Primary Root Excision Induces ERF071, Which Mediates the Development of Lateral Roots in Makapuno Coconut (Cocos nucifera)
Abstract
1. Introduction
2. Results
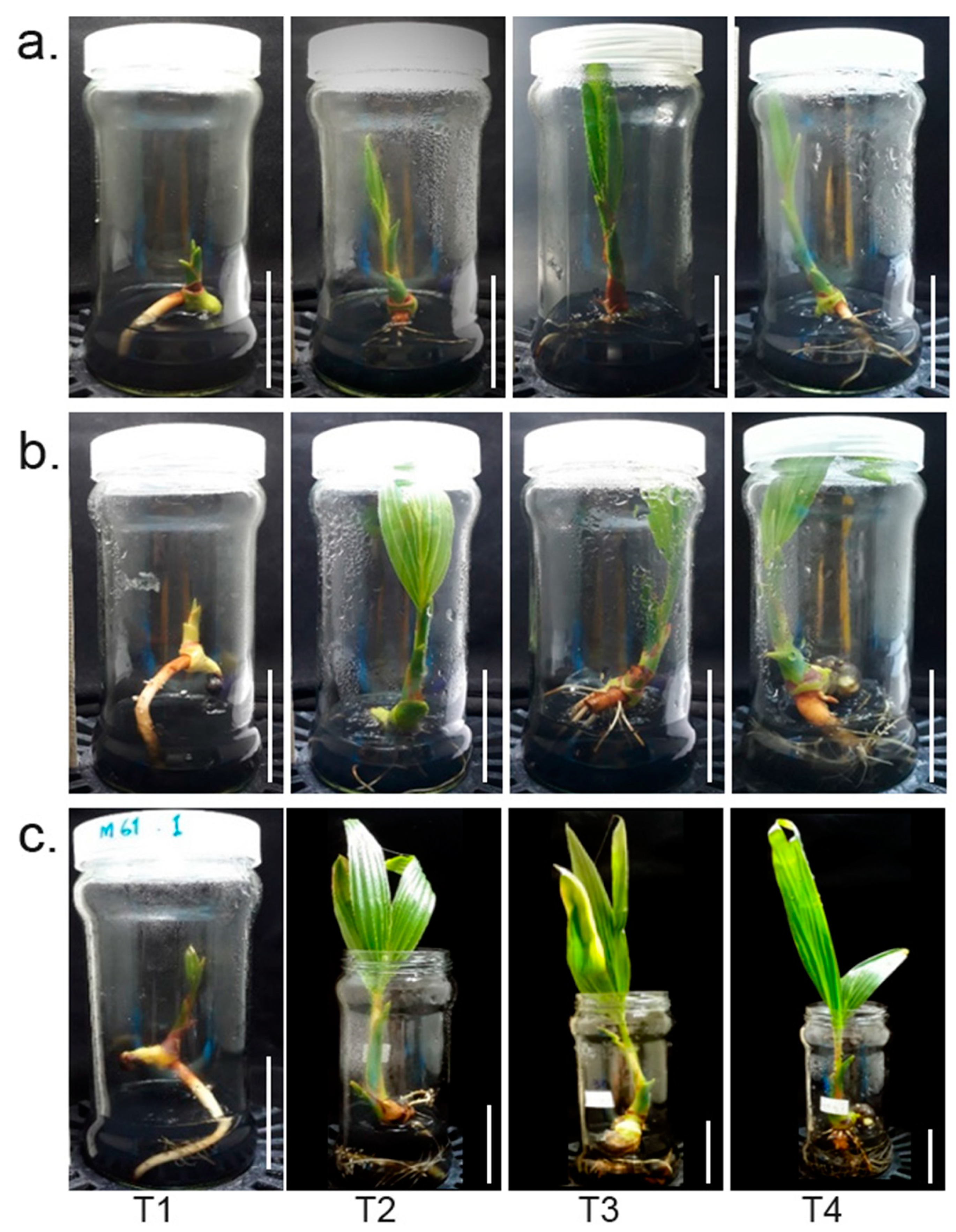
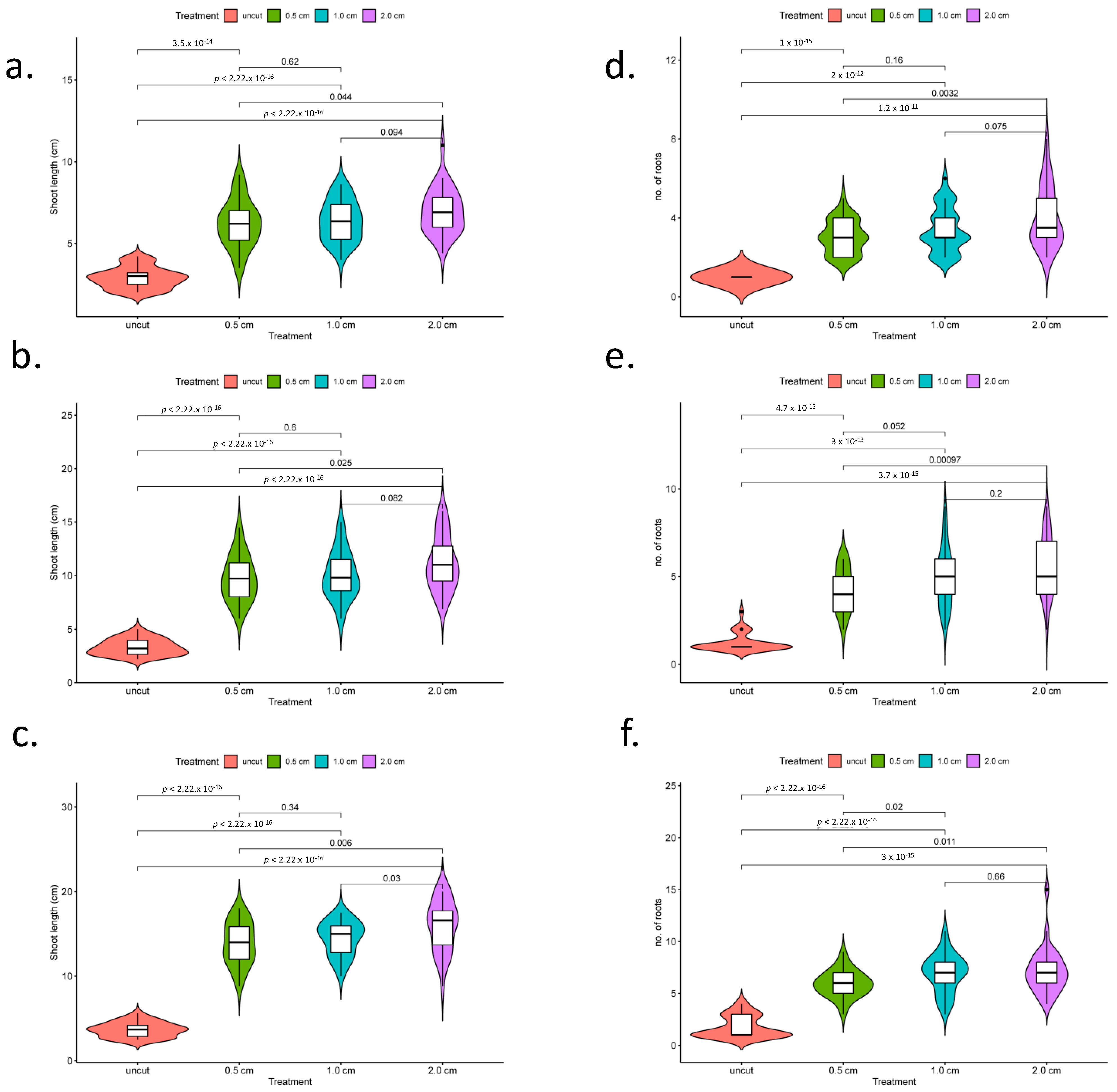
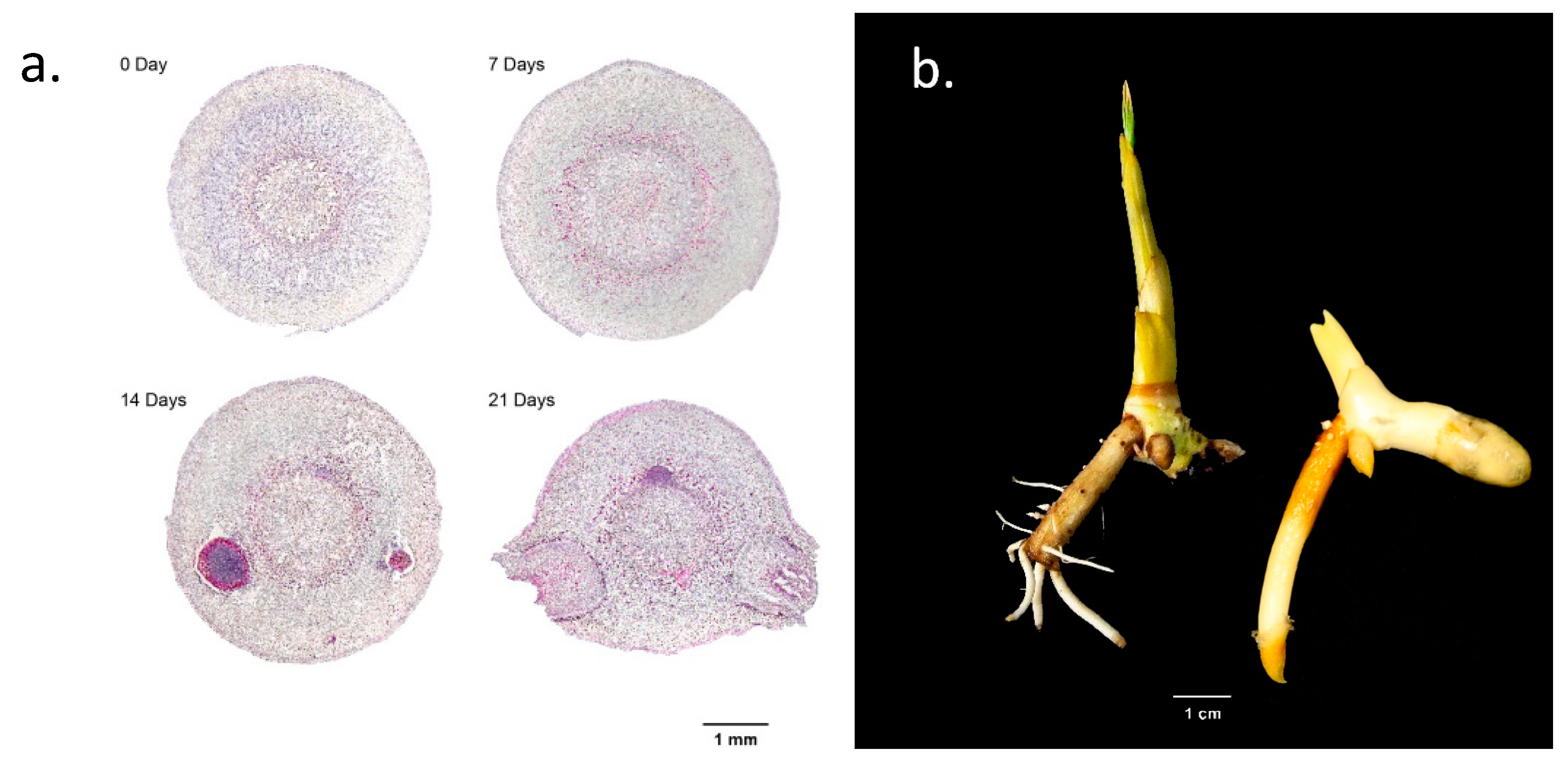
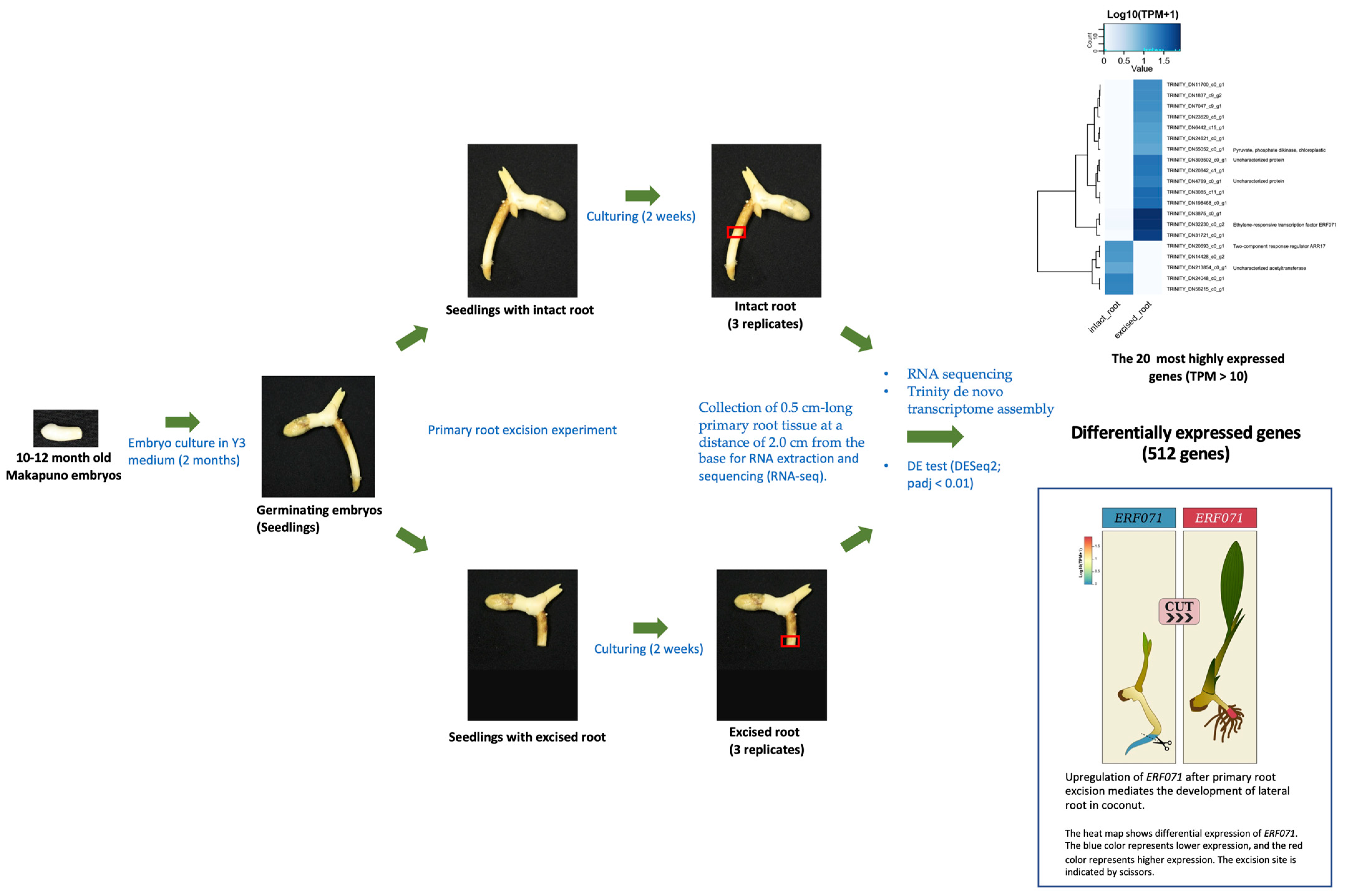
2.1. Makapuno Embryo Culture and Investigation of the Impact of Primary-Root Excision on Lateral-Root Development and Shoot Growth
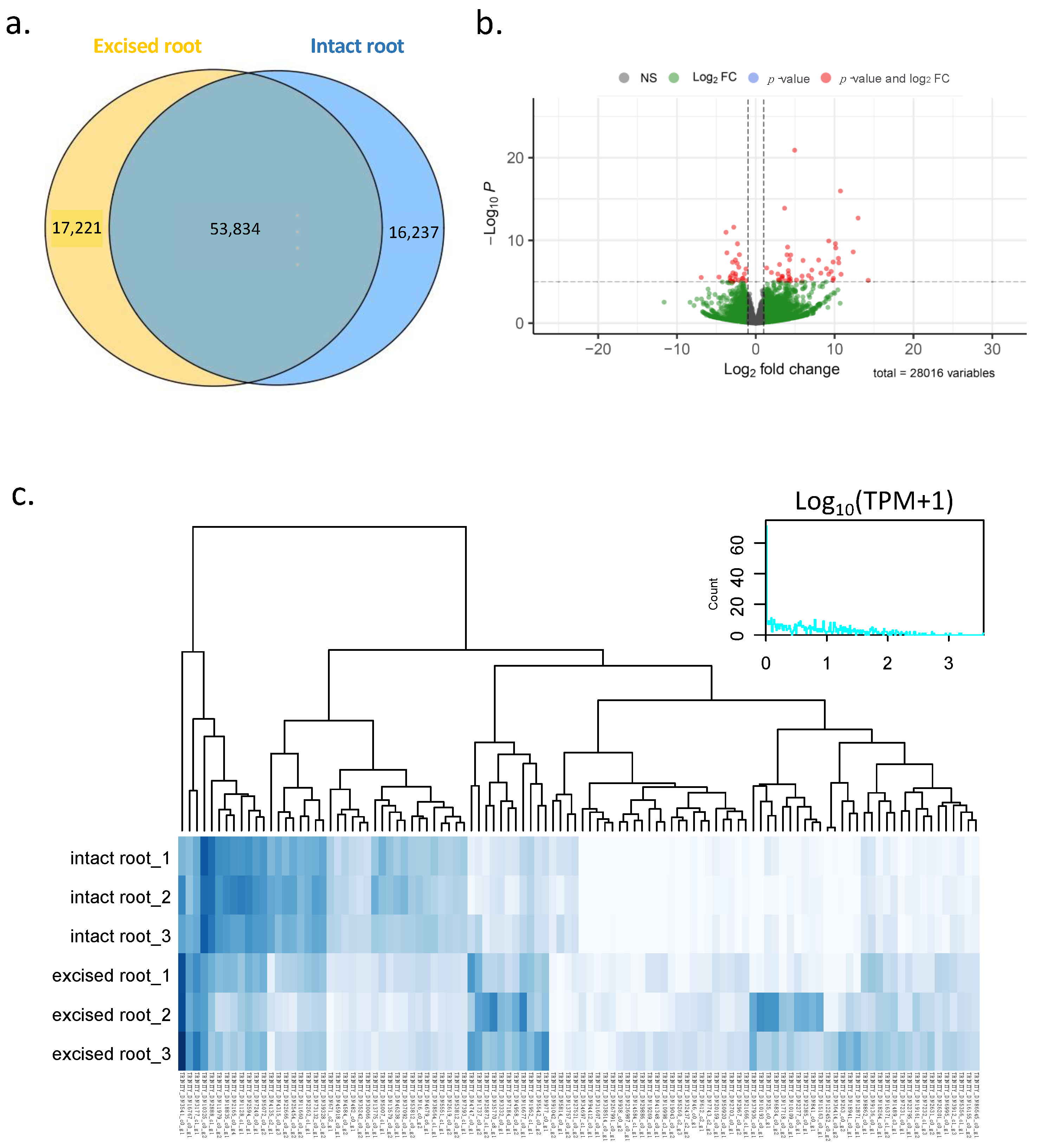
2.2. RNA Sequencing and Transcriptome-Assembly Quality Statistics
2.3. Differential Gene Expression Analysis for Excised and Intact Roots
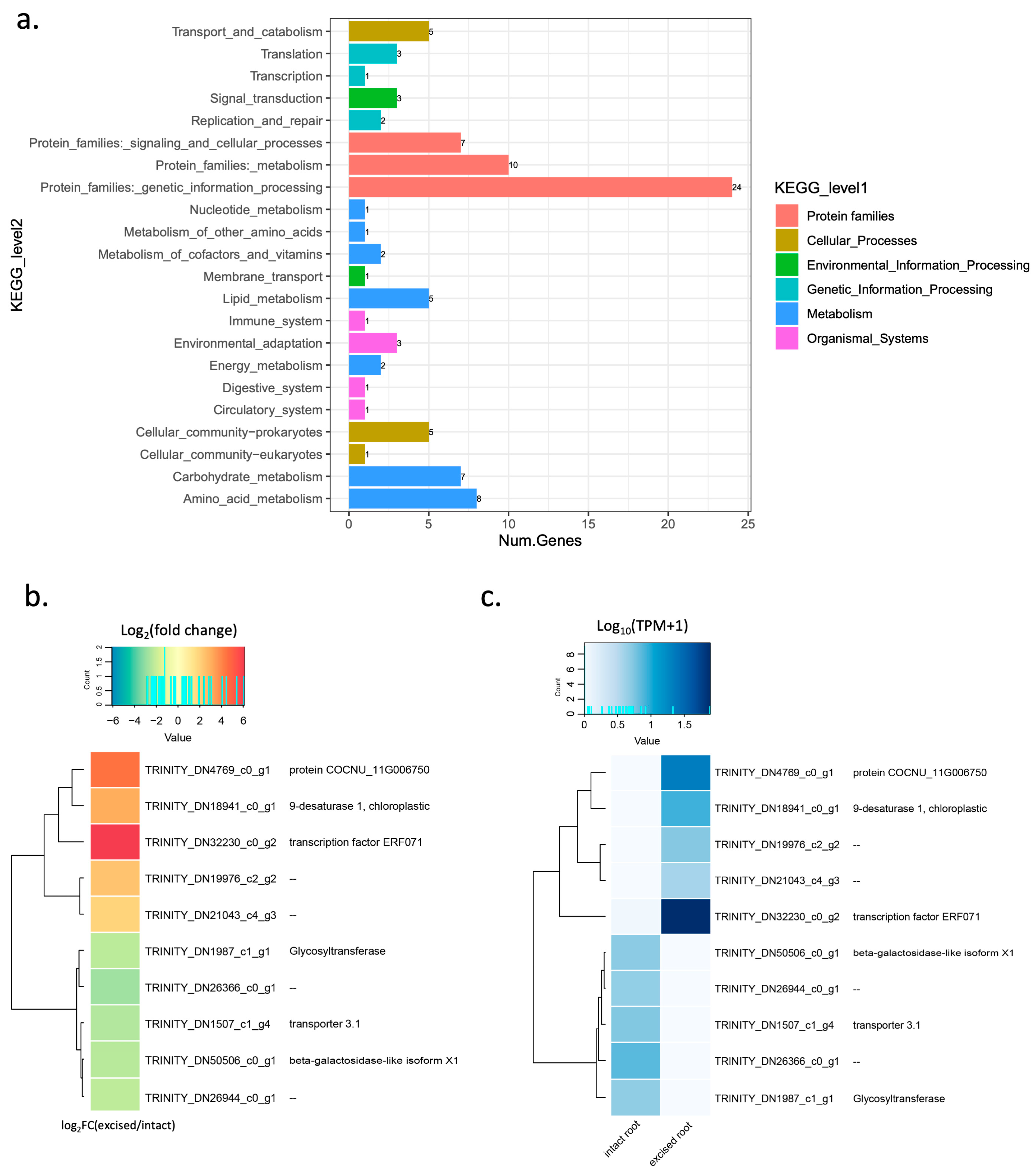
2.4. Gene Annotation, Classification, and Pathway Analysis
2.5. Microscopic Observation of Lateral Root Formation
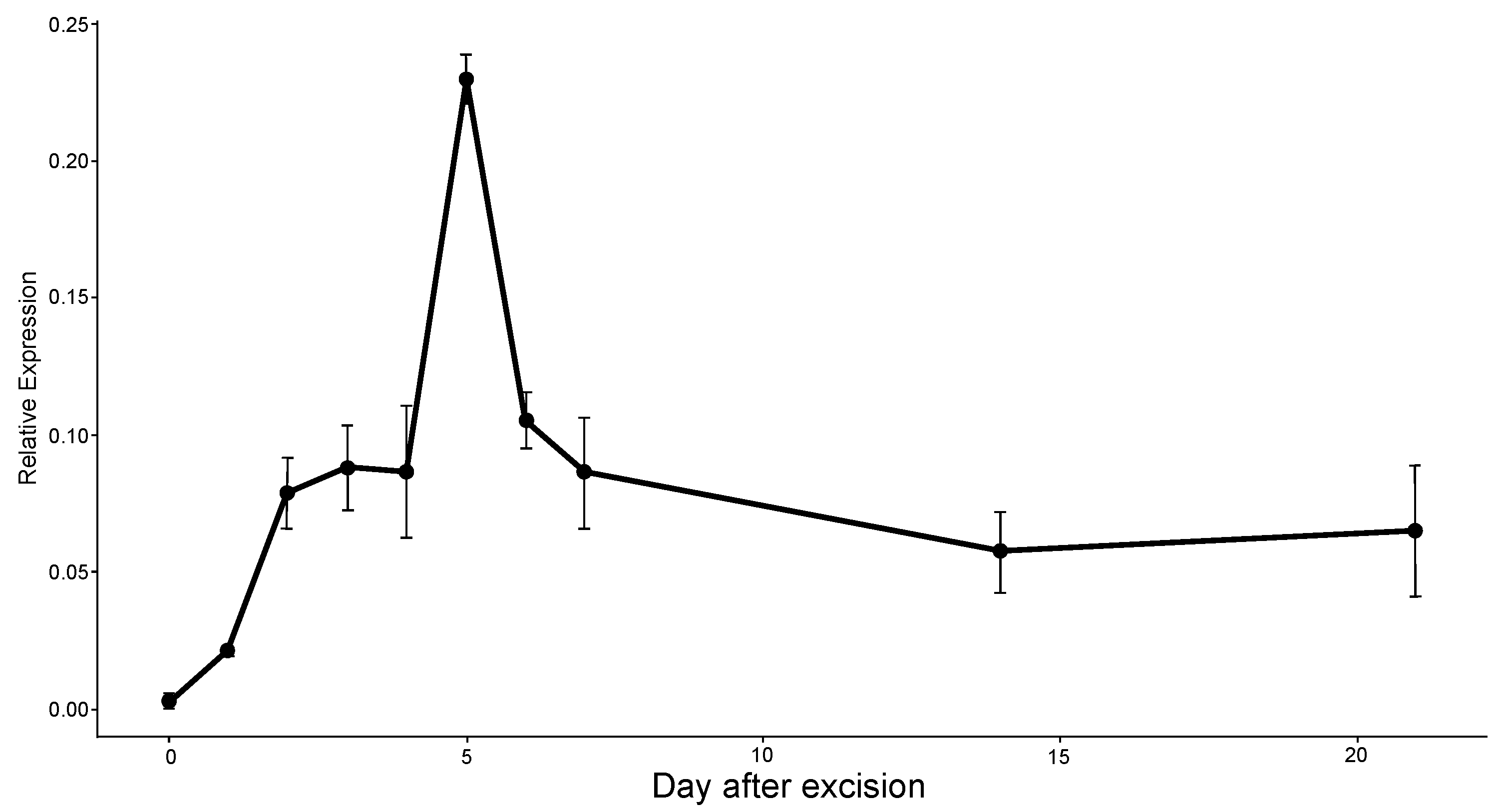
2.6. Expression Analysis of ERF071 Using qRT-PCR
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Embryo Culture Growth Conditions and Root Excision Treatments
4.3. RNA Extraction, RNA Sequencing (RNA-seq), and De Novo Transcriptome Assembly
4.4. Gene Annotation, Classification, and Pathway Analysis
4.5. Read Abundance Quantification and Differential Gene Expression Analysis
4.6. Microscopic Observation
4.7. Expression Analysis by Quantitative Reverse Transcription PCR (qRT-PCR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, Q.T.; Bandupriya, H.D.D.; Foale, M.; Adkins, S.W. Biology, propagation and utilization of elite coconut varieties (makapuno and aromatics). Plant Physiol. Biochem. 2016, 109, 579–589. [Google Scholar] [CrossRef] [PubMed]
- De Guzman, E.V. The growth and development of coconut ‘Makapuno’embryos in vitro. I. The induction of rooting. Philipp. Agric. 1970, 53, 65–78. [Google Scholar]
- White, P.R. A Handbook of Plant Tissue Culture; LWW: Philadelphia, PA, USA, 1943; Volume 56, ISBN 0038-075X. [Google Scholar]
- Engelmann, F.; Batugal, P. Background on the development and implementation of the coconut embryo in vitro culture project. In Coconut Embryo In Vitro Culture: Part 2; IPGRI: Rome, Italy; APO: Serdang, Malaysia, 2002; pp. 1–4. ISBN 92-9043-536-4. [Google Scholar]
- Rillo, E.P.; Cueto, C.A.; Medes, W.R.; Areza-Ubaldo, M.B. Development of an improved embryo culture protocol for coconut in the Philippines. In Proceedings of the Second International on Embryo Culture Workshop, Mérida, Yucatán, Mexico, 14–17 March 2000; International Plant Genetic Resources Institute (IPGRI): Rome, Italy, 2002. [Google Scholar]
- Samosir, Y.M.S.; Adkins, S. Improving acclimatization through the photoautotrophic culture of coconut (Cocos nucifera) seedlings: An in vitro system for the efficient exchange of germplasm. In Vitro Cell. Dev. Biol.-Plant 2014, 50, 493–501. [Google Scholar] [CrossRef]
- Sisunandar; Alkhikmah; Husin, A.; Julianto, T.; Yuniaty, A.; Rival, A.; Adkins, S.W. Ex vitro rooting using a mini growth chamber increases root induction and accelerates acclimatization of Kopyor coconut (Cocos nucifera L.) embryo culture-derived seedlings. In Vitro Cell. Dev. Biol.-Plant 2018, 54, 508–517. [Google Scholar] [CrossRef]
- Stupendick, J.T.; Shepherd, K.R. Root regeneration of root-pruned Pinus radiata seedlings. II. Effects of root pruning on photosynthesis and translocation. NZJ For. Sci 1980, 10, 148–158. [Google Scholar]
- WIGHTMAN, F.; SCHNEIDER, E.A.; THIMANN, K.V. Hormonal factors controlling the initiation and development of lateral roots: II. Effects of exogenous growth factors on lateral root formation in pea roots. Physiol. Plant. 1980, 49, 304–314. [Google Scholar] [CrossRef]
- Biddington, N.L.; Dearman, A.S. Shoot and root growth of lettuce seedlings following root pruning. Ann. Bot. 1984, 53, 663–668. [Google Scholar] [CrossRef]
- Andersen, L.; Rasmussen, H.N.; Brander, P.E. Regrowth and dry matter allocation in Quercus robur (L.) seedlings root pruned prior to transplanting. New For. 2000, 19, 205–214. [Google Scholar] [CrossRef]
- Zhang, R.; Peng, F.-R.; Yan, P.; Cao, F.; Liu, Z.-Z.; Le, D.-L.; Tan, P.-P. Effects of root pruning on germinated pecan seedlings. HortScience 2015, 50, 1549–1552. [Google Scholar] [CrossRef]
- Xu, D.; Miao, J.; Yumoto, E.; Yokota, T.; Asahina, M.; Watahiki, M. YUCCA9-Mediated Auxin Biosynthesis and Polar Auxin Transport Synergistically Regulate Regeneration of Root Systems Following Root Cutting. Plant Cell Physiol. 2017, 58, 1710–1723. [Google Scholar] [CrossRef]
- Matosevich, R.; Cohen, I.; Gil-Yarom, N.; Modrego, A.; Friedlander-Shani, L.; Verna, C.; Scarpella, E.; Efroni, I. Local auxin biosynthesis is required for root regeneration after wounding. Nat. Plants 2020, 6, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Pop, T.I.; Pamfil, D.D.; Bellini, C.C. Auxin control in the formation of adventitious roots. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 307–316. [Google Scholar] [CrossRef]
- Dastidar, M.G.; Jouannet, V.; Maizel, A. Root branching: Mechanisms, robustness, and plasticity. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 329–343. [Google Scholar] [CrossRef]
- Lavenus, J.; Goh, T.; Roberts, I.; Guyomarc’h, S.; Lucas, M.; De Smet, I.; Fukaki, H.; Beeckman, T.; Bennett, M.; Laplaze, L. Lateral root development in Arabidopsis: Fifty shades of auxin. Trends Plant Sci. 2013, 18, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; He, L.; Huang, R. The coordination of ethylene and other hormones in primary root development. Front. Plant Sci. 2019, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Ivanchenko, M.G.; Muday, G.K. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J. 2008, 55, 175–187. [Google Scholar] [CrossRef]
- Trupiano, D.; Yordanov, Y.; Regan, S.; Meilan, R.; Tschaplinski, T.; Scippa, G.S.; Busov, V. Identification, characterization of an AP2/ERF transcription factor that promotes adventitious, lateral root formation in Populus. Planta 2013, 238, 271–282. [Google Scholar] [CrossRef]
- Ivanchenko, M.G.; Muday, G.K.; Dubrovsky, J.G. Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J. 2008, 55, 335–347. [Google Scholar] [CrossRef]
- Péret, B.; De Rybel, B.; Casimiro, I.; Benková, E.; Swarup, R.; Laplaze, L.; Beeckman, T.; Bennett, M.J. Arabidopsis lateral root development: An emerging story. Trends Plant Sci. 2009, 14, 399–408. [Google Scholar] [CrossRef]
- Casero, P.J.; Casimiro, I.; Lloret, P.G. Lateral root initiation by asymmetrical transverse divisions of pericycle cells in four plant species: Raphanus sativus, Helianthus annuus, Zea mays, and Daucus carota. Protoplasma 1995, 188, 49–58. [Google Scholar] [CrossRef]
- De Smet, I.; Vanneste, S.; Inzé, D.; Beeckman, T. Lateral root initiation or the birth of a new meristem. Plant Mol. Biol. 2006, 60, 871–887. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Hwang, E.Y.; Seok, H.-Y.; Tarte, V.N.; Jeong, M.S.; Jang, S.B.; Moon, Y.-H. Arabidopsis AtERF71/HRE2 functions as transcriptional activator via cis-acting GCC box or DRE/CRT element and is involved in root development through regulation of root cell expansion. Plant Cell Rep. 2015, 34, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Bandupriya, H.D.D.; López-Villalobos, A.; Sisunandar, S.; Foale, M.; Adkins, S.W. Tissue culture and associated biotechnological interventions for the improvement of coconut (Cocos nucifera L.): A review. Planta 2015, 242, 1059–1076. [Google Scholar] [CrossRef]
- Carandang, E.V. Makapuno embryo culture: The Philippine Coconut Research and Development Foundation experience. In Proceedings of the Second International on Embryo Culture Workshop, Mérida, Yucatán, Mexico, 14–17 March 2000; International Plant Genetic Resources Institute (IPGRI): Rome, Italy, 2002. [Google Scholar]
- León, J.; Rojo, E.; Sánchez-Serrano, J.J. Wound signalling in plants. J. Exp. Bot. 2001, 52, 1–9. [Google Scholar] [CrossRef]
- Eysholdt-Derzsó, E.; Sauter, M. Root Bending Is Antagonistically Affected by Hypoxia and ERF-Mediated Transcription via Auxin Signaling. Plant Physiol. 2017, 175, 412–423. [Google Scholar] [CrossRef]
- Eysholdt-Derzsó, E.; Sauter, M. Hypoxia and the group VII ethylene response transcription factor HRE2 promote adventitious root elongation in Arabidopsis. Plant Biol. 2019, 21 (Suppl. S1), 103–108. [Google Scholar] [CrossRef]
- Feng, K.; Hou, X.-L.; Xing, G.-M.; Liu, J.-X.; Duan, A.-Q.; Xu, Z.-S.; Li, M.-Y.; Zhuang, J.; Xiong, A.-S. Advances in AP2/ERF super-family transcription factors in plant. Crit. Rev. Biotechnol. 2020, 40, 750–776. [Google Scholar] [CrossRef]
- Heyman, J.; Canher, B.; Bisht, A.; Christiaens, F.; De Veylder, L. Emerging role of the plant ERF transcription factors in coordinating wound defense responses and repair. J. Cell Sci. 2018, 131, jcs208215. [Google Scholar] [CrossRef]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006, 140, 411–432. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Wang, H.; Xin, H.; Yang, X.; Yan, J.; Li, J.; Tran, L.-S.P.; Shinozaki, K.; Yamaguchi-Shinozaki, K.; et al. Genome-wide analysis of ZmDREB genes and their association with natural variation in drought tolerance at seedling stage of Zea mays L. PLoS Genet. 2013, 9, e1003790. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.-Y.; Tran, H.T.; Lee, S.-Y.; Moon, Y.-H. AtERF71/HRE2, an Arabidopsis AP2/ERF Transcription Factor Gene, Contains Both Positive and Negative Cis-Regulatory Elements in Its Promoter Region Involved in Hypoxia and Salt Stress Responses. Int. J. Mol. Sci. 2022, 23, 5310. [Google Scholar] [CrossRef] [PubMed]
- Girardi, C.L.; Rombaldi, C.V.; Dal Cero, J.; Nobile, P.M.; Laurens, F.; Bouzayen, M.; Quecini, V. Genome-wide analysis of the AP2/ERF superfamily in apple and transcriptional evidence of ERF involvement in scab pathogenesis. Sci. Hortic. 2013, 151, 112–121. [Google Scholar] [CrossRef]
- Ren, B.; Liang, Y.; Deng, Y.; Chen, Q.; Zhang, J.; Yang, X.; Zuo, J. Genome-wide comparative analysis of type-A Arabidopsis response regulator genes by overexpression studies reveals their diverse roles and regulatory mechanisms in cytokinin signaling. Cell Res. 2009, 19, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-B.; Liu, G.; Liu, J.; Zhang, B.; Meng, W.; Müller, B.; Hayashi, K.-I.; Zhang, X.; Zhao, Z.; De Smet, I.; et al. Synergistic action of auxin and cytokinin mediates aluminum-induced root growth inhibition in Arabidopsis. EMBO Rep. 2017, 18, 1213–1230. [Google Scholar] [CrossRef] [PubMed]
- Torrey, J.G. Endogenous and exogenous influences on the regulation of lateral root formation. In New Root Formation in Plants and Cuttings; Jackson, M.B., Ed.; Springer: Dordrecht, The Netherland, 1986; pp. 31–66. ISBN 978-94-009-4358-2. [Google Scholar]
- Beeckman, T.; Burssens, S.; Inzé, D. The peri-cell-cycle in Arabidopsis. J. Exp. Bot. 2001, 52, 403–411. [Google Scholar] [CrossRef]
- Casimiro, I.; Beeckman, T.; Graham, N.; Bhalerao, R.; Zhang, H.; Casero, P.; Sandberg, G.; Bennett, M.J. Dissecting Arabidopsis lateral root development. Trends Plant Sci. 2003, 8, 165–171. [Google Scholar] [CrossRef]
- Smith, S.; De Smet, I. Root system architecture: Insights from Arabidopsis and cereal crops. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 1441–1452. [Google Scholar] [CrossRef]
- Casimiro, I.; Marchant, A.; Bhalerao, R.P.; Beeckman, T.; Dhooge, S.; Swarup, R.; Graham, N.; Inzé, D.; Sandberg, G.; Casero, P.J.; et al. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 2001, 13, 843–852. [Google Scholar] [CrossRef]
- Fukaki, H.; Okushima, Y.; Tasaka, M. Auxin-mediated lateral root formation in higher plants. Int. Rev. Cytol. 2007, 256, 111–137. [Google Scholar] [CrossRef]
- Adamowski, M.; Friml, J. PIN-dependent auxin transport: Action, regulation, and evolution. Plant Cell 2015, 27, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Eeuwens, C.J. Mineral Requirements for Growth and Callus Initiation of Tissue Explants Excised from Mature Coconut Palms (Cocos nucifera) and Cultured in vitro. Physiol. Plant. 1976, 36, 23–28. [Google Scholar] [CrossRef]
- Saensuk, C.; Wanchana, S.; Choowongkomon, K.; Wongpornchai, S.; Kraithong, T.; Imsabai, W.; Chaichoompu, E.; Ruanjaichon, V.; Toojinda, T.; Vanavichit, A.; et al. De novo transcriptome assembly and identification of the gene conferring a “pandan-like” aroma in coconut (Cocos nucifera L.). Plant Sci. 2016, 252, 324–334. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef] [PubMed]
- Patro, R.; Duggal, G.; Kingsford, C. Accurate, fast, and model-aware transcript expression quantification with Salmon. BioRxiv 2015, 10, 021592. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
| Statistics | Root (Excised + Intact) |
|---|---|
| Number of total contigs | 541,700 |
| Number of contigs hit reference genes | 215,347 |
| Total nucleotides (bp) | 801,856,735 |
| N50 length (bp) | 1897 |
| Average contig length (bp) | 975.30 |
| Min. contig length (bp) | 178 |
| Max. contig length (bp) | 21,353 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thuzar, M.; Sae-lee, Y.; Saensuk, C.; Pitaloka, M.K.; Dechkrong, P.; Aesomnuk, W.; Ruanjaichon, V.; Wanchana, S.; Arikit, S. Primary Root Excision Induces ERF071, Which Mediates the Development of Lateral Roots in Makapuno Coconut (Cocos nucifera). Plants 2023, 12, 105. https://doi.org/10.3390/plants12010105
Thuzar M, Sae-lee Y, Saensuk C, Pitaloka MK, Dechkrong P, Aesomnuk W, Ruanjaichon V, Wanchana S, Arikit S. Primary Root Excision Induces ERF071, Which Mediates the Development of Lateral Roots in Makapuno Coconut (Cocos nucifera). Plants. 2023; 12(1):105. https://doi.org/10.3390/plants12010105
Chicago/Turabian StyleThuzar, Mya, Yonlada Sae-lee, Chatree Saensuk, Mutiara K. Pitaloka, Punyavee Dechkrong, Wanchana Aesomnuk, Vinitchan Ruanjaichon, Samart Wanchana, and Siwaret Arikit. 2023. "Primary Root Excision Induces ERF071, Which Mediates the Development of Lateral Roots in Makapuno Coconut (Cocos nucifera)" Plants 12, no. 1: 105. https://doi.org/10.3390/plants12010105
APA StyleThuzar, M., Sae-lee, Y., Saensuk, C., Pitaloka, M. K., Dechkrong, P., Aesomnuk, W., Ruanjaichon, V., Wanchana, S., & Arikit, S. (2023). Primary Root Excision Induces ERF071, Which Mediates the Development of Lateral Roots in Makapuno Coconut (Cocos nucifera). Plants, 12(1), 105. https://doi.org/10.3390/plants12010105






