Ultraviolet-B Irradiation Increases Antioxidant Capacity of Pakchoi (Brassica rapa L.) by Inducing Flavonoid Biosynthesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Impacts of UV-B Radiation on Plant Growth and Biomass in Pakchoi
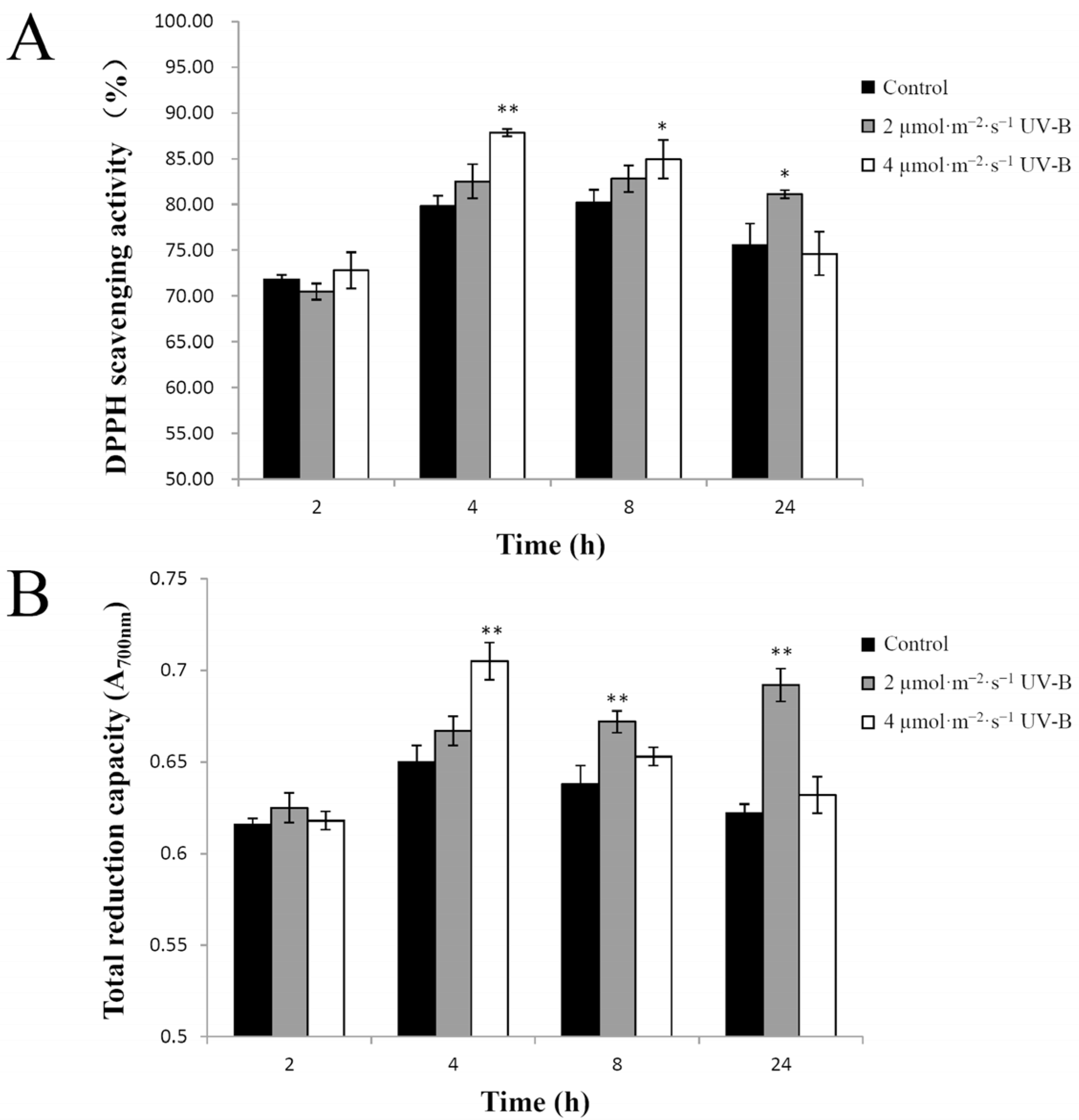
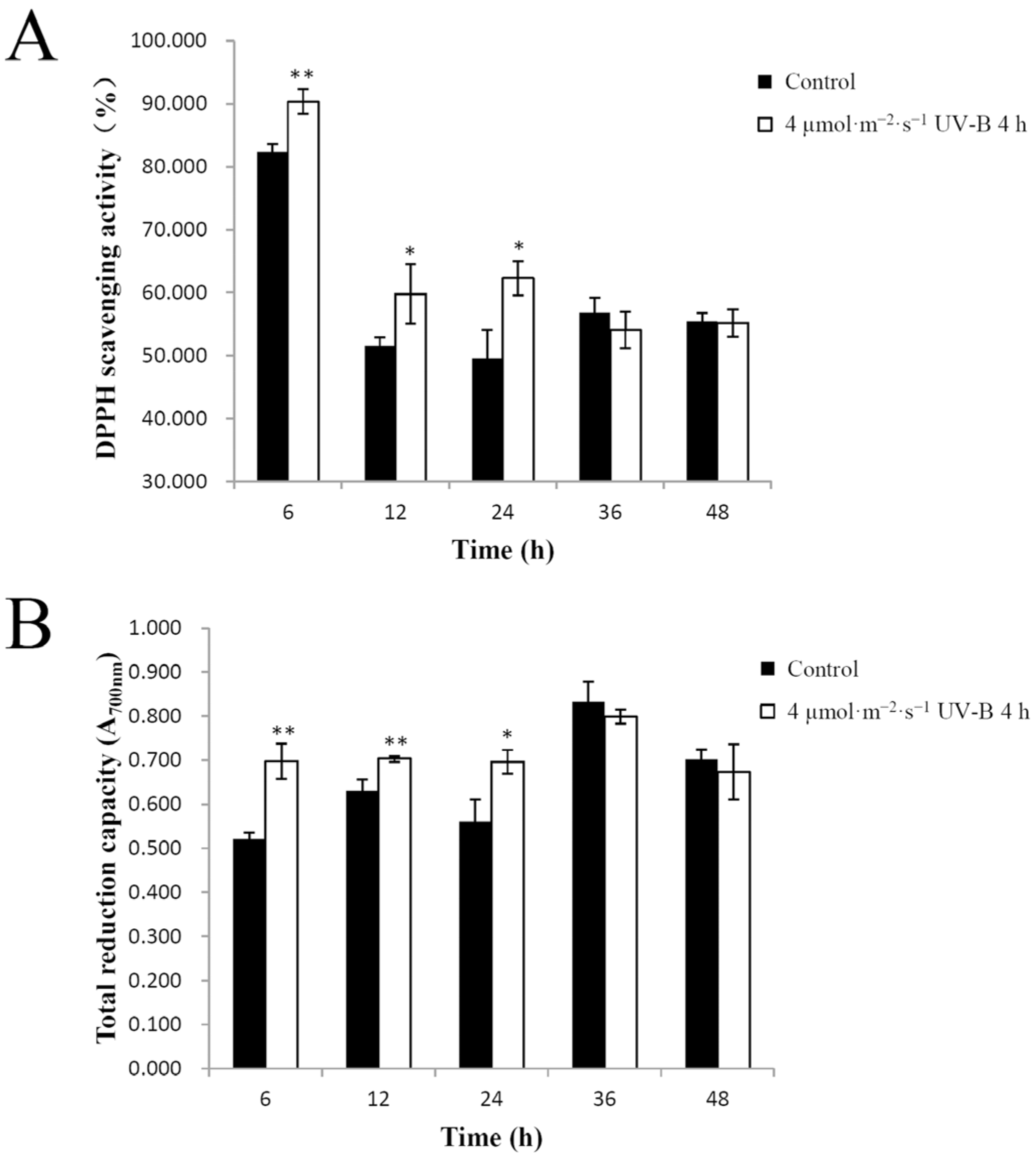
2.2. UV-B Irradiation Effect on Total Antioxidant Capacity in Pakchoi
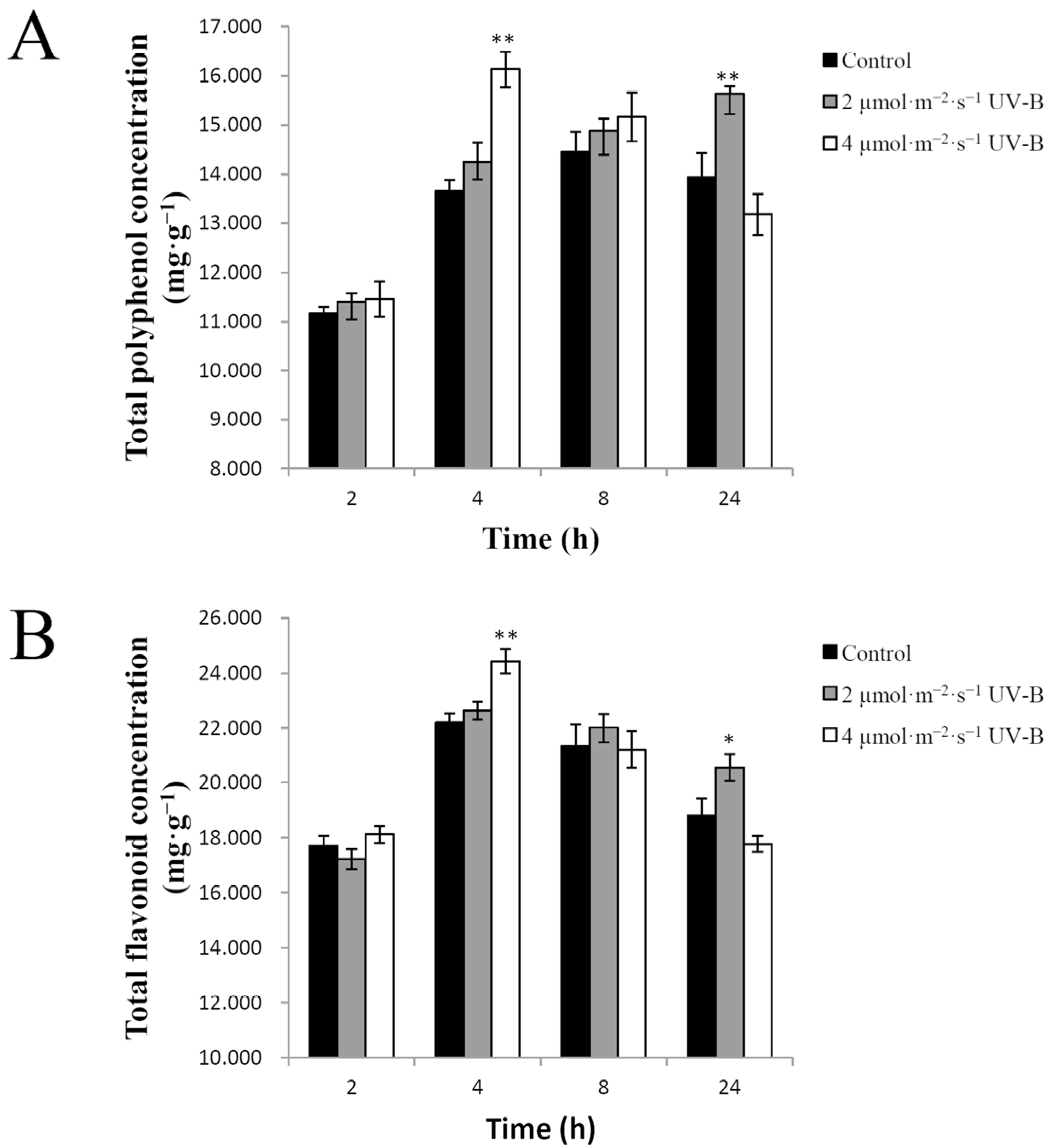
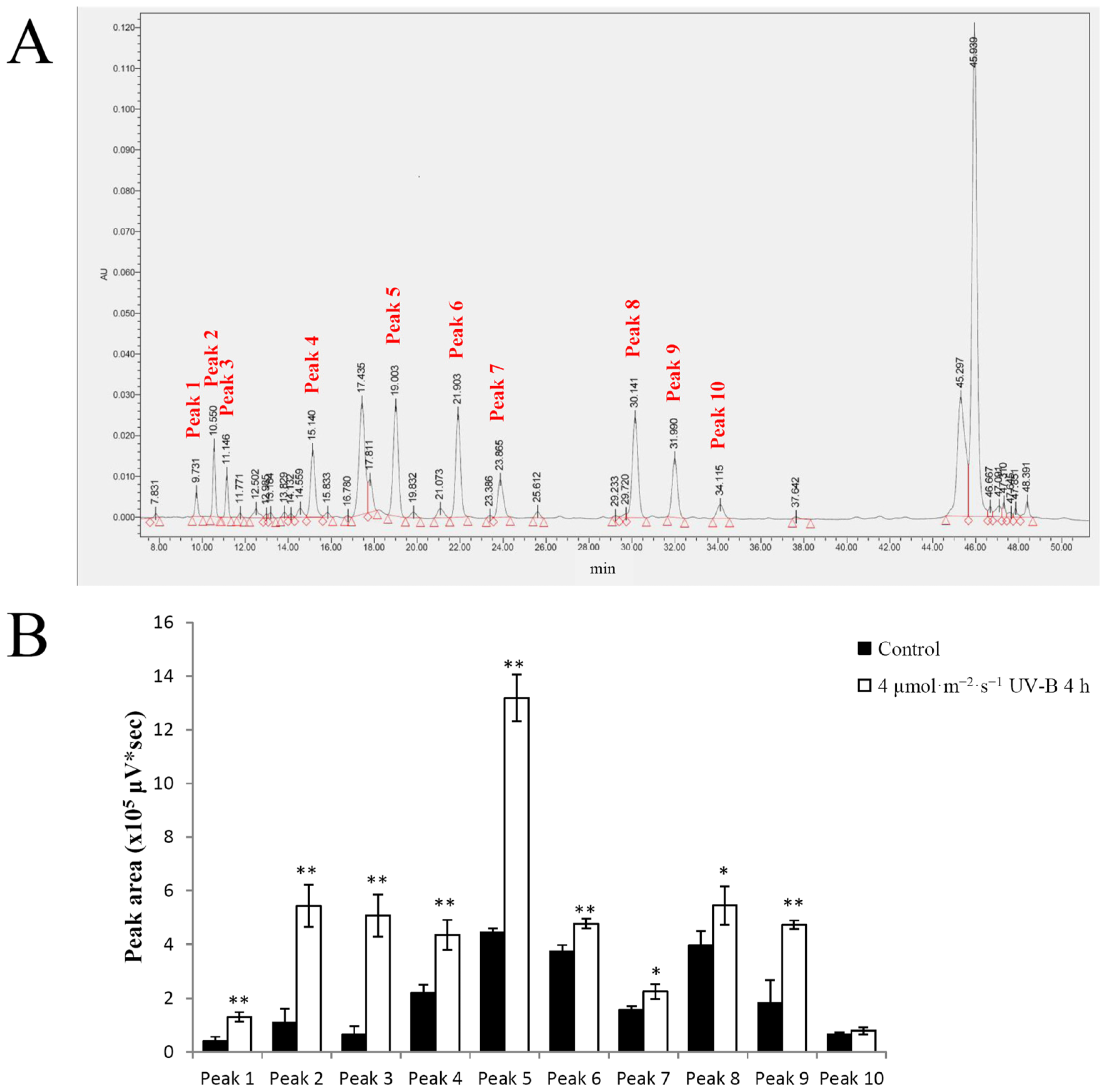
2.3. Effects of UV-B Irradiation on Non-Enzymatic Antioxidants
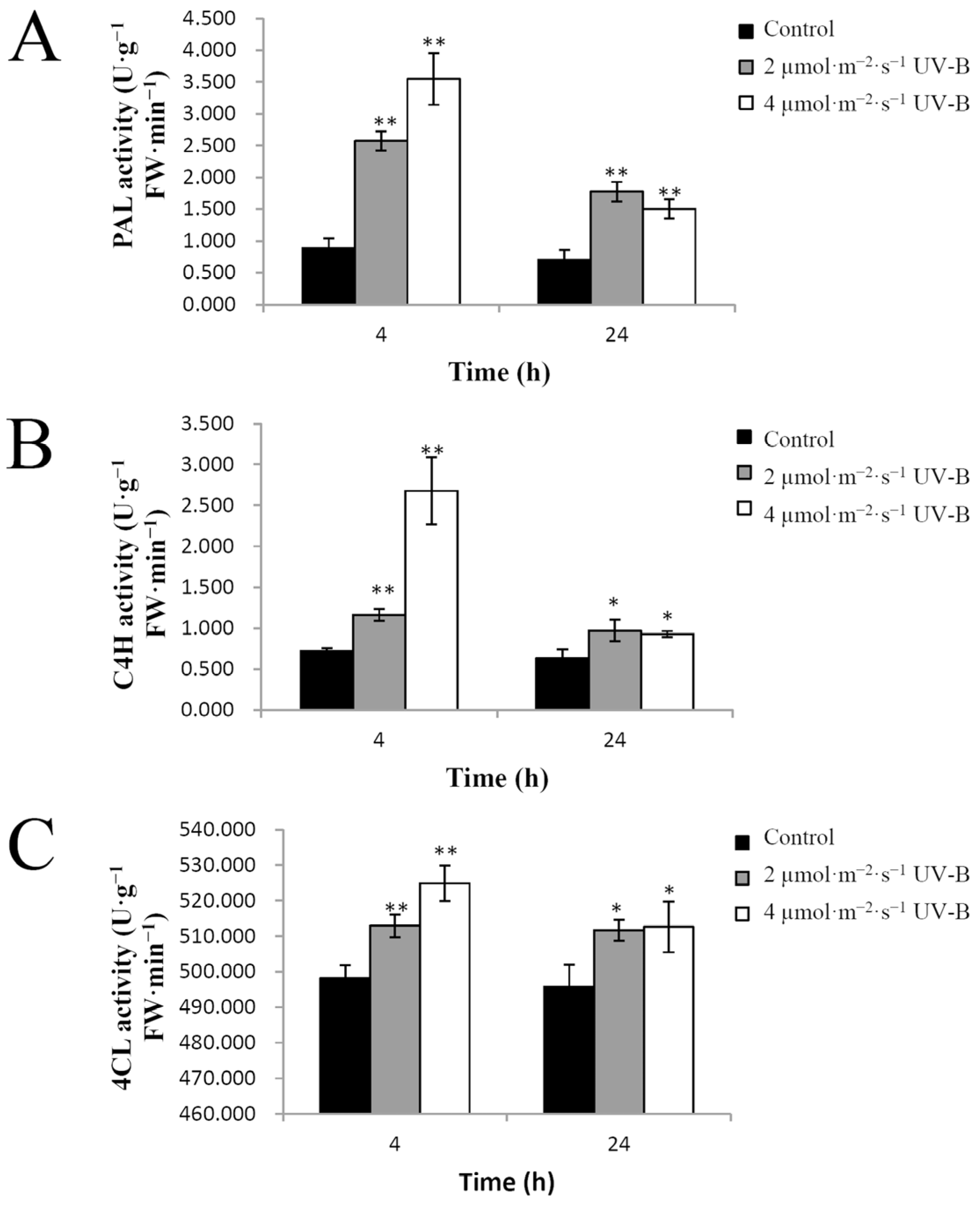
2.4. Effects of UV-B Radiation on Flavonoid Biosynthesis Enzymes
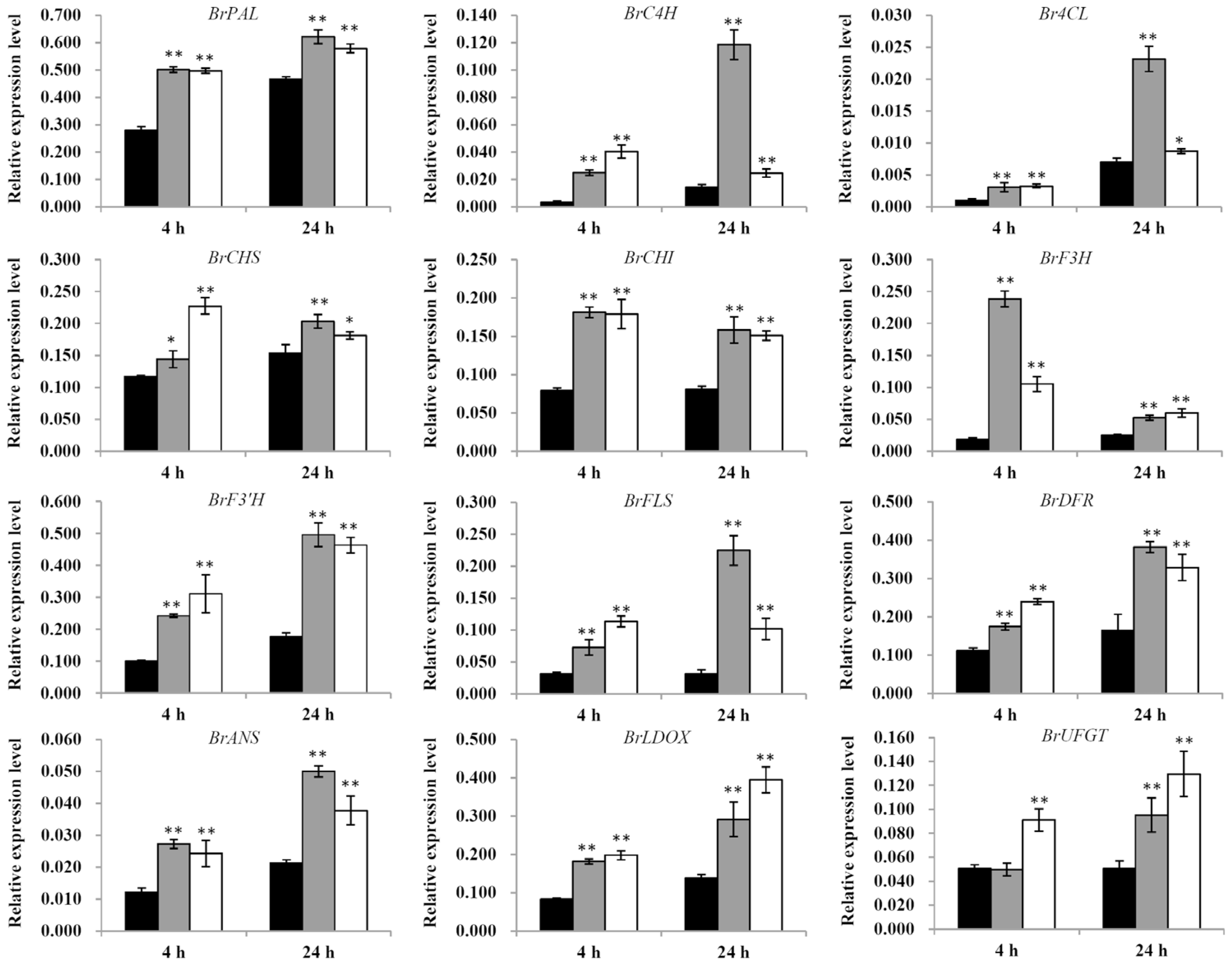
2.5. UV-B Effect on the Expression of Flavonoid Biosynthesis Genes
3. Materials and Methods
3.1. Plant Materials and Growth Conditions
3.2. Radiation Procedure
3.3. DPPH Scavenging Assay
3.4. Determination of Total Reduction Capacity
3.5. Analysis of Flavonoids by HPLC and LC–MS
3.6. Assay of Flavonoid Biosynthesis Enzyme Activities
3.7. RNA Extraction and Quantitative Reverse Transcription PCR (qRT–PCR) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef] [Green Version]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Neugart, S.; Klaring, H.P.; Zietz, M.; Schreiner, M.; Rohn, S.; Kroh, L.W.; Krumbein, A. The effect of temperature and radiation on flavonol aglycones and flavonol glycosides of kale (Brassica oleracea var. sabellica). Food Chem. 2012, 133, 1456–1465. [Google Scholar] [CrossRef]
- Neugart, S.; Majer, P.; Schreiner, M.; Hideg, É. Blue light treatment but not green light treatment after pre-exposure to UV-B stabilizes flavonoid glycoside changes and corresponding biological effects in three different Brassicaceae sprouts. Front. Plant Sci. 2021, 11, 611247. [Google Scholar] [CrossRef]
- Seong, G.U.; Hwang, I.W.; Chung, S.K. Antioxidant capacities and polyphenolics of Chinese cabbage (Brassica rapa L. ssp. Pekinensis) leaves. Food Chem. 2016, 199, 612–618. [Google Scholar] [CrossRef]
- Rokayya, S.; Li, C.J.; Zhao, Y.; Li, Y.; Sun, C.H. Cabbage (Brassica oleracea L. var. capitata) phytochemicals with antioxidant and anti-inflammatory potential. Asian Pac. J. Cancer Prev. 2014, 14, 6657–6662. [Google Scholar] [CrossRef] [Green Version]
- Heinze, M.; Hanschen, F.S.; Wiesner-Reinhold, M.; Baldermann, S.; Gräfe, J.; Schreiner, M.; Neugart, S. Effects of developmental stages and reduced UVB and low UV conditions on plant secondary metabolite profiles in pak choi (Brassica rapa subsp. chinensis). J. Agric. Food Chem. 2018, 66, 1678–1692. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Hanschen, F.S.; Neugart, S.; Schreiner, M.; Vargas, S.A.; Gutschmann, B.; Baldermann, S. Boiling and steaming induced changes in secondary metabolites in three different cultivars of pak choi (Brassica rapa subsp. chinensis). J. Food Compos. Anal. 2019, 82, 103232. [Google Scholar] [CrossRef]
- Jansen, M.A.; Bornman, J.F. UV-B radiation: From generic stressor to specific regulator. Physiol. Plant 2012, 145, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Reifenrath, K.; Muller, C. Species-specific and leaf-age dependent effects of ultraviolet radiation on two Brassicaceae. Phytochemistry 2007, 68, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Harbaum-Piayda, B.; Walter, B.; Bengtsson, G.B.; Hubbermann, E.M.; Bilger, W.; Schwarz, K. Influence of pre-harvest UV-B irradiation and normal or controlled atmosphere storage on flavonoid and hydroxycinnamic acid contents of pak choi (Brassica campestris L. ssp chinensis var. communis). Postharvest Biol. Tech. 2010, 56, 202–208. [Google Scholar] [CrossRef]
- Neugart, S.; Bumke-Vogt, C. Flavonoid glycosides in Brassica species respond to UV-B depending on exposure time and adaptation time. Molecules 2021, 26, 494. [Google Scholar] [CrossRef] [PubMed]
- Wiesner-Reinhold, M.; Gomas, J.V.D.; Herz, C.; Tran, H.T.T.; Baldermann, S.; Neugart, S.; Filler, T.; Glaab, J.; Einfeldt, S.; Schreiner, M.; et al. Subsequent treatment of leafy vegetables with low doses of UVB-radiation does not provoke cytotoxicity, genotoxicity, or oxidative stress in a human liver cell model. Food Biosci. 2021, 43, 101327. [Google Scholar] [CrossRef]
- Hung, H.C.; Joshipura, K.J.; Jiang, R.; Hu, F.B.; Hunter, D.; Smith-Warner, S.A.; Colditz, G.A.; Rosner, B.; Spiegelman, D.; Willett, W.C. Fruit and vegetable intake and risk of major chronic disease. J. Natl. Cancer Inst. 2004, 96, 1577–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.Q.; Huang, B.A.; Hu, W.Y.; Chen, Y.; Mao, M.C.; Yao, L.P. The impact of greenhouse vegetable farming duration and soil types on phytoavailability of heavy metals and their health risk in eastern China. Chemosphere 2014, 103, 121–130. [Google Scholar] [CrossRef]
- Mormile, P.; Rippa, M.; Graziani, G.; Ritieni, A. Use of greenhouse-covering films with tailored UV-B transmission dose for growing ‘medicines’ through plants: Rocket salad case. J. Sci. Food Agric. 2019, 99, 6931–6936. [Google Scholar] [CrossRef]
- Yoshida, H.; Nishikawa, T.; Hikosaka, S.; Goto, E. Effects of nocturnal UV-B irradiation on growth, flowering, and phytochemical concentration in leaves of greenhouse-grown red perilla. Plants 2021, 10, 1252. [Google Scholar] [CrossRef]
- Harbaum, B.; Hubbermann, E.M.; Wolff, C.; Herges, R.; Zhu, Z.; Schwarz, K. Identification of flavonoids and hydroxycinnamic acids in pak choi varieties (Brassica campestris L. ssp. chinensis var. communis) by HPLC-ESI-MSn and NMR and their quantification by HPLC-DAD. J. Agric. Food Chem. 2007, 55, 8251–8260. [Google Scholar] [CrossRef]
- Harbaum, B.; Hubbermann, E.M.; Zhu, Z.; Schwarz, K. Free and bound phenolic compounds in leaves of pak choi (Brassica campestris L. ssp. chinensis var. communis) and Chinese leaf mustard (Brassica juncea Coss). Food chem. 2008, 110, 838–846. [Google Scholar] [CrossRef]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Merillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH•) tests. J. Am. Oil Chem. Soc. 2002, 79, 1191. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, B.A.; Jenkins, G.I. UV-B signaling pathways with different fluence-rate response profiles are distinguished in mature Arabidopsis leaf tissue by requirement for UVR8, HY5, and HYH. Plant Physiol. 2008, 146, 576–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, N.N.; Lu, Y.W.; Wu, Q.; Liu, Y.Y.; Xia, Y.; Xia, K.; Cui, J. UV-B-induced anthocyanin accumulation in hypocotyls of radish sprouts continues in the dark after irradiation. J. Sci. Food Agric. 2016, 96, 886–892. [Google Scholar] [CrossRef]
- Usano-Alemany, J.; Panjai, L. Effects of increasing doses of UV-B on main phenolic acids content, antioxidant activity and estimated biomass in lavandin (Lavandula x intermedia). Nat. Prod. Commun. 2015, 10, 1269–1272. [Google Scholar] [CrossRef] [Green Version]
- Takshak, S.; Agrawal, S.B. Defence strategies adopted by the medicinal plant Coleus forskohlii against supplemental ultraviolet-B radiation: Augmentation of secondary metabolites and antioxidants. Plant Physiol. Biochem. 2015, 97, 124–138. [Google Scholar] [CrossRef]
- Singh, V.P.; Srivastava, P.K.; Prasad, S.M. Differential effect of UV-B radiation on growth, oxidative stress and ascorbate-glutathione cycle in two cyanobacteria under copper toxicity. Plant Physiol. Biochem. 2012, 61, 61–70. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Nascimento, L.B.; Leal-Costa, M.V.; Menezes, E.A.; Lopes, V.R.; Muzitano, M.F.; Costa, S.S.; Tavares, E.S. Ultraviolet-B radiation effects on phenolic profile and flavonoid content of Kalanchoe pinnata. J. Photochem. Photobiol. B. 2015, 148, 73–81. [Google Scholar] [CrossRef]
- Sun, M.Y.; Gu, X.D.; Fu, H.W.; Zhang, L.; Chen, R.Z.; Cui, L.; Zheng, L.H.; Zhang, D.W.; Tian, J.K. Change of secondary metabolites in leaves of Ginkgo biloba L. in response to UV-B induction. Innov. Food Sci. Emerg. Technol. 2010, 11, 672–676. [Google Scholar] [CrossRef]
- Martinez-Luscher, J.; Torres, N.; Hilbert, G.; Richard, T.; Sanchez-Diaz, M.; Delrot, S.; Aguirreolea, J.; Pascual, I.; Gomes, E. Ultraviolet-B radiation modifies the quantitative and qualitative profile of flavonoids and amino acids in grape berries. Phytochemistry 2014, 102, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Avena-Bustillos, R.J.; Du, W.X.; Woods, R.; Olson, D.; Breksa, A.P., 3rd; McHugh, T.H. Ultraviolet-B light treatment increases antioxidant capacity of carrot products. J. Sci. Food Agric. 2012, 92, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The flavonoid biosynthetic pathway in Arabidopsis: Structural and genetic diversity. Plant Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Jiao, J.; Gai, Q.Y.; Wang, W.; Luo, M.; Gu, C.B.; Fu, Y.J.; Ma, W. Ultraviolet radiation-elicited enhancement of isoflavonoid accumulation, biosynthetic gene expression, and antioxidant activity in Astragalus membranaceus hairy root cultures. J. Agric. Food Chem. 2015, 63, 8216–8224. [Google Scholar] [CrossRef]
- Guo, N.; Cheng, F.; Wu, J.; Liu, B.; Zheng, S.N.; Liang, J.L.; Wang, X.W. Anthocyanin biosynthetic genes in Brassica rapa. BMC genom. 2014, 15, 426. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.T.T.; Lim, S.; Lee, J.G.; Lee, E.J. VcBBX, VcMYB21, and VcR2R3MYB transcription factors are involved in UV–B-induced anthocyanin biosynthesis in the peel of harvested blueberry fruit. J. Agric. Food Chem. 2017, 65, 2066–2073. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.; Soliman, M.H.; Ameta, K.L.; El-Esawi, M.A.; Elkelish, A. Changes in ecophysiology, osmolytes, and secondary metabolites of the medicinal plants of Mentha piperita and Catharanthus roseus subjected to drought and heat stress. Biomolecules 2020, 10, 43. [Google Scholar] [CrossRef] [Green Version]
- Loayza, F.E.; Brecht, J.K.; Simonne, A.H.; Plotto, A.; Baldwin, E.A.; Bai, J.; Lon-Kan, E. A brief hot-water treatment alleviates chilling injury symptoms in fresh tomatoes. J. Sci. Food Agric. 2021, 101, 54–64. [Google Scholar] [CrossRef]
- Jia, Z.S.; Tang, M.C.; Wu, J.M. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Ni, J.; Hao, J.; Jiang, Z.F.; Zhan, X.R.; Dong, L.X.; Yang, X.L.; Sun, Z.H.; Xu, W.Y.; Wang, Z.K.; Xu, M.J. NaCl induces flavonoid biosynthesis through a putative novel pathway in post-harvest Ginkgo leaves. Front. Plant Sci. 2017, 8, 920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, C.J.; Rubery, P.H. A spectrophotometric assay for trans-cinnamic acid 4-hydroxylase activity. Anal. Biochem. 1975, 68, 554–561. [Google Scholar] [CrossRef]
- Knobloch, K.H.; Hahlbrock, K. Isoenzymes of p-coumarate: CoA ligase from cell suspension cultures of Glycine max. Eur. J. Biochem. 1975, 52, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Lou, P.P.; Han, Y.D.; Chen, Z.H.; Chen, J.M.; Ni, J.; Yang, Y.J.; Jiang, Z.F.; Xu, M.J. GrTCP11, a cotton TCP transcription factor, inhibits root hair elongation by down-regulating jasmonic acid pathway in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 769675. [Google Scholar] [CrossRef]
- Cheng, F.; Liu, S.Y.; Wu, J.; Fang, L.; Sun, S.L.; Liu, B.; Li, P.X.; Hua, W.; Wang, X.W. BRAD, the genetics and genomics database for Brassica plants. BMC Plant Biol. 2011, 11, 136. [Google Scholar] [CrossRef] [Green Version]
- Long, L.; Yang, W.W.; Liao, P.; Guo, Y.W.; Kumar, A.; Gao, W. Transcriptome analysis reveals differentially expressed ERF transcription factors associated with salt response in cotton. Plant Sci. 2019, 281, 72–81. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, J.; Lou, P.; Han, Y.; Zheng, L.; Lu, J.; Chen, Z.; Ni, J.; Yang, Y.; Xu, M. Ultraviolet-B Irradiation Increases Antioxidant Capacity of Pakchoi (Brassica rapa L.) by Inducing Flavonoid Biosynthesis. Plants 2022, 11, 766. https://doi.org/10.3390/plants11060766
Hao J, Lou P, Han Y, Zheng L, Lu J, Chen Z, Ni J, Yang Y, Xu M. Ultraviolet-B Irradiation Increases Antioxidant Capacity of Pakchoi (Brassica rapa L.) by Inducing Flavonoid Biosynthesis. Plants. 2022; 11(6):766. https://doi.org/10.3390/plants11060766
Chicago/Turabian StyleHao, Juan, Panpan Lou, Yidie Han, Lijun Zheng, Jiangjie Lu, Zhehao Chen, Jun Ni, Yanjun Yang, and Maojun Xu. 2022. "Ultraviolet-B Irradiation Increases Antioxidant Capacity of Pakchoi (Brassica rapa L.) by Inducing Flavonoid Biosynthesis" Plants 11, no. 6: 766. https://doi.org/10.3390/plants11060766
APA StyleHao, J., Lou, P., Han, Y., Zheng, L., Lu, J., Chen, Z., Ni, J., Yang, Y., & Xu, M. (2022). Ultraviolet-B Irradiation Increases Antioxidant Capacity of Pakchoi (Brassica rapa L.) by Inducing Flavonoid Biosynthesis. Plants, 11(6), 766. https://doi.org/10.3390/plants11060766





