Aphids on Aphid-Susceptible Cultivars Have Easy Access to Turnip Mosaic Virus, and Effective Inoculation on Aphid-Resistant Cultivars of Oilseed Rape (Brassica napus)
Abstract
:1. Introduction
2. Results
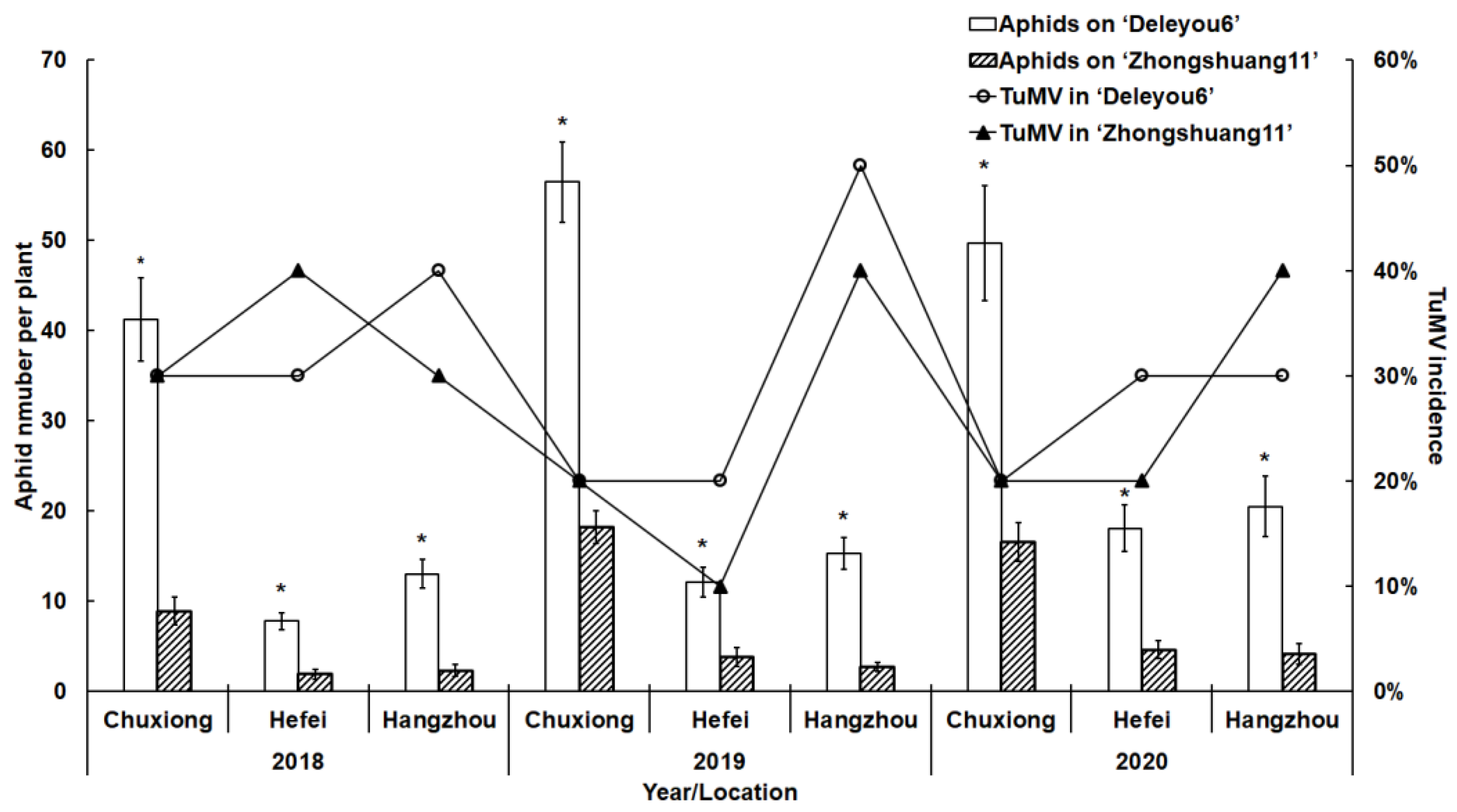
2.1. Aphid and TuMV Incidence in the Filed
2.2. Identification of TuMV-Resistance of Plants
2.3. Identification of Aphid-Resistance of Plants
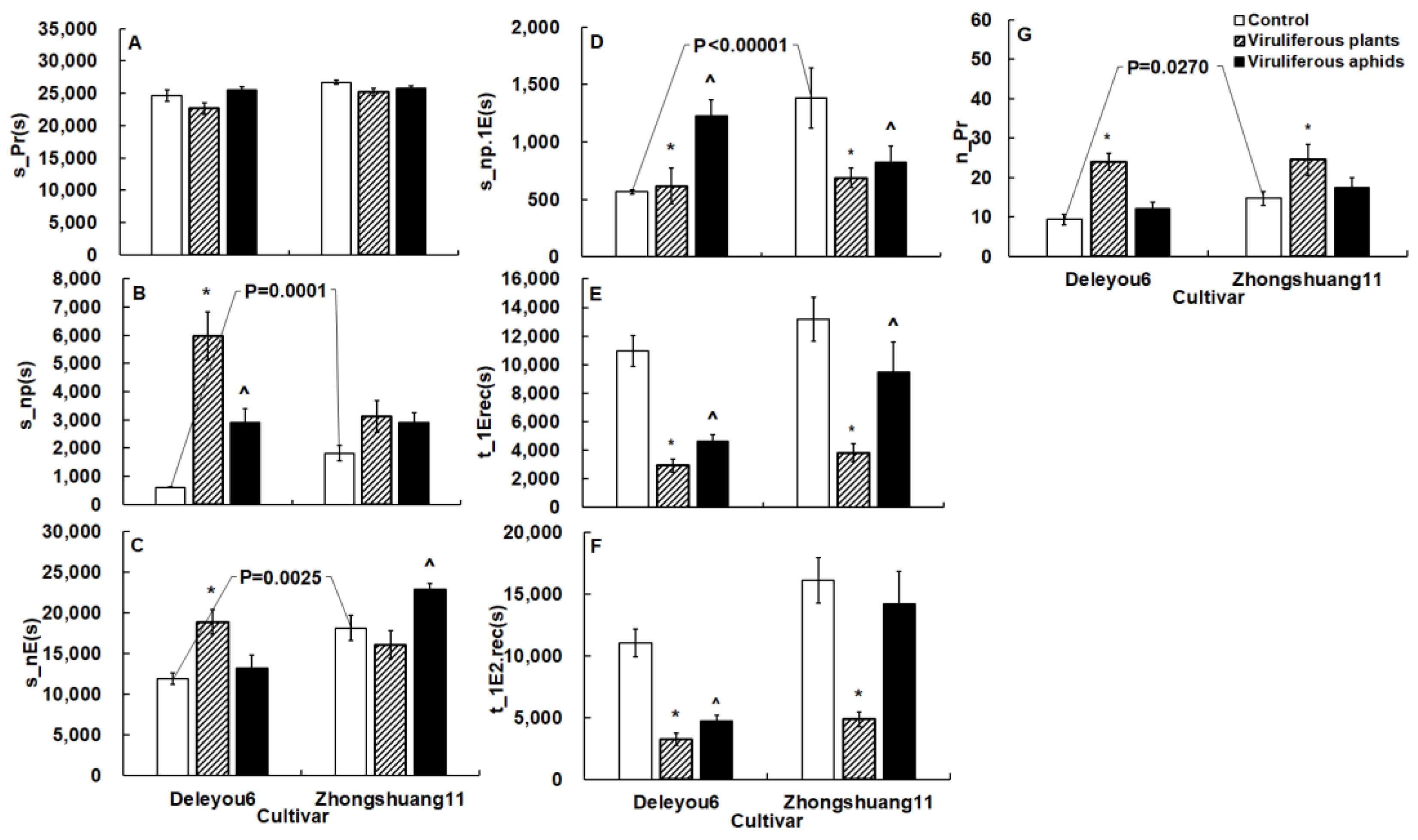
2.4. TuMV Indirectly Modifies Cabbage Aphid Probing Behavior by Infecting Oilseed Rape
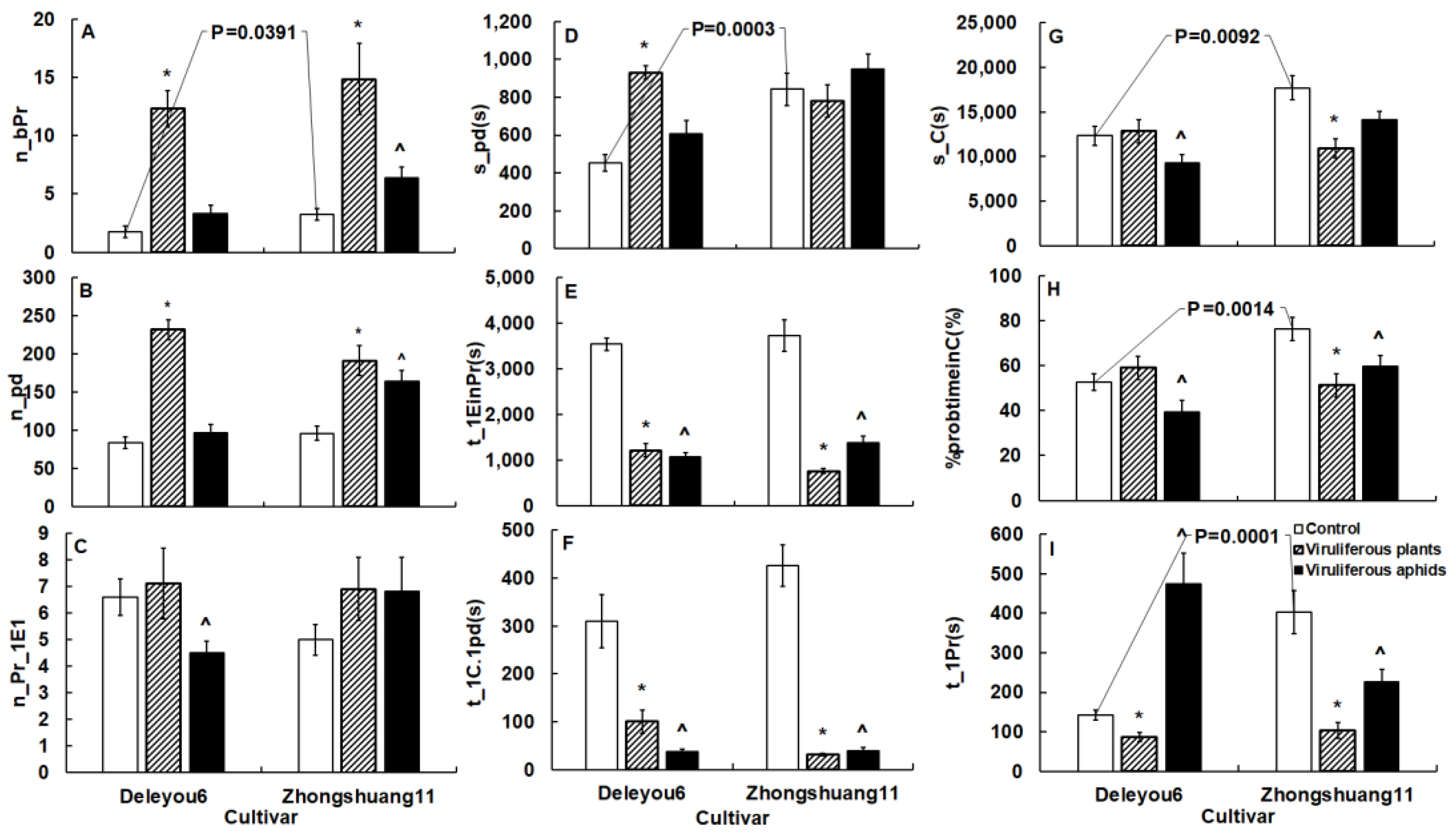
2.5. TuMV Directly Modifies Cabbage Aphid Probing Behavior by Infecting Aphids
3. Discussion
3.1. Assessment of TuMV-Resistance
3.2. Assessment of Aphid Resistance
3.3. Indirect Effects of TuMV Infection
3.4. Direct Effects of TuMV Infection
3.5. Cultivar Effects of TuMV Transmission
4. Materials and Methods
4.1. Biomaterials
4.2. Field Investigation of Aphid and TuMV Incidence
4.3. Identification of TuMV-Resistance of Plants by Artificial Inoculation
4.4. Microscopic Observation of Leaf Surface
4.5. EPG Experiments
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitfield, A.E.; Falk, B.W.; Rotenberg, D. Insect vector-mediated transmission of plant viruses. Virology 2015, 479–480, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Fingu-Mabola, J.C.; Francis, F. Aphid–plant–phytovirus pathosystems: Influencing factors from vector behaviour to virus spread. Agriculture 2021, 11, 502. [Google Scholar] [CrossRef]
- Bosque-Pérez, N.A.; Eigenbrode, S.D. The influence of virus-induced changes in plants on aphid vectors: Insights from luteovirus pathosystems. Virus Res. 2011, 159, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Carmo-Sousa, M.; Moreno, A.; Garzo, E.; Fereres, A. A non-persistently transmitted-virus induces a pull–push strategy in its aphid vector to optimize transmission and spread. Virus Res. 2014, 186, 38–46. [Google Scholar] [CrossRef]
- Gadhave, K.R.; Dutta, B.; Coolong, T.; Srinivasan, R. A non-persistent aphid-transmitted potyvirus differentially alters the vector and non-vector biology through host plant quality manipulation. Sci. Rep. 2019, 9, 2503. [Google Scholar] [CrossRef]
- McMenemy, L.S.; Hartley, S.E.; MacFarlane, S.A.; Karley, A.J.; Shepherd, T.; Johnson, S.N. Raspberry viruses manipulate the behaviour of their insect vectors. Entomol. Exp. Appl. 2012, 144, 56–68. [Google Scholar] [CrossRef]
- Hodge, S.; Powell, G. Conditional facilitation of an aphid vector, Acyrthosiphon pisum, by the plant pathogen, pea enation mosaic virus. J. Insect Sci. 2010, 10, 155. [Google Scholar] [CrossRef]
- Mauck, K.E.; Moraes, C.D.; Mescher, M.C. Effects of pathogens on sensory-mediated interactions between plants and insect vectors. Curr. Opin. Plant Biol. 2016, 32, 53–61. [Google Scholar] [CrossRef]
- Westwood, J.H.; Groen, S.C.; Du, Z.; Murphy, A.M.; Anggoro, D.T.; Tungadi, T.; Luang-In, V.; Lewsey, M.G.; Rossiter, J.T.; Powell, G.; et al. A trio of viral proteins tunes aphid-plant interactions in Arabidopsis thaliana. PLoS ONE 2013, 8, e83066. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Biochemical and physiological mechanisms underlying effects of cucumber mosaic virus on host-plant traits that mediate transmission by aphid vectors. Plant. Cell Environ. 2014, 37, 1427–1439. [Google Scholar] [CrossRef]
- Gadhave, K.R.; Gautam, S.; Rasmussen, D.A.; Srinivasan, R. Aphid transmission of potyvirus: The largest plant-infecting RNA virus genus. Viruses 2020, 12, 773. [Google Scholar] [CrossRef] [PubMed]
- Belliure, B.; Janssen, A.; Maris, P.C.; Peters, D.; Sabelis, M.W. Herbivore arthropods benefit from vectoring plant viruses. Ecol. Lett. 2005, 8, 70–79. [Google Scholar] [CrossRef]
- Jiu, M.; Zhou, X.P.; Tong, L.; Xu, J.; Yang, X.; Wan, F.H.; Liu, S.S. Vector-virus mutualism accelerates population increase of an invasive whitefly. PLoS ONE 2007, 2, e182. [Google Scholar] [CrossRef] [PubMed]
- Mauck, K.E.; Moraes, C.D.; Mescher, M.C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl. Acad. Sci. USA 2010, 107, 3600–3605. [Google Scholar] [CrossRef]
- Lung, M.C.; Pirone, T.P. Acquisition factor required for aphid transmission of purified cauliflower mosaic virus. Virology 1974, 60, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Hoh, F.; Uzest, M.; Drucker, M.; Plisson-Chastang, C.; Bron, P.; Blanc, S.; Dumas, C. Structural insights into the molecular mechanisms of cauliflower mosaic virus transmission by its insect vector. J. Virol. 2010, 84, 4706–4713. [Google Scholar] [CrossRef]
- Plisson, C.; Uzest, M.; Drucker, M.; Froissart, R.; Dumas, C.; Conway, J.; Thomas, D.; Blanc, S.; Bron, P. Structure of the mature P3-virus particle complex of cauliflower mosaic virus revealed by cryo-electron microscopy. J. Mol. Biol. 2005, 346, 267–277. [Google Scholar] [CrossRef]
- Uzest, M.; Gargani, D.; Dombrovsky, A.; Cazevieille, C.; Cot, D.; Blanc, S. The“acrostyle”: A newly described anatomical structure in aphid stylets. Arthropod Struct. Dev. 2010, 39, 221–229. [Google Scholar] [CrossRef]
- Berthelot, E.; Macia, J.-L.; Martinière, A.; Morisset, A.; Gallet, R.; Blanc, S.; Khelifa, M.; Drucker, M. Pharmacological analysis of transmission activation of two aphid-vectored plant viruses, turnip mosaic virus and cauliflower mosaic virus. Sci. Rep. 2019, 9, 9374. [Google Scholar] [CrossRef]
- Jeger, M.J. The epidemiology of plant virus disease: Towards a new synthesis. Plants 2020, 9, 1768. [Google Scholar] [CrossRef]
- Cai, L.; Xu, Z.; Chen, K.; Yan, L.; Hou, M. Surveys of virus diseases of oilseed rape and serological diagnosis of the viruses in Hubei and Anhui Provinces. Plant Protect. 2007, 33, 88–90. [Google Scholar]
- Li, G.; Lv, H.; Zhang, S.; Zhang, S.; Li, F.; Zhang, H.; Qian, W.; Fang, Z.; Sun, R. TuMV management for brassica crops through host resistance: Retrospect and prospects. Plant Pathol. 2019, 68, 1035–1044. [Google Scholar] [CrossRef]
- Li, L.L.; Huang, Z.H.; Wang, S.Y. Infectivity, distribution, and transport regularity of TuMV in rapeseed. Chin. J. Oil Crop Sci. 1991, 4, 59–61. [Google Scholar]
- Wang, L. Studies on Resistance Mechanism of Rape to Lipaphis erysimi (Homoptera: Aphidae). Master’s Thesis, Sichuan Agricultural University, Yaan, China, 2012. [Google Scholar]
- Lu, X.; Huang, W.; Zhang, S.; Li, F.; Zhang, H.; Sun, R.; Li, G.; Zhang, S. Resistance management through Brassica crop–TuMV–aphid interactions: Retrospect and prospects. Horticulturae 2022, 8, 247. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Electrical nature of recorded signals during stylet penetration by aphids. Entomol. Exp. Appl. 1985, 38, 177–186. [Google Scholar] [CrossRef]
- Hao, Z.P.; Hou, S.M.; Hu, B.C.; Huang, F.; Dang, X.L. Assessment of probing behavior of the cabbage aphid, Brevicoryne brassicae (Hemiptera: Aphididae), on three Brassica napus cultivars at three developmental stages using Electropenetrography (EPG). J. Kansas Entomol. Soc. 2017, 90, 11–23. [Google Scholar] [CrossRef]
- Hao, Z.P.; Zhan, H.X.; Gao, L.L.; Huang, F.; Zhu, L.N.; Hou, S.M. Possible effects of leaf tissue characteristics of oilseed rape Brassica napus on probing and feeding behaviors of cabbage aphids. Arthropod-Plant Interact. 2020, 14, 733–744. [Google Scholar] [CrossRef]
- Chen, K.-R.; Cai, L.; Xu, Z.-Y.; Yan, L.-Y.; Wang, G.-P. Resistance evaluation of cultivars and germplasms to turnip mosaic virus in oilseed rape. Chin. J. Oil Crop Sci. 2006, 28, 350–353. [Google Scholar]
- Traicevski, V.; Ward, S.A. Probing behaviour of Aphis craccivora Koch on host plants of different nutritional quality. Ecol. Entomol. 2002, 27, 213–219. [Google Scholar] [CrossRef]
- Powell, G.; Tosh, C.R.; Hardie, J. Host plant selection by aphids: Behavioral, evolutionary, and applied perspectives. Annu. Rev. Entomol. 2006, 51, 309–330. [Google Scholar] [CrossRef]
- Jiang, W.; Cheng, Q.; Lu, C.; Chen, W.; Zhao, D.; He, Y. Different host plants distinctly influence the adaptability of Myzus persicae (Hemiptera: Aphididae). Agriculture 2022, 12, 2162. [Google Scholar] [CrossRef]
- Werker, E. Trichome diversity and development. Adv. Bot. Res. 2000, 31, 1–35. [Google Scholar]
- Simmons, A.T.; Gurr, G.M. Trichome-based host plant resistance of Lycopersicon species and the biocontrol agent Mallada signata: Are they compatible? Entomol. Exp. Appl. 2004, 113, 95–101. [Google Scholar] [CrossRef]
- Prado, E.; Tjallingii, W.F. Effects of previous plant infestation on sieve element acceptance by two aphids. Entomol. Exp. Appl. 1997, 82, 189–200. [Google Scholar] [CrossRef]
- Han, B.Y.; Chen, Z.M. Difference in probing behavior of tea aphid on vegetative parts of tea plant and non-host plants. Entomol. Sin. 2000, 7, 337–343. [Google Scholar]
- Yan, F.M.; Wang, M.Q. Insect Electrical Penetration Graph and Its Application, 1st ed.; Henan Science and Technology Press: Zhengzhou, China, 2017. [Google Scholar]
- Tjallingii, W.F. Salivary secretions by aphids interacting with proteins of phloem wound responses. J. Exp. Bot. 2006, 57, 739–745. [Google Scholar] [CrossRef]
- Dreyer, D.L.; Jones, K.C.; Jurd, L.; Campbell, B.C. Feeding deterrency of some 4-hydroxycoumarins and related compounds relationship to host-plant resistance of alfalfa towards pea aphid Acyrthosiphon pisum. J. Chem. Ecol. 1987, 13, 925–930. [Google Scholar] [CrossRef]
- Karley, A.J.; Douglas, A.E.; Parker, W.E. Amino acid composition and nutritional quality of potato leaf phloem sap for aphids. J. Exp. Biol. 2002, 205, 3009–3018. [Google Scholar] [CrossRef]
- Matsumura, E.E.; Kormelink, R. Small talk: On the possible role of trans-kingdom small RNAs during plant–virus–vector tritrophic communication. Plants 2023, 12, 1411. [Google Scholar] [CrossRef]
- Peng, Q.; Li, W.; Zhou, X.; Sun, C.; Hou, Y.; Hu, M.; Fu, S.; Zhang, J.; Kundu, J.K.; Lei, L. Genetic diversity analysis of Brassica yellows virus causing aberrant color symptoms in oilseed rape. Plants 2023, 12, 1008. [Google Scholar] [CrossRef]
- Bak, A.; Patton, M.F.; Perilla-Henao, L.M.; Aegerter, B.J.; Casteel, C.L. Ethylene signaling mediates potyvirus spread by aphid vectors. Oecologia 2019, 190, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Casteel, C.L.; Yang, C.L.; Nanduri, A.C.; Jong, H.N.D.; Whitham, S.A.; Jander, G. The NIa-Pro protein of Turnip mosaic virus improves growth and reproduction of the aphid vector, Myzus persicae (green peach aphid). Plant J. 2014, 77, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Weldegergis, B.T.; Li, J.; Jung, C.; Qu, J.; Sun, Y.; Qian, H.; Tee, C.; van Loon, J.J.A.; Dicke, M.; et al. Virulence factors of geminivirus interact with MYC2 to subvert plant resistance and promote vector performance. Plant Cell 2014, 26, 4991–5008. [Google Scholar] [CrossRef]
- Wu, X.; Xu, S.; Zhao, P.; Zhang, X.; Yao, X.; Sun, Y.; Fang, R.; Ye, J. The Orthotospovirus nonstructural protein NSs suppresses plant MYC-regulated jasmonate signaling leading to enhanced vector attraction and performance. PLoS Pathog. 2019, 15, e1007897. [Google Scholar] [CrossRef] [PubMed]
- Madden, L.V.; Raccah, B.; Pirone, T.P. Modeling plant disease increase as a function of vector numbers: Nonpersistent viruses. Res. Popul. Ecol. 1990, 32, 47–65. [Google Scholar] [CrossRef]
- Madden, L.V.; Jeger, M.J.; van den Bosch, F. A theoretical assessment of the effects of vector–virus transmission mechanism on plant virus disease epidemics. Phytopathology 2000, 90, 576–594. [Google Scholar] [CrossRef]
- Collar, J.L.; Avilla, C.; Fereres, A. New correlations between aphid stylet paths and nonpersistent virus transmission. Environ. Entomol. 1997, 26, 537–544. [Google Scholar] [CrossRef]
- Powell, G. Intracellular salivation is the aphid activity associated with inoculation of non-persistently transmitted viruses. J. Gen. Virol. 2005, 86, 469–472. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Bosque-Pérez, N.A.; Davis, T.S. Insect-borne plant pathogens and their vectors: Ecology, evolution, and complex interactions. Annu. Rev. Entomol. 2018, 63, 169–191. [Google Scholar] [CrossRef]
- Mauck, K.E. Variation in virus effects on host plant phenotypes and insect vector behavior: What can it teach us about virus evolution? Curr. Opin. Virol. 2016, 21, 114–123. [Google Scholar] [CrossRef]
- He, W.B.; Li, j.; Liu, S.S. Differential profiles of direct and indirect modification of vector feeding behaviour by a plant virus. Sci. Rep. 2015, 5, 7682. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.J.; Jiang, S.L.; Wang, W.J.; Li, G.Q.; Tao, X.R.; Pan, W.D.; Sword, G.A.; Chen, F.J. Rice stripe virus counters reduced fecundity in its insect vector by modifying insect physiology, primary endosymbionts and feeding behavior. Sci. Rep. 2015, 5, 12527. [Google Scholar] [CrossRef] [PubMed]
- Stafford, C.A.; Walker, G.P.; Ullman, D.E. Infection with a plant virus modifies vector feeding behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 9350–9355. [Google Scholar] [CrossRef]
- Ren, G.; Wang, X.; Chen, D.; Wang, X.; Fan, X.; Liu, X. Potato virus Y-infected tobacco affects the growth, reproduction, and feeding behavior of a vector aphid, Myzus persicae (Hemiptera: Aphididae). Appl. Entomol. Zool. 2015, 50, 239–243. [Google Scholar] [CrossRef]
- Fereres, A.; Blua, M.J.; Perring, T.M. Retention and transmission characteristics of zucchini yellow mosaic virus by Aphis gossypii and Myzus persicae (Homoptera: Aphididae). J. Econ. Entomol. 1992, 85, 759–765. [Google Scholar] [CrossRef]
- Bak, A.; Cheung, A.L.; Yang, C.; Whitham, S.A.; Casteel, C.L. A viral protease relocalizes in the presence of the vector to promote vector performance. Nat. Commun. 2017, 8, 14493. [Google Scholar] [CrossRef]
- Casteel, C.L.; De Alwis, M.; Bak, A.; Dong, H.; Whitham, S.A.; Jander, G. Disruption of ethylene responses by Turnip mosaic virus mediates suppression of plant defense against the green peach aphid vector. Plant Physiol. 2015, 169, 209–218. [Google Scholar] [CrossRef]
- Jing, P.; Bai, S.F.; Liu, F. Effects of rice resistance on the feeding behavior and subsequent virus transmission efficiency of Laodelphasx striatellus. Arthropod-Plant Interact. 2015, 9, 97–105. [Google Scholar] [CrossRef]
- Werner, B.J. Behavioral Responses of the Green Peach Aphid to Virus-Induced Volatiles Produced by Potatoes Infected with Potato Leafroll Virus. Master’s Thesis, University of Idaho, Moscow, Russia, 2006. [Google Scholar]
- Medina-Ortega, K.J.; Bosque-Pérez, N.A.; Ngumbi, E.; Jiménez-Martínez, E.S.; Eigenbrode, S.D. Rhopalosiphum padi (Hemiptera: Aphididae) responses volatile cues from barley yellow dwarf virus-infected wheat. Environ. Entomol. 2009, 38, 836–845. [Google Scholar] [CrossRef]
- Martín, B.; Collar, J.L.; Tjallingii, W.F.; Fereres, A. Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J. Gen. Virol. 1997, 78, 2701–2705. [Google Scholar] [CrossRef]
- Wang, B.; Chen, J.-Q.; Zhang, P.-F.; Ma, L.; Wang, Y.-M. Effects of post-acquisition fast on cucumber mosaic virus transmission by the cotton aphid, Aphis gossypii. Acta Entomol. Sin. 2003, 46, 259–266. [Google Scholar]
- Alvarez, A.E.; Tjallingii, W.F.; Garzo, E.; Vleeshouwers, V.; Dicke, M.; Vosman, B. Location of resistance factors in the leaves of potato and wild tuber-bearing Solanum species to the aphid Myzus persicae. Entomol. Exp. Appl. 2006, 121, 145–157. [Google Scholar] [CrossRef]
- Hao, Z.P.; Zhan, H.X.; Wang, Y.L.; Hou, S.M. How cabbage aphids Brevicoryne brassicae (L.) make a choice to feed on Brassica napus cultivars. Insects 2019, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L. Identification of turnip mosaic virus strains on oilseed rape. Chin. J. Oil Crop Sci. 1989, 2, 51–55, 65. [Google Scholar]
- Li, L.L. Studies on purification and properties of turnip mosaic virus CRl strain in rapeseed in China. Chin. J. Oil Crop Sci. 1990, 4, 17–22. [Google Scholar]
- Hao, Z.-P.; Sheng, L.; Feng, Z.-B.; Fei, W.-X.; Hou, S.-M. Turnip mosaic virus infection differentially modifies cabbage aphid probing behavior in spring and winter oilseed rape (Brassica napus). Insects 2022, 13, 791. [Google Scholar] [CrossRef]

| Cultivar | Average Disease Incidence (%) | Average Disease Index (%) | Resistance Rank |
|---|---|---|---|
| Deleyou6 | 25.80 ± 2.83 | 29.48 ± 3.87 | medium resistance |
| Zhongshuang11 | 20.30 ± 2.98 | 16.25 ± 3.12 * | medium resistance |
| Variable | Cultivar | |
|---|---|---|
| Deleyou6 | Zhongshuang11 | |
| Thickness of upper epidermis (μm) | 19.06 ± 0.95 | 29.75 ± 1.51 * |
| Trichome length (μm) | 824.75 ± 48.73 | 711.23 ± 28.67 |
| Trichome density on the upper surface | 15.33 ± 3.04 | 14.00 ± 1.15 |
| Trichome density on the lower surface | 27.40 ± 2.38 | 40.50 ± 1.50 * |
| Main Variables Associated with Behavior Modification | Cultivar | |||||
|---|---|---|---|---|---|---|
| Deleyou6 | Zhongshuang11 | |||||
| Treatment I | Treatment II | Treatment III | Treatment I | Treatment II | Treatment III | |
| Epidermis | ||||||
| t_1Pr (s) | 142.12 ± 12.01 | 86.20 ± 12.03 | 474.58 ± 77.17 | 402.78 ± 53.98 | 102.84 ± 19.71 | 227.37 ± 29.55 |
| Mesophyll | ||||||
| n_bPr | 1.75 ± 0.47 | 12.30 ± 1.56 | —— | 3.25 ± 0.50 | 14.85 ± 3.08 | 6.40 ± 0.91 |
| n_pd | —— | 231.70 ± 12.53 | —— | —— | 191.30 ± 19.77 | 163.85 ± 14.60 |
| s_pd (s) | 453.27 ± 43.70 | 930.79 ± 36.81 | —— | 841.95 ± 85.15 | —— | —— |
| t_1C.1pd (s) | —— | 100.00 ± 24.23 | 37.49 ± 5.69 | —— | 32.28 ± 2.63 | 40.01 ± 6.17 |
| t_1EinPr (s) | —— | 1211.67 ± 143.29 | 1074.91 ± 86.78 | —— | 754.98 ± 50.53 | 1383.98 ± 136.41 |
| s_C (s) | 12,325.42 ± 1094.15 | —— | 9293.24 ± 944.29 | 17,707.84 ± 1346.55 | 10,904.90 ± 1092.13 | —— |
| %probtimeinC | 52.61 ± 3.76 | 39.41 ± 5.19 | 76.27 ± 5.14 | 51.22 ± 5.30 | 59.56 ± 4.93 | |
| Phloem | ||||||
| n_E1 | —— | 10.15 ± 0.89 | 3.90 ± 0.56 | —— | 7.95 ± 1.08 | 5.95 ± 1.08 |
| s_E1 (s) | 88.98 ± 5.85 | 345.11 ± 32.56 | 195.45 ± 28.35 | 314.60 ± 32.10 | 236.15 ± 38.27 | 742.74 ± 145.31 |
| %_E1/E12 | 0.81 ± 0.07 | 5.36 ± 0.82 | —— | 29.39 ± 5.95 | 3.62 ± 0.90 | —— |
| d_E1followedby1sE2 (s) | —— | 33.37 ± 2.15 | 43.72 ± 1.40 | —— | 19.66 ± 0.60 | 45.42 ± 1.89 |
| s_E1followedbysE2 (s) | 95.96 ± 7.50 | —— | —— | 150.44 ± 7.88 | 58.71 ± 8.72 | —— |
| %probtimeinE1 | 0.37 ± 0.05 | 1.70 ± 0.17 | 0.82 ± 0.12 | 0.95 ± 0.16 | —— | 2.35 ± 0.62 |
| s_E2 (s) | 13,141.15 ± 952.43 | 8669.17 ± 1425.51 | —— | 7411.50 ± 1392.52 | —— | —— |
| s_longestE2 (s) | 11,905.99 ± 805.27 | 4853.35 ± 937.68 | —— | 6906.80 ± 1400.80 | —— | —— |
| E2index | 85.85 ± 2.58 | 37.26 ± 5.44 | —— | 57.57 ± 7.29 | —— | 30.40 ± 2.40 |
| %sE2/E2 | 97.44 ± 1.24 | 39.15 ± 4.48 | 71.67 ± 5.83 | 54.63 ± 8.29 | —— | —— |
| Resistance assessment | Aphid-susceptible | TuMV-medium resistant | Aphid-resistant | TuMV-medium resistant | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Z.-P.; Feng, Z.-B.; Sheng, L.; Fei, W.-X.; Hou, S.-M. Aphids on Aphid-Susceptible Cultivars Have Easy Access to Turnip Mosaic Virus, and Effective Inoculation on Aphid-Resistant Cultivars of Oilseed Rape (Brassica napus). Plants 2023, 12, 1972. https://doi.org/10.3390/plants12101972
Hao Z-P, Feng Z-B, Sheng L, Fei W-X, Hou S-M. Aphids on Aphid-Susceptible Cultivars Have Easy Access to Turnip Mosaic Virus, and Effective Inoculation on Aphid-Resistant Cultivars of Oilseed Rape (Brassica napus). Plants. 2023; 12(10):1972. https://doi.org/10.3390/plants12101972
Chicago/Turabian StyleHao, Zhong-Ping, Zeng-Bei Feng, Lei Sheng, Wei-Xin Fei, and Shu-Min Hou. 2023. "Aphids on Aphid-Susceptible Cultivars Have Easy Access to Turnip Mosaic Virus, and Effective Inoculation on Aphid-Resistant Cultivars of Oilseed Rape (Brassica napus)" Plants 12, no. 10: 1972. https://doi.org/10.3390/plants12101972





