Abstract
In this work, we studied the effects of in vitro oxidative stress applied by H2O2 to maize pollen germination and cytosolic Ca2+, taken as an experimental model to test the biological activity of extracts of emmer (Triticum turgidum L. spp. dicoccum (Schrank ex Shubler) Thell.) wheatgrass obtained from grains sprouted with distilled water, or salinity (50 mM) or selenium (45 mg L−1 of Na2SeO3). Wheatgrass extracts were obtained in two ways: by direct extraction in methanol, which represented the free phenolic fraction of extracts (Ef), and by residual content after alkaline digestion, which made it possible to obtain extracts with the bound fraction (Eb). Comparative tests on maize pollen were carried out by differently combining H2O2 and either wheatgrass extracts or pure phenolic acids (4-HO benzoic, caffeic, p-coumaric and salicylic). The cytosolic Ca2+ of maize pollen was influenced by either H2O2 or pure phenolic acids or Ef, but not by Eb. The negative effect of H2O2 on maize pollen germination and cytosolic Ca2+ was mitigated by Ef and, slightly, by Eb. The extent of the biological response of Ef depended on the sprouting conditions (i.e., distilled water, salinity or selenium). The extracts of Se-biofortified wheatgrass were the most effective in counteracting the oxidative stress.
1. Introduction
Wheatgrass (1–2 weeks old seedlings of Graminaceae species) is recognized as a great source of phytochemicals, i.e., the secondary metabolites of plants having antioxidant activity and related benefits in human health [1,2]. Wheatgrass from Triticum species has been extensively studied, and recent trends on healthy diets have been promoting the rediscovery of ancient species, often grown in low-input systems and organic farming [3]. In particular, emmer (Triticum turgidum L. spp. dicoccum (Schrank ex Schübler) Thell.) has been found to be rich in phenolic compounds, especially phenolic acids (PAs) [4,5], which would also contribute to the abiotic stress tolerance of this species [6,7].
Phytochemicals may have complementary and/or overlapping mechanisms of action [8] and this may explain why the biological activity of wheatgrass is not easy to study and may be different from that expected from each purified molecule it contains. Moreover, phenolic compounds, similarly to other phytochemicals, exist in either free forms or bound forms (covalently conjugated through ester bonds to cell wall components such as cellulose, pectin and polysaccharides), which have different fates and healthy properties after ingestion [9,10].
A body of recent literature demonstrates that the production of phytochemicals in sprouts and wheatgrass of many species can be boosted by appropriate elicitation techniques [1,3,11], with changes also occurring in the proportion between free and bound phenolics. As far as emmer sprouts and wheatgrass are concerned, the application of moderate salt stress was found to increase the total content of phenolic compounds, especially the free ones [6]. On the other hand, bio-fortification with selenium (Se), which has not been tested yet on emmer wheatgrass, has been found to increase phenolic acids and antioxidant activity in sprouts of maize [12] and rice [13], confirming that Se is a powerful protecting agent against abiotic stresses [14].
Although sprouts and wheatgrass of many cereal species have been characterized for the kinds and amounts of phenolic compounds, very few studies have dealt with testing the effects of these matrices on biological systems. In this regard, the measurement of cytosolic Ca2+ and germination of maize pollen has proven to be an effective, simple and cheap tool for evaluating the effect of a plant matrix in a biological system [15,16]. In fact, calcium (Ca2+) plays an important role in the signal transduction, growth and development of plants [17,18]. Ca2+ homeostasis is maintained by keeping the cytosolic ion concentration below 0.1 µM. When cells are stimulated, raising cytosolic Ca2+ levels above 200 nM, they activate a molecular signal to trigger downstream responses [19,20,21]. Furthermore, pollen represents a good experimental model, because it can be easily labeled with the FURA 2AM fluorescent probe and kept in suspension, which is a strategy that is not always possible with plant cells. This model is also useful to evaluate the occurrence of oxidative stress, since the increase in reactive oxygen species (ROS) in the cells would alter molecular signals including cytosolic Ca2+ [22,23,24]. Oxidative stress is a harmful process that can negatively affect several cellular structures, such as membranes, lipids, proteins, lipoproteins and DNA, and this is the case for both plant [25] and animal cells [26]. With regard to the study of oxidative stress, a body of literature reports the use of hydrogen peroxide (H2O2) in in vitro experiments. This is because, among the three primary ROS (superoxide anion, hydroxyl radical and hydrogen peroxide), only H2O2 has a half-life long enough (few seconds) to be used for inducing oxidative stress in vitro [27,28]. Recently, Del Pino et al. [16] demonstrated that extracts of emmer wheatgrass grown with distilled water, or in the presence of salinity (i.e., 50 mM) or Se (45 mg L−1), affected cytosolic Ca2+ and maize pollen germination. In that case, maize pollen was germinated in optimal conditions and the effects of emmer extracts were evaluated considering only the extracts of free phenolic compounds (i.e., those obtained by extraction with methanol).
The present work follows up the work by Del Pino et al. [16], by using the same biological model (maize pollen germination and cytosolic Ca2+) to test the effect of either free or bound extracts of emmer wheatgrass grown with distilled water, or salinity or selenium, and to evaluate the protective effect against the oxidative stress caused in pollen by the application of H2O2.
2. Results
2.1. Total Selenium Content in Extracts of Emmer Wheatgrass
The Se content in EfSe and EbSe was 10 and 2.4 times higher, respectively, than in Efc, Efs, Ebc and Ebs (Table 1).

Table 1.
Total selenium content in the free and bound extracts of emmer wheatgrass grown with distilled water as a control (Efc), or in the presence of salinity as NaCl 50 mM (Efs) or selenium as 45 mg L−1 of Na2SeO3 (EfSe). All analyses were performed in triplicate.
2.2. Cytosolic Ca2+ of Maize Pollen
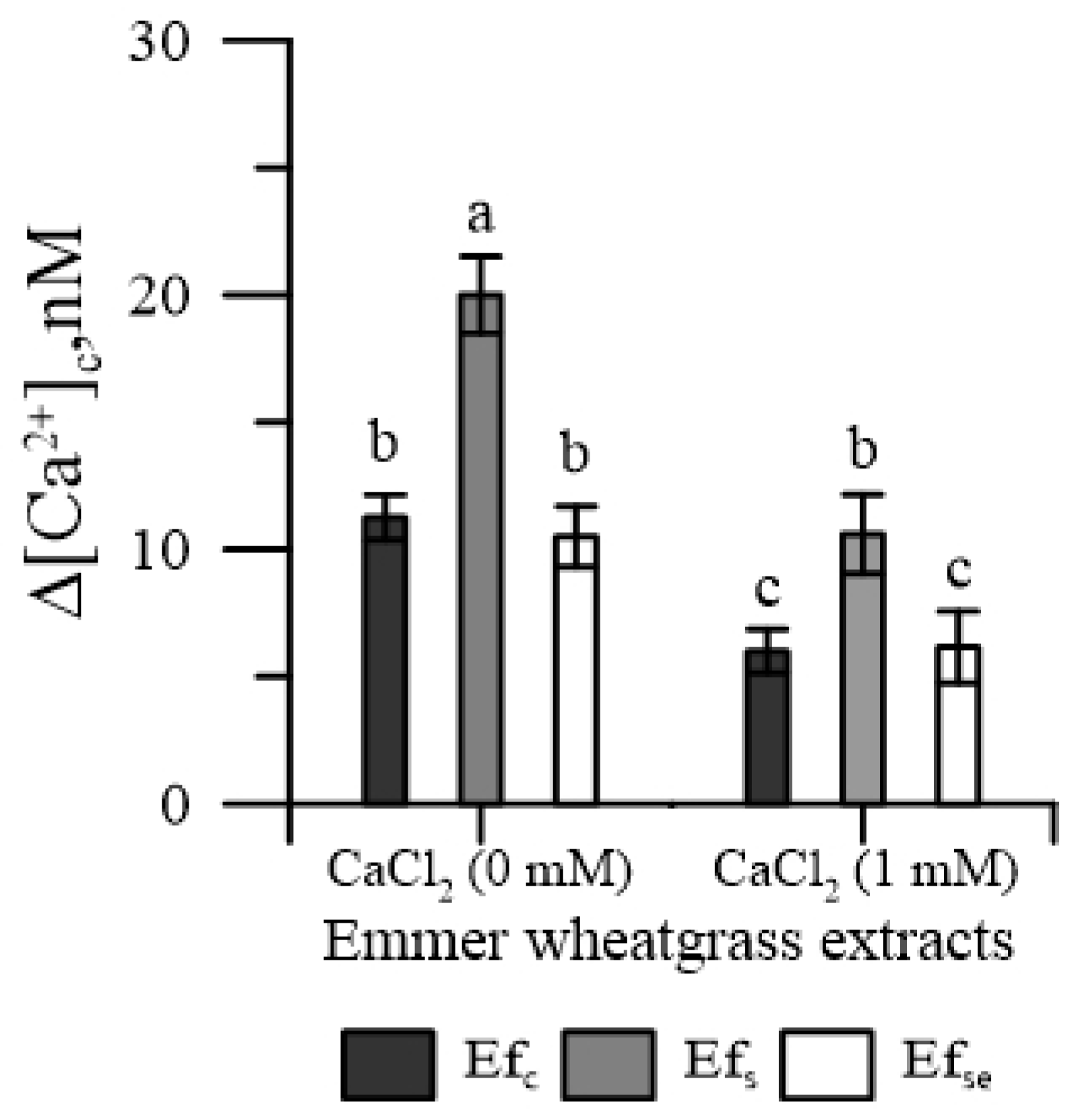
The concentration of cytosolic Ca2+ of pollen [Ca2+]cp increased with each kind of free extract, both in the absence and in the presence of CaCl2 in the incubation medium; however, Efs caused the greatest increase (Figure 1). On the contrary, none of the bound extracts affected [Ca2+]cp (data not shown).
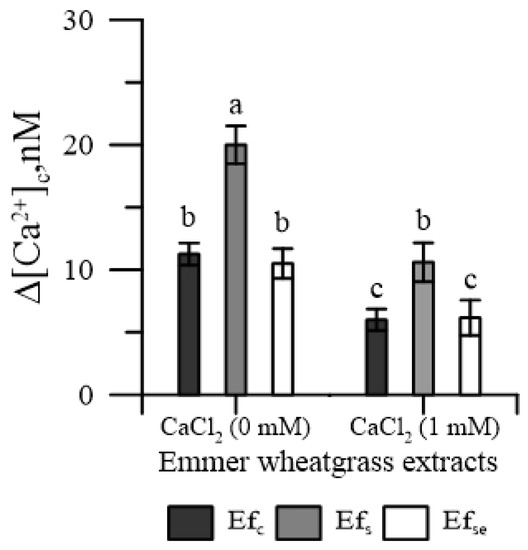
Figure 1.
Effects of 50 mg of free extracts (Ef) of emmer wheatgrass obtained with distilled water as a control (Efc), or with NaCl (Efs) 50 mM or Na2SeO3 0.45 mg L−1 (EfSe), on the cytosolic Ca2+ of maize pollen. The measurements were carried out in the absence of Ca2+ and in the presence of Ca2+ (1 mM CaCl2) in the incubation medium. Data are expressed as means ± SEM from four independent tests. Different letters indicate significant differences for p < 0.05.
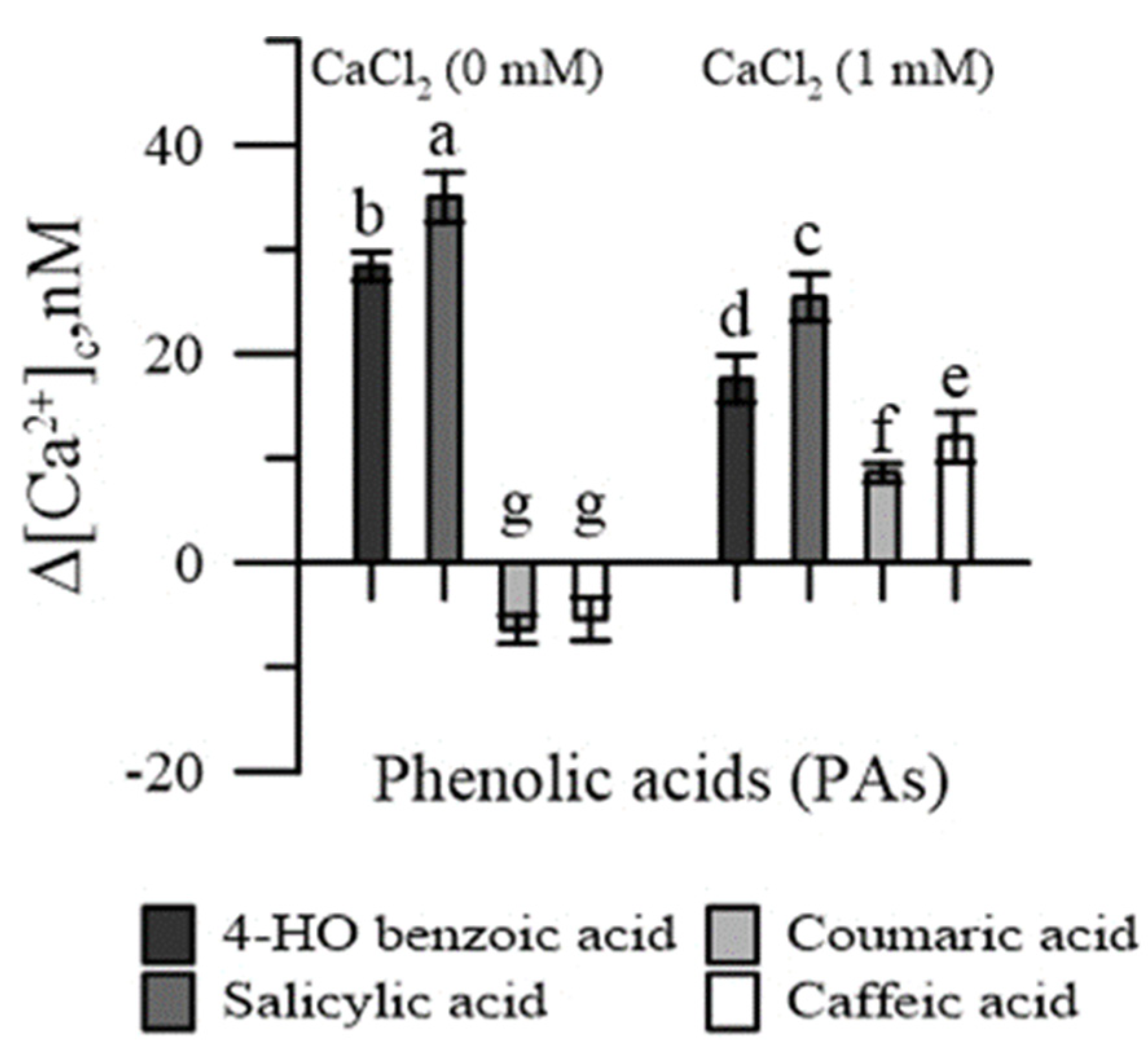
In the absence of CaCl2 in the incubation medium, salicylic and 4-HO benzoic acids increased [Ca2+]cp, while coumaric and caffeic acids reduced it (Figure 2). With the addition of CaCl2 in the incubation medium, an increase in [Ca2+]cp was observed with all PAs, although it was slighter for p-coumaric and caffeic acids.
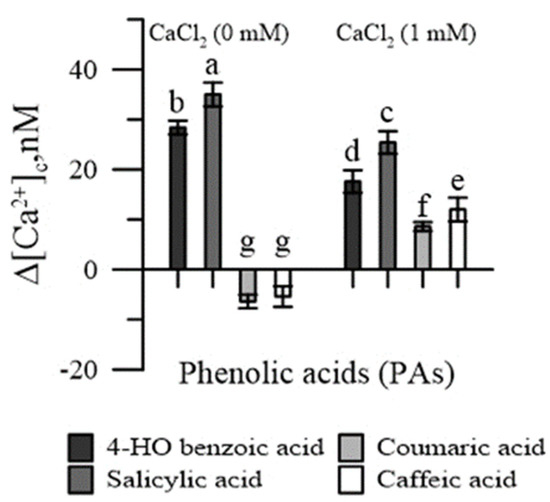
Figure 2.
Effects of 4-HO benzoic, caffeic, p-coumaric and salicylic acids on cytosolic Ca2+ of maize pollen, in Ca2+-free conditions and in the presence of CaCl2. Data are expressed as means ± SEM from five independent tests. Different letters indicate significant differences for p < 0.05.
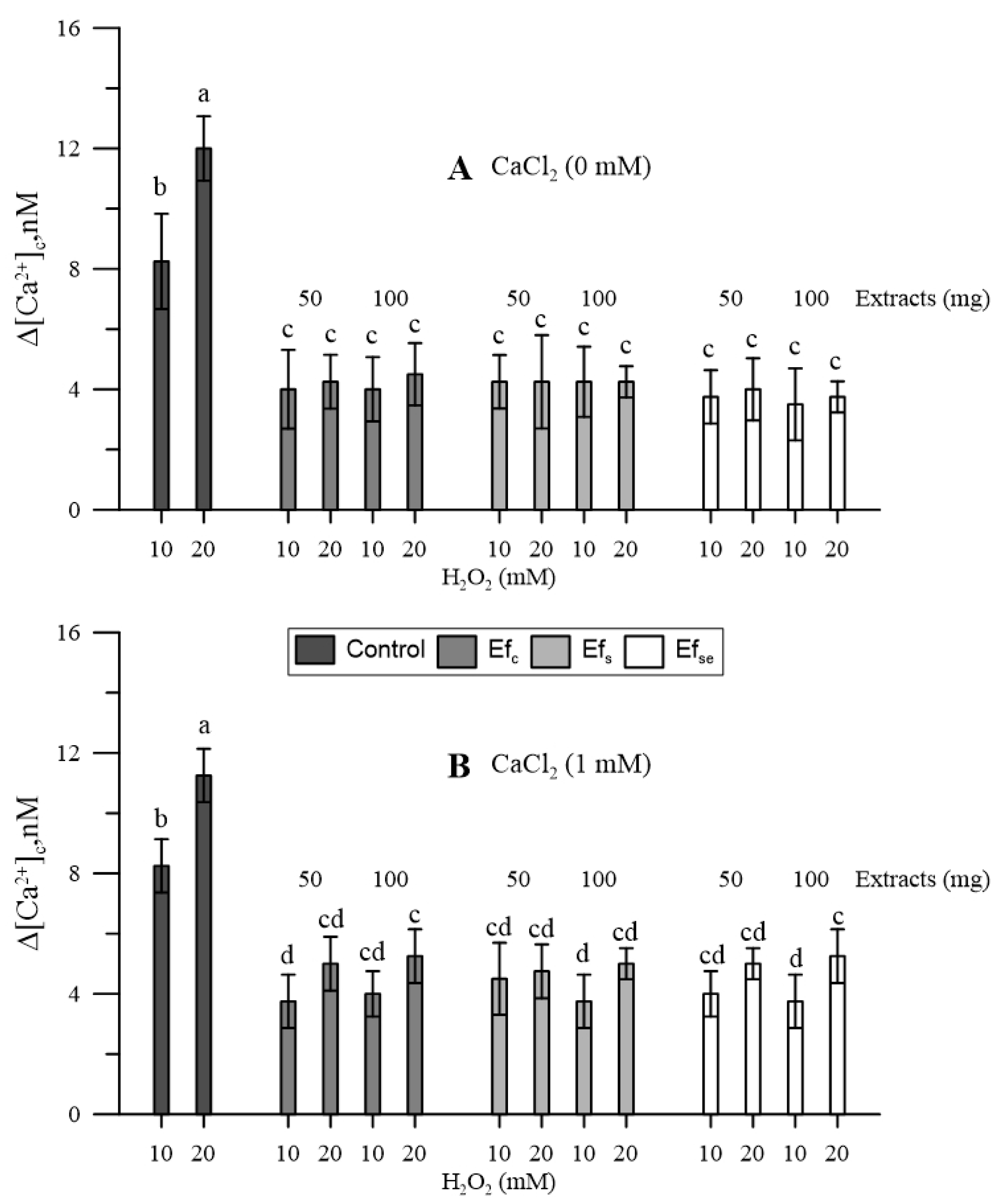
Hydrogen peroxide increased [Ca2+]cp; the higher the dose, the higher the increase (Figure 3).
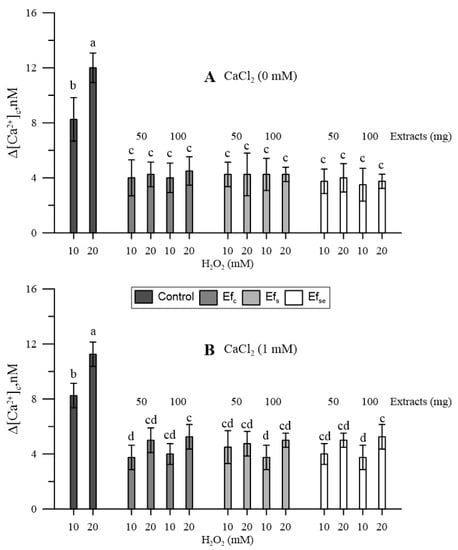
Figure 3.
Effects of H2O2 (10 and 20 mM) on the cytosolic Ca2+ of maize pollen pre-treated with 50 or 100 mg of free extracts of emmer wheatgrass grown with distilled water as a control (Efc), or in the presence of salinity as NaCl 50 mM (Efs) or selenium as 45 mg L−1 of Na2SeO3 (EfSe). Maize pollen was (A) in the absence or (B) in the presence of 1 mM CaCl2 in the growing medium. All analyses were performed in triplicate. Data are expressed as means ± SEM from four independent tests. Different letters indicate significant differences for p < 0.05.
Since the bound extracts had not affected the [Ca2+]cp, only free extracts (Ef) were tested for their effect against oxidative conditions. All three types of free extracts antagonized the effects of H2O2 on cytosolic Ca2+. The effect did not substantially change in the absence (Figure 3A) or presence (Figure 3B) of CaCl2 in the incubation medium, although, in the presence of CaCl2, the highest H2O2 caused further and generally non-significant increases in [Ca2+]cp (Figure 3B).
2.3. Germination of Maize Pollen
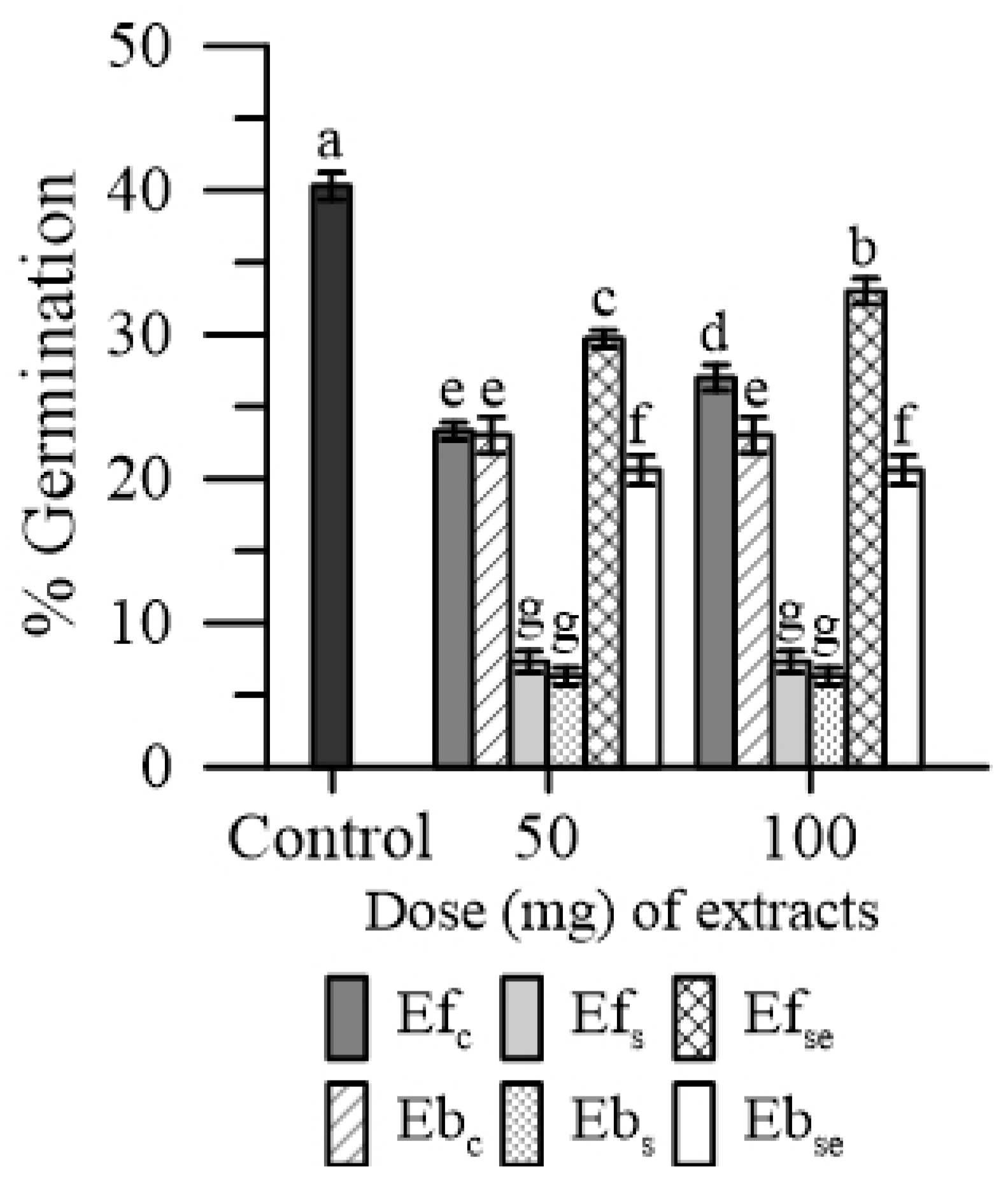
Both Ef and Eb reduced the germination rate of maize pollen. The magnitude of this effect was different between treatments: (Efs and Ebs) > EbSe > (Efc and Ebc,) > EfSe (Figure 4).
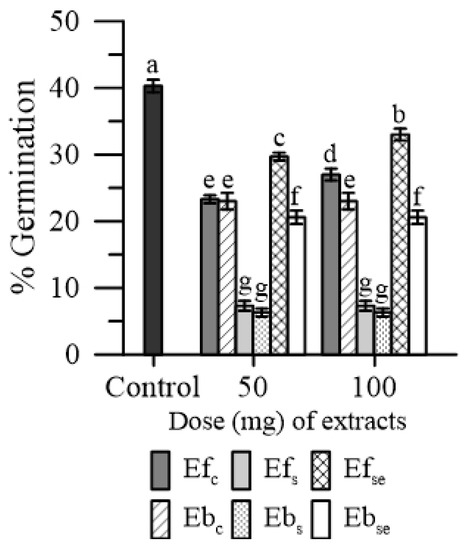
Figure 4.
Germination of maize pollen in the presence of 50 or 100 mg of free (Ef) and bound (Eb) extracts of emmer wheatgrass grown with distilled water as a control (Efc, Ebc), or in the presence of salinity as NaCl 50 mM (Efs, Ebs) or selenium as 45 mg L−1 of Na2SeO3 (EfSe, EbSe). Data are expressed as means ± SEM from five independent tests. Different letters indicate significant differences for p < 0.05.
Considering the Ef and Eb groups, the lowest inhibiting effect on pollen germination was caused by EfSe (−25%) and EbSe (−45%). No substantial dose-dependent effect was observed in both Ef and Eb.
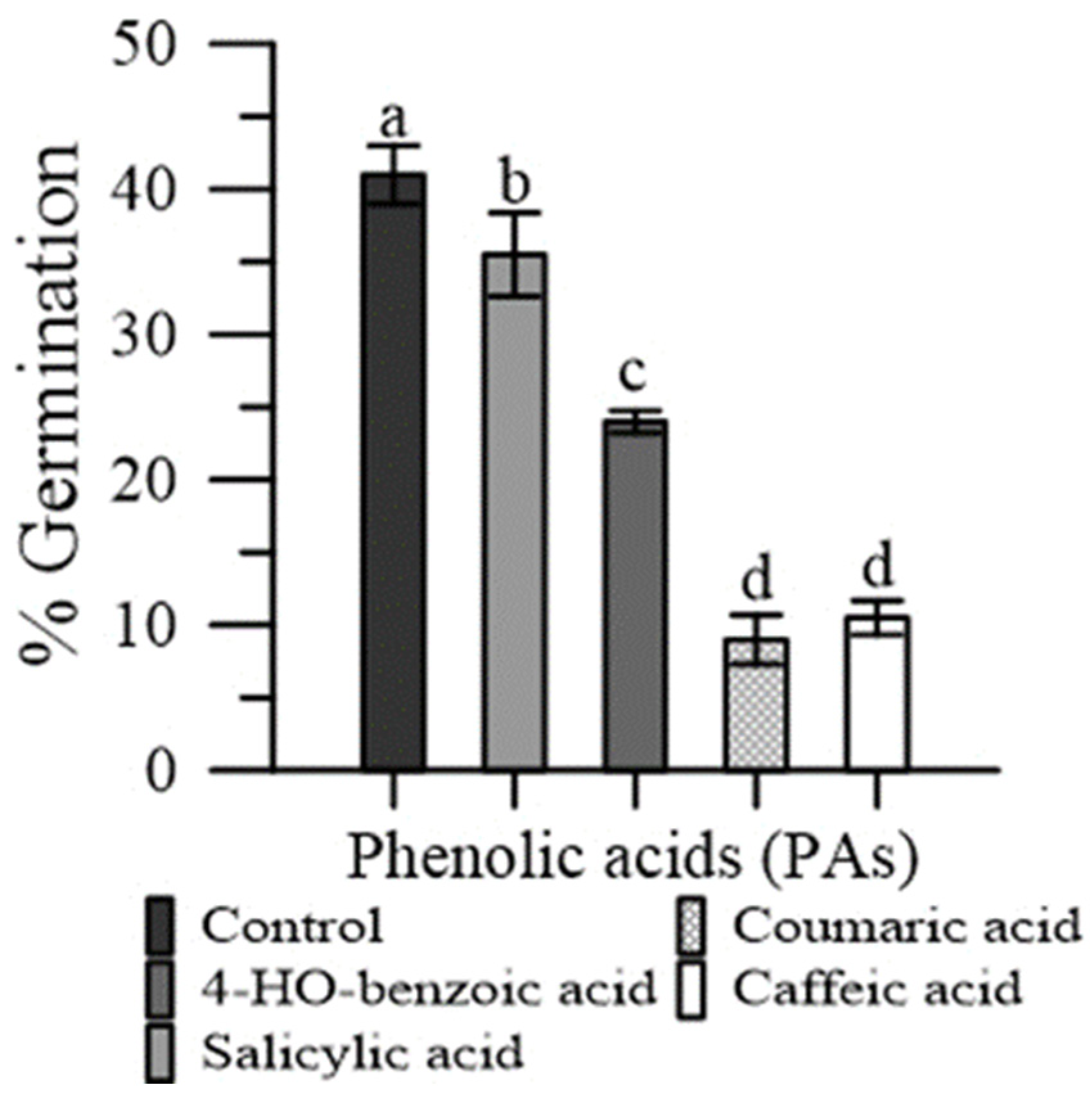
The p-coumaric and caffeic acid severely depressed pollen germination (−81% and −76%, respectively), while the effect was less marked with 4-HO benzoic acid (−32%) and salicylic acid (−15%) (Figure 5).
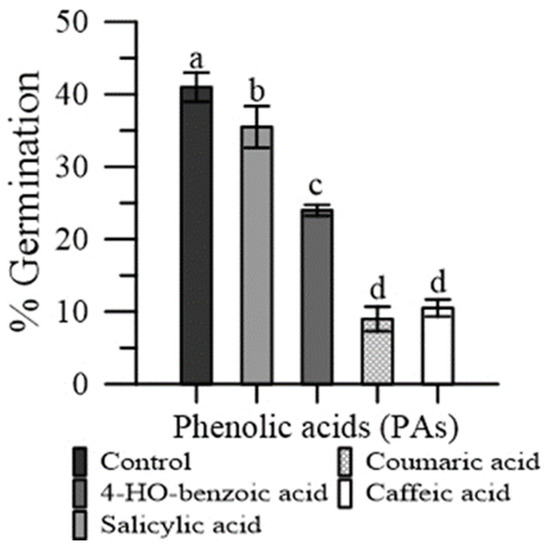
Figure 5.
Germination of maize pollen grains in the presence of pure PAs: 4-HO benzoic, caffeic, p-coumaric and salicylic. Data are expressed as means ± SEM from four independent tests. Different letters indicate significant differences for p < 0.05.
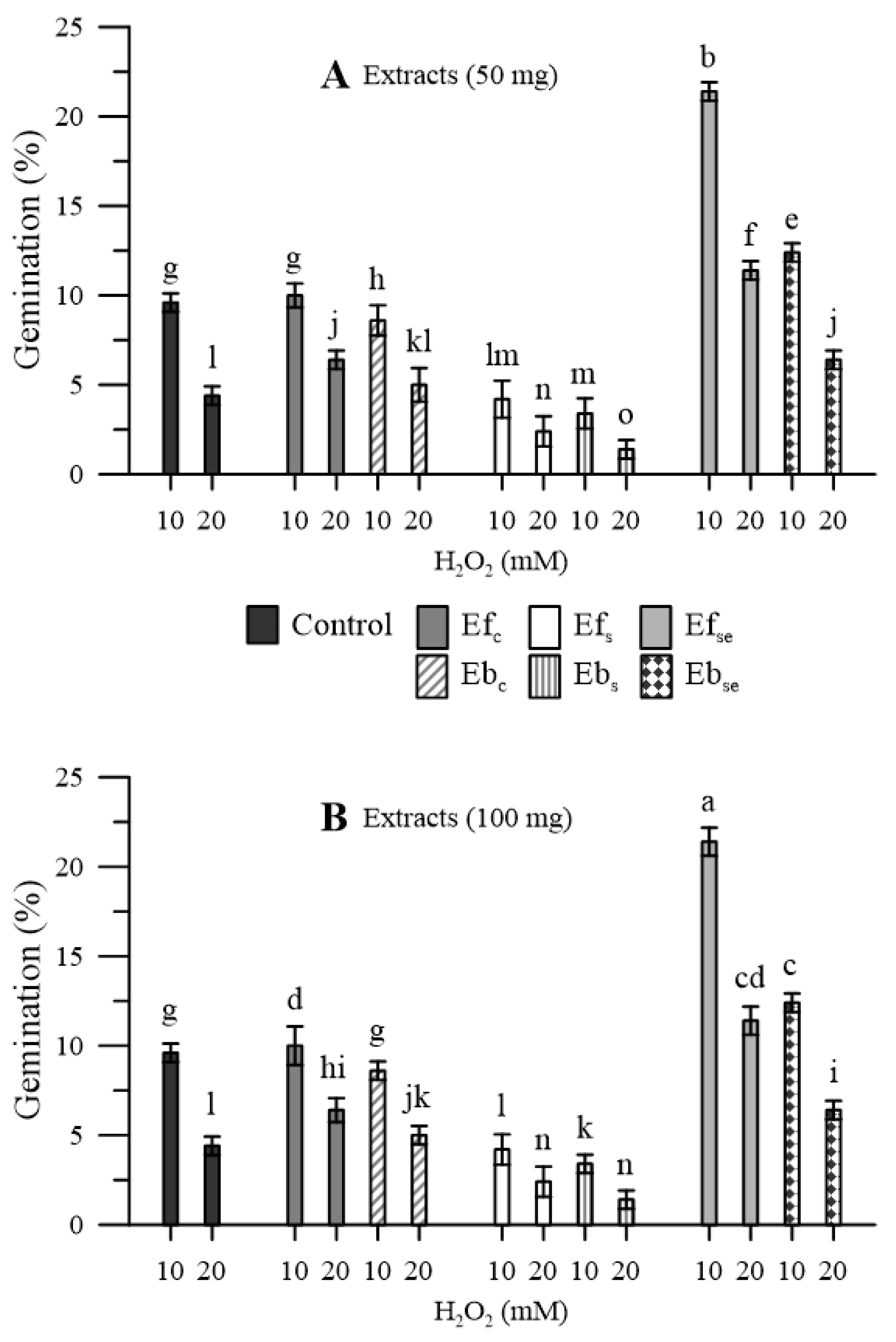
In the pollen that was not pre-treated with wheatgrass extracts, the oxidative stress induced, with H2O2, a marked reduction in the germination rate (−78% and −92% with 10 and 20 mM H2O2, respectively) (Figure 6).
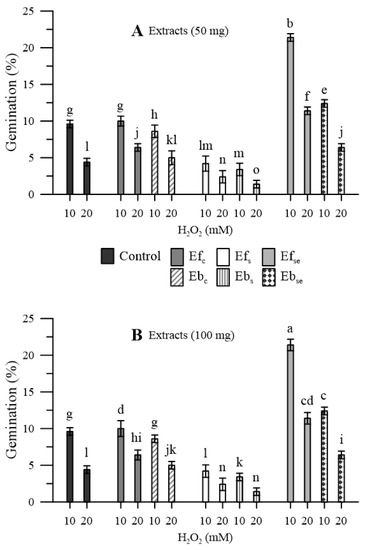
Figure 6.
Germination of maize pollen during oxidative stress induced by 10 and 20 mM of H2O2 as affected by pre-treating the pollen with (A) 50 mg or (B) 100 mg of free (Ef) and bound (Eb) extracts of emmer wheatgrass grown with distilled water as a control (Efc, Ebc) or in the presence of salinity as NaCl 50 mM (Efs, Ebs) or selenium as 45 mg L−1 of Na2SeO3 (EfSe, EbSe). Data are expressed as means ± SEM from five independent tests. Different letters indicate significant differences for p < 0.05.
A similar situation was observed in the case of pollen pre-treated with extracts of wheatgrass grown in distilled water, while the situation was even worse in the case of wheatgrass grown under salinity. On the contrary, extracts of wheatgrass biofortified with Se allowed an appreciable recovery of the germination percentage, especially in the case of free extracts (EfSe) and milder oxidative stress (10 mM H2O2). For all three kinds of wheatgrass, free extracts allowed better germination performances that bound extracts. No relevant dose-dependent effects were observed for both free and bound extracts.
3. Discussion
The high phytochemical content and antioxidant activity of sprouts and wheatgrass are well documented, whereas the supposed benefits for living organisms have rarely been ascertained. Maize pollen germination and cytosolic Ca2+ were used in this work as a model to evaluate the actual effect of emmer wheatgrass and its role in mitigating the oxidative stress caused by H2O2. Of course, evidence obtained with this model cannot be used to speculate on the effect of wheatgrass on other kind of cells, least of all animal cells. Anyway, the use of this model represents a means to observe integrated effects of the wheatgrass matrix instead of focusing on single compounds. The results obtained in this experiment give rise to some reasoning.
In the absence of oxidative stress, all three types of free extracts of emmer wheatgrass (i.e., either obtained with distilled water, or salinity or selenium) had a negative effect on both the cytosolic Ca2+ and germination of maize pollen (Figure 1 and Figure 4), while bound extracts affected only pollen germination. Moreover, the wheatgrass obtained under salinity had the most negative effect (Figure 1).
It is not easy to explain why cytosolic Ca2+ was affected only by free extracts, while pollen germination was affected by both free and bound extracts. Assuming that phenolic compounds of extracts might have a role in pollen performances, it is worth pointing out that bound forms are the most represented in emmer wheatgrass [4,5,6] but, differently from free phenolics, they are not readily available to exert their activity.
Moreover, other undetected phytochemicals could take over pollen germination, as this represents a complex biological event to which many effectors, in addition to cytosolic Ca2+, may play a role [22,29,30]. We only examined changes in cytosolic Ca2+ because germination is activated by Ca2+ signals [29,30]. The specific analytical methods used for the identification of PAs in free and bound extracts could not detect the presence of other active molecules [31]. Finally, the severity of the method used to extract bound phenolics might further explain the low activity of these forms [10].
We actually tested four of the most represented PAs in emmer wheatgrass (Figure 2 and Figure 5), two hydroxy-cinnamic (caffeic and p-coumaric) and two hydroxy-benzoic (4-HO benzoic and salicylic) [4,5,6]. All the four PAs perturbed the cytosolic Ca2+ homeostasis (Figure 2), the two hydroxybenzoic acids by having an agonist activity, and the two hydroxycinnamic by having a chelating activity. This would suggest that these PAs might be responsible, among other compounds, for the effect of free extracts. It is worth noting that in the presence of external CaCl2 1 mM, the concentration of cytosolic Ca2+ was lower than in the absence of external CaCl2. This was expected, because the entrance of Ca2+ from outside the cell depends on the Ca2+ depletion in intracellular stores. Since, in normal conditions, the Ca2+ concentration in the cytosol is in the order of nM, while the Ca2+ concentration in the extracellular medium was 1 mM, any agent altering the cytosolic ion concentration could affect the molecular mechanisms of homeostasis. In our case, phenolic acids increased cytosolic Ca2+ (due to agonist or chelating activity) and depleted the internal stores, causing a higher entrance of the extracellular Ca2+ to restore the basal conditions. In particular, the chelating activity of hydroxy-cinnamic acids could explain their higher inhibitory effect on germination. The worsening of pollen performances observed with extracts of wheatgrass grown under salinity (Figure 1 and Figure 4) could be a consequence of the increased content of free phenolics caused by this elicitor, as observed by Stagnari et al. [6] for emmer wheatgrass obtained with 50 mM NaCl in the growing medium.
In the presence of oxidative stress, all three types of free extracts antagonized the effects of H2O2 on cytosolic Ca2+ (Figure 3). There is a well-known and documented bidirectional relationship between ROS, which can modulate calcium-dependent cellular networks, and calcium signaling, which plays a key role in ROS assembly [22,23,32]. ROS behave as Ca2+ agonists, stimulate ion mobilization from internal reserves and activate the entry of Ca2+ from the extracellular medium [23,24,33]. Although both H2O2 and free extracts affected cytosolic Ca2+, it is rational to assume that the two agents acted with different molecular mechanisms. Free extracts had a transient effect on the cytosolic Ca2+ of the pollen, which was then restored, while H2O2 caused a prolonged increase in cytosolic Ca2+ over time, a depletion of pollen Ca2+ reserves and a persistent loss of Ca2+ homeostasis. In fact, free extracts mitigated the effects of H2O2 on cytosolic Ca2+, allowing the recovery of Ca2+ homeostasis and Ca2+ signal function. Bound extracts had no effect. Actually, previous works demonstrated that the highest antioxidant activity in emmer wheatgrass is associated with free phenolics [5], while bound phenolics were found to have low antioxidant activity in both rice [34] and in rapeseed sprouts [35].
Only the free extract of the Se-biofortified wheatgrass allowed an appreciable recovery of pollen germination and only at the lowest level of oxidative stress (10 mM H2O2) (Figure 6). A very slight effect was observed also with bound extracts of Se-biofortified wheatgrass. It is reasonable to assume that the positive effect of the Se-enriched extracts was mainly due to their higher Se contents. In fact, biofortification was successful, as demonstrated by the higher Se content in both the free and bound extracts (Table 1). Selenium is known as a protective agent in oxidative stress [36,37]. The protective effect on the homeostasis of cytosolic Ca2+ is one of the positive effects of Se in plants [22,23,28]. Indeed, some studies conducted on maize and olive pollen subjected to oxidative stress reported that Se restored Ca2+ homeostasis and improved pollen germination [15,38]. On the other hand, a recent study by Benincasa et al. [12] reported that endogenous Se is a promoter of phenolic compounds and antioxidant activity mainly in combination with other factors, such as salinity. However, the effect of Se-biofortification on phenolic content may be contradictory, depending on the plant species and on the Se dose, as reviewed by D’Amato et al. [14]. Since 100 mg of extract gave no further benefit in the recovery of pollen germination, we can deduce that the amount of Se contained in 50 mg of extract was enough to achieve the maximum effect in terms of mitigation of the stress caused by H2O2 (saturation effect). Further research with lower doses of Se-biofortified wheatgrass extracts (e.g., including treatments with 10, 20, 30 and 40 mg, besides the treatments with 0 and 50 mg) is needed to ascertain the minimum rate required to achieve the maximum stress mitigation.
4. Materials and Methods
4.1. Reagents
Hydrogen peroxide (30% w/v) and nitric acid (65%, w/v) were purchased from Suprapur Reagents Merck (Darmstadt, Germany). 4-hydroxybenzoic acid (4-OHBA), caffeic acid (CA), p-coumaric acid (p-CA) and salicylic acid (SA), were purchased from Sigma Aldrich (St. Louis, MO, USA). All standards were prepared as a stock solution at 5 mg mL−1 in methanol and stored at −20 °C in the dark. FURA 2-AM (FURA-2-pentakis (acetoxymethyl ester)), Triton X-100 (t-octylphenoxypolyethoxyethanol), EGTA (ethylene glycol-bis (β-aminoethyl ether) −N, N, N′, N′-tetracetic acid), sodium selenite (Na2SeO3), NaCl, KCl, MgCl2, Hepes, dimethyl sulfoxide (DMSO) and CaCl2 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other reagents (reagent grade) were obtained from common commercial sources.
4.2. Emmer Wheatgrass Production
Emmer wheatgrass was obtained by germinating grains and growing seedlings with distilled water, or salinity or selenium as described in Del Pino et al. [16]. Briefly, grains of emmer (Triticum turgidum L. spp. dicoccum (Schrank ex Shubler) Thell., cv. Zefiro) were incubated in plastic trays (20 g per tray) containing sterile cotton and filter paper wetted with distilled water as a control (C), or with a solution containing NaCl 50 mM (S) or 45 mg L−1 of Na2SeO3 (Se), according to a completely randomized block design with four replicates (trays). To maintain the air circulation while preventing dehydration, the trays were covered by a drilled top and then they were incubated in a growth chamber at 20 °C in the dark. After germination, trays were moved in a light–dark regime of 10:14 h, with a light intensity at 200 μmol photons m−2 s−1. Distilled water was periodically added to trays to restore initial tray weights, considering the change in seedling biomass as negligible, and thus, approximately keeping the initial NaCl and Se concentrations of these treatments [6,7]. Wheatgrass from C treatment was collected 8 days after sowing (DAS), while wheatgrass of S and Se treatments were collected when they reached the same seedling growth stage as in C (9 DAS for S and 11 DAS for Se treatments), because either S or Se slowed seedling growth compared to C. Only shoots were harvested, and replicates of each treatment were re-grouped two by two for the chemical analysis, performed in triplicate. Samples were stored at −20 °C until extraction.
4.3. Preparation of Emmer Wheatgrass Extracts
Emmer wheatgrass extracts were obtained, using methanol as a solvent, as described in Del Pino et al. [16] and according to the method of Krygier et al. [39], with slight modifications. Two grams of frozen wheatgrass were mixed with 20 mL of MeOH and homogenized on ice using an Ultraturrax three times, alternating 30 s homogenization and 30 s pause to prevent the material from heating. The solution was then kept in agitation for 24 h and centrifuged at 5000 rpm for 10 min. The supernatant (free fraction) was recovered and evaporated to dryness using a rotary evaporator. The dry extracts were suspended in 2 mL of methanol. This represented the extract of free phenolics (Ef).
The remaining solid residue was mixed with 10 mL of NaOH (5 N) for 1 h and then HCl (5 M) was added until pH = 2. Samples were mixed with 10 mL of ethyl acetate, vortexed and centrifuged at 3000 rpm for 10 min and the supernatant was then recovered (bound fraction). This extraction was performed three times, and the supernatants were pooled and evaporated to dryness using a rotary evaporator. The dry residue was suspended in 2 mL of methanol. This represented the extract of bound phenolics (Eb).
Preliminary tests were carried out on the wheatgrass extracts (Ef and Eb) to evaluate the most suitable concentration for the measurement of cytosolic Ca2+ and the germination of maize pollen. Based on the preliminary tests mentioned above, the treatments tested in this study were: free (Ef) and bound (Eb) extracts from wheatgrass grown in distilled water (Efc and Ebc), and with a solution of 50 mM NaCl (Efs and Ebs) or 45 mg L−1 Na2SeO3 (EfSe and EbSe).
4.4. Determination of Total Selenium in Wheatgrass Extracts
Measurements of the total selenium content were performed in all emmer wheatgrass extracts (Efc, Efs, EfSe and Ebc, Ebs, EbSe) following the method of D’Amato et al. [40]. Wheatgrass extracts (200 µg) were microwave-digested (ETHOS one high-performance microwave digestion system; Mile-stone Inc., Sorisole, Bergamo, Italy) with 8 mL of ultrapure concentrated nitric acid (65% w/w) and 2 mL of hydrogen peroxide (30% w/w). The heating program for the digestion procedure was 30 min at 1000 W and 200 °C. After cooling down, the digests were diluted with water up to 20 mL and passed through 0.45-μm filters. The samples were analyzed by ICP-MS (Agilent 7900, Agilent Technologies, Santa Clara, CA, USA) with an Octopole Reaction System (ORS). Total Se standard solutions were prepared by diluting the corresponding stock solutions (Se standard 1000 mg L−1 for AAS TraceCert, 89498, Sigma-Aldrich, Milan, Italy) with HPLC-grade water. Results were expressed as micrograms per kilograms. This method was accurately validated with a recovery test (n = 3) by adding a Se standard solution (4 mg L−1) into the mixture of Se-enriched sample and nitric acid prior to digestion in tubes and after appropriate dilution.
4.5. Measurement of Cytosolic Ca2+ with the Addition of Emmer Wheatgrass Extracts, Pure Phenolic Acids and H2O2 for an Oxidative Stress Induced In Vitro
Cytosolic Ca2+ levels were determined spectrofluorometrically using the FURA-2AM probe according to Del Pino et al. [16]. Aliquots (100 mg) of maize pollen, stored in the dark at 5 °C until use, were suspended in 10 mL of PBS and hydrated for 2 days at 25 °C. Hydrated pollens were harvested by centrifugation at 1000 g for 4 min and then resuspended in 2 mL Ca2+-free HBSS buffer (120 mM NaCl, 5.0 mM KCl, MgCl2 1 mM, 5 mM glucose, 25 mM Hepes, pH 7.4). Pollen suspensions were incubated in the dark with FURA-2 (2 µL of a 2 mM solution in DMSO) for 120 min, and then centrifuged at 1000 g for 4 min. Pollens were then harvested and suspended in ~10 mL of Ca2+-free HBSS containing 0.1 mM EGTA, which was included to rule out or, at least, minimize a potential background due to contaminating ions (so as to obtain a suspension of 1 × 106 pollen granules hydrated per mL).
Fluorescence was measured in a PerkinElmer LS 50 B spectrofluorometer (ex. 340 and 380 nm, em. 510 nm), set with 10 and a 7.5 nm slit widths in the excitation and emission windows, respectively. Fluorometric readings were normally taken after 300–400 s. In detail, the determination of cytosolic Ca2+ started after placing the pollen suspension labelled with FURA 2AM in the cuvette and lasted for 100 s.
After determining the basal cytosolic Ca2+ content of the pollen, the following agents were added, singularly or in different combinations according to specific purposes. In detail, aliquots (50 mg) of each of the three free (Efc, Efs, EfSe) and three bound (Ebc, Ebs, EbSe) extracts, and aliquots of pure phenolic acids (0.250 mg of 4-HO benzoic, caffeic, p-coumaric, and salicylic acid) were used in the assay to determine the effect of wheatgrass extract and of most representative wheatgrass PAs on the cytosolic Ca2+ of maize pollen labelled with the FURA 2AM fluorescent probe. The measurements were carried out in the absence of Ca2+ (Ca2+-free) and in the presence of Ca2+ (1 mM CaCl2) in the incubation medium.
Oxidative stress was induced in vitro with hydrogen peroxide at 10 and 20 mM, and in the absence of Ca2+ (Ca2+-free) or presence of Ca2+ (1 mM CaCl2) in the incubation medium, and in the absence or presence of two doses (50 and 100 mg) of each of the three free extracts.
After the addition of each test agent, changes in cytosolic calcium were monitored for another 200–300 s. Cytosolic calcium concentrations of pollen ([Ca2+]cp) were calculated following Grynkiewicz et al. [41]. The extent of the determined variations was expressed as Δ[Ca2+]cp, nM.
4.6. Germination of Maize Pollen Grains with the Addition of Emmer Wheatgrass Extracts, Pure Phenolic Acids and H2O2 for an Oxidative Stress Induced In Vitro
Fresh pollen samples from each plot were hydrated in a humid chamber at room temperature for 30 min [42], and then transferred to 6-well culture Corning plates (1 mg of pollen per plate) containing 3 mL of an agar-solidified growing medium composed of 1.2% agar, 10%, sucrose, 0.03% boric acid and 0.15% calcium chloride (pH 5.5) [43]. Pollen suspensions were incubated for 24–48 h in a growth chamber at 27 °C with gentle shaking to ensure homogeneous distribution of the samples in the wells.
Two doses (50 or 100 mg) of free and bound (Ef and Eb) extracts, and aliquots of pure phenolic acids (0.250 mg of 4-HO benzoic, caffeic, p-coumaric and salicylic acid) were applied to test, in vitro, the germination on maize pollen grains.
Oxidative stress was induced in vitro with hydrogen peroxide at 10 and 20 mM, and in the absence or presence of two doses (50 and 100 mg) of each of the three free or bound extracts.
Germinated and non-germinated pollen grains were counted under a 10x magnification microscope. Germination rates were calculated based on three replicates, each of which consisted of 100 grains. Germination of grains was confirmed when the pollen tube had grown longer than the grain’s diameter [43].
4.7. Statistical Analysis
Statistical evaluations were performed using the OriginPro software [44]. Variance assessments included homogeneity analysis using the Levene test and normality analysis using the D’Agostino–Pearson test. Significance of differences was assessed by the Fisher’s least significant differences test, after the analysis of variance according to the randomized complete split-plot with five (Figure 2, Figure 4 and Figure 6) or four replicates (Figure 1 and Figure 3), and with randomized complete one-way design with four replicates for Figure 5. The results obtained are expressed as mean values ± standard error of the mean (SEM). Differences with p < 0.05 were considered statistically significant.
5. Conclusions
Maize pollen germination and cytosolic Ca2+ were confirmed to be a good model to test the integrated effects of the wheatgrass matrix. Cytosolic Ca2+ was affected only by free extracts of emmer wheatgrass, while the germination of maize pollen was affected by both free and bound extracts. Based on the effects observed by applying pure phenolic acids, it is reasonable to assume that these compounds may partly contribute to the effects of the extracts, but other compounds are likely involved, which deserve to be further studied. The extracts, mainly the free ones, were able to counteract the perturbation of Ca2+ homeostasis caused by H2O2 and to slightly mitigate its depressive effect on pollen germination. The mitigation effect depended on the wheatgrass growing conditions (i.e., distilled water, salinity or selenium). The best results were obtained with Se-biofortified wheatgrass, likely due to the Se itself rather than to its boosting effect on phenolic compounds.
Author Contributions
Conceptualization, C.A.P. and P.B.; methodology, C.A.P., A.M.D.P. and P.B.; formal analysis, A.M.D.P., B.F. and R.D.; investigation, A.M.D.P. and C.A.P.; resources, P.B., C.A.P. and R.D.; data curation, A.M.D.P. and C.A.P.; writing—original draft preparation A.M.D.P., C.A.P., P.B. and B.F.; writing—review and editing, P.B., C.A.P., A.M.D.P. and D.B.; supervision, C.A.P. and P.B.; project administration, P.B.; funding acquisition, P.B. and D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Project “Ricerca di Base 2020” of the Department of Agricultural, Food and Environmental Sciences of the University of Perugia (Principal Investigator: Paolo Benincasa).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the last author (C.A.P.) on reasonable request.
Acknowledgments
We gratefully acknowledge Silvano Locchi for technical assistance in the production of emmer sprouts and maize pollen.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted grains: A comprehensive review. Nutrients 2019, 11, 421. [Google Scholar]
- Tufail, T.; Saeed, F.; Afzaal, M.; Suleria, H.A.R. Wheatgrass juice: A nutritional weapon against various maladies. In Human Health Benefits of Plant Bioactive Compounds; Apple Academic Press: Palm Bay, FL, USA, 2019; pp. 245–253. [Google Scholar]
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and microgreens: Trends, opportunities, and horizons for novel research. Agronomy 2020, 10, 1424. [Google Scholar]
- Benincasa, P.; Galieni, A.; Manetta, A.C.; Pace, R.; Guiducci, M.; Pisante, M.; Stagnari, F. Phenolic compounds in grains, sprouts and wheatgrass of hulled and non-hulled wheat species. J. Sci. Food Agric. 2015, 95, 1795–1803. [Google Scholar] [PubMed]
- Benincasa, P.; Tosti, G.; Farneselli, M.; Maranghi, S.; Bravi, E.; Marconi, O.; Falcinelli, B.; Guiducci, M. Phenolic content and antioxidant activity of einkorn and emmer sprouts and wheatgrass obtained under different radiation wavelengths. Ann. Agric. Sci. 2020, 65, 68–76. [Google Scholar]
- Stagnari, F.; Galieni, A.; D’Egidio, S.; Falcinelli, B.; Pagnani, G.; Pace, R.; Pisante, M.; Benincasa, P. Effects of sprouting and salt stress on polyphenol composition and antiradical activity of einkorn, emmer and durum wheat. Ital. J. Agron. 2017, 12, 293–301. [Google Scholar]
- Falcinelli, B.; Benincasa, P.; Calzuola, I.; Gigliarelli, L.; Lutts, S.; Marsili, V. Phenolic content and antioxidant activity in raw and denatured aqueous extracts from sprouts and wheatgrass of einkorn and emmer obtained under salinity. Molecules 2017, 22, 2132. [Google Scholar]
- Holst, B.; Williamson, G. Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. Curr. Opin. Biotechnol. 2008, 19, 73–82. [Google Scholar]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in food, a review. Food Chem. 2014, 152, 46–55. [Google Scholar]
- Shahidi, F.; Yeo, J. Insoluble-bound phenolics in food. Molecules 2016, 21, 1216. [Google Scholar]
- Liu, H.; Kang, Y.; Zhao, X.; Liu, Y.; Zhang, X.; Zhang, S. Effects of elicitation on bioactive compounds and biological activities of sprouts. J. Funct. Foods 2019, 53, 136–145. [Google Scholar]
- Benincasa, P.; D’Amato, R.; Falcinelli, B.; Troni, E.; Fontanella, M.C.; Frusciante, S.; Guiducci, M.; Beone, G.M.; Businelli, D.; Diretto, G. Grain endogenous selenium and moderate salt stress work as synergic elicitors in the enrichment of bioactive compounds in maize sprouts. Agronomy 2020, 10, 735. [Google Scholar] [CrossRef]
- D’Amato, R.; Fontanella, M.C.; Falcinelli, B.; Beone, G.M.; Bravi, E.; Marconi, O.; Benincasa, P.; Businelli, D. Selenium biofortification in rice (Oryza sativa L.) sprouting: Effects on Se yield and nutritional traits with focus on phenolic acid profile. J. Agric. Food Chem. 2018, 66, 4082–4090. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C.; et al. Current knowledge on selenium biofortification to improve the nutraceutical profile of food: A comprehensive review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef] [PubMed]
- Del Pino, A.M.; Guiducci, M.; D’Amato, R.; Di Michele, A.; Tosti, G.; Datti, A.; Palmerini, C.A. Selenium maintains cytosolic Ca2+ homeostasis and preserves germination rates of maize pollen under H2O2-induced oxidative stress. Sci. Rep. 2019, 9, 13502. [Google Scholar] [CrossRef]
- Del Pino, A.M.; Falcinelli, B.; D’Amato, R.; Businelli, D.; Benincasa, P.; Palmerini, C.A. Extracts of emmer wheatgrass grown with distilled water, salinity or selenium differently affect germination and cytosolic Ca2+ of maize pollen. Agronomy 2021, 11, 633. [Google Scholar]
- Thor, K. Calcium-Nutrient and Messenger. Front. Plant Sci. 2019, 10, 440–446. [Google Scholar] [CrossRef]
- Fox, T.C.; Guerinot, M.L. Molecular biology of cation transport in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 669–696. [Google Scholar]
- Dodd, A.N.; Kudla, J.; Sanders, D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef]
- Ordenes, V.R.; Reyes, F.C.; Wolff, D.; Orellana, A. A thapsigargin sensitive Ca2+ pump is present in the pea Golgi apparatus membrane. Plant Physiol. 2002, 129, 1820–1828. [Google Scholar]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. New Phytol. 2018, 218, 414–431. [Google Scholar]
- Steinhorst, L.; Kudla, J. Calcium—A central regulator of pollen germination and tube growth. Biochim. Biophys. Acta 2013, 1833, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Calcium and mitochondria in the regulation of cell death. Biochem. Biophys. Res. Commun. 2015, 460, 72–81. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Collin, F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Michard, E.; Alves, F.; Feijò, J.A. The role of ion fluxes in polarized cell growth and morphogenesis: The pollen tube as an experimental paradigm. Int. J. Dev. Biol. 2009, 53, 1609–1622. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.Y.; Wu, H.M. Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu. Rev. Plant Biol. 2008, 59, 547–572. [Google Scholar] [CrossRef]
- Kim, K.-H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Proietti, P.; Marinucci, M.T.; Del Pino, A.M.; D′Amato, R.; Regni, L.; Acuti, G.; Chiaradia, E.; Palmerini, C.A. Selenium maintains Ca2+ homeostasis in sheep lymphocytes challenged by oxidative stress. PLoS ONE 2018, 13, e0201523. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Calì, T.; Ottolini, D.; Carofoli, E. Intracellular calcium homeostasis and signaling. In Metallomics and the Cell; Metal Ions in Life Sciences; Banci, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 12, pp. 119–168. [Google Scholar]
- Ti, H.; Li, Q.; Zhang, R.; Zhang, M.; Deng, Y.; Wei, Z.; Chi, Z.; Zhang, Y. Free and bound phenolic profiles and antioxidant activity of milled fractions of different indica rice varieties cultivated in southern China. Food Chem. 2014, 159, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, B.; Sileoni, V.; Marconi, O.; Perretti, G.; Quinet, M.; Lutts, S.; Benincasa, P. Germination under moderate salinity increases phenolic content and antioxidant activity in rapeseed (Brassica napus var oleifera Del.) sprouts. Molecules 2017, 22, 1377. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef]
- Prins, C.N.; Hantzis, L.J.; Quinn, C.F.; Pilon-Smits, E.A.H. Effects of selenium accumulation on reproductive functions in Brassica juncea and Stanleya pinnata. J. Exp. Bot. 2011, 62, 5633–5640. [Google Scholar] [CrossRef]
- Del Pino, A.M.; Regni, L.; D’Amato, R.; Tedeschini, E.; Businelli, B.; Proietti, P.; Palmerini, C.A. Selenium-enriched pollen grains of Olea europaea L.: Ca2+ signaling and germination under oxidative stress. Front. Plant Sci. 2019, 10, 1611. [Google Scholar] [CrossRef] [PubMed]
- Krygier, K.; Sosulski, F.; Hogge, L. Free, esterified, and insoluble-bound phenolic acids. Extraction and purification procedure. J. Agric. Food Chem. 1982, 30, 330–334. [Google Scholar] [CrossRef]
- D’Amato, R.; De Feudis, M.; Guiducci, M.; Businelli, D. Zea mays L. grain: Increase in nutraceutical and antioxidant properties due to se fortification in low and high water regimes. J. Agric. Food Chem. 2019, 67, 7050–7059. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef]
- Rejo´n, J.D.; Zienkiewicz, A.; Rodríguez-García, M.I.; Castro, A.J. Profiling and functional classification of esterases in olive (Olea europaea) pollen during germination. Ann. Bot. 2012, 110, 1035–1045. [Google Scholar] [CrossRef]
- Martins, E.S.; Davide, L.M.C.; Miranda, G.J.; de Oliveira Barizon, J.; de Assis Souza, F.; de Carvalho, R.P.; Gonçalves, M.C. In vitro pollen viability of maize cultivars at different times of collection. Ciênc. Rural 2017, 47, e20151077. [Google Scholar] [CrossRef]
- OriginPro, version 2019b; OriginLab Corporation: Northampton, MA, USA, 2019.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).