Response of Three Miscanthus × giganteus Cultivars to Toxic Elements Stress: Part 2, Comparison between Two Growing Seasons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pot Experiment Preparations
2.2. Pot Experimental Design
2.3. Miscanthus Sampling and Preparation
2.3.1. TE Concentrations in the Collected Leaves
2.3.2. Antioxidant Enzymatic Activities Assays
2.3.3. Photosynthetic Pigments and Secondary Metabolism Molecules Quantification
2.4. Statistical Analysis
3. Results
3.1. Miscanthus Leaf TE Concentrations at the End of the Second Growing Season
3.2. Stem Height, Diameter, and Number of Tillers per Plant at the End of the Second Growing Season
3.3. SOD, APX, and GR Activity Determination
3.4. Photosynthetic Pigment Quantification
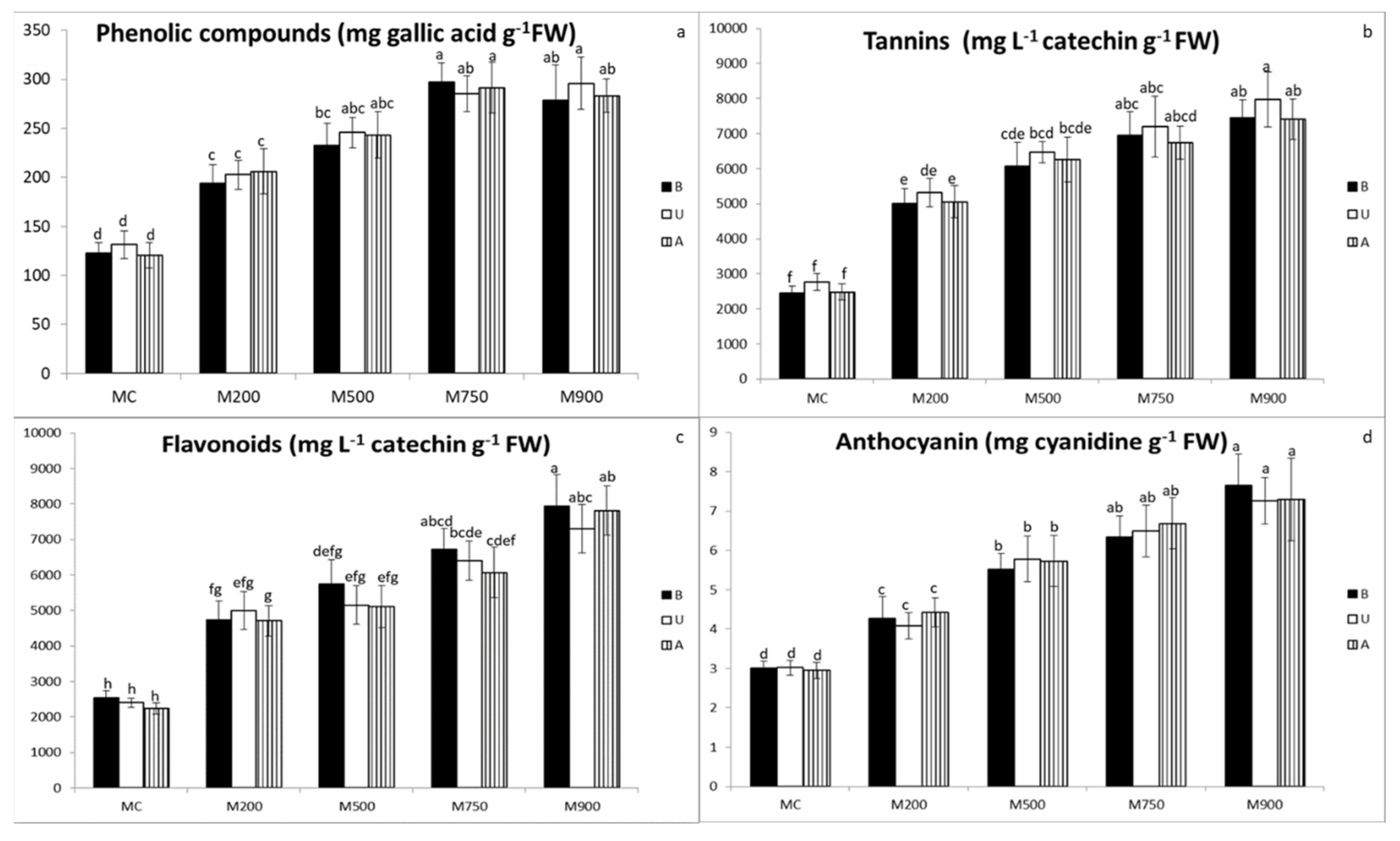
3.5. Secondary Metabolites Quantification
4. Discussion
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antoniadis, V.; Golia, E.E.; Liu, Y.-T.; Wang, S.-L.; Shaheen, S.M.; Rinklebe, J. Soil and Maize Contamination by Trace Elements and Associated Health Risk Assessment in the Industrial Area of Volos, Greece. Environ. Int. 2019, 124, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Agyeman, P.C.; John, K.; Kebonye, N.M.; Borůvka, L.; Vašát, R.; Drábek, O.; Němeček, K. Human Health Risk Exposure and Ecological Risk Assessment of Potentially Toxic Element Pollution in Agricultural Soils in the District of Frydek Mistek, Czech Republic: A Sample Location Approach. Environ. Sci. Eur. 2021, 33, 137. [Google Scholar] [CrossRef]
- Douay, F.; Pelfrêne, A.; Planque, J.; Fourrier, H.; Richard, A.; Roussel, H.; Girondelot, B. Assessment of Potential Health Risk for Inhabitants Living near a Former Lead Smelter. Part 1: Metal Concentrations in Soils, Agricultural Crops, and Homegrown Vegetables. Environ. Monit. Assess. 2013, 185, 3665–3680. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Al Souki, K.S.; Waterlot, C.; Douay, F.; Pelfrêne, A.; Ridošková, A.; Louvel, B.; Pourrut, B. Potentials of Miscanthus × Giganteus for Phytostabilization of Trace Element-Contaminated Soils: Ex Situ Experiment. Ecotoxicol. Environ. Saf. 2021, 214, 112125. [Google Scholar] [CrossRef]
- Hu, Y.; Nan, Z.; Su, J.; Wang, N. Heavy Metal Accumulation by Poplar in Calcareous Soil with Various Degrees of Multi-Metal Contamination: Implications for Phytoextraction and Phytostabilization. Environ. Sci. Pollut. Res. 2013, 20, 7194–7203. [Google Scholar] [CrossRef] [PubMed]
- Pelfrêne, A.; Kleckerová, A.; Pourrut, B.; Nsanganwimana, F.; Douay, F.; Waterlot, C. Effect of Miscanthus Cultivation on Metal Fractionation and Human Bioaccessibility in Metal-Contaminated Soils: Comparison between Greenhouse and Field Experiments. Environ. Sci. Pollut. Res. 2015, 22, 3043–3054. [Google Scholar] [CrossRef]
- Dary, M.; Chamber-Perez, M.A.; Palomares, A.J.; Pajuelo, E. “In Situ” Phytostabilisation of Heavy Metal Polluted Soils Using Lupinus Luteus Inoculated with Metal Resistant Plant-Growth Promoting Rhizobacteria. J. Hazard. Mater. 2010, 177, 323–330. [Google Scholar] [CrossRef]
- Epelde, L.; Becerril, J.M.; Mijangos, I.; Garbisu, C. Evaluation of the Efficiency of a Phytostabilization Process with Biological Indicators of Soil Health. J. Environ. Qual. 2009, 38, 2041–2049. [Google Scholar] [CrossRef]
- Bidar, G.; Pruvot, C.; Garcon, G.; Verdin, A.; Shirali, P.; Douay, F. Seasonal and Annual Variations of Metal Uptake, Bioaccumulation, and Toxicity in Trifolium Repens and Lolium Perenne Growing in a Heavy Metal-Contaminated Field. Environ. Sci. Pollut. Res. 2009, 16, 42–53. [Google Scholar] [CrossRef]
- Bidar, G.; Garcon, G.; Pruvot, C.; Dewaele, D.; Cazier, F.; Douay, F.; Shirali, P. Behavior of Trifolium Repens and Lolium Perenne Growing in a Heavy Metal Contaminated Field: Plant Metal Concentration and Phytotoxicity. Environ. Pollut. 2007, 147, 546–553. [Google Scholar] [CrossRef]
- Lopareva-Pohu, A.; Pourrut, B.; Waterlot, C.; Garcon, G.; Bidar, G.; Pruvot, C.; Shirali, P.; Douay, F. Assessment of Fly Ash-Aided Phytostabilisation of Highly Contaminated Soils after an 8-Year Field Trial: Part 1. Influence on Soil Parameters and Metal Extractability. Sci. Total Environ. 2011, 409, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Pourrut, B.; Lopareva-Pohu, A.; Pruvot, C.; Garcon, G.; Verdin, A.; Waterlot, C.; Bidar, G.; Shirali, P.; Douay, F. Assessment of Fly Ash-Aided Phytostabilisation of Highly Contaminated Soils after an 8-Year Field Trial—Part 2. Influence on Plants. Sci. Total Environ. 2011, 409, 4504–4510. [Google Scholar] [CrossRef] [PubMed]
- Bidar, G.; Waterlot, C.; Verdin, A.; Proix, N.; Courcot, D.; Detriche, S.; Fourrier, H.; Richard, A.; Douay, F. Sustainability of an in Situ Aided Phytostabilisation on Highly Contaminated Soils Using Fly Ashes: Effects on the Vertical Distribution of Physicochemical Parameters and Trace Elements. J. Environ. Manage. 2016, 171, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Nsanganwimana, F.; Pourrut, B.; Mench, M.; Douay, F. Suitability of Miscanthus Species for Managing Inorganic and Organic Contaminated Land and Restoring Ecosystem Services. A Review. J. Environ. Manage. 2014, 143, 123–134. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Pourrut, B.; Waterlot, C.; Louvel, B.; Bidar, G.; Labidi, S.; Fontaine, J.; Muchembled, J.; Sahraoui, A.L.-H.; Fourrier, H.; et al. Metal Accumulation and Shoot Yield of Miscanthus × Giganteus Growing in Contaminated Agricultural Soils: Insights into Agronomic Practices. Agric. Ecosyst. Environ. 2015, 213, 61–71. [Google Scholar] [CrossRef]
- Al Souki, K.S.; Liné, C.; Louvel, B.; Waterlot, C.; Douay, F.; Pourrut, B. Miscanthus × Giganteus Culture on Soils Highly Contaminated by Metals: Modelling Leaf Decomposition Impact on Metal Mobility and Bioavailability in the Soil–Plant System. Ecotoxicol. Environ. Saf. 2020, 199, 110654. [Google Scholar] [CrossRef]
- Al Souki, K.S.; Burdová, H.; Trubač, J.; Štojdl, J.; Kuráň, P.; Kříženecká, S.; Machová, I.; Kubát, K.; Popelka, J.; Auer Malinská, H.; et al. Enhanced Carbon Sequestration in Marginal Land upon Shift towards Perennial C4 Miscanthus × giganteus: A Case Study in North-Western Czechia. Agronomy 2021, 11, 293. [Google Scholar] [CrossRef]
- Al Souki, K.S.; Burdová, H.; Mamirova, A.; Kuráň, P.; Kříženecká, S.; Oravová, L.; Tolaszová, J.; Nebeská, D.; Popelka, J.; Ust’ak, S.; et al. Evaluation of the Miscanthus × Giganteus Short Term Impacts on Enhancing the Quality of Agricultural Soils Affected by Single and/or Multiple Contaminants. Environ. Technol. Innov. 2021, 24, 101890. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Waterlot, C.; Louvel, B.; Pourrut, B.; Douay, F. Metal, Nutrient and Biomass Accumulation during the Growing Cycle of Miscanthus Established on Metal-Contaminated Soils. J. Plant Nutr. Soil Sci. 2016, 179, 257–269. [Google Scholar] [CrossRef]
- Al Souki, K.S.; Louvel, B.; Douay, F.; Pourrut, B. Assessment of Miscanthus × Giganteus Capacity to Restore the Functionality of Metal-Contaminated Soils: Ex Situ Experiment. Appl. Soil Ecol. 2017, 115, 44–52. [Google Scholar] [CrossRef]
- Arduini, I.; Masoni, A.; Mariotti, M.; Ercoli, L. Low Cadmium Application Increase Miscanthus Growth and Cadmium Translocation. Environ. Exp. Bot. 2004, 52, 89–100. [Google Scholar] [CrossRef]
- Guo, H.; Hong, C.; Chen, X.; Xu, Y.; Liu, Y.; Jiang, D.; Zheng, B. Different Growth and Physiological Responses to Cadmium of the Three Miscanthus Species. PLoS ONE 2016, 11, e0153475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Yang, S.; Huang, Y.; Zhou, S. The Tolerance and Accumulation of Miscanthus sacchariflorus (Maxim.) Benth., an Energy Plant Species, to Cadmium. Int. J. Phytoremediation 2015, 17, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Al Souki, K.S.; Liné, C.; Douay, F.; Pourrut, B. Response of Three Miscanthus × Giganteus Cultivars to Toxic Elements Stress: Part 1, Plant Defence Mechanisms. Plants 2021, 10, 2035. [Google Scholar] [CrossRef] [PubMed]
- Sterckeman, T.; Douay, F.; Proix, N.; Fourrier, H.; Perdrix, E. Assessment of the Contamination of Cultivated Soils by Eighteen Trace Elements around Smelters in the North of France. Water Air Soil Pollut. 2002, 135, 173–194. [Google Scholar] [CrossRef]
- Waterlot, C.; Bidar, G.; Pelfrêne, A.; Roussel, H.; Fourrier, H.; Douay, F. Contamination, Fractionation and Availability of Metals in Urban Soils in the Vicinity of Former Lead and Zinc Smelters, France. Pedosphere 2013, 23, 143–159. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Dringen, R.; Gutterer, J.M. Glutathione Reductase from Bovine Brain. Methods Enzymol. 2002, 348, 281–288. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Fernando, A.; Oliveira, J.S. Effects on Growth, Productivity and Biomass Quality of Miscanthus × giganteus of Soils Contaminated with Heavy Metals. In Proceedings of the 2nd World Conference on Biomass for Energy, Industry and Climate Protection, Rome, Italy, 10–14 May 2004. [Google Scholar]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead Uptake, Toxicity, and Detoxification in Plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar] [PubMed] [Green Version]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-Metal-Induced Reactive Oxygen Species: Phytotoxicity and Physicochemical Changes in Plants. Rev. Environ. Contam. Toxicol. 2014, 232, 1–44. [Google Scholar] [PubMed]
- Scebba, F.; Arduini, I.; Ercoli, L.; Sebastiani, L. Cadmium Effects on Growth and Antioxidant Enzymes Activities in Miscanthus Sinensis. Biol. Plant 2006, 50, 688–692. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Arduini, I.; Ercoli, L.; Mariotti, M.; Masoni, A. Response of Miscanthus to Toxic Cadmium Applications during the Period of Maximum Growth. Environ. Exp. Bot. 2006, 55, 29–40. [Google Scholar] [CrossRef]
- Pajević, S.; Borišev, M.; Nikolić, N.; Arsenov, D.D.; Orlović, S.; Župunski, M. Phytoextraction of Heavy Metals by Fast-Growing Trees: A Review. In Phytoremediation, Management of Environmental Contaminants; Springer: Berlin/Heidelberg, Germany, 2016; Volume 3. [Google Scholar]
- Vijayaraghavan, K.; Arockiaraj, J.; Kamala-Kannan, S. Portulaca Grandiflora as Green Roof Vegetation: Plant Growth and Phytoremediation Experiments. Int. J. Phytoremediation 2017, 19, 537–544. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, M.N.V.; Sytar, O. Lead Toxicity, Defense Strategies and Associated Indicative Biomarkers in Talinum Triangulare Grown Hydroponically. Chemosphere 2012, 89, 1056–1065. [Google Scholar] [CrossRef]
- Hamels, F.; Malevé, J.; Sonnet, P.; Kleja, D.B.; Smolders, E. Phytotoxicity of Trace Metals in Spiked and Field-Contaminated Soils: Linking Soil-Extractable Metals with Toxicity. Environ. Toxicol. Chem. 2014, 33, 2479–2487. [Google Scholar] [CrossRef]


| MC | M200 | M500 | M750 | M900 | |
|---|---|---|---|---|---|
| Cd (mg kg−1) | 0.3 ± 0.0 | 3.8 ± 0.2 | 9.0 ± 0.2 | 13.5 ± 0.3 | 16.0 ± 0.3 |
| Pb (mg kg−1) | 37.3 ± 1.3 | 260.3 ± 2.0 | 528.6 ± 5.3 | 747.1 ± 16.9 | 898.6 ± 16.3 |
| Zn (mg kg−1) | 54.6 ± 3.1 | 388.0 ± 14.8 | 537.0 ± 10.9 | 906.0 ± 16.8 | 1116.0 ± 1.7 |
| Cd (mg kg−1) | Pb (mg kg−1) | Zn (mg kg−1) | ||
|---|---|---|---|---|
| B | MC | 0.4 ± 0.0 h | 6.9 ± 0.8 f | 37.2 ± 3.1 h |
| M200 | 0.7 ± 0.1 g | 12.6 ± 1.4 e | 76.1 ± 5.3 f | |
| M500 | 1.4 ± 0.1 e | 19.8 ± 1.7 cd | 90.9 ± 4.8 de | |
| M750 | 2.4 ± 0.1 c | 25.9 ± 3.8 b | 114.7 ± 4.5 b | |
| M900 | 3.1 ± 0.1 a | 31.0 ± 2.1 a | 138.2 ± 4.1 a | |
| U | MC | 0.4 ± 0.0 h | 5.9 ± 0.4 f | 29.0 ± 3.7 h |
| M200 | 0.7 ± 0.1 g | 11.2 ± 2.0 ef | 66.2 ± 4.4 g | |
| M500 | 1.1 ± 0.1 f | 18.0 ± 1.7 d | 82.2 ± 4.6 ef | |
| M750 | 2.1 ± 0.1 d | 23.1 ± 1.9 bc | 104.7 ± 4.0 c | |
| M900 | 2.7 ± 0.1 b | 27.4 ± 3.0 ab | 116.3 ± 3.3 b | |
| A | MC | 0.4 ± 0.0 h | 7.0 ± 0.9 f | 41.5 ± 6.0 h |
| M200 | 0.7 ± 0.0 g | 12.8 ± 1.1 e | 77.1 ± 5.2 f | |
| M500 | 1.2 ± 0.1 e | 19.5 ± 1.4 cd | 93.5 ± 2.1 d | |
| M750 | 2.3 ± 0.2 c | 25.6 ± 2.6 b | 120.4 ± 4.3 b | |
| M900 | 3.1 ± 0.1 a | 31.7 ± 3.9 a | 140.7 ± 4.9 a |
| Tillers Number | Height (cm) | Diameter (mm) | ||
|---|---|---|---|---|
| B | MC | 16.3 + 1.5 bc | 109.3 ± 9.1 a | 9.3 ± 1.8 a |
| M200 | 10.0 + 2.9 def | 99.2 ± 4.3 ab | 8.7 ± 1.2 a | |
| M500 | 9.4 + 2.3 def | 97.6 ± 7.1 abc | 9.0 ± 1.4 a | |
| M750 | 6.0 + 1.7 f | 96.0 ± 5.9 abc | 8.4 ±1.6 a | |
| M900 | 6.0 + 1.9 f | 94.8 ± 4.1 abc | 8.7 ± 1. a | |
| U | MC | 27.3 + 4.2 a | 92.7 ± 5.9 abc | 7.3 ± 0.7 a |
| M200 | 12.0 + 1.2 bcd | 87.6 ± 7.2 bc | 8.2 ± 0.7 a | |
| M500 | 11.2 + 2.2 bcde | 86.2 ± 4.1 bc | 8.1 ± 0.6 a | |
| M750 | 9.0 + 2.8 def | 86.0 ± 7.9 bc | 7.8 ± 1.0 a | |
| M900 | 7.4 + 2.7 def | 84.4 ± 5.4 c | 8.3 ± 0.4 a | |
| A | MC | 17.0 + 2.0 b | 106.3 ± 6.8 a | 9.2 ± 0.3 a |
| M200 | 10.8 + 2.6 cdef | 100.2 ± 5.9 ab | 8.7 ±0.8 a | |
| M500 | 10.0 + 2.3 def | 98.8 ± 6.9 ab | 8.7 ± 0.7 a | |
| M750 | 6.4 + 1.6 ef | 97.2 ± 9.3 abc | 8.4 ± 1.4 a | |
| M900 | 6.0 + 0.9 f | 95.2 ± 9.2 abc | 8.8 ± 1.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Souki, K.S.; Liné, C.; Moravec, J.; Douay, F.; Pourrut, B. Response of Three Miscanthus × giganteus Cultivars to Toxic Elements Stress: Part 2, Comparison between Two Growing Seasons. Plants 2022, 11, 945. https://doi.org/10.3390/plants11070945
Al Souki KS, Liné C, Moravec J, Douay F, Pourrut B. Response of Three Miscanthus × giganteus Cultivars to Toxic Elements Stress: Part 2, Comparison between Two Growing Seasons. Plants. 2022; 11(7):945. https://doi.org/10.3390/plants11070945
Chicago/Turabian StyleAl Souki, Karim Suhail, Clarisse Liné, Jiří Moravec, Francis Douay, and Bertrand Pourrut. 2022. "Response of Three Miscanthus × giganteus Cultivars to Toxic Elements Stress: Part 2, Comparison between Two Growing Seasons" Plants 11, no. 7: 945. https://doi.org/10.3390/plants11070945






