Photosynthetic Plasticity and Stomata Adjustment in Chromosome Segment Substitution Lines of Rice Cultivar KDML105 under Drought Stress
Abstract
:1. Introduction
2. Results
2.1. Drought Responses of KDML105-CSSLs Lines
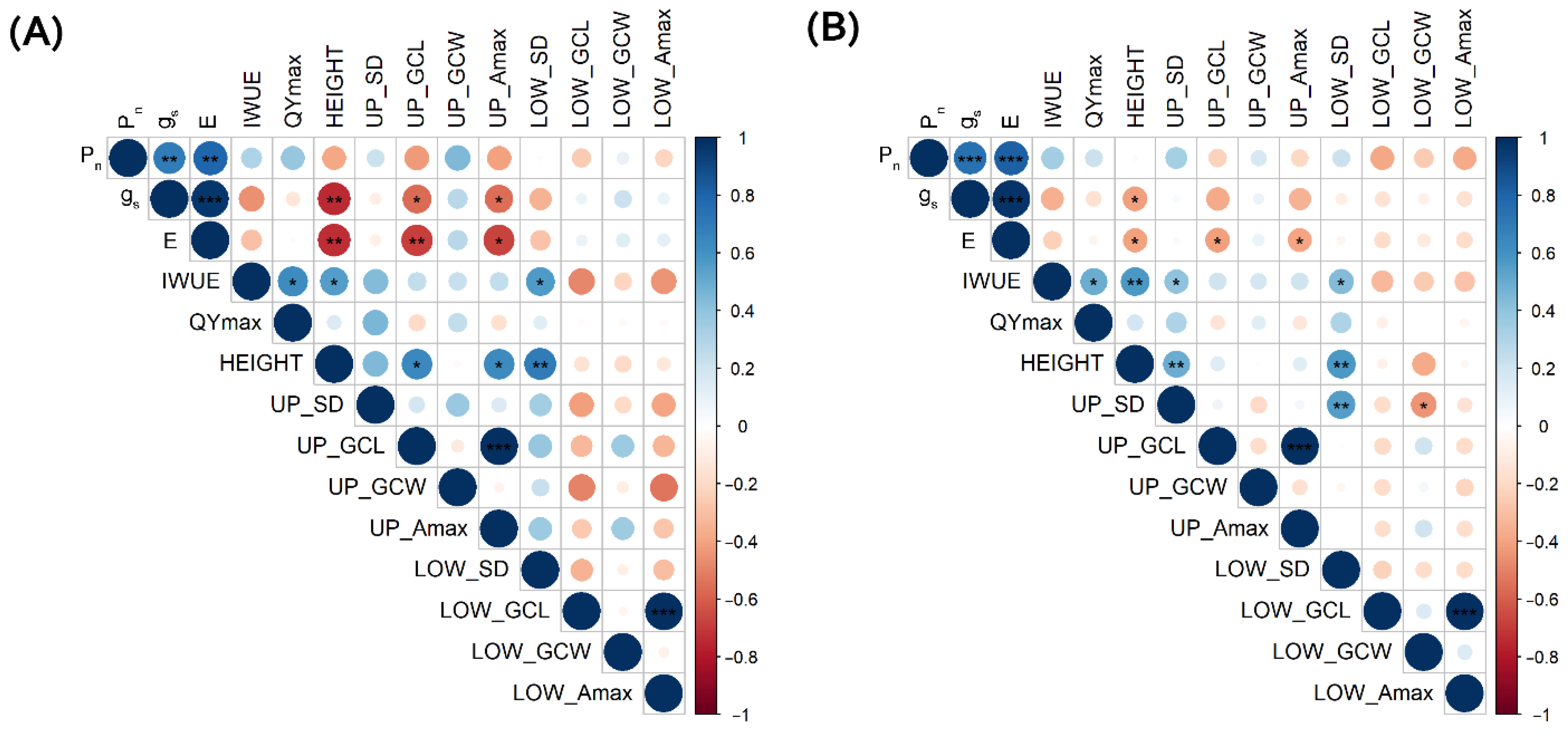
2.2. Relationship among the Evaluated Parameters
2.3. Principal Component Analysis (PCA)
2.4. Bulk Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materails and Growth Conditions
4.2. Physiological Traits
4.3. Plant Phenotyping
4.4. Stomatal Traits
4.5. Statistical Analysis
- DSmean = mean value in drought stress conditions.
- WWmean = mean value in well-watered conditions.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimeates. PNAS 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [Green Version]
- Rockström, J.; Falkenmark, M.; Karlberg, L.; Hoff, H.; Rost, S.; Gerten, D. Future water availability for global food production: The potential of green water for increasing resilience to global change. Water Resour. Res. 2009, 45, W00A12. [Google Scholar] [CrossRef] [Green Version]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.C.; Mahat, V.; Ramirez, J.A. Adaptation to future water shortages in the United States caused by population growth and climate change. Earths Future 2019, 7, 219–234. [Google Scholar] [CrossRef] [Green Version]
- Lafitte, H.R.; Yongsheng, G.; Yan, S.; Li, Z.-K. Whole plant responses, key processes, and adaptation to drought stress: The case of rice. J. Exp. Bot. 2007, 58, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Rousset, N. The impact of climate change, water security and the implications for agriculture. China Perspects 2007, 2007, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, J. Thailand’s Rice Farmers Adapt to Climate Change. United Nation University. Available online: https://ourworld.unu.edu/en/climate-change-adaptation-for-thailands-rice-farmers (accessed on 17 July 2021).
- Napasinuwong, O.; Pray, C. Adoption of drought-tolerant rice in Thailand: Participatory varietal selection and implications for breeding programs. J. Dev. Agric. Econ. 2014, 6, 394–404. [Google Scholar]
- Vikram, P.; Mallikarjuna Swamy, B.P.; Dixit, S.; Singh, R.; Singh, B.P.; Miro, B.; Kohli, A.; Henry, A.; Singh, N.K.; Kumar, A. Drought susceptibility of modern tice varieties: An effect of linkage of drought tolerance with undesirable traits. Sci. Rep. 2015, 5, 14799. [Google Scholar] [CrossRef] [Green Version]
- Polthanee, A.; Promkhambut, A. Impact of climate change on rice-based cropping systems and farmers’ adaptation strategies in Northeast Thailand. Asian J. Crop Sci. 2014, 6, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Thaiturapaisan, T. Thailand’s Drought Crisis 2016: Understanding It without the Panic. The Siam Commercial Bank Public Company Limited. Available online: https://www.scbeic.com/en/detail/product/2127 (accessed on 20 June 2021).
- Velikova, V.; Arena, C.; Izzao, L.G.; Tsonev, T.; Koleva, D.; Tattini, M.; Roeva, O.; Maio, A.D.; Loreto, F. Functional and Structural Leaf Plasticity Determine Photosynthetic Performance during Drought Stress and Recovery in Two Platanus orientalis Populations from Contrasting Habitats. Int. J. Mol. Sci. 2020, 21, 3912. [Google Scholar] [CrossRef]
- Panda, D.; Mishra, S.S.; Behera, P.K. Drought tolerance in rice: Focus on recent mechanisms and approaches. Rice Sci. 2021, 28, 119–132. [Google Scholar] [CrossRef]
- Zhu, R.; Wu, F.Y.; Zhou, S.; Hu, T.; Huang, J.; Gao, Y. Cumulative effects of drought-flood abrupt alternation on the photosynthetic characteristics of rice. Environ. Exp. Bot. 2020, 169, 103901. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of stomatal density and morphology on water-use-efficiency in a changing world. Front. Plant Sci. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Buckley, T.N. The control of stomata by water balance. New Phytol. 2005, 168, 275–292. [Google Scholar] [CrossRef]
- Buckley, C.R.; Caine, R.S.; Gray, J.E. Pores for thought: Can genetic manipulation of stomatal density protect future rice yields? Front. Plant Sci. 2020, 10, 1783. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sugano, S.S.; Shimada, T.; Hara-Nishimura, I. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytol. 2013, 198, 757–764. [Google Scholar] [CrossRef]
- Muchow, R.C.; Sinclair, T.R. Epidermal conductance, stomatal density and stomatal size among genotypes of Sorghum bicolor (L.) Moench. Plant Cell Environ. 1989, 12, 425–431. [Google Scholar] [CrossRef]
- Kanjoo, V.; Jearakongman, S.; Punyawaew, K.; Siangliw, J.L.; Siangliw, M.; Vanavichit, A.; Toojinda, T. Co-location of quantitative trait loci for drought and salinity tolerance in rice. Thai J. Genet. 2011, 4, 126–138. [Google Scholar]
- Ruangsiri, M.; Vejchasarn, P.; Saengwilai, P.; Lynch, J.; Bennett, M.J.; Brown, K.M.; Chutteang, C.; Boonruangrod, R.; Shearman, J.; Toojinda, T.; et al. Genetic control of root architectural traits in KDML105 chromosome segment substitution lines under well-watered and drought stress conditions. Plant Prod. Sci. 2021, 24, 512–529. [Google Scholar] [CrossRef]
- Galeano, E.; Vasconcelos, T.S.; Oliveira, P.N.; Carrer, H. Physiological and molecular responses to drought stress in teak (Tectona grandis L.f.). PLoS ONE 2019, 14, e0221571. [Google Scholar] [CrossRef]
- Chatterjee, J.; Thakur, V.; Nepomuceno, R.; Coe, R.A.; Dionora, J.; Elmido-Mabilangan, A.; Llave, A.D.; Reyes, A.M.D.; Monroy, A.N.; Canicosa, I.; et al. Natural diversity in stomatal features of cultivated and wild Oryza species. Rice 2020, 13, 1–20. [Google Scholar] [CrossRef]
- Giuliani, R.; Koteyeva, N.; Voznesenskaya, E.; Evans, M.A.; Cousins, A.B.; Edwards, G.E. Coordination of Leaf Photosynthesis, Transpiration, and Structural Traits in Rice and Wild Relatives (Genus Oryza). Plant Physiol. 2013, 162, 1632–1651. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, J.; Yang, C.; Du, S.; Yang, W. Photosynthetic performance of soybean plants to water deficit under high and low light intensity. South Afr. J. Bot. 2016, 105, 279–287. [Google Scholar] [CrossRef]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, Y.; Kjelgren, R.; Liu, X. Response of stomatal density and bound gas exchange in leaves of maize to soil water deficit. Acta Physiol. Plant 2015, 37, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pitaloka, M.K.; Caine, R.S.; Hepworth, C.; Harrison, E.L.; Sloan, J.; Chutteang, C.; Phunthong, C.; Nongngok, R.; Toojinda, T.; Ruengphayak, S. Induced genetic variations in stomatal density and size of rice strongly affects water use efficiency and responses to drought stresses. Front. Plant Sci. 2022, 13, 801706. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, S.; Li, Y. Increase rate of light-induced stomatal conductance is related to stomatal size in the genus Oryza. J. Exp. Bot. 2019, 70, 5259–5269. [Google Scholar] [CrossRef]
- Franks, P.J.; Beerling, D.J. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. PNAS 2009, 106, 10343–10347. [Google Scholar] [CrossRef] [Green Version]
- Kondamudi, R.; Swamy, K.N.; Rao, Y.V.; Kiran, T.V.; Suman, K.; Rao, D.S.; Rao, P.R.; Subrahmanyam, D.; Sarla, N.; Kumari, B.R.; et al. Gas exchange, carbon balance and stomatal traits in wild and cultivated rice (Oryza sativa L.) genotypes. Acta Physiol. Plant 2016, 38, 1–9. [Google Scholar] [CrossRef]
- Wei, H.; Meng, T.; Li, C.; Xu, K.; Huo, Z.; Wei, H.; Guo, B.; Zhang, H.; Dai, Q. Comparisons of grain yield and nutrient accumulation and translocation in high-yielding japonica/indica hybrids, indica hybrids, and japonica conventional varieties. Field Crops Res. 2017, 204, 101–109. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, H.; Blumwald, E.; Li, H.; Cheng, J.; Dai, Q.; Huo, Z.; Xu, K.; Guo, B. Different characteristics of high yield formation between inbred japonica super rice and inter-sub-specific hybrid super rice. Field Crops Res. 2016, 198, 179–187. [Google Scholar] [CrossRef]
- Thaylor, S.H.; Franks, P.J.; Hulme, S.P.; Spriggs, E.; Christin, P.A.; Edwards, E.J.; Woodward, F.I.; Osborne, C.P. Photosynthetic pathway and ecological adaptation explain stomatal trait diversity amongst grasses. New Phytol. 2011, 193, 387–396. [Google Scholar] [CrossRef] [PubMed]
- de Boer, H.J.; Price, C.A.; Wagner-Cremer, F.; Dekker, S.C.; Franks, P.J.; Veneklaas, E.J. Optimal allocation of leaf epidermal area for gas exchange. New Phytol. 2016, 210, 1219–1228. [Google Scholar] [CrossRef] [Green Version]
- Dittberner, H.; Korte, A.; Mettler-Altmann, T.; Weber, A.P.M.; Monroe, G.; de Meaux, J. Natural variation in stomata size contributes to the local adaptation of water-use efficiency in Arabidopsis thaliana. Mol. Ecol. 2018, 27, 4052–4065. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, J.; Liu, L.; Wang, Z.; Li, Y.; Guo, L.; Li, Y.; Zhang, X.; Ren, S.; Zhao, B.; et al. SITLFP8 reduces water loss to improve water-use-efficiency by modulating cell size and stomatal density via endoreduplication. Plant Cell Environ. 2020, 43, 2666–2679. [Google Scholar] [CrossRef]
- Tari, I. Abaxial and adaxial stomatal density, stomatal conductances and water status of bean primary leaves as affected by paclobutrazol. Biol. Plant. 2004, 47, 215–220. [Google Scholar] [CrossRef]
- Hossain, M.; Lam, H.M.; Zhang, J. Responses in gas exchange and water status between drought-tolerant and -susceptible soybean genotypes with ABA application. Crop J. 2015, 3, 501–506. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Zhu, C.; Liu, W.; Lou, F.; Mi, J.; Ren, Y.; Li, J.; Sang, T. High photosynthetic rate and water use efficiency of Miscanthus lutarioriparius characterize an energy crop in the semiarid temperate region. Glob. Chang. Biol. Bioenergy 2015, 7, 207–218. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and challenges in a changing climate. Plant Sci. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hong, T.; Lin, H.; He, D. Characteristics and correlations of leaf stomata in different Aleurites montana provenances. PLoS ONE 2018, 13, e0208899. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cao, Q.; Sun, L.; Yang, X.; Yang, W.; Zhang, H. Stomatal conductance and morphology of Arbuscular Mycorrhizal wheat plants response to elevated to CO2 and NaCl stress. Front. Plant Sci. 2018, 9, 1363. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fulita, M. Physiological, Biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Piveta, L.B.; Roma-Burgos, N.; Noldin, J.A.; Viana, V.E.; de Oliveira, C.; Lamego, F.P.; de Avila, L.A. Molecular and physiological responses of rice and weedy rice to heat and drought stress. Agriculture 2021, 11, 9. [Google Scholar] [CrossRef]
- Dingkuhn, M.; Cruz, R.T.; O’Toole, J.C.; D÷rffling, K. Net photosynthesis, water use efficiency, leaf water potential and leaf rolling as affected by water deficit in tropical upland. Aust. J. Agric. Res. 1989, 40, 1171–1181. [Google Scholar] [CrossRef]
- Reddy, S.H.; Singhal, R.K.; DaCosta, M.V.J.; Kambalimath, S.K.; Rajanna, M.P.; Muthurajan, R.; Sevanthi, A.M.; Mohapatra, T.; Sarla, N.; Chinnusamy, V.; et al. Leaf mass area determines water use efficiency through its influence on carbon gain in rice mutants. Physiol. Plant. 2020, 169, 194–213. [Google Scholar] [CrossRef]
- Kusumi, K.; Hirotsuka, S.; Kumamaru, T.; Iba, K. Increased leaf photosynthesis caused by elevated stomatal conductance in a rice mutant deficient in SLAC1, a guard cell anion channel protein. J. Exp. Bot. 2012, 63, 5635–5644. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Liu, H.; Hua, L.; Luo, Q.; Lin, Y.; He, P.; Feng, S.; Liu, J.; Ye, Q. Differential responses of stomata and photosynthesis to elevated temperature in two co-occuring subtropical forest tree species. Front. Plant Sci. 2018, 9, 467. [Google Scholar] [CrossRef] [Green Version]
- Soares, A.S.; Driscoll, S.P.; Olmos, E.; Harbinson, J.; Arrabaҫa, M.C.; Foyer, C.H. Adaxial/abaxial specification in the regulation of photosynthesis and stomatal opening with respect to light orientation and growth with CO2 enrichment in the C4 species Paspalum dilatatum. New Phytol. 2007, 177, 186–198. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–8998. [Google Scholar] [CrossRef] [Green Version]
- Franks, P.J.; Farquhar, G.D. The effect of exogenous abscisic acid on stomatal development, stomatal mechanisms, and leaf gas exchange in Tradescantia virginiana. Plant Physiol. 2001, 125, 935–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franks, P.J.; Farquhar, G.D. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 2007, 125, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Simko, V. R package “corrplot”: Visualization of a Correlation Matrix. Available online: https://github.com/taiyun/corrplot (accessed on 1 December 2022).
- Kassambara, A. Practical Guide to Principal Component Methods in R; Createspace Independent Publishing: Scotts Valley, CA, USA, 2017; pp. 12–50, STHDA; Available online: www.sthda.com (accessed on 15 October 2022).


| Traits | TRT | Mean | LSD | Min | Max | CV | G Effect | TRT Effect |
|---|---|---|---|---|---|---|---|---|
| Pn | WW | 12.33 | 3.48 | 10.33 | 15.03 | 13.08 | ns | ** |
| DS | 7.49 | 3.32 | 6.04 | 9.4 | 20.54 | ns | ||
| Plasticity | −37.69 | 38.82 | −58.07 | −15.42 | −47.69 | ns | ||
| gs | WW | 0.14 | 0.051 | 0.112 | 0.17 | 16.85 | ns | ** |
| DS | 0.083 | 0.024 | 0.068 | 0.106 | 13.07 | * | ||
| Plasticity | −38.63 | 32.1 | −55.038 | −8.497 | −38.47 | ns | ||
| E | WW | 0.0035 | 0.0012 | 0.0028 | 0.0042 | 15.85 | ns | ** |
| DS | 0.0021 | 0.0006 | 0.0017 | 0.0027 | 13.23 | * | ||
| Plasticity | −38.41 | 33.01 | −51.76 | −9.3248 | −39.77 | ns | ||
| IWUE | WW | 89.06 | 19.05 | 75.96 | 106.69 | 9.9 | ns | ns |
| DS | 90.89 | 29.4 | 70.24 | 121.33 | 14.97 | ns | ||
| Plasticity | 3.62 | 37.51 | −15.97 | 45.36 | 479.73 | ns | ||
| QYmax | WW | 0.7565 | 0.0711 | 0.7195 | 0.7846 | 4.3517 | ns | ** |
| DS | 0.681 | 0.1257 | 0.5717 | 0.7596 | 8.5444 | ns | ||
| Plasticity | −9.84 | 17.81 | −25.06 | −0.2037 | −83.81 | ns | ||
| HEIGHT | WW | 695.33 | 162.98 | 632.3 | 788.7 | 10.85 | ns | ** |
| DS | 568.34 | 71.81 | 442.4 | 675.5 | 5.85 | ** | ||
| Plasticity | −17.69 | 21.19 | −31.77 | −0.59 | −55.45 | ns | ||
| UP_SD | WW | 512.16 | 99.19 | 455.56 | 587.65 | 8.96 | ns | ns |
| DS | 497.1 | 130.34 | 453.08 | 562.96 | 12.14 | ns | ||
| Plasticity | −1.99 | 38.04 | −14.19 | 17.2 | −885.82 | ns | ||
| UP_GCL | WW | 12.44 | 1.8 | 11.3 | 13.37 | 6.69 | ns | ** |
| DS | 13.26 | 1.7 | 12.55 | 14.07 | 5.93 | ns | ||
| Plasticity | 7.13 | 21.17 | −1.35 | 18.22 | 137.42 | ns | ||
| UP_GCW | WW | 3.88 | 0.54 | 3.55 | 4.37 | 6.46 | ns | ns |
| DS | 3.78 | 0.56 | 3.55 | 3.97 | 6.88 | ns | ||
| Plasticity | −2.06 | 48.52 | −13.7 | 9.27 | −1,087.87 | ns | ||
| UP_Amax | WW | 31.09 | 9.43 | 25.71 | 35.76 | 14.05 | ns | ns |
| DS | 35.15 | 9.8 | 31.56 | 39.48 | 12.9 | ns | ||
| Plasticity | 15.29 | 48.52 | −2.19 | 42.43 | 146.87 | ns | ||
| LOW_SD | WW | 654.51 | 143.81 | 560.49 | 706.17 | 10.17 | ns | ns |
| DS | 642.17 | 109.9 | 603.7 | 676.54 | 7.92 | ns | ||
| Plasticity | −1.07 | 24.97 | −14.32 | 18.25 | −1,083.73 | ns | ||
| LOW_GCL | WW | 12.2 | 1.79 | 11.33 | 13.52 | 6.81 | ns | ns |
| DS | 11.95 | 2.47 | 10.06 | 14.13 | 9.56 | ns | ||
| Plasticity | −1.67 | 21.7 | −13.71 | 11.06 | −601.25 | ns | ||
| LOW_GCW | WW | 3.89 | 0.7 | 3.59 | 4.16 | 8.33 | ns | *** |
| DS | 3.47 | 0.58 | 3.2 | 3.95 | 7.79 | ns | ||
| Plasticity | −10.17 | 25.11 | −18.66 | 3.25 | −114.27 | ns | ||
| LOW_Amax | WW | 29.94 | 8.61 | 25.74 | 26.26 | 13.32 | ns | ns |
| DS | 28.73 | 13.18 | 19.99 | 40.6 | 21.24 | ns | ||
| Plasticity | −2.74 | 46.64 | −25.43 | 24.53 | −788 | ns |
| LINES | TRT | Pn | gs | E | IWUE | QYmax | HEIGHTT | UP_SD | UP_GCL | UP_GCW | UP_Amax | LOW_SD | LOW_GCL | LOW_GCW | LOW_Amax |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSSL 26 | WW | 12.40085 | 0.149529 | 0.003687 | 83.2152 | 0.743846 | 701 | 470.3704 | 12.35515 | 4.033852 | 31.19718 | 606.1728 | 12.57833 | 4.018169 | 31.63711 |
| CSSL 28 | WW | 13.27751 | 0.146094 | 0.003673 | 90.78073 | 0.761377 | 689.5 | 506.1728 | 12.85801 | 3.770606 | 33.72576 | 706.1728 | 12.19166 | 3.593803 | 29.94588 |
| CSSL 29 | WW | 10.82491 | 0.142655 | 0.003648 | 75.96116 | 0.758405 | 674.7 | 455.5556 | 11.83199 | 3.799463 | 28.17341 | 696.2963 | 11.49074 | 4.028734 | 26.40059 |
| CSSL 37 | WW | 10.48667 | 0.13565 | 0.003459 | 76.79568 | 0.746339 | 652.9 | 508.642 | 11.35872 | 3.947241 | 25.87774 | 670.3704 | 11.78924 | 3.609668 | 27.64564 |
| CSSL 54 | WW | 12.74923 | 0.154158 | 0.003859 | 83.00898 | 0.768806 | 679.2 | 480.2469 | 12.53246 | 3.54823 | 31.14717 | 560.4938 | 13.52053 | 4.164308 | 36.36476 |
| CSSL 62 | WW | 10.33348 | 0.122282 | 0.00291 | 84.72687 | 0.762349 | 742.1 | 500 | 13.37501 | 3.918357 | 35.64489 | 671.6049 | 11.32786 | 3.904699 | 25.73798 |
| CSSL 119 | WW | 12.04496 | 0.11365 | 0.002917 | 106.6908 | 0.783779 | 700.3 | 517.284 | 12.46539 | 3.896942 | 31.05866 | 629.6296 | 11.44258 | 3.827262 | 26.01624 |
| CSSL 123 | WW | 12.43124 | 0.13139 | 0.003306 | 94.62477 | 0.784559 | 695.5 | 579.0123 | 11.3036 | 3.660179 | 25.70711 | 675.3086 | 12.11961 | 3.882777 | 29.42015 |
| CSSL 128 | WW | 12.28607 | 0.136865 | 0.003385 | 90.97505 | 0.759657 | 653.7 | 527.1605 | 11.7867 | 3.989174 | 27.62395 | 698.7654 | 13.09047 | 3.80141 | 34.12915 |
| CSSL 136 | WW | 10.99383 | 0.111877 | 0.002818 | 97.83279 | 0.764345 | 730.6 | 475.3086 | 13.34637 | 3.667335 | 35.75589 | 632.0988 | 12.3559 | 3.738654 | 30.72173 |
| DH 103 | WW | 14.3001 | 0.17023 | 0.004185 | 86.35481 | 0.724581 | 632.3 | 587.6543 | 13.37404 | 4.284816 | 35.69122 | 655.5556 | 11.89782 | 3.913337 | 28.39954 |
| DH 212 | WW | 15.03306 | 0.159582 | 0.00385 | 94.90108 | 0.757108 | 698.8 | 529.6296 | 13.07897 | 4.372234 | 33.9856 | 638.2716 | 12.895 | 4.152852 | 33.75207 |
| KDML 105 | WW | 13.08381 | 0.142357 | 0.003616 | 91.91278 | 0.719508 | 788.7 | 520.9877 | 11.99898 | 3.564857 | 28.5624 | 667.9012 | 11.85631 | 3.888481 | 29.01944 |
| CSSL 26 | DS | 8.535008 | 0.082756 | 0.002137 | 102.8125 | 0.711213 | 592.3 | 464.1975 | 13.50081 | 3.725274 | 36.48579 | 637.037 | 11.20324 | 3.82478 | 25.07327 |
| CSSL 28 * | DS | 9.39688 | 0.105755 | 0.002732 | 86.99507 | 0.738096 | 473.5 | 454.321 | 12.65097 | 3.966569 | 31.79215 | 603.7037 | 11.80988 | 3.54298 | 27.66314 |
| CSSL 29 | DS | 6.110094 | 0.068145 | 0.001761 | 89.40127 | 0.721516 | 580 | 530.8642 | 12.97404 | 3.711418 | 33.30658 | 675.3086 | 10.06432 | 3.253083 | 19.98689 |
| CSSL 37 | DS | 6.035887 | 0.070819 | 0.001739 | 85.2332 | 0.739112 | 568.7 | 516.0494 | 13.38775 | 3.687008 | 36.50275 | 634.5679 | 12.11951 | 3.726083 | 29.14734 |
| CSSL 54 | DS | 8.278924 | 0.06961 | 0.001904 | 121.33 | 0.747777 | 675.5 | 495.0617 | 14.01411 | 3.875013 | 39.29486 | 655.5556 | 11.66471 | 3.446318 | 27.09667 |
| CSSL 62 * | DS | 8.331205 | 0.081988 | 0.002115 | 102.4924 | 0.759609 | 576.65 | 479.0123 | 13.17886 | 3.661818 | 35.03889 | 661.7284 | 12.1971 | 3.199996 | 30.04031 |
| CSSL 119 | DS | 6.116743 | 0.068462 | 0.001673 | 89.65746 | 0.624153 | 598.5 | 480.2469 | 14.06655 | 3.551428 | 39.47764 | 676.5432 | 11.76777 | 3.951293 | 27.56656 |
| CSSL 123 | DS | 7.60206 | 0.083981 | 0.001941 | 90.58027 | 0.588482 | 604 | 562.963 | 12.66098 | 3.750572 | 31.78102 | 645.679 | 11.53657 | 3.387278 | 26.64317 |
| CSSL 128 | DS | 8.062722 | 0.100935 | 0.002419 | 79.96742 | 0.571675 | 442.4 | 453.0864 | 12.63332 | 3.72549 | 31.56042 | 648.1481 | 11.833 | 3.492691 | 28.0137 |
| CSSL 136 * | DS | 8.752824 | 0.100977 | 0.002521 | 85.7431 | 0.706101 | 549.2 | 508.642 | 13.25533 | 3.932211 | 34.7958 | 622.2222 | 12.13645 | 3.292633 | 29.69243 |
| DH 103 | DS | 6.061651 | 0.08681 | 0.002091 | 70.24229 | 0.631991 | 546.3 | 551.2346 | 13.90681 | 3.696656 | 38.3 | 617.284 | 13.12586 | 3.230416 | 34.62566 |
| DH 212 | DS | 6.858925 | 0.075496 | 0.001958 | 91.05952 | 0.646964 | 570.3 | 466.6667 | 13.61416 | 3.928358 | 36.86224 | 606.1728 | 14.13031 | 3.411843 | 40.59869 |
| KDML 105 | DS | 7.218214 | 0.084343 | 0.002093 | 86.09545 | 0.666582 | 611.1 | 500 | 12.54595 | 3.868894 | 31.80701 | 664.1975 | 11.78291 | 3.309272 | 27.31333 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lertngim, N.; Ruangsiri, M.; Klinsawang, S.; Raksatikan, P.; Thunnom, B.; Siangliw, M.; Toojinda, T.; Siangliw, J.L. Photosynthetic Plasticity and Stomata Adjustment in Chromosome Segment Substitution Lines of Rice Cultivar KDML105 under Drought Stress. Plants 2023, 12, 94. https://doi.org/10.3390/plants12010094
Lertngim N, Ruangsiri M, Klinsawang S, Raksatikan P, Thunnom B, Siangliw M, Toojinda T, Siangliw JL. Photosynthetic Plasticity and Stomata Adjustment in Chromosome Segment Substitution Lines of Rice Cultivar KDML105 under Drought Stress. Plants. 2023; 12(1):94. https://doi.org/10.3390/plants12010094
Chicago/Turabian StyleLertngim, Narawitch, Mathurada Ruangsiri, Suparad Klinsawang, Pimpa Raksatikan, Burin Thunnom, Meechai Siangliw, Theerayut Toojinda, and Jonaliza Lanceras Siangliw. 2023. "Photosynthetic Plasticity and Stomata Adjustment in Chromosome Segment Substitution Lines of Rice Cultivar KDML105 under Drought Stress" Plants 12, no. 1: 94. https://doi.org/10.3390/plants12010094
APA StyleLertngim, N., Ruangsiri, M., Klinsawang, S., Raksatikan, P., Thunnom, B., Siangliw, M., Toojinda, T., & Siangliw, J. L. (2023). Photosynthetic Plasticity and Stomata Adjustment in Chromosome Segment Substitution Lines of Rice Cultivar KDML105 under Drought Stress. Plants, 12(1), 94. https://doi.org/10.3390/plants12010094










