Susceptibility of Cider Apple Accessions to European Canker—Comparison between Evaluations in Field Planted Trees and Rapid Screening Tests
Abstract
1. Introduction
2. Results
2.1. Field Phenotyping in Young and Mature Apple Trees
2.2. Isolates Identification and Pathogenicity Tests
2.3. Evaluation of Cultivar Resistance on Detached Shoots
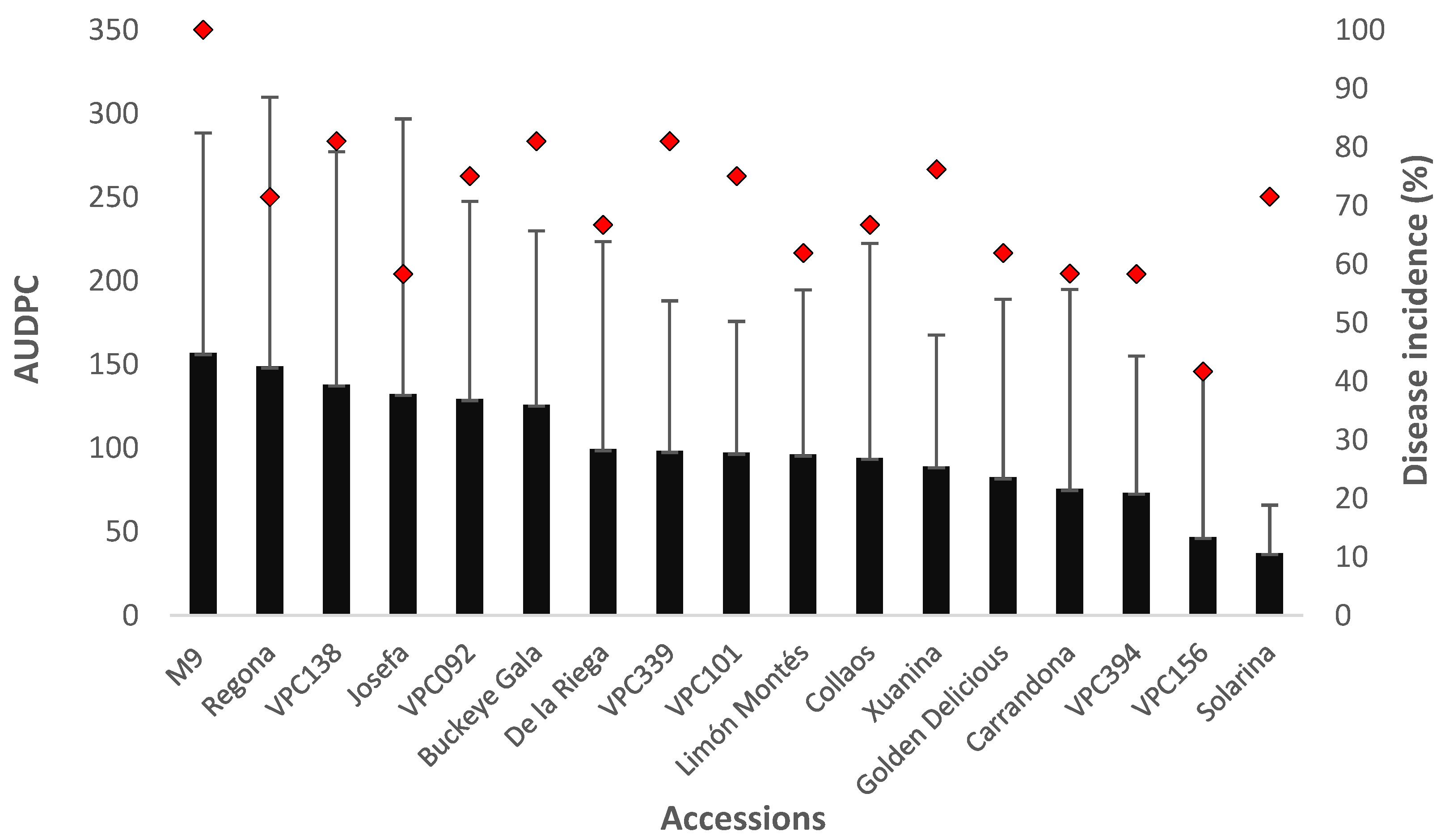
2.4. Evaluation of Cultivar Resistance on Potted Trees
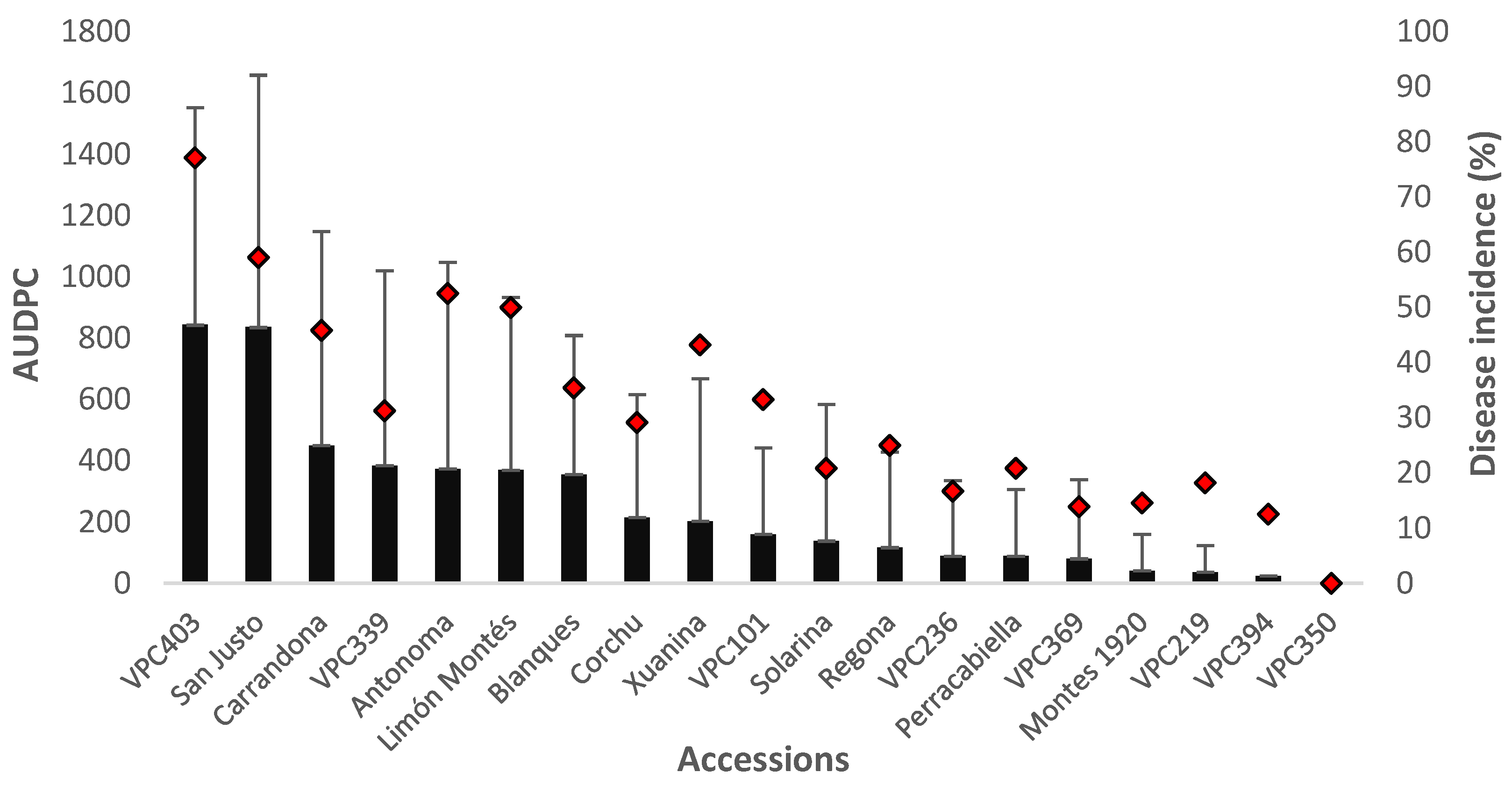
2.5. Evaluation of Cultivar Resistance Determined by Inoculating Adult Trees under Field Conditions
2.6. Comparison between Phenotyping Methods for Evaluating Partial Resistance to European Canker
3. Discussion
3.1. Pathogenicity of Local N. ditissima Isolates
3.2. Sources for Relative Resistance against N. ditissima among an Extensive Collection of Local Apple Accessions
3.3. Reliability of Phenotyping Methods and Resistance Parameters
4. Materials and Methods
4.1. Field Phenotyping in Young and Mature Apple Trees
4.1.1. Plant Material and Experimental Conditions
4.1.2. Phenotypic Susceptibility Assessment after Natural Infections
4.2. Isolates Identification and Pathogenicity Tests
4.2.1. Isolates Identification and Inoculum Preparation
4.2.2. Pathogenicity and Virulence of Local Isolates
4.3. Evaluation of Cultivar Resistance on Detached Shoots
4.4. Evaluation of Cultivar Resistance on Potted Trees
4.4.1. Inoculation Using In Vitro Conidial Production from a Single Isolate
4.4.2. Inoculation Using Field-Collected Inoculum
4.5. Evaluation of Cultivar Resistance Determined by Inoculating Adult Trees under Field Conditions
4.6. Experimental Design and Disease Evaluation Methods
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeller, S.M. European Canker of Pomaceous Fruit Trees; Oregon Agricultural College Experiment Station: Corvallis, OR, USA, 1926. [Google Scholar]
- Swinburne, T.R. The Seasonal Release of Spores of Nectria galligena from Apple Cankers in Northern Ireland. Ann. Appl. Biol. 1971, 69, 97–104. [Google Scholar] [CrossRef]
- Cooke, L.R. The Influence of Fungicide Sprays on Infection of Apple Cv. Bramley’s Seedling by Nectria galligena. Eur. J. Plant Pathol. 1999, 105, 783–790. [Google Scholar] [CrossRef]
- Weber, R.W.S. Biology and Control of the Apple Canker Fungus Neonectria ditissima (Syn. N. galligena) from a Northwestern European Perspective. Erwerbs-Obstbau 2014, 56, 95–107. [Google Scholar] [CrossRef]
- Bus, V.G.M.; Scheper, R.W.A.; Walter, M.; Campbell, R.E.; Kitson, B.; Turner, L.; Fisher, B.M.; Johnston, S.L.; Wu, C.; Deng, C.H.; et al. Genetic Mapping of the European Canker (Neonectria ditissima) Resistance Locus Rnd1 from Malus ‘Robusta 5’. Tree Genet. Genomes 2019, 15, 25. [Google Scholar] [CrossRef]
- Saville, R.; Berrie, A.; Olivieri, L.; Xu, X. European apple canker: Developing novel control strategies. In Proceedings of the 11th International IOBC-WPRS Workshop on Pome Fruit Diseases IOBC-WPRS, Jūrmala, Latvia, 26–30 June 2017. [Google Scholar]
- Xu, X.; Robinson, J. Effects of Fruit Maturity and Wetness on the Infection of Apple Fruit by Neonectria galligena. Plant Pathol. 2010, 59, 542–547. [Google Scholar] [CrossRef]
- Castlebury, L.A.; Rossman, A.Y.; Hyten, A.S. Phylogenetic Relationships of Neonectria/Cylindrocarpon on Fagus in North America. Botany 2006, 84, 1417–1433. [Google Scholar]
- Chaverri, P.; Salgado, C.; Hirooka, Y.; Rossman, A.; Samuels, G. Delimitation of Neonectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and related genera with Cylindrocarpon-like anamorphs. Stud. Mycol. 2011, 68, 57–78. [Google Scholar] [CrossRef]
- Weber, R.W.; Børve, J. Infection Biology as the Basis of Integrated Control of Apple Canker (Neonectria ditissima) in Northern Europe. CABI Agric. Biosci. 2021, 2, 5. [Google Scholar] [CrossRef]
- Grove, G. Nectria Canker. Compend. Apple Dis. 1990, 35–36. [Google Scholar]
- Beresford, R.M.; Kim, K.S. Identification of Regional Climatic Conditions Favorable for Development of European Canker of Apple. Phytopathology 2011, 101, 135–146. [Google Scholar] [CrossRef]
- Dubin, H.; English, H. Factors Affecting Apple Leaf Scar Infection by Nectria galligena Conidia. Phytopathology 1974, 64, 1201–1203. [Google Scholar] [CrossRef]
- Latorre, B.A.; Rioja, M.E.; Lillo, C.; Muñoz, M. The Effect of Temperature and Wetness Duration on Infection and a Warning System for European Canker (Nectria galligena) of Apple in Chile. Crop Prot. 2002, 21, 285–291. [Google Scholar] [CrossRef]
- Xu, X.-M.; Ridout, M. The Effects of Inoculum Dose, Duration of Wet Period, Temperature and Wound Age on Infection by Nectria galligena of Pruning Wounds on Apple. Eur. J. Plant Pathol. 1998, 104, 511–519. [Google Scholar] [CrossRef]
- Amponsah, N.; Beresford, M.W.R.; Scheper, R. Seasonal Wound Presence and Susceptibility to Neonectria ditissima Infection in New Zealand Apple Trees. N. Z. Plant Prot. 2015, 68, 250–256. [Google Scholar] [CrossRef]
- Cooke, L.; Watters, B.; Brown, A. The Effect of Fungicide Sprays on the Incidence of Apple Canker (Nectria galligena) in Bramley’s Seedling. Plant Pathol. 1993, 42, 432–442. [Google Scholar] [CrossRef]
- Cross, J. Guidelines for Integrated Production of Pome Fruits in Europe; IOBC-WPRS: Bellegarde, France, 2002; p. 25. [Google Scholar]
- Gómez-Cortecero, A.; Saville, R.J.; Scheper, R.W.A.; Bowen, J.K.; Agripino De Medeiros, H.; Kingsnorth, J.; Xu, X.; Harrison, R.J. Variation in Host and Pathogen in the Neonectria/Malus Interaction; toward an Understanding of the Genetic Basis of Resistance to European Canker. Front. Plant Sci. 2016, 7, 1365. [Google Scholar] [CrossRef]
- Dapena, E.; Blázquez, M.D.; Fernández, M. Recursos Fitogenéticos Del Banco de Germoplasma de Manzano Del SERIDA. Tecnol. Agroaliment. Boletín Inf. SERIDA 2006, 3, 34–39. [Google Scholar]
- Delgado, A.; Egea, J.A.; Luedeling, E.; Dapena, E. Agroclimatic Requirements and Phenological Responses to Climate Change of Local Apple Cultivars in Northwestern Spain. Sci. Hortic. 2021, 283, 110093. [Google Scholar] [CrossRef]
- Dapena, E.; Fernandez-Ceballos, A. Estudio Del Cambio Climático y Sus Implicaciones En El Cultivo Del Manzano En Asturias. Tecnol. Agroaliment. Boletín Inf. SERIDA 2007, 4, 18–24. [Google Scholar]
- Alston, F. Response of Apple Cultivars to Canker, Nectria galligena. East Malling Res. Stn. Annu. Rep. 1970, 147–148. [Google Scholar]
- Borecki, Z.; Czynczyk, A. Susceptibility of Apple Cultivars to Bark Canker Diseases. Acta Agrobot. 1985, 38, 49–59. [Google Scholar] [CrossRef]
- Van de Weg, W.E. Screening for Resistance to Nectria galligena Bres. in Cut Shoots of Apple. Euphytica 1989, 42, 233–240. [Google Scholar] [CrossRef]
- Dapena, E. Comportamiento Agronómico y Tecnológico de Variedades de Manzano Asturianas. Ph.D. Thesis, Universidad de Oviedo, Asturias, Spain, 1996. [Google Scholar]
- Garkava-Gustavsson, L.; Zborowska, A.; Sehic, J.; Rur, M.; Nybom, H.; Englund, J.; Lateur, M.; Van de Weg, E.; Holefors, A. Screening of Apple Cultivars for Resistance to European Canker, Neonectria ditissima. Acta Hortic. 2013, 976, 529–536. [Google Scholar] [CrossRef]
- Ghasemkhani, M.; Liljeroth, E.; Sehic, J.; Zborowska, A.; Nybom, H. Cut-off Shoots Method for Estimation of Partial Resistance in Apple Cultivars to Fruit Tree Canker Caused by Neonectria ditissima. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2015, 65, 412–421. [Google Scholar] [CrossRef]
- Umpleby, E.; Swarbrick, T. The Incidence of Canker in Young Cider Apple Trees. Ann. Rep. Long Ashton. Agric. Hortic. Res. Stn. For. 1936, 1935, 98–103. [Google Scholar]
- Zagaja, S.; Millikan, D.; Kaminski, W.; Myszka, T. Field Resistance to Nectria Canker in Apple. Plant Report. 1971, 55, 445–447. [Google Scholar]
- Lateur, M.; Populer, C. Screening Fruit Tree Genetic Resources in Belgium for Disease Resistance and Other Desirable Characters. Euphytica 1994, 77, 147–153. [Google Scholar] [CrossRef]
- Kazlouskaya, Z.A.; Marchuk, Y.G. Evaluation of Susceptibility to Nectria Canker as Initial Material for Apple Breeding. Acta Hortic. 2013, 549–554. [Google Scholar] [CrossRef]
- Lateur, M. Evaluation of Apple (Malus domestica Borkh.) Genetic Resources for European Canker (Nectria galligena Bres.) Resistance Methodological Study. Ph.D. Thesis, Faculté des Sciences Agronomiques de Gembloux, Gembloux, Belgium, 2001. [Google Scholar]
- Scheper, R.W.A.; Fisher, B.M.; Taylor, T.; Hedderley, D.I. Detached Shoot Treatments Cannot Replace Whole-Tree Assays When Phenotyping for Apple Resistance to Neonectria ditissima. N. Z. Plant Prot. 2018, 71, 151–157. [Google Scholar] [CrossRef]
- Gómez-Cortecero, A. The Molecular Basis of Pathogenicity of N. ditissima. Ph.D. Thesis, University of Reading, Reading, UK, 2020. [Google Scholar]
- Langrell, S.; Barbara, D. Magnetic Capture Hybridisation for Improved PCR Detection Of Nectria galligena from Lignified Apple Extracts. Plant Mol. Biol. Report. 2001, 19, 5–11. [Google Scholar] [CrossRef]
- Saville, R.; Olivieri, L.; Xu, X.; Fountain, M. Fungal Diseases of Fruit: Apple Cankers in Europe. In Integrated Management of Diseases and Insect Pests of Tree Fruit; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 59–83. [Google Scholar]
- Campos, J.D.S.; Bogo, A.; Sanhueza, R.M.V.; Casa, R.T.; da Silva, F.N.; da Cunha, I.C.; Kuhnem, P.R. European Apple Canker: Morphophysiological Variability and Pathogenicity in Isolates of Neonectria ditissima in Southern Brazil. Ciência Rural 2017, 47, e20160288. [Google Scholar] [CrossRef]
- Bramel, P.; Volk, G.M. A Global Strategy for the Conservation and Use of Apple Genetic Resources; Global Crop Diversity Trust: Bonn, Germany, 2019. [Google Scholar]
- Dapena, E.; Blázquez, M. Descripción de Las Variedades de Manzana de La DOP Sidra de Asturias; SERIDA: Villaviciosa, Spain, 2009. [Google Scholar]
- Wenneker, M.; Goedhart, P.; Van der Steeg, P.; Van de Weg, W.; Schouten, H. Methods for the Quantification of Resistance of Apple Genotypes to European Fruit Tree Canker Caused by Neonectria ditissima. Plant Dis. 2017, 101, 2012–2019. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garkava-Gustavsson, L.; Ghasemkhani, M.; Zborowska, A.; Englund, J.-E.; Lateur, M.; van de Weg, E. Approaches for Evaluation of Resistance to European Canker (Neonectria ditissima) in Apple. Acta Hortic. 2016, 1127, 75–82. [Google Scholar] [CrossRef]
- Karlström, A.; Gómez-Cortecero, A.; Nellist, C.F.; Ordidge, M.; Dunwell, J.M.; Harrison, R.J. Identification of Novel Genetic Regions Associated with Resistance to European Canker in Apple. bioRxiv 2021, in press. [Google Scholar]
- Swinburne, T.; Cartwright, J.; Flack, N.J.; Brown, A. The control of apple canker (Nectria galligena) in a young orchard with established infections. Ann. Appl. Biol. 1975, 81, 61–73. [Google Scholar] [CrossRef]
- Saure, M. Untersuchungen Ueber Die Voraussetzungen Fuer Ein Epidemisches Auftreten Des Obstbaumkrebses (Nectria galligena Bres). Ph.D. Thesis, Technische Universität, Berlin Germany, 1961. [Google Scholar]
- Petrini, O. Endophytische Pilze in Epiphytischen Areceae, Promeliaceae Und Orchidaceae. Sydowia 1981, 34, 135–148. [Google Scholar]
- McCracken, A.R.; Berrie, A.; Barbara, D.J.; Locke, T.; Cooke, L.R.; Phelps, K.; Swinburne, T.R.; Brown, A.E.; Ellerker, B.; Langrell, S.R.H. Relative Significance of Nursery Infections and Orchard Inoculum in the Development and Spread of Apple Canker (Nectria galligena) in Young Orchards. Plant Pathol. 2003, 52, 553–566. [Google Scholar] [CrossRef]
- Walter, M.; Stevenson, O.; Amponsah, N.; Scheper, R.; Rainham, D.; Hornblow, C.; Kerer, U.; Dryden, G.; Latter, I.; Butler, R. Control of Neonectria ditissima with Copper Based Products in New Zealand. N. Z. Plant Prot. 2015, 68, 241–249. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 5 February 2022).
- De Mendiburu, F.; Yaseen, M. Agricolae: Statistical Procedures for Agricultural Research; R Package Version 1.4.0; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]


| Susceptibility Group | Field Resistance Classification | Range of Scores for Disease Severity | Number of Cultivars | Mean ± Standard Error | Percentage of the Collection |
|---|---|---|---|---|---|
| 1 | Very resistant | 0–0.5 | 22 | 0.34 ± 0.04 | 5.5 |
| 2 | Resistant | 0.51–1 | 43 | 0.88 ± 0.02 | 10.75 |
| 3 | Moderately resistant | 1.01–1.5 | 51 | 1.43 ± 0.02 | 12.75 |
| 4 | Intermediate | 1.51–2 | 75 | 1.88 ± 0.01 | 18.75 |
| 5 | Moderately susceptible | 2.01–2.75 | 94 | 2.5 ± 0.02 | 23.5 |
| 6 | Susceptible | 2.76–3.25 | 82 | 3.08 ± 0.01 | 20.5 |
| 7 | Highly susceptible | 3.26–5 | 33 | 3.68 ± 0.03 | 8.25 |
| Isolate Name | Location | Year of Collection | Host | Disease Incidence (%) | AUDPC (mm) |
|---|---|---|---|---|---|
| OPC304 | Oles | 2017 | Local accession | 66.7 a | 201.83 a |
| P112 | Priesca | 2017 | ‘Carla’ | 60.6 a | 59.25 b |
| BGV344 | Villaviciosa | 2018 | ‘Urtebi’ | 72.7 a | 136.17 ab |
| Test | Variable | Df | n | p |
|---|---|---|---|---|
| Potted trees (single isolate inoculum) | AUDPC | 16 | 300 | <0.001 |
| Disease incidence (%) | 16 | 108 | 0.004 | |
| Potted trees (field-collected inoculum) | AUDPC | 15 | 300 | 0.011 |
| Disease incidence (%) | 15 | 140 | 0.05 | |
| Adult trees (field-collected inoculum) | AUDPC | 18 | 850 | <0.001 |
| Disease incidence (%) | 18 | 221 | <0.001 |
| Field Assessment (NI) | Detached Shoots (SI) | Potted Trees (SI) | Potted Trees (FI) | Adult Trees (FI) | |
|---|---|---|---|---|---|
| Field assessment (NI) | |||||
| Detached shoots (SI) | 0.23 (0.26) | ||||
| Potted trees (SI) | 0.15 (0.06) | 0.57 (0.50) | |||
| Potted trees (FI) | 0.13 (−0.38) | 0.06 (0.41) | 0.23 (0.24) | ||
| Adult trees (FI) | 0.41 (0.51) | 0.69 (0.49) | 0.14 (0.33) | −0.21 (0.36) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado, Á.; García-Fernández, B.; Gómez-Cortecero, A.; Dapena, E. Susceptibility of Cider Apple Accessions to European Canker—Comparison between Evaluations in Field Planted Trees and Rapid Screening Tests. Plants 2022, 11, 1145. https://doi.org/10.3390/plants11091145
Delgado Á, García-Fernández B, Gómez-Cortecero A, Dapena E. Susceptibility of Cider Apple Accessions to European Canker—Comparison between Evaluations in Field Planted Trees and Rapid Screening Tests. Plants. 2022; 11(9):1145. https://doi.org/10.3390/plants11091145
Chicago/Turabian StyleDelgado, Álvaro, Belén García-Fernández, Antonio Gómez-Cortecero, and Enrique Dapena. 2022. "Susceptibility of Cider Apple Accessions to European Canker—Comparison between Evaluations in Field Planted Trees and Rapid Screening Tests" Plants 11, no. 9: 1145. https://doi.org/10.3390/plants11091145
APA StyleDelgado, Á., García-Fernández, B., Gómez-Cortecero, A., & Dapena, E. (2022). Susceptibility of Cider Apple Accessions to European Canker—Comparison between Evaluations in Field Planted Trees and Rapid Screening Tests. Plants, 11(9), 1145. https://doi.org/10.3390/plants11091145






