Use of Gamma Radiation for the Genetic Improvement of Underutilized Plant Varieties
Abstract
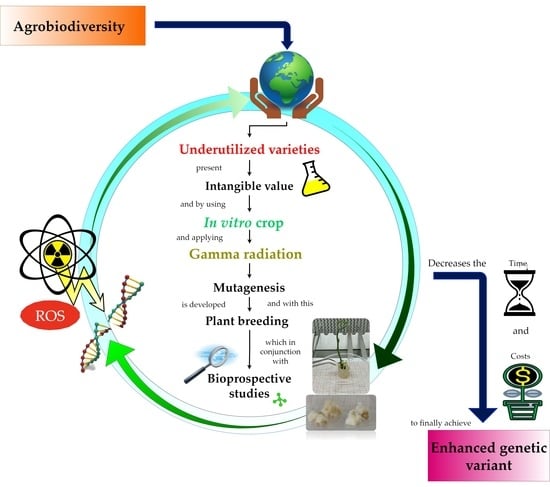
1. Introduction
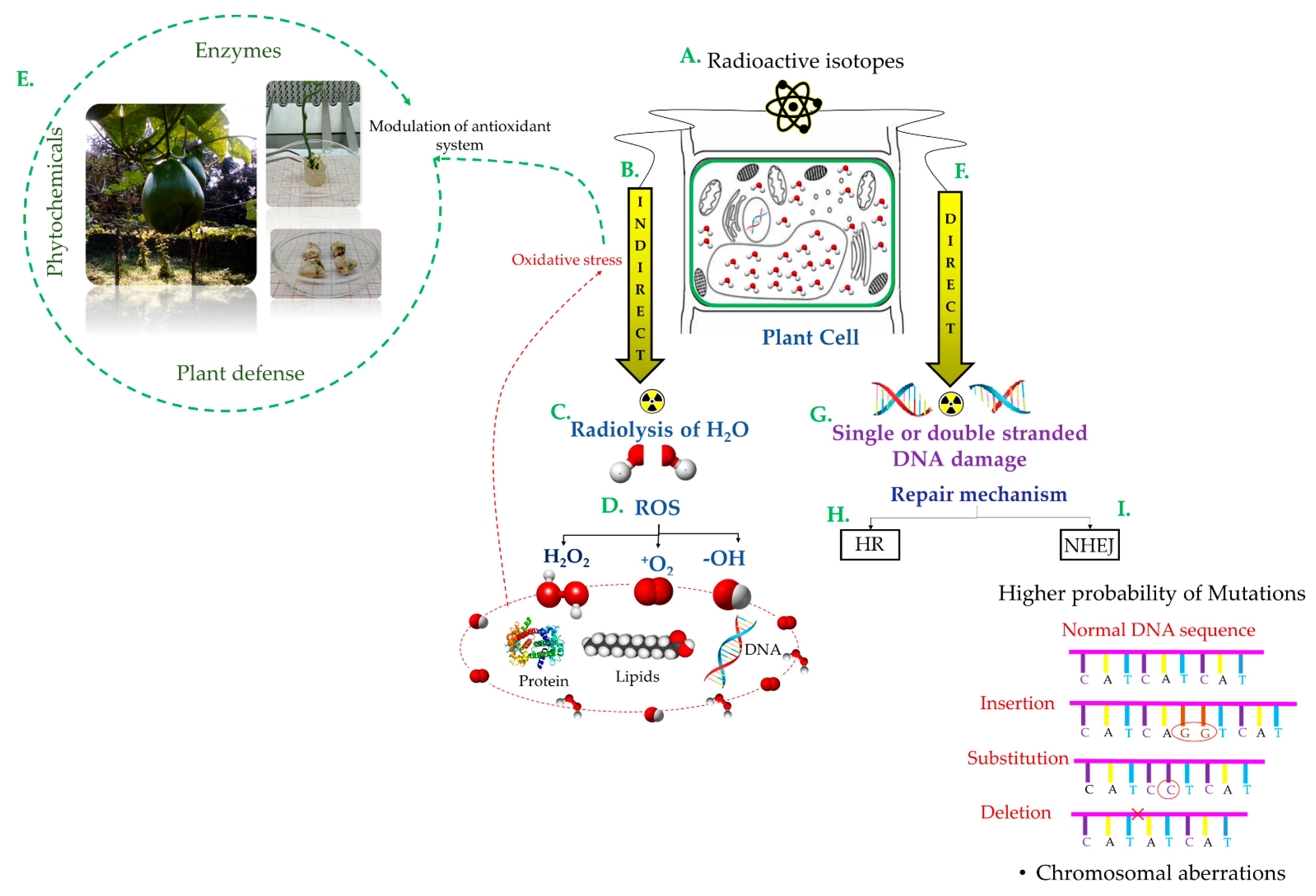
2. Radiosensitivity
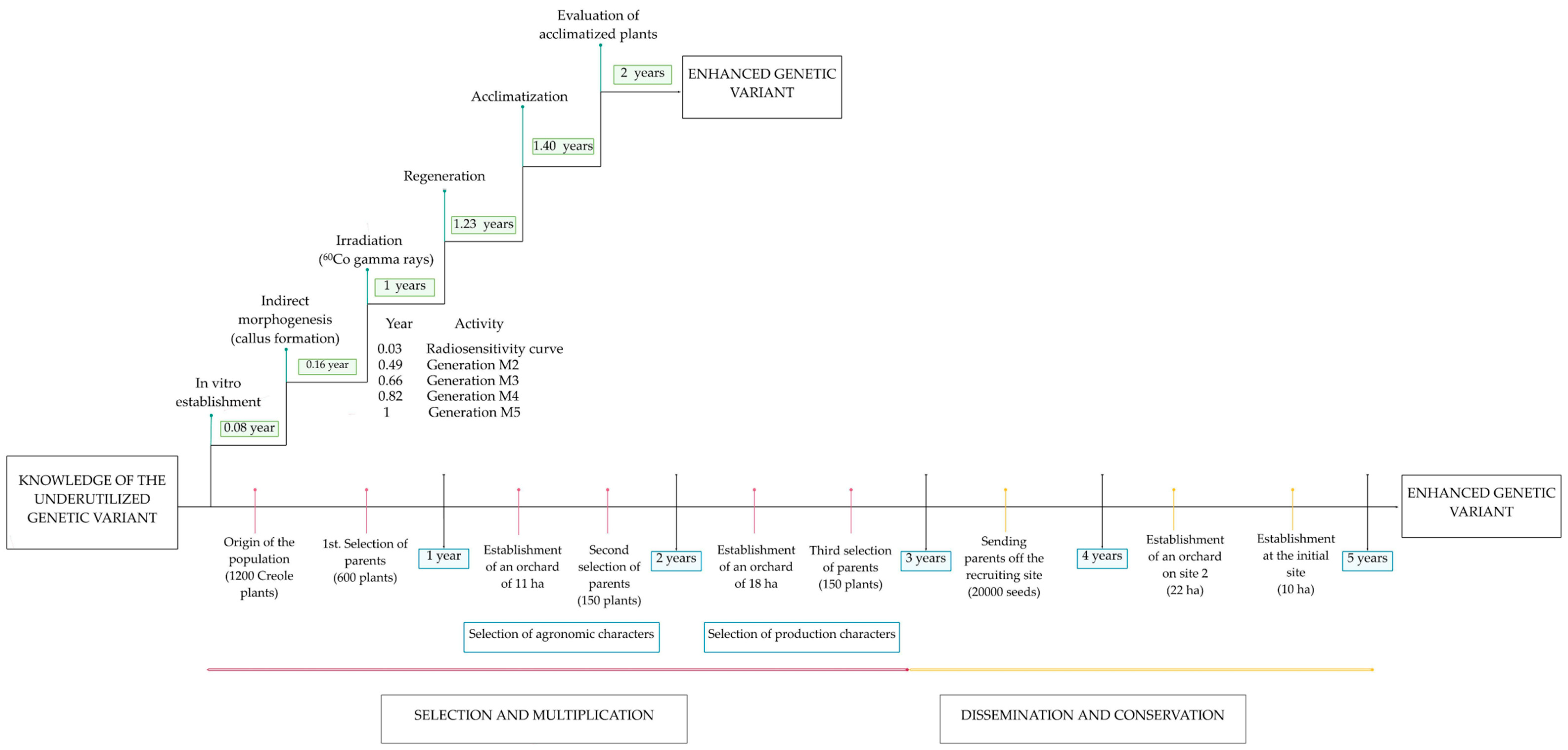
3. Gamma Radiation and In Vitro Culture
4. Gamma Radiation as a Tool for Plant Breeding
5. Effect of Gamma Radiation on the Concentration of Secondary Metabolites
6. Molecular Analysis for the Identification and Screening of Mutants
7. Prospects for the Application of Gamma Radiation in Underutilized Genetic Varieties
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spencer, L.M.M.; Forster, B.P.; Jankuloski, L. Manual on Mutation Breeding, 3rd ed.; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2018. [Google Scholar]
- Ludovici, G.M.; Oliveira de Souza, S.; Chierici, A.; Cascone, M.G.; d’Errico, F.; Malizia, A. Adaptation to Ionizing Radiation of Higher Plants: From Environmental Radioactivity to Chernobyl Disaster. J. Environ. Radioact. 2020, 222, 106375. [Google Scholar] [CrossRef] [PubMed]
- Obodovskiy, I. Chapter 2-Nuclei and Nuclear Radiations. Radiation 2019, 41–62. [Google Scholar] [CrossRef]
- Jan, S.; Parween, T.; Siddiqi, T.O.; Mahmooduzzafar. Effect of Gamma Radiation on Morphological, Biochemical, and Physiological Aspects of Plants and Plant Products. Environ. Rev. 2012, 20, 17–39. [Google Scholar] [CrossRef]
- Caplin, N.; Willey, N. Ionizing Radiation, Higher Plants, and Radioprotection: From Acute High Doses to Chronic Low Doses. Front. Plant Sci. 2018, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz, T.E.; Hasbach, G.P.; Andrade, J.M.G.; Sánchez, C.M.; Jiménez, J.G.; Bárcenas, T.F.; Arriaga, O.V. The genus Chenopodium: A potential food source. In Biotechnology of Neglected and Underutilized Crops; Springer: Dordrecht, The Netherlands, 2013; pp. 3–31. [Google Scholar]
- Bravo, M.; Arteaga, M.I.; Herrera, F.F. Bioinventario de especies subutilizadas comestibles y medicinales en el norte de Venezuela. Boletín Latinoam. Caribe Plantas Med. Aromát. 2017, 16, 347–360. [Google Scholar]
- Khan, S.A.; Rahman, L.; Verma, R.; Shanker, K. Physical and Chemical Mutagenesis in Stevia Rebaudiana: Variant Generation with Higher UGT Expression and Glycosidic Profile but with Low Photosynthetic Capabilities. Acta Physiol. Plant 2016, 38, 1–12. [Google Scholar] [CrossRef]
- Hernández, M.S.; Pedraza, S.M.E.; Antonio, L.P.; Gómez, S.J.M.; Morales, G.J.L. Mutagenesis in the Improvement of Ornamental Plants. Rev. Chapingo Ser. Hortic. 2019, 25, 151–167. [Google Scholar] [CrossRef]
- Wi, S.G.; Chung, B.Y.; Kim, J.H.; Baek, M.H.; Yang, D.H.; Lee, J.W.; Kim, J.S. Ultrastructural changes of cell organelles in Arabidopsis stems after gamma irradation. J. Plant Biol. 2005, 48, 195–200. [Google Scholar] [CrossRef]
- Datta, S.K.; Silva, J.A. Role of Induced Mutagenesis for Development of New Flower Colour and Type in Ornamentals. In Floriculture, Ornamental and Plant Biotechnology: Advances and Topical, 1st ed.; Global Science Books: Carrollton, GA, USA, 2006; pp. 640–645. Available online: http://www.globalsciencebooks.info/Journals/GSBJournals.html (accessed on 10 January 2022).
- Álvarez, H.A.; Morales, N.R.C.; Avendaño, A.H.C.; Corrales, L.R.; Villarrea, G.F.; Santellano, E.E.; Gomez, S.Y. Mean Lethal Dose (LD50) and Growth Reduction (GR (50)) Due to Gamma Radiation in Wilman Lovegrass (Eragrostis superba). Rev. Mex. Cienc. Pecu. 2019, 10, 227–238. [Google Scholar]
- Surakshitha, N.C.; Soorianathasundaram, K. Determination of Mutagenic Sensitivity of Hardwood Cuttings of Grapes ‘Red Globe’and ‘Muscat’ (Vitis vinifera L.) to gamma rays. Sci. Hortic. 2017, 226, 152–156. [Google Scholar] [CrossRef]
- Chakravarty, B.; Sen, S. Enhancement of Regeneration Potential and Variability by γ-Irradiation in Cultured Cells of Scilla Indica. Biol. Plant. 2001, 44, 189–193. [Google Scholar] [CrossRef]
- FAO. Laboratory Training Manual on the Use of Isotopes and Radiation in Entomology; FAO: Rome, Italy, 1977; Volume 61, p. 271. [Google Scholar]
- Al-Safadi, B.; Ayyoubi, Z.; Jawdat, D. The Effect of Gamma Irradiation on Potato Microtuber Production in vitro. Plant Cell Tissue Organ Cult. 2000, 61, 183–187. [Google Scholar] [CrossRef]
- Ernest, F.P.; Noëlle, M.A.H.; Godswill, N.N.; Thiruvengadam, M.; Simon, O.A.; Bille, N.H.; Shariati, M.A. Radiosensitivity of Two Varieties of Watermelon (Citrullus lanatus) to Different Doses of Gamma Irradiation. Braz. J. Bot. 2020, 43, 897–905. [Google Scholar] [CrossRef]
- Quintana, V.; Alvarado, L.; Alvarado, L.; Saravia, D.; Saravia, D.; Borjas, R.; Borjas, R.; Gómez, L.; Castro, C.V.; Julca, O.A.; et al. Gamma Radiosensitivity of Coffee (Coffea arabica L. Var. Typica). Peruv. J. Agron. 2019, 3, 74. [Google Scholar] [CrossRef]
- Álvarez, H.A.; Corrales, L.R.; Morales, N.C.R.; Avendaño, A.C.H.; Villarreal, G.F. Dosis Óptima de Irradiación Gamma Con Co 60 Para Inducción de Mutagénesis En Pastos. Nova Sci. 2017, 9, 65–82. [Google Scholar] [CrossRef]
- Ángeles, E.A.; Valencia, B.A.J.; Virgen, C.G.; Ramírez, S.C.; Paredes, G.L.; Hurtado-De la Peña, S. Determinación de la dosis letal (dl50) con CO60 en vitroplántulas de Agave Tequilana Var. Azul. Rev. Fitotec. Mex. 2013, 36, 381. [Google Scholar]
- Veitía, N.; García, L.R.; Bermúdez, C.I.; Orellana, P.; Padrón, Y.; Torres, D. Efecto de Las Radiaciones Gamma Sobre Callos de Papa Var. ‘Desirée’. Biotecnol. Veg. 2007, 7, 57–61. [Google Scholar]
- Mba, C.; Afza, R.; Shu, Q.Y. Mutagenic radiations: X-rays, ionizing particles and ultraviolet. In Plant Mutation Breeding and Biotechnology; Shu, Q.Y., Forster, B.F., Nakagawa, H., Eds.; CAB International and FAO: Rome, Italy, 2012; pp. 83–106. [Google Scholar]
- Esnault, M.A.; Legue, F.; Chenal, C. Ionizing Radiation: Advances in Plant Response. Environ. Exp. Bot. 2010, 68, 231–237. [Google Scholar] [CrossRef]
- Caro, M.D.P.; Estupiñán, R.S.Y.; Rache, C.L.Y.; Pacheco, M.J.C. Effect of Gamma Rays on Vegetative Buds of Physalis peruviana L. Acta Agronómica 2012, 61, 305–314. [Google Scholar]
- Zaka, R.; Chenal, C.; Misset, M.T. Study of External Low Irradiation Dose Effects on Induction of Chromosome Aberrations in Pisum Sativum Root Tip Meristem. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2002, 517, 87–99. [Google Scholar] [CrossRef]
- Wang, L.; Wu, J.; Lan, F.; Gao, P. Morphological, Cytological and Molecular Variations Induced by Gamma Rays in Chrysanthemum Morifolium ‘Donglinruixue’. Folia Hortic. 2020, 32, 87–96. [Google Scholar] [CrossRef]
- Balero, A.V.; Pérez, P.A.O.; Rodríguez, N.V.; Rodríguez, D.T. Crecimiento, Regeneración y Radiosensibilidad de Callos de Caña de Azúcar (Saccharum spp. Híbrido Var. “SP 70-1284”) Tratados Con Radiación Gamma Fuente 60Co. Biotecnol. Veg. 2004, 4, 165–169. [Google Scholar]
- Suprasanna, P.; Vitthal, S.B.; Yadav, P.V. In vitro Mutagenesis and Selection in Plant Tissue Cultures and Their Prospects for Crop Improvement. Bioremediat. Biodivers. Bioavailab. 2012, 6, 6–14. [Google Scholar]
- Al-Safadi, B.; Elias, R. Improvement of Caper (Capparis spinosa L.) Propagation Using in vitro Culture and Gamma Irradiation. Sci. Hortic. 2011, 127, 290–297. [Google Scholar] [CrossRef]
- Hasbullah, N.A.; Taha, R.M.; Saleh, A.; Mahmad, N. Irradiation Effect on in vitro Organogenesis, Callus Growth and Plantlet Development of Gerbera Jamesonii. Hortic. Bras. 2012, 30, 252–257. [Google Scholar] [CrossRef]
- Kapare, V.; Satdive, R.; Fulzele, D.P.; Malpathak, N. Impact of Gamma Irradiation Induced Variation in Cell Growth and Phytoecdysteroid Production in Sesuvium portulacastrum. J. Plant Growth Regul. 2017, 36, 919–930. [Google Scholar] [CrossRef]
- Billore, V.; Mirajkar, S.J.; Suprasanna, P.; Jain, M. Gamma Irradiation Induced Effects on in vitro Shoot Cultures and Influence of Monochromatic Light Regimes on Irradiated Shoot Cultures of Dendrobium Sonia Orchid. Biotechnol. Rep. 2019, 22, e00343. [Google Scholar] [CrossRef] [PubMed]
- Surya, M.I.; Ismaini, L.; Lailaty, I.Q. ‘In vitro’regeneration and Induction of Mutation in Loquat (‘Eriobotrya japonica’ L.). Aust. J. Crop Sci. 2019, 13, 701. [Google Scholar] [CrossRef]
- Due, M.S.; Susilowati, A.; Yunus, A. The Effect of Gamma Rays Irradiation on Diversity of Musa Paradisiaca Var. Sapientum as Revealed by ISSR Molecular Marker. Biodiversitas 2019, 20, 1416–1422. [Google Scholar] [CrossRef][Green Version]
- Pérez, J.M.; Tallón, C.I.; Pérez, T.O. Inducing Mutations in Citrus spp.: Sensitivity of Different Sources of Plant Material to Gamma Radiation. Appl. Radiat. Isot. 2020, 157, 109030. [Google Scholar] [CrossRef]
- Borzouei, A.; Kafi, M.; Khazaei, H.; Naseriyan, B.; Majdabadi, A. Effects of Gamma Radiation on Germination and Physiological Aspects of Wheat (Triticum aestivum L.) seedlings. Pak. J. Bot. 2010, 42, 2281–2290. [Google Scholar]
- Amri-Tiliouine, W.; Laouar, M.; Abdelguerfi, A.; Jankowicz-Cieslak, J.; Jankuloski, L.; Till, B.J. Genetic variability induced by gamma rays and preliminary results of low-cost TILLING on M2 generation of chickpea (Cicer arietinum L.). Front. Plant Sci. 2018, 9, 1568. [Google Scholar] [CrossRef] [PubMed]
- Shuryak, I.; Tkavc, R.; Matrosova, V.Y.; Volpe, R.P.; Grichenko, O.; Klimenkova, P.; Conze, I.H.; Balygina, I.A.; Gaidamakova, E.K.; Daly, M.J. Chronic gamma radiation resistance in fungi correlates with resistance to chromium and elevated temperatures, but not with resistance to acute irradiation. Sci. Rep. 2019, 9, 11361. [Google Scholar] [CrossRef]
- FAO/IAEA. MVD-Search. (s. f.). Available online: https://mvd.iaea.org/#!Search (accessed on 21 January 2021).
- Yasmeen, S.; Khan, M.T.; Khan, I.A. Revisiting the Physical Mutagenesis for Sugarcane Improvement: A Stomatal Prospective. Sci. Rep. 2020, 10, 16003. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, Y.; Zhao, L.; Mo, B.; Luo, T. Effect of Gamma Rays on Sophora Davidii and Detection of DNA Polymorphism through ISSR Marker. Biomed Res. Int. 2017, 2017, 8576404. [Google Scholar]
- Yaycili, O.; Alikamanoğlu, S. Induction of Salt-Tolerant Potato (Solanum tuberosum L.) Mutants with Gamma Irradiation and Characterization of Genetic Variations via RAPD-PCR Analysis. Turk. J. Biol. 2012, 36, 405–412. [Google Scholar]
- Abdelnour-Esquivel, A.; Perez, J.; Rojas, M.; Vargas, W.; Gatica-Arias, A. Use of Gamma Radiation to Induce Mutations in Rice (Oryza sativa L.) and the Selection of Lines with Tolerance to Salinity and Drought. Vitr. Cell. Dev. Biol.-Plant 2020, 56, 88–97. [Google Scholar] [CrossRef]
- Mabrouk, Y.; Charaabi, K.; Mahiout, D.; Rickauer, M.; Belhadj, O. Evaluation of Chickpea (Cicer arietinum L.) Irradiation-Induced Mutants for Resistance to Ascochyta Blight in Controlled Environment. Braz. J. Bot. 2018, 41, 311–318. [Google Scholar] [CrossRef]
- Rachmawati, D.; Hanifah, W.N.; Parjanto; Yunus, A. Selection of Short Stem Mentik Susu Rice M3 from Gamma Ray Irradiation. IOP Conf. Ser. Earth Environ. Sci. 2019, 250, 012020. [Google Scholar] [CrossRef]
- Vardhan, P.V.; Shukla, L.I. Gamma Irradiation of Medicinally Important Plants and the Enhancement of Secondary Metabolite Production. Int. J. Radiat. Biol. 2017, 93, 967–979. [Google Scholar] [CrossRef]
- Choi, H.I.; Han, S.M.; Jo, Y.D.; Hong, M.J.; Kim, S.H.; Kim, J.B. Effects of Acute and Chronic Gamma Irradiation on the Cell Biology and Physiology of Rice Plants. Plants 2021, 10, 439. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ahmed, O.K.; El-Desouky, W. Effect of Low Doses γ-Irradiation on Oxidative Stress and Secondary Metabolites Production of Rosemary (Rosmarinus officinalis L.) Callus Culture. Radiat. Phys. Chem. 2011, 80, 968–976. [Google Scholar] [CrossRef]
- Moghaddam, S.S.; Jaafar, H.; Ibrahim, R.; Rahmat, A.; Aziz, M.A.; Philip, E. Effects of Acute Gamma Irradiation on Physiological Traits and Flavonoid Accumulation of Centella asiatica. Molecules 2011, 16, 4994–5007. [Google Scholar] [CrossRef]
- Mohajer, S.; Taha, R.M.; Lay, M.M.; Esmaeili, A.K.; Khalili, M. Stimulatory Effects of Gamma Irradiation on Phytochemical Properties, Mitotic Behaviour, and Nutritional Composition of Sainfoin (Onobrychis viciifolia Scop.). Sci. World J. 2014, 2014, 854093. [Google Scholar] [CrossRef]
- Ashouri Sheikhi, A.; Hassanpour, H.; Jonoubi, P.; Ghorbani Nohooji, M.; Nadimifar, M.S. The Effect of Gamma Irradiation on In vitro Total Phenolic Content and Antioxidant Activity of Ferula gummosa Bioss. J. Med. Plants 2016, 3, 122–131. [Google Scholar]
- Muhallilin, I.; Aisyah, S.I.; Sukma, D. The Diversity of Morphological Characteristics and Chemical Content of Celosia Cristata Plantlets Due to Gamma Ray Irradiation. Biodiversitas 2019, 20, 862–866. [Google Scholar] [CrossRef]
- Magdy, A.M.; Fahmy, E.M.; Al-Ansary, A.E.R.M.F.; Awad, G. Improvement of 6-Gingerol Production in Ginger Rhizomes (Zingiber officinale Roscoe) Plants by Mutation Breeding Using Gamma Irradiation. Appl. Radiat. Isot. 2020, 162, 109193. [Google Scholar] [CrossRef]
- Azeez, H.; Ibrahim, K.; Pop, R.; Pamfil, D.; Hârţa, M.; Bobiș, O. Changes Induced by Gamma Ray Irradiation on Biomass Production and Secondary Metabolites Accumulation in Hypericum Triquetrifolium Turra Callus Cultures. Ind. Crops Prod. 2017, 108, 183–189. [Google Scholar] [CrossRef]
- Parchin, R.A.; Ghomi, A.A.N.; Badi, H.N.; Eskandari, A.; Navabpour, S.; Mehrafarin, A. Growth Characteristics and Phytochemical Responses of Iranian Fenugreek (Trigonella foenum-graecum L.) Exposed to Gamma Irradiation. Ind. Crops Prod. 2019, 139, 111593. [Google Scholar] [CrossRef]
- Mariadoss; Satdive, R.; Fulzele, D.P.; Ramamoorthy, S.; Doss, G.P.; Zayed, H.; Younes, S.; Rajasekaran. Enhanced Production of Anthraquinones by Gamma-Irradiated Cell Cultures of Rubia Cordifolia in a Bioreactor. Ind. Crops Prod. 2020, 145, 111987. [Google Scholar] [CrossRef]
- Topuz, A.; Ozdemir, F. Influences of Gamma Irradiation and Storage on the Capsaicinoids of Sun-Dried and Dehydrated Paprika. Food Chem. 2004, 86, 509–515. [Google Scholar] [CrossRef]
- Khalil, S.A.; Ahmad, N.; Zamir, R. Gamma Radiation Induced Variation in Growth Characteristics and Production of Bioactive Compounds during Callogenesis in Stevia rebaudiana (Bert.). New Negat. Plant Sci. 2015, 1–2, 1–5. [Google Scholar] [CrossRef]
- Kim, D.S.; Song, M.; Kim, S.H.; Jang, D.S.; Kim, J.B.; Ha, B.K.; Kim, S.H.; Lee, K.J.; Kang, S.Y.; Jeong, I.Y. The Improvement of Ginsenoside Accumulation in Panax ginseng as a Result of γ-Irradiation. J. Ginseng Res. 2013, 37, 332–340. [Google Scholar] [CrossRef]
- Fulzele, D.P.; Satdive, R.; Kamble, S.; Singh, S.; Singh, S. Improvement of Anticancer Drug Camptothecin Production by Gamma Irradiation on Callus Cultures of Nothapodytes Foetida. Int. J. Pharm. Res. Allied Sci. 2015, 4, 19–27. [Google Scholar]
- Wang, X.; Ma, R.; Cui, D.; Cao, Q.; Shan, Z.; Jiao, Z. Physio-Biochemical and Molecular Mechanism Underlying the Enhanced Heavy Metal Tolerance in Highland Barley Seedlings Pre-Treated with Low-Dose Gamma Irradiation. Sci. Rep. 2017, 7, 14233. [Google Scholar] [CrossRef]
- Li, F.; Shimizu, A.; Nishio, T.; Tsutsumi, N.; Kato, H. Comparison and Characterization of Mutations Induced by Gamma-Ray and Carbon-Ion Irradiation in Rice (Oryza sativa L.) Using Whole-Genome Resequencing. G3 Genes Genomes Genet. 2019, 9, 3743–3751. [Google Scholar] [CrossRef]
- Ishak, I. Identification of the Second Mutation of BADH2 Gene Derived from Rice Mutant Lines Induced by Gamma Rays. At. Indones. 2016, 42, 39. [Google Scholar] [CrossRef][Green Version]
- Tan, C.; Zhang, X.Q.; Wang, Y.; Wu, D.; Bellgard, M.I.; Xu, Y.; Shu, X.; Zhou, G.; Li, C. Characterization of Genome-Wide Variations Induced by Gamma-Ray Radiation in Barley Using RNA-Seq. BMC Genom. 2019, 20, 783. [Google Scholar] [CrossRef]
- González, T.M.; Prat, M. Desarrollo de Una Plataforma de Tilling En Melón (Cucumis melo L.). Doctoral Thesis, Universitat Autònoma de Barcelona, Bellaterra, Spain, 2014. [Google Scholar]
- Cho, H.Y.; Park, S.J.; Kim, D.S.; Jang, C.S. A TILLING Rice Population Induced by Gamma-Ray Irradiation and Its Genetic Diversity. Korean J. Breed. Sci. 2010, 42, 365–373. [Google Scholar]
- Hwang, S.G.; Kim, J.H.; Jang, C.S. A Major QTL and a Candidate Gene for Heading Date in an Early Maturing Rice Mutant Induced by Gamma Ray Irradiation. Genes Genom. 2016, 38, 747–756. [Google Scholar] [CrossRef]
- Kang, R.; Seo, E.; Park, A.; Kim, W.J.; Kang, B.H.; Lee, J.H.; Kim, S.H.; Kang, S.Y.; Ha, B.K. A Comparison of the Transcriptomes of Cowpeas in Response to Two Different Ionizing Radiations. Plants 2021, 10, 567. [Google Scholar] [CrossRef]
- Kim, D.G.; Lyu, J.I.; Lim, Y.J.; Kim, J.M.; Hung, N.N.; Eom, S.H.; Kim, S.H.; Kim, J.B.; Bae, C.H.; Kwon, S.J. Differential Gene Expression Associated with Altered Isoflavone and Fatty Acid Contents in Soybean Mutant Diversity Pool. Plants 2021, 10, 1037. [Google Scholar] [CrossRef]
- Domínguez García, I.A. El Marco Jurídico Para el Aprovechamiento, Conservación y Promoción de los Recursos Fitogenéticos Para la Alimentación y la Agricultura; Chapingo: Texcoco, Mexico, 2014. [Google Scholar]
- FAO. Primer Informe Sobre El Estado de Los Recursos Fitogenéticos en el Mundo; FAO: Roma, Italy, 1996. [Google Scholar]
- Arora, R.K. Diversity in Underutilized Plant Species: An Asia-Pacific Perspective; Bioversity International: New Delhi, India, 2014. [Google Scholar]
- Caetano, C.M.; Peña Cuellar, R.D.; Maigual Juajibioy, J.L.; Vásquez Ávila, L.N.; Caetano Nunes, D.G.; Caetano Nunes de Pazdiora, B.R. Mejoramiento Participativo: Herramienta Para La Conservación de Cultivos Subutilizados y Olvidados. Acta Agron. 2015, 64 (Suppl. S3), 307–327. [Google Scholar] [CrossRef][Green Version]
- Padulosi, S.; Hodgkin, T.; Williams, J.; Haq, N.; Engles, J.M.M.; Rao, V.R.; Jackson, M.T. 30 underutilized crops: Trends, challenges and opportunities in the 21st century. Manag. Plant Genet. Divers. 2002, 323. Available online: https://eprints.soton.ac.uk/53786/ (accessed on 10 January 2022).
- González, S.R.; Cadena, I.J.; Morales, F.F.J.; Ruiz, V.V.M.; Pimentel, L.J.; Peña, L.A. Model for the conservation and sustainable use of plant genetic resources in México. Wulfenia J. 2015, 22, 333–353. [Google Scholar]
- Linares, E.; Bye, R. Las especies subutilizadas de la milpa. Rev. Digit. Univ. 2015, 16, 22. [Google Scholar]
- Luna, C.L.M.; González, E.A.R. La chincuya (Annona purpurea Moc. & Sessé ex Dunal): Una planta mesoamericana. In Anonáceas; Universidad Autónoma Chapingo: Texcoco, Mexico, 2015; p. 229. [Google Scholar]
- De la Cruz Torres, E. Aplicación de la Energía Nuclear en el Mejoramiento de Pseudocereales Nativos de México. 2010. Available online: https://xdoc.mx/documents/aplicacion-de-la-energia-nuclear-en-el-mejoramiento-de-5e9229009c7b4 (accessed on 10 January 2022).
- FAO. Segundo Plan de Acción Mundial Para los Recursos Fitogenéticos Para la Alimentación y la Agricultura; FAO: Roma, Italy, 2011; 108p. [Google Scholar]
- Iñiguez, L.M.I.; Cadena, I.J.; Soto, H.R.M.; Morales, F.F.J.; Cortes, C.M.; Watanabe, K.N.; Cadena, Z.J.D. Bioprospecting of Sechium spp. varieties for the selection of characters with pharmacological activity. Sci. Rep. 2021, 11, 6185. [Google Scholar] [CrossRef]
- Avendaño, A.C.H.; Cadena, I.J.; Arévalo, G.M.L.C.; Cisneros, S.V.M.; Morales, F.F.J.; Ruiz, P.L.M. Mejoramiento genético participativo en chayote. Agroproductividad 2014, 7, 6. [Google Scholar]

| Basic and Industrial | Fruit Trees | Vegetables | Impulse | Ornamental |
|---|---|---|---|---|
| Agave spp. | Persea spp. | Capsicum spp. | Bixa orellana L. | Bromeliaceae |
| Gossypium barbadense L. | Theobroma spp. | Solanum lycopersicum L. | Suaeda acuminata (C. A. Mey.) Moq. | Cactaceae |
| Phaseolus vulgaris L. | Juglans spp. | Cucurbita spp. | Portulaca oleracea L. | Tagetes spp. |
| Helianthus annuus L. | Carica spp. | Ipomoea spp. | Yucca spp. | Dahlia spp. |
| Jatropha curcas L. | Vitis spp. | Sechium spp. | Echeveria spp. | |
| Zea mays L. | Annona spp. | Solanum cardiophyllum Lindl. | Hymenocallis spp. | |
| Vanilla spp. | Spondias spp. | Physalis spp. | Euphorbiaceae | |
| Amaranthus spp. | Psidium guajava L. | Orchidaceae | ||
| Simmondsia chinensis (Link) C. K. Schneid | Byrsonima crassifolia (L.)Kunth | Beaucarnea recurvata Lem. | ||
| Opuntia spp. | Tigridia spp. | |||
| Philodendron spp. | ||||
| Pouteria spp. | ||||
| Crataegus spp. |
| Common Name | Scientific Name | Irradiated Tissue Material | Treatment | LD50 | Observations | Reference |
|---|---|---|---|---|---|---|
| Watermelon | Citrullus lanatus (Thunb.) Matsum. & Nakai var. Kaolack and var. Crimson sweet | Seeds | 100, 200, 300, 400, and 600 Gy | Kaolack 225.40 Gy and Crimson sweet 221.56 Gy | Radiosensitivity of the two most frequently cultivated varieties in Cameroon and determination of LD50. | [16] |
| Coffee plant | Coffea arabica L. var. typica | Seeds | 0, 50, 100, and 150 Gy | 100 Gy | Determination of LD50 and morphological changes in plant | [17] |
| Wilman lovegrass | Eragrostis superba Peyr. | Seeds | 100, 200, 300, 450, 600, 900, 1400, 2000, and 4000 Gray | 2486 Gy | Determination of LD50. | [18] |
| Grasses: llorón, buffel, banderita, and navajita | Lloron (Eragrostis curvula), buffel (Pennisetum ciliare), banderita (Bouteloua curtipendula), and navajita (Bouteloua gracilis) | Seeds | 100, 200, 300, 450, 600, and 900 Gray | Pasto lloron 628 Gy, buffel 712 Gy, banderita 698 Gy, and navajita 411 Gy | Determination and comparison of LD50 in pastures. | [19] |
| Agave | Agave tequilana Weber var. Azul | Callus cultures and seedlings | 10, 20, 30, 40, and 50 Gy | seedlings 20–25 Gy; Callus 16 Gy | Determination of LD50 and comparison between plant material. | [20] |
| Potato | Solanum tuberosum L. var. Désirée | Callus cultures | 5, 10, 15, 20, and 30 Gy | 10 Gy | Determination of mean lethal dose. | [21] |
| Golden berry/Uchuva | Physalis peruviana L. | Axillary buds | 50, 100, 200, and 300 Gy | Higher percentage of cells with chromosomal alterations. | [24] | |
| Chrysanthemum | Chrysanthemum morifolium (Ramat.) “Donglinruixue” | Seeds | 0, 15, 20, 25, 30, and 35 Gy * | 35 Gy | The seeds will form genomic and chromosomal abnormalities during anaphase. | [26] |
| Sugar cane | Saccharum spp. Híbrido var. “SP 70-1284” | Callus cultures | 10, 20, 30, 40, 50, 60, 70, and 80 Gy | 30 Gy | Determination of LD50. | [27] |
| Gerbera | Gerbera jamesonii H. Bolus | In vitro explant growth, callus cultures and seedlings | 10, 20, 30, 40, 50, and 60 Gy | 20 gy | Callus fresh weight decrease response. | [30] |
| Beach purslane | Sesuvium portulacastrum L. | Shoots | 5 to 40 Gy | 20 Gy | Increased concentration of ecdisteroid 20-hydroxyecdysone. | [31] |
| Orchid | Dendrobium sonia | Shoots | 15–45 Gy | 30 GY | GR decreased shoot length, fresh weight, and leaf area, but its combination with yellow light increased shoot survival and length, fresh weight, and chlorophyll content | [32] |
| Loquat | Eriobotrya japonica L. | Callus cultures and seedlings | (0, 10, 30, and 50 Gy) | 10 Gy | Response in growth traits: callus diameter, callus height, number of shoots, number of leaves, and height of seedlings. | [33] |
| Banana | Musa paradisiaca L. | In vitro sprout seedlings | 10 Gy, 20 Gy, and 30 Gy | 10 and 20 Gy | Seedling morphological properties. Bases of mass propagation. | [34] |
| Citrus | Citrus spp. (several varieties: ‘Alemow’ and sour orange as citrus rootstock, lemon cv. ‘Fino 49’ and ‘Verna 51’, tangerine cv. ‘Nova’, and lime cv. ‘Bearss’) | Seeds, buds, and nodal segments | Seeds 0, 50, 100, 150, 200, and 250 Gy Buds 0, 25, 50, 75, and 100 Gy Nodal segments 0, 10, 20, 30, 40, and 50 Gy | Seeds (LD50 of 127 Gy in Alemow, and 156 Gy in sour orange). Buds (LD50 around 50 Gy for all cultivars) and nodal segments (LD50 around 25 Gy for both lemon cultivars). | Difficult-breeding species. | [35] |
| Wheat | Triticum aestivum L. | Seeds | 100, 200, 300 and 400 Gy | 100 Gy | 85% increase in proline concentration and higher chlorophyll a concentration in seedlings. | [36] |
| Chickpea | Cicer arietinum L. | Seeds | 50 a 750 Gy (frequency of 50 Gy) with a dose rate of 10.606 Gy min−1 | 150 Gy | Lines resistant to Ascochyta rabiei. | [44] |
| Rice | Oryza sativa L. var. Mentik Susu | M3 Seeds | 200 gGy | Mutants with short plant height, high productivity, higher seed yield, and short harvest age. | [45] | |
| Asiatic spark | Centella asiática (L.) Urb. | Axillary buds | 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, and 120 Gy | 20 and 30 Gy | Higher concentrations of total flavonoids. | [49] |
| Esparceta, Sainfoin | Onobrychis viciifolia Scop. Syn. Onobrychis sativa L. | Seeds | 30, 60, 90, and 120 Gy | 90 Gy | Remarkable increase in the phenolic content of the leaf extract and increase of alkaloid Berberine. | [50] |
| Barijeh | Ferula gummosa Boiss. | Callus cultures | 0 to 25 Gy | Of 20 and 25 Gy | Increased phenolic content. | [51] |
| Jengger Ayam | Celosia cristata L. | Seedlings | 0, 25, 50, and 75 Gy | 25 Gy | The C1U3 2.3.1 mutant presents triterpenic compounds that were not found in the controls. | [52] |
| Curled-leaved St. John’s-wort | Hypericum triquetrifolium Turra | Callus cultures | 10, 20, 40, and 50 Gy | 10 Gy | Higher content of phytochemicals than in the control samples. | [54] |
| Fenugreek | Trigonella foenum-graecum L. | Seeds | 0, 100, 200, 300, and 400 Gy | 100 Gy | 7% and 9% increases in trigonelline and nicotinic acid. | [55] |
| Common madder or Indianmadder | Rubia cordifolia L. | Callus cultures | 2, 4, 6, 8, 10, 12, 14, and 16 Gy | 8 Gy | Radiation dose for kinetic study of cell growth and anthraquinone content. They accumulated a maximum level of alizarin and glitter that were 6 and 11 times higher than the non-irradiated callus cultures. | [56] |
| Barley | Hordeum vulgare L. | Seedlings | 50–300 Gy | 50 Gy | High concentration of proline and antioxidant enzyme activity. Heavy metal stress resistance. | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riviello-Flores, M.d.l.L.; Cadena-Iñiguez, J.; Ruiz-Posadas, L.d.M.; Arévalo-Galarza, M.d.L.; Castillo-Juárez, I.; Soto Hernández, M.; Castillo-Martínez, C.R. Use of Gamma Radiation for the Genetic Improvement of Underutilized Plant Varieties. Plants 2022, 11, 1161. https://doi.org/10.3390/plants11091161
Riviello-Flores MdlL, Cadena-Iñiguez J, Ruiz-Posadas LdM, Arévalo-Galarza MdL, Castillo-Juárez I, Soto Hernández M, Castillo-Martínez CR. Use of Gamma Radiation for the Genetic Improvement of Underutilized Plant Varieties. Plants. 2022; 11(9):1161. https://doi.org/10.3390/plants11091161
Chicago/Turabian StyleRiviello-Flores, María de la Luz, Jorge Cadena-Iñiguez, Lucero del Mar Ruiz-Posadas, Ma. de Lourdes Arévalo-Galarza, Israel Castillo-Juárez, Marcos Soto Hernández, and Carlos Roman Castillo-Martínez. 2022. "Use of Gamma Radiation for the Genetic Improvement of Underutilized Plant Varieties" Plants 11, no. 9: 1161. https://doi.org/10.3390/plants11091161
APA StyleRiviello-Flores, M. d. l. L., Cadena-Iñiguez, J., Ruiz-Posadas, L. d. M., Arévalo-Galarza, M. d. L., Castillo-Juárez, I., Soto Hernández, M., & Castillo-Martínez, C. R. (2022). Use of Gamma Radiation for the Genetic Improvement of Underutilized Plant Varieties. Plants, 11(9), 1161. https://doi.org/10.3390/plants11091161