yoshie-Stark, Y.; Nakada, R.; Kirchhoff, E. Functional properties and chemical composition of fractionated brown and yellow linseed meal (Linum usitatissimum L.). J. Food Eng. 2020, 98, 453–460. [Google Scholar] [CrossRef]
- Naran, R.; Chen, G.; Carpita, N. Novel rhamnogalacturonan I and arabinoxylan polysaccharides of flax seed mucilage. Plant Physiol. 2008, 148, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Porokhovinova, E.A.; Pavlov, A.V.; Brach, N.B.; Morvan, C. Carbohydrate composition of flax mucilage and its relation to morphological characters. Agric. Biol. 2017, 52, 161–171. [Google Scholar]
- Miart, F.; Fournet, F.; Dubrulle, N.; Petit, E.; Demailly, H.; Dupont, L.; Zabijak, L.; Marcelo, P.; Boudaoud, A.; Pineau, C.; et al. Cytological approaches combined with chemical analysis reveals the layered nature of flax mucilage. Front. Plant Sci. 2019, 10, 684. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Paynel, F.; Morvan, C.; Lerouge, P.; Driouich, A.; Ray, B. Characterization of mucilage polysaccharides, arabinogalactanproteins and cell-wall hemicellulosic polysaccharides isolated from flax seed meal: A wealth of structural moieties. Carbohydr. Polym. 2013, 93, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Fry, S.C.; York, W.S.; Albersheim, P.; Darvill, A.; Hayashi, T.; Joseleau, J.P.; Kato, Y.; Lorences, E.P.; Maclachlan, G.A.; McNeil, M.; et al. An unambiguous nomenclature for xyloglucan-derived oligosaccharides. Plant Physiol. 1993, 89, 1–3. [Google Scholar] [CrossRef]
- Salem, M.A.; Ezzat, S.M.; Giavalisco, P.; Sattar, E.A.; El Tanbouly, N. Application of a comprehensive metabolomics approach for the selection of flaxseed varieties with the highest nutritional and medicinal attributes. J. Food Drug Anal. 2021, 29, 214–239. [Google Scholar] [CrossRef]
- Heldt, H.W. Plant Bioshemistry; Binom: Moscow, Russia, 2011; 463p. [Google Scholar]
- Gorshkova, T.A. Plant Cell as a Dynamic System; Nauka: Moscow, Russia, 2007; 429p. [Google Scholar]
- Seifert, G.J.; Roberts, K. The biology of arabinogalactan proteins. Annu. Rev. Plant Biol. 2007, 58, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Porokhovinova, E.A.; Shelenga, T.V.; Kosykh, L.A.; Sanin, A.A.; Kazarina, A.V.; Kutuzova, S.N.; Pavlov, A.V.; Brach, N.B. Biochemical diversity of fatty acid composition in flax from VIR’s genetic collection and effect of environment on its development. Russ. J. Genet. Appl. Res. 2017, 7, 626–639. [Google Scholar] [CrossRef]
- Tavarini, S.; Castagna, A.; Conte, G.; Foschi, L.; Sanmartin, C.; Incrocci, L.; Ranieri, A.; Serra, A.; Angelini, L.G. Evaluation of chemical composition of two linseed varieties as sources of health-beneficial substances. Molecules 2019, 16, 3729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sargi, S.C.; Silva, B.C.; Santos, H.M.; Montanher, P.F.; Boeing, J.S.; Júnior, O.; Souza, N.E.; Visentainer, J.V. Antioxidant capacity and chemical composition in seeds rich in omega-3: Chia, flax, and perilla. Food Sci. Technol. Camp. 2013, 33, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Bhatty, R.S. Nutrient composition of whole flaxseed and flaxseed meal. In Flaxseed in Human Nutrition; Cunnane, S.C., Thompson, L.U., Eds.; AOCS Press: Champaign, IL, USA, 1995; pp. 22–42. [Google Scholar]
- Oomah, B.D.; Mazza, G.; Kenaschuk, E.O. Flavonoid content of the flaxseed, influence of cultivar and environment. Euphytica 1996, 90, 163–167. [Google Scholar] [CrossRef]
- Oomah, B.D.; Kenaschuk, E.O.; Mazza, G.D. Phenolic acids in flaxseed. J. Agric. Food Chem. 1995, 43, 2016–2019. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Qiu, C.; Ye, Y.; Guo, X.; Chen, G.; Li, T.; Wang, Y.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles and health benefits in fiber and oil flaxseeds (Linum usitatissimum L.). Food Chem. 2017, 214, 227–233. [Google Scholar] [CrossRef]
- Sharova, E.I. Cell Wall of Plants; Publishing House of St. Petersburg State University: St. Petersburg, Russia, 2004; 156p. [Google Scholar]
- Dzuvor, C.K.O.; Taylor, J.T.; Acquah, C.; Pan, S.; Agyei, D. Bioprocessing of functional ingredients from flaxseed. Molecules 2018, 23, 2444. [Google Scholar] [CrossRef] [Green Version]
- Gui, B.; Shim, Y.Y.; Datla, R.S.S.; Covello, P.S.; Stone, S.L.; Reaney, M.J.T. Identification and quantification of cyclolinopeptides in five flaxseed cultivars. J. Agric. Food Chem. 2012, 60, 8571–8579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.W.; Tschaplinski, T.J.; Wang, L.; Glazebrook, J.; Greenberg, J.T. Priming in systemic plant immunity. Science 2009, 324, 89–91. [Google Scholar] [CrossRef]
- Chupakhina, G.N. System of Ascorbic Acid of Plants; Kaliningrad University: Kaliningrad, Russia, 1997; 120p. [Google Scholar]
- Chen, Y.C.; Holmes, E.C.; Rajniak, J.; Kim, J.-G.; Tang, S.; Fischer, C.R.; Mudgett, M.B.; Sattely, E.S. N-hydroxy-pipecolic acid is a mobile metabolite that induces systemic disease resistance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E4920–E4929. [Google Scholar] [CrossRef] [Green Version]
- Vandenberghe, L.P.S.; Karp, S.G.; de Oliveira, P.Z.; de Carvalho, J.C.; Rodrigues, C.; Soccol, C.R. Chapter 18—Solid-state fermentation for the production of organic acids. In Current Advances in Solid-State Fermentation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 415–434. [Google Scholar]
- Singh, O.V.; Kumar, R. Biotechnological production of gluconic acid: Future implications. Appl. Microbiol. Biotechnol. 2007, 75, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Botov, A.Y.; Severin, A.P.; Yatsuk, V.Y.; Siplivaya, L.E. Researching of carbohydrate structure of some family Asteraceae plants. I.P. Pavlov Russ. Med. Biol. Her. 2012, 4, 142–145. [Google Scholar] [CrossRef]
- Vrinten, P.; Hu, Z.; Munchinsky, M.A.; Rowland, G.; Qui, X. Two FAD3 desaturase genes control the level of linolenic acid in flax seed. Plant Physiol. 2005, 1, 79–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Struijs, K.; Vincken, J.P.; Verhoef, R.; van Oostveen-van Casteren, W.H.M.; Voragen, A.G.J.; Gruppen, H. The flavonoid herbacetin diglucoside as a constituent of the lignan macromolecule from flaxseed hulls. Phytochemistry 2007, 68, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Porokhovinova, E. Genetic control of morphological characters of seedlings, bolls, and seeds in flax (Linum usitatissimum L.). Vavilov J. Genet. Breed. 2012, 16, 936–947. [Google Scholar]
- Shtark, O.; Puzanskiy, R.; Avdeeva, G.; Yemelyanov, V.; Shavarda, A.; Romanyuk, D.; Kliukova, M.; Kirpichnikova, A.; Tikhonovich, I.; Zhukov, V.; et al. Metabolic alterations in pisum sativum roots during plant growth and arbuscular mycorrhiza development. Plants 2021, 10, 1033. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Schauer, N. Metabolomics-assisted breeding: A viable option for crop improvement? Trends Genet. 2009, 25, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Ivanter, E.V.; Korosov, A.V. Leading to Quantitative Biology; Petrozavodsk State University: Petrozavodsk, Russia, 2003; p. 302. (In Russian) [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 2nd ed.; Freeman: New York, NY, USA, 1995; 850p. [Google Scholar]
- Rostova, N.S. Comparison Analyses of Correlation Structures. Evaluation of Biological Systems by Mathematical Methods; Biological Institute of Leningrad State University: Leningrad, Russia, 1985; pp. 37–54. (In Russian) [Google Scholar]
- StatSoft, Inc. Electronic Statistics Textbook; StatSoft: Tulsa, OK, USA, 2013; Available online: http://statsoft.ru/home/textbook/default.htm (accessed on 15 October 2021).
 —All substances are related to each other 0.5 > r > 0.9, individual correlations are not shown.
—All substances are related to each other 0.5 > r > 0.9, individual correlations are not shown.  r > 0.9,
r > 0.9,  0.9 > r > 0.7,
0.9 > r > 0.7,  0.7 > r > 0.5,
0.7 > r > 0.5,  r < 0.
r < 0.
 —All substances are related to each other 0.5 > r > 0.9, individual correlations are not shown.
—All substances are related to each other 0.5 > r > 0.9, individual correlations are not shown.  r > 0.9,
r > 0.9,  0.9 > r > 0.7,
0.9 > r > 0.7,  0.7 > r > 0.5,
0.7 > r > 0.5,  r < 0.
r < 0.




| Abbreviation | Substance | Average ± Se | Abbreviation | Substance | Average ± Se |
|---|---|---|---|---|---|
| Sugars, glyceraldehyde | Secondary metabolites | ||||
| Xyl | xylose | 17.4 ± 4.2 | campstrl | campesterol | 38.2 ± 4.1 |
| Ara | arabinose | 54.8 ± 8.2 | sitostrl | sitosterol | 62.7 ± 3.2 |
| Lyx | lyxose | 1.72 ± 0.30 | PhtSt | total phytosterols | 101 ± 5 |
| Rib | ribose | 27.3 ± 4.6 | coniferol | coniferol | 15.9 ± 2.6 |
| Pentose | total pentoses | 101 ± 16 | kaemph | kaempferol | 26.2 ± 4.8 |
| Fruct | fructose | 38.4 ± 6.6 | caffeic | caffeic acid | 0.89 ± 0.28 |
| Sorb | sorbose | 9.97 ± 2.84 | vanillic | vanillic acid | 0.46 ± 0.09 |
| Gal | galactose | 10.7 ± 3.6 | ferulic | ferulic acid | 96.2 ± 19.5 |
| Glc | glucose | 53.7 ± 8.7 | PhSb | total phenolic substances | 140 ± 19 |
| Man | mannose | 60.3 ± 8.0 | quinoline | quinoline | 3.63 ± 0.86 |
| methGluD | methyl glucose | 3.06 ± 0.31 | Iquinoline | isoquinoline | 2.50 ± 0.53 |
| Hexose | total hexoses | 176 ±20 | HCArom | total heterocyclic aromaticsubstances | 6.13 ± 0.74 |
| Msug | total monosaccharides | 277 ± 32 | SecMet | total secondary metabolites | 247 ± 19 |
| Suc | sucrose | 3263 ± 162 | Amino acids, carbamide | ||
| Melib | melibiose | 981 ± 113 | Ala | alanine | 14.2 ± 1.6 |
| Malt | maltose | 26.9 ± 4.3 | Gly | glycine | 10.4 ± 1.0 |
| Dsug | total disaccharides | 4271 ± 229 | Pro | proline | 20.0 ± 3.0 |
| Raf | raffinose | 3080 ± 171 | Hyp | hydroxyproline | 12.5 ± 1.4 |
| Tur | turanoseglucopyranoside | 41.0 ± 10.7 | Val | valin | 6.93 ± 0.62 |
| Tsug | total trisaccharides | 3121 ± 179 | Leu | leucine | 4.66 ± 1.74 |
| Psug | total polysaccharides | 7392 ± 390 | Ser | serine | 2.72 ± 0.45 |
| 2OgliDgal | 2-O-glycerol-d-galactopyranoside | 8.92 ± 6.12 | Thr | threonine | 5.99 ± 0.52 |
| glAld | glyceraldehyde | 2.59 ± 0.39 | Asp | aspartic acid | 21.0 ± 2.1 |
| SugGlAld | total sugars | 7672 ± 413 | Orn | ornithine | 0.90 ± 0.17 |
| Fatty acids, glicerol, paraffin | Glu | glutamic acid | 19.6 ± 3.7 | ||
| pel | Pelargonic acid | 1.00 ± 0.13 | GABA | gamma-aminobutyric acid | 0.49 ± 0.16 |
| lau | lauric acid | 0.29 ± 0.05 | Phe | phenylalanine | 9.83 ± 1.70 |
| pal | palmitic acid | 94.1 ± 9.5 | His | histidine | 1.12 ± 0.25 |
| lio | linoleic acid | 296 ± 27 | Tyr | tyrosine | 2.55 ± 0.46 |
| ole | oleic acid | 339 ± 37 | carbam | carbamide | 0.63 ± 0.13 |
| lin | alpha-linolenic acid | 349 ± 51 | AACarb | total amino acids and carbamid | 134 ± 11 |
| vac | vaccenic acid | 24.6 ± 3.5 | Organic acidsand phosphate | ||
| ste | stearic acid | 51.1 ± 6.8 | lactic | lactic acid | 2.93 ± 0.25 |
| eic | eicosanoic acid | 7.79 ± 0.70 | pyruvic | pyruvic acid | 0.71 ± 0.14 |
| glin | gamma-linolenic acid | 40.6 ± 9.6 | methylmalonic | methylmalonic acid | 0.76 ± 0.21 |
| lign | lignoceric acid | 7.09 ± 1.19 | oxalic | oxalic acid | 3.48 ± 0.22 |
| FA | total fatty acids | 1210 ± 128 | 3hydroxypr | 3-hydroxypropionic acid | 0.98 ± 0.08 |
| MAG1 | monoacylglycerol 16:0 | 8.39 ± 1.36 | phosph | phosphate | 40.1 ± 3.7 |
| MAG2 | monoacylglycerol 18:2 | 35.5 ± 8.1 | methph | methyl phosphate | 1.00 ± 0.06 |
| MAG3 | monoacylglycerol 18:0 | 6.55 ± 0.99 | benzoic | benzoic acid | 0.40 ± 0.08 |
| DAG | diacylglycerol | 32.9 ± 3.2 | PHBA | 4-Hydroxybenzoic acid | 1.14 ± 0.40 |
| Gll | total glycerols | 83.3 ± 11.5 | nicotinic | nicotinic acid | 0.22 ± 0.03 |
| parC18 | paraffin wax C18 | 1.65 ± 0.23 | succinic | succinic acid | 1.63 ± 0.29 |
| FAGllPf | total fatty acids and glycerols | 1295 ± 132 | glyceric | glyceric acid | 0.62 ± 0.08 |
| Polyhydric alcohols | 5hydroxypipecolic | 5-hydroxypipecolic acid | 0.43 ± 0.05 | ||
| erythl | erythritol | 26.8 ± 1.6 | malic | malic acid | 10.2 ± 1.1 |
| glycerol | glycerol | 141 ± 30 | Salicylic | salicylic acid | 0.61 ± 0.10 |
| glyclphs | glycerol-phosphate | 16.4 ± 2.0 | Threonate | threonic acid | 0.89 ± 0.09 |
| xyltl | xylitol | 19.2 ± 4.6 | Tartaric | tartaric acid | 3.53 ± 0.52 |
| mannitol | mannitol | 12.2 ± 5.5 | azelaic | azelaic acid | 8.73 ± 1.01 |
| dulcl | dulcitol | 5.34 ± 0.77 | ribonic | ribonic acid | 2.13 ± 0.26 |
| mInosl | myo-Inositol | 27.3 ± 2.0 | gluALactn | gluconic acid 1,5-lactone | 10.8 ± 2.9 |
| Inosl | inositol | 132 ± 6 | gallic | gallic acid | 2.02 ± 0.32 |
| galtl | galactinol | 67.3 ± 10.8 | gluconic | gluconic acid | 1.16 ± 0.16 |
| PhAlc | total polyhydric alcohols | 447 ± 37 | IdPUA | L-iduronic acid | 1.01 ± 0.26 |
| OrA | total organic acids | 95.4 ± 8.7 | |||
| All | Total | 9899 ± 480 | |||
| Substance | Morphological Character Presence | Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | U-Mann-Whitney | t-Student | |||||||
| n | Mean ± Se | Rank Sum | n | Mean ± Se | Rank Sum | Z Adjusted | p-Level * | t-Value | p-Level * | |
| Brown seeds | ||||||||||
| Glu | 6 | 8.2 ± 3.8 | 28 | 10 | 26.5 ± 4.3 | 109 | 2.57 | 0.01 | 2.91 | 0.01 |
| Yellow seeds | ||||||||||
| Iquinoline | 6 | 4.6 ± 0.8 | 80 | 10 | 1.2 ± 0.3 | 56 | −3.15 | 0.002 | 4.77 | 0.0003 |
| White petals | ||||||||||
| 3hydroxypr | 4 | 0.66 ± 0.09 | 15 | 12 | 1.09 ± 0.05 | 121 | 2.30 | 0.02 | 2.71 | 0.02 |
| phosph | 4 | 26 ± 2 | 14 | 12 | 45 ± 4 | 122 | 2.43 | 0.02 | 2.50 | 0.03 |
| ribonic | 4 | 1.10 ± 0.21 | 13 | 12 | 2.48 ± 0.28 | 123 | 2.55 | 0.01 | 2.71 | 0.02 |
| OrA | 4 | 61 ± 6 | 14 | 12 | 107 ± 9 | 122 | 2.43 | 0.02 | 2.66 | 0.02 |
| Asp | 4 | 14 ± 2 | 16 | 12 | 24 ± 2 | 120 | 2.18 | 0.03 | 2.38 | 0.03 |
| sitostrl | 4 | 47 ± 4 | 10 | 12 | 68 ± 3 | 126 | 2.91 | 0.004 | 3.84 | 0.002 |
| Raf | 4 | 2312 ± 256 | 13 | 12 | 3336 ± 154 | 123 | 2.55 | 0.01 | 3.36 | 0.005 |
| Line | Pedigree | Genes | Seeds Color | Characters |
|---|---|---|---|---|
| gc-2 | l-1 from k-48, Altgauzen breeding, Russia | red-brown | wild type, earliness, high iodine number of oil | |
| gc-65 | l-3 from k-3178, local Russia, Tver region | ora1 | speckled | orange anthers, earliness |
| gc-432 | l-5 from k-4043, Deep Pink, The Netherlands | pf-d | black | pink petals, earliness |
| gc-109 | l-3-2 from k-6099, Macovi M.A.G., Argentina | wf1 | red-brown | white petals, early flowering |
| gc-119 | l-2-3 from k-6210, NP (RR) 38, India | dlb3-e, ora3 | red-brown | light blue petals, orange anthers, a lot of ole and few of lin in oil |
| gc-121 | l-1-1 from k-6272, L.Dominion, N. Ireland | sfc1, rs1 | light-yellow-brown | Violet petals, high yield |
| gc-124 | l-1 from k-6284, Stormont Motley, N. Ireland | fe | spotted | earliness, dilution of anthocyanin pigmentation in all plant |
| gc-129 | l-2 from k-6392, Bolley Golden, USA | pf-ad yspf1 | yellow | pink petals, high iodine number of oil |
| gc-132 | l-1 from k-6608, Currong, Australia | sfbs1 | red-brown | no anthocyanins in hypocotyl and flower, white deformated petals, rust resistance, a lot of arabinoxilans in mucilage |
| gc-136 | l-1 from k-6634, Mermilloid, Czech | s1 | yellow | no anthocyanins in plant, white deformated petals, a lot of ste and lin in oil, high iodine number of oil, a lot of arabinoxilans, Glc, low amount of pectins in mucilage |
| gc-141 | l-1 from k-6815, K-6, Russia | pf1 | dark yellow-brown | pink petals |
| gc-159 | l-1-1 from k-7659, Bionda, Germany | YSED1 | yellow | a lot of arabinoxilans in mucilage, low amount of pectins and Gal in mucilage |
| gc-173 | l-1 from i-548145, 48254, Ottawa 2152, Germany | sgh1, sfc3-2, ysed2 | dark-yellow | violet petals, a lot of ste and lio in oil, a lot of arabinoxilans in mucilage |
| k-210 | l-1 ɜ i-588294, B-125, Lithuania | ygp1 | red-brown | light blue petals, yellow-green color of the plant, a lot of arabinoxilans in mucilage |
| gc-391 | l-1-2 from i-601679, Eyre, Australia | sfbs1, YSED1, lufad3a, lufad3b | yellow | no anthocyanins in the plant, white deformated petals, low content of lin, a lot of arabinoxilans in mucilage |
| gc-473 | l-1 from i-606307, B-200, Lithuania | red-brown | yellow-green color of the plant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porokhovinova, E.A.; Shelenga, T.V.; Kerv, Y.A.; Khoreva, V.I.; Konarev, A.V.; Yakusheva, T.V.; Pavlov, A.V.; Slobodkina, A.A.; Brutch, N.B. Features of Profiles of Biologically Active Compounds of Primary and Secondary Metabolism of Lines from VIR Flax Genetic Collection, Contrasting in Size and Color of Seeds. Plants 2022, 11, 750. https://doi.org/10.3390/plants11060750
Porokhovinova EA, Shelenga TV, Kerv YA, Khoreva VI, Konarev AV, Yakusheva TV, Pavlov AV, Slobodkina AA, Brutch NB. Features of Profiles of Biologically Active Compounds of Primary and Secondary Metabolism of Lines from VIR Flax Genetic Collection, Contrasting in Size and Color of Seeds. Plants. 2022; 11(6):750. https://doi.org/10.3390/plants11060750
Chicago/Turabian StylePorokhovinova, Elizaveta A., Tatyana V. Shelenga, Yulia A. Kerv, Valentina I. Khoreva, Alexey V. Konarev, Tamara V. Yakusheva, Andrey V. Pavlov, Anastasia A. Slobodkina, and Nina B. Brutch. 2022. "Features of Profiles of Biologically Active Compounds of Primary and Secondary Metabolism of Lines from VIR Flax Genetic Collection, Contrasting in Size and Color of Seeds" Plants 11, no. 6: 750. https://doi.org/10.3390/plants11060750
APA StylePorokhovinova, E. A., Shelenga, T. V., Kerv, Y. A., Khoreva, V. I., Konarev, A. V., Yakusheva, T. V., Pavlov, A. V., Slobodkina, A. A., & Brutch, N. B. (2022). Features of Profiles of Biologically Active Compounds of Primary and Secondary Metabolism of Lines from VIR Flax Genetic Collection, Contrasting in Size and Color of Seeds. Plants, 11(6), 750. https://doi.org/10.3390/plants11060750







