Resistance of Primary Photosynthesis to Photoinhibition in Antarctic Lichen Xanthoria elegans: Photoprotective Mechanisms Activated during a Short Period of High Light Stress
Abstract
:1. Introduction
2. Results
2.1. Photoinhibition-Induced Decrease in PSII Functioning and Consequent Recovery
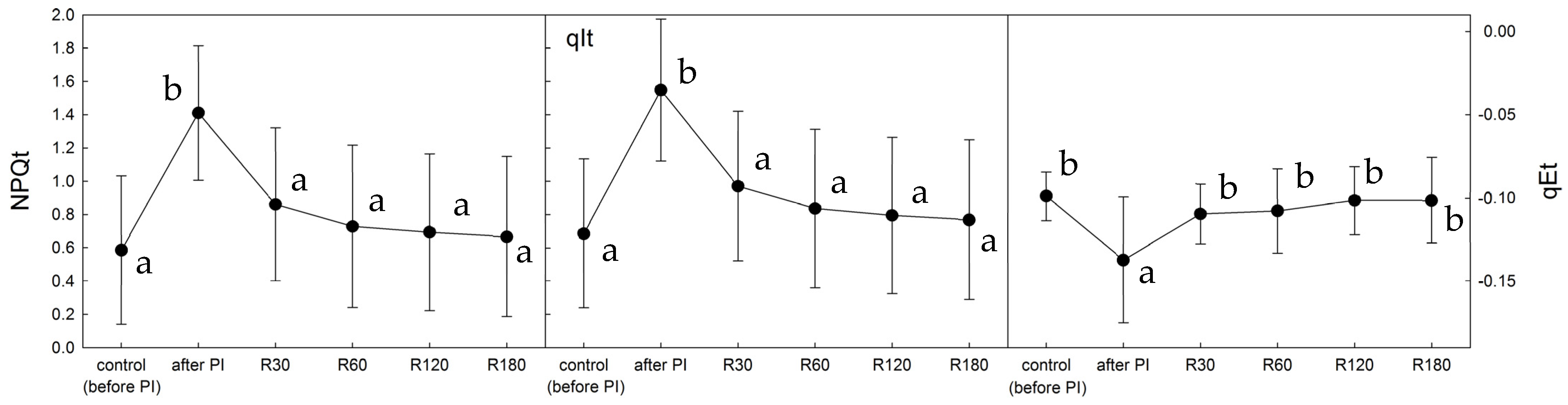
2.2. NPQ Activation by Light in Control, Photoinhibited, and Recovered Thalli
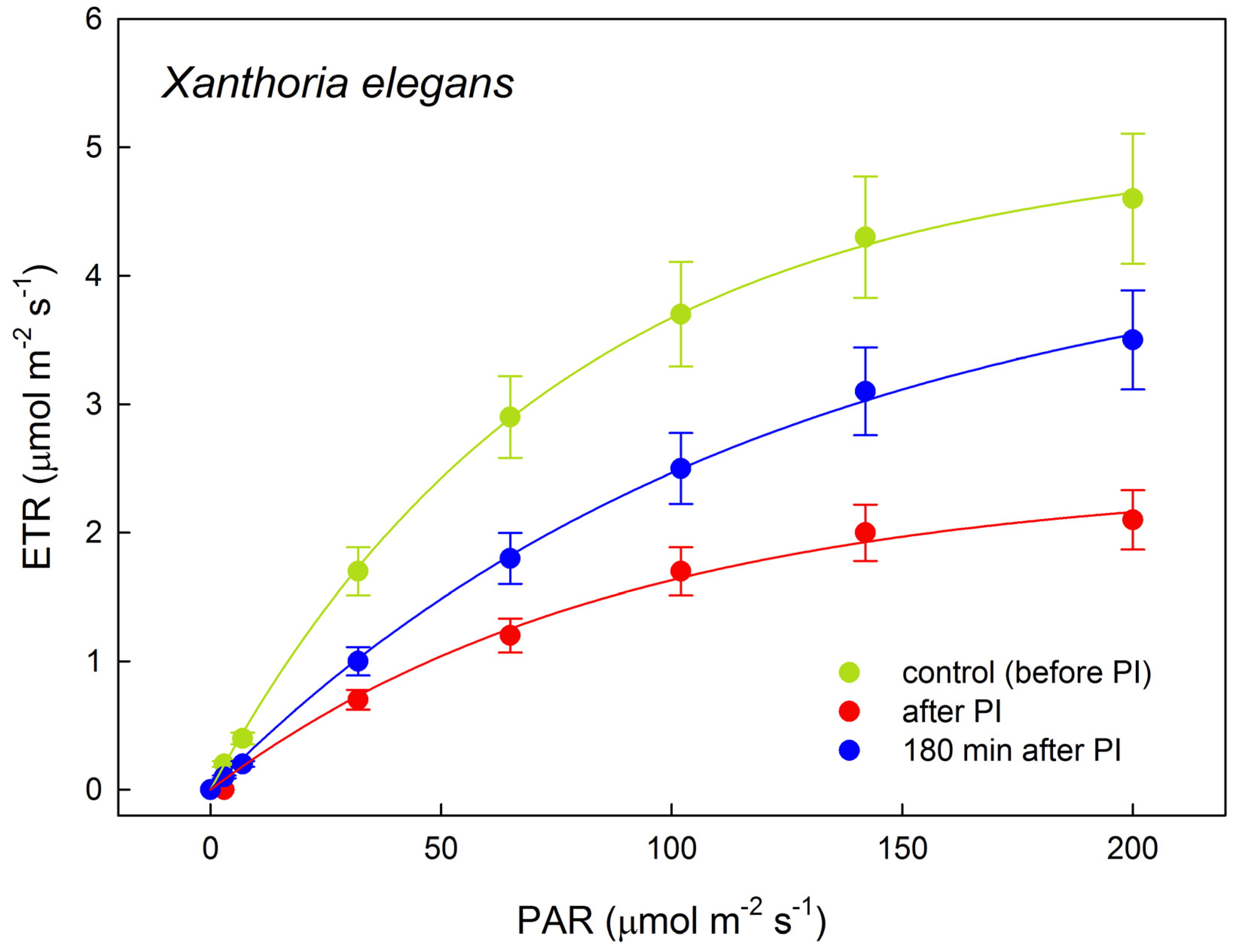
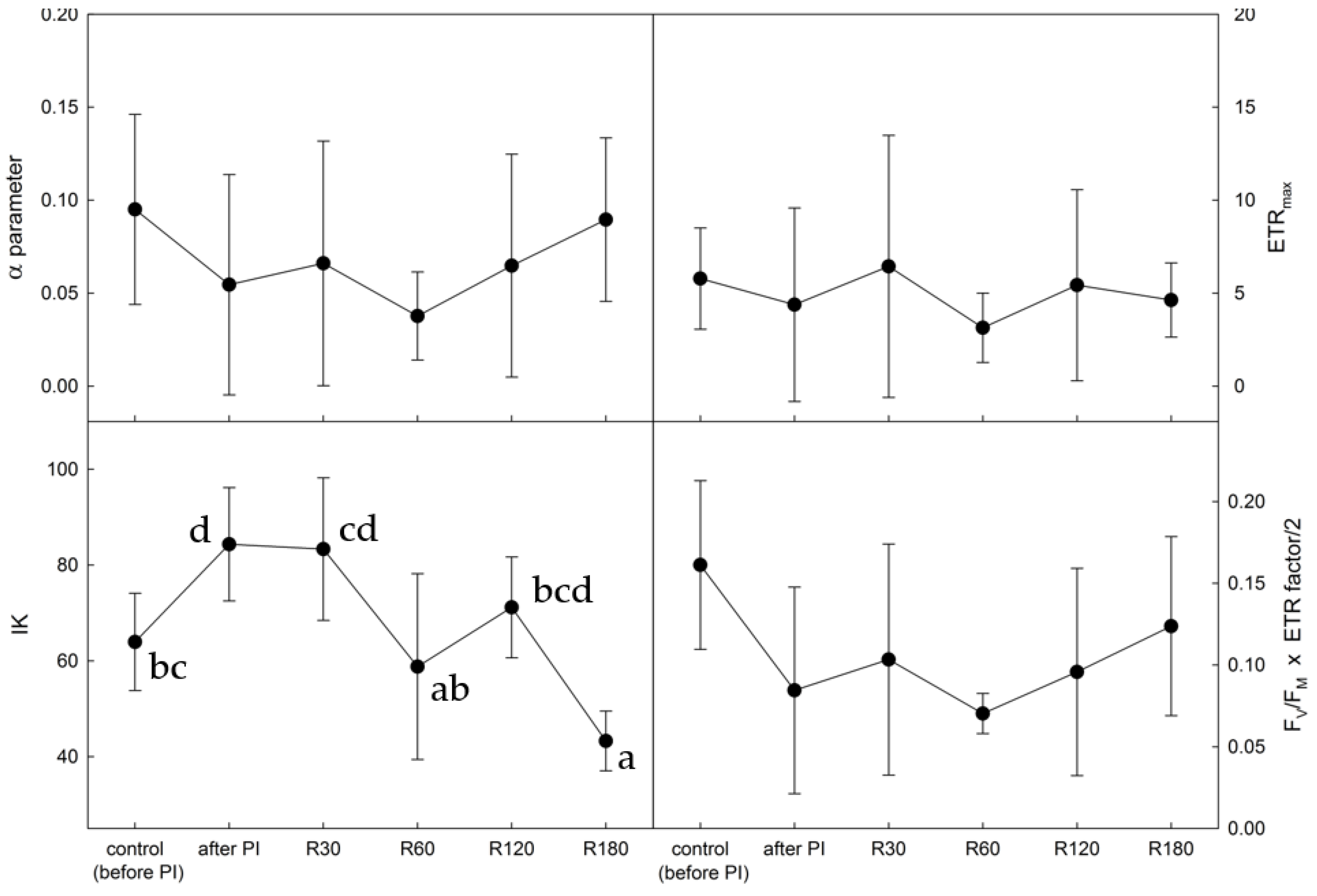
2.3. Photosynthetic Rapid Light Response Curves of Electron Transport
3. Discussion
4. Material and Methods
4.1. Collection and Handling of Samples
4.2. Photoinhibitory Treatment
4.3. Photoinhibition and Recovery of PSII
4.4. Induction Kinetics of NPQ
4.5. Rapid Light Response Curves of Photosynthetic Electron Transport
4.6. Statistical Analysis
5. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Tyystjärvi, E. Photoinhibition of Photosystem II. Int. Rev. Cell Mol. Biol. 2013, 300, 243–303. [Google Scholar] [CrossRef]
- Vítek, P.; Mishra, K.B.; Mishra, A.; Veselá, B.; Findurová, H.; Svobodová, K.; Oravec, M.; Sahu, P.P.; Klem, K. Non-destructive insights into photosynthetic and photoprotective mechanisms in Arabidopsis thaliana grown under two light regimes. Spectrochim. Scta. Part A Mol. Biomol. Spectrosc. 2022, 281, 121531. [Google Scholar] [CrossRef] [PubMed]
- Mafole, T.C.; Solhaug, K.A.; Minibayeva, F.V.; Beckett, R.P. Tolerance to photoinhibition within lichen species is higher in melanised thalli. Photosynthetica 2019, 57, 96–102. [Google Scholar] [CrossRef]
- Beckett, R.; Minibayeva, F.; Solhaug, K.; Roach, T. Photoprotection in lichens: Adaptations of photobionts to high light. Lichenologist 2021, 53, 21–33. [Google Scholar] [CrossRef]
- Mattila, H.; Mishra, K.B.; Kuusisto, I.; Mishra, A.; Novotná, K.; Šebela, D.; Tyystjärvi, E. Effects of low temperature on photoinhibition and singlet oxygen production in four natural accessions of Arabidopsis. Planta 2020, 252, 19. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.; Sotoudehnia, P.; Kuuslampi, T.; Stracke, R.; Mishra, K.B.; Tyystjärvi, E. Singlet oxygen, flavonols and photoinhibition in green and senescing silver birch leaves. Trees 2021, 35, 1267–1282. [Google Scholar] [CrossRef]
- Puhovkin, A.; Bezsmertna, O.; Parnikoza, I. Interspecific differences in desiccation tolerance of selected Antarctic lichens: Analysis of photosystem II effectivity and quenching mechanisms. Czech Polar Rep. 2022, 12, 31–43. [Google Scholar] [CrossRef]
- Hájek, J.; Puhovkin, A.; Giordano, D.; Sekerák, J., Jr. What does critical temperature tell us about the resistance of polar lichens to freezing stress? Applicability of linear cooling method to ecophysiological studies. Czech Polar Rep. 2022, 12, 246–255. [Google Scholar] [CrossRef]
- Murchie, E.H.; Ruban, A.V. Dynamic non-photochemical quenching in plants: From molecular mechanism to productivity. Plant J. 2019, 101, 885–896. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, M.H.; Goltsev, V.N.; Żuk-Golaszewska, K.; Zivcak, M.; Brestic, M. Chlorophyll Fluorescence. Understanding Crop Performance—Basics and Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Cao, S.; Zhang, X.; Xu, D.; Fan, X.; Mou, S.; Wang, Y.; Ye, N.; Wang, W. A transthylakoid proton gradient and inhibitors induce a non-photochemical fluorescence quenching in unicellular algae Nannochloropsis sp. FEBS Lett. 2013, 587, 1310–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Štepigová, J.; Vráblíková, H.; Lang, J.; Večeřová, K.; Barták, M. Glutathione and zeaxanthin formation during high light stress in foliose lichens. Plant Soil Environ. 2007, 53, 340–344. [Google Scholar] [CrossRef] [Green Version]
- Vráblíková, H.; Barták, M.; Wonisch, A. Changes in glutathione and xanthophyll cycle pigments in the high light-stressed lichens Umbilicaria antarctica and Lasallia pustulata. J. Photochem. Photobiol. B-Biol. 2005, 79, 35–41. [Google Scholar] [CrossRef]
- Mishra, K.B.; Mishra, A.; Kubásek, J.; Urban, O.; Heyer, A.G. Govindjee Low temperature induced modulation of photosynthetic induction in non-acclimated and cold-acclimated Arabidopsis thaliana: Chlorophyll a fluorescence and gas-exchange measurements. Photosynth. Res. 2019, 139, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Mishra, K.B.; Surá, K.; Veselá, B.; Klem, K.; Urban, O. Non-photochemical quenching in natural accessions of Arabidopsis thaliana during cold acclimation. Environ. Exp. Bot. 2023, 211, 105372. [Google Scholar] [CrossRef]
- Ohnishi, N.; Allakhverdiev, S.I.; Takahashi, S.; Higashi, S.; Watanabe, M.; Nishiyama, Y.; Murata, N. Two-step mechanism of photodamage to photosystem II: Step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center. Biochemistry 2005, 44, 8494–8499. [Google Scholar] [CrossRef]
- Phinney, N.H.; Gauslaa, Y.; Solhaug, K.A. Why chartreuse? The pigment vulpinic acid screens blue light in the lichen Letharia vulpina. Planta 2019, 249, 709–718. [Google Scholar] [CrossRef]
- Bianchi, E.; Paoli, L.; Colzi, I.; Coppi, A.; Gonnelli, C.; Lazzaro, L.; Loppi, S.; Papini, A.; Vannini, A.; Benesperi, R. High-light stress in wet and dry thalli of the endangered Mediterranean lichen Seirophora villosa (Ach.) Frödén: Does size matter? Mycol. Progress 2019, 18, 463–470. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Solhaug, K.A. Fungal melanins as a sun screen for symbiotic green algae in the lichen Lobaria pulmonaria. Oecologia 2001, 126, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ranjan, S.; Nayaka, S.; Pathre, U.V.; Shirke, P.A. Functional characteristics of a fruticose type of lichen, Stereocaulon foliolosum Nyl. in response to light and water stress. Acta Physiol. Plant. 2013, 35, 1605–1615. [Google Scholar] [CrossRef]
- Solhaug, K. Low-light recovery effects on assessment of photoinhibition with chlorophyll fluorescence in lichens. Lichenologist 2018, 50, 139–145. [Google Scholar] [CrossRef]
- Barták, M.; Hájek, J.; Očenášová, P. Photoinhibition of photosynthesis in Antarctic lichen Usnea antarctica. I. Light intensity- and light duration-dependent changes in functioning of photosystem II. Czech Polar Rep. 2012, 2, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Očenášová, P.; Barták, M.; Hájek, J. Photoinhibition of photosynthesis in Antarctic lichen Usnea antarctica. II. Analysis of non-photochemical quenching mechanisms activated by low to medium light doses. Czech Polar Rep. 2014, 4, 90–99. [Google Scholar] [CrossRef]
- Balarinová, K.; Barták, M.; Hazdrová, J.; Hájek, J.; Jílková, J. Changes in photosynthesis, pigment composition and glutathione contents in two Antarctic lichens during a light stress and recovery. Photosynthetica 2014, 52, 538–547. [Google Scholar] [CrossRef]
- White, A.J.; Critchley, C. Rapid light curves: A new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth. Res. 1999, 59, 63–72. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Campbell, S.; Miller, C.; Steven, A.; Stephens, A. Photosynthetic responses of two temperate seagrasses across a water quality gradient using chlorophyll fluorescence. J. Exp. Mar. Biol. Ecol. 2003, 291, 57–78. [Google Scholar] [CrossRef]
- Huang, M.Y.; Wong, S.L.; Weng, J.H. Rapid light-response curve of chlorophyll fluorescence in terrestrial plants: Relationship to CO2 exchange among five woody and four fern species adapted to different light and water regimes. Plants 2021, 10, 445. [Google Scholar] [CrossRef] [PubMed]
- Serôdio, J.; Vieira, S.; Cruz, S.; Coelho, H. Rapid light-response curves of chlorophyll fluorescence in microalgae: Relationship to steady-state light curves and non-photochemical quenching in benthic diatom-dominated assemblages. Photosynth. Res. 2006, 90, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Houliez, E.; Lefebvre, S.; Lizon, F.; Schmitt, F.G. Rapid light curves (RLC) or non-sequential steady-state light curves (N-SSLC): Which fluorescence-based light response curve methodology robustly characterizes phytoplankton photosynthetic activity and acclimation status? Mar. Biol. 2017, 164, 175. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.M.; Lee, H.; Hong, S.G.; Lee, J. Study of ecophysiological responses of the antarctic fruticose lichen Cladonia borealis using the PAM fluorescence system under natural and laboratory conditions. Plants 2020, 9, 85. [Google Scholar] [CrossRef] [Green Version]
- Li, X.P.; Muller-Moule, P.; Gilmore, A.M.; Niyogi, K.K. PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc. Natl. Acad. Sci. USA 2002, 99, 15222–15227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dall’Osto, L.; Caffarri, S.; Bassi, R. A mechanism of nonphotochemical energy dissipation, independent from PsbS, revealed by a conformational change in the antenna protein CP26. Plant Cell 2005, 17, 1217–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, M.D.; Sylak-Glassman, E.J.; Fleming, G.R.; Niyogi, K.K. A thioredoxin-like/β-propeller protein maintains the efficiency of light harvesting in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, E2733–E2740. [Google Scholar] [CrossRef] [Green Version]
- Vráblíková, H.; McEvoy, M.; Solhaug, K.A.; Barták, M.; Gauslaa, Y. Annual variation in photo acclimation and photoprotection of the photobiont in the foliose lichen Xanthoria parietina. J. Photochem. Photobiology. B Biol. 2006, 83, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Mkhize, K.; Minibayeva, F.; Beckett, R. Adaptions of photosynthesis in sun and shade in populations of some Afromontane lichens. Lichenologist 2022, 54, 319–329. [Google Scholar] [CrossRef]
- Mkhize, K.G.W.; Minibayeva, F.; Beckett, R.P. Lichen photobionts can be hardened to photoinhibition by pretreatment with light. Acta Physiol. Plant. 2022, 44, 122. [Google Scholar] [CrossRef]
- Heber, U.; Bilger, W.; Bligny, R.; Lange, O.L. Phototolerance of lichens, mosses and higher plants in an alpine environment: Analysis of photoreactions. Planta 2000, 211, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Heber, U.; Bukhov, N.G.; Shuvalov, V.A.; Kobayashi, Y.; Lange, O.L. Protection of the photosynthetic apparatus against damage by excessive illumination in homoiohydric leaves and poikilohydric mosses and lichens. J. Exp. Bot. 2001, 52, 1999–2006. [Google Scholar] [CrossRef] [Green Version]
- Barták, M.; Hájek, J.; Vráblíková, H.; Dubová, J. High-light stress and photoprotection in Umbilicaria antarctica monitored by chlorophyll fluorescence imaging and changes in zeaxanthin and glutathione. Plant Biol. 2004, 6, 333–341. [Google Scholar] [CrossRef]
- Barták, M.; Vrábliková, H.; Hájek, J. Sensitivity of photosystem 2 of Antarctic lichens to high irradiance stress: Fluorometric study of fruticose (Usnea antarctica) and foliose (Umbilicaria decussata) species. Photosynthetica 2003, 41, 497–504. [Google Scholar] [CrossRef]
- Hájek, J.; Barták, M.; Gloser, J. Effects of thallus temperature and hydration on photosynthetic parameters of Cetraria islandica from contrasting habitats. Photosynthetica 2001, 39, 427–435. [Google Scholar] [CrossRef]
- Barták, M.; Váczi, P.; Hájek, J.; Smykla, J. Low-temperature limitation of primary photosynthetic processes in Antarctic lichens Umbilicaria antarctica and Xanthoria elegans. Polar Biol. 2007, 31, 47–51. [Google Scholar] [CrossRef]
- Míguez, F.; Fernández-Marín, B.; Becerril, J.M.; García-Plazaola, J.I. Diversity of winter photoinhibitory responses: A case study in co-occurring lichens, mosses, herbs and woody plants from subalpine environments. Physiol. Plant. 2017, 160, 282–296. [Google Scholar] [CrossRef] [Green Version]
- Ndhlovu, N.T.; Solhaug, K.A.; Minibayeva, F.; Beckett, R.P. Melanisation in boreal lichens is accompanied by variable changes in non-photochemical quenching. Plants 2022, 11, 2726. [Google Scholar] [CrossRef]
- Serôdio, J.; Lavaud, J. A model for describing the light response of the nonphotochemical quenching of chlorophyll fluorescence. Photosynth Res. 2011, 108, 61–76. [Google Scholar] [CrossRef]
- Xu, J.; Wu, X.; Yang, Y.; Xu, S.; Kang, Y.; Fu, X.; Yue, H.; Shi, J.; Wu, Z. Changes in growth, photosynthesis and chlorophyll fluorescence in the freshwater dinoflagellate Peridinium umbonatum (Peridiniales, Pyrrophyta) in response to different temperatures. Phycologia 2016, 55, 469–477. [Google Scholar] [CrossRef]
- Láska, K.; Barták, M.; Hájek, J.; Prošek, P.; Bohuslavová, O. Climatic and ecological characteristics of deglaciated area of James Ross Island, Antarctica, with a special respect to vegetation cover. Czech Polar Rep. 2011, 1, 49–62. [Google Scholar] [CrossRef]
- Marečková, M.; Barták, M. Effects of short-term low temperature stress on chlorophyll fluorescence transients in Antarctic lichen species. Czech Polar Rep. 2016, 6, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta (BBA)-Bioenerg. 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.E. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Bilger, W.; Schreiber, U.; Bock, M. Determination of the quantum efficiency of photosystem II and of non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia 1995, 102, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Tietz, S.; Hall, C.C.; Cruz, J.A.; Kramer, D.M. NPQ(T): A chlorophyll fluorescence parameter for rapid estimation and imaging of non-photochemical quenching of excitons in photosystem-II-associated antenna complexes. Plant Cell Environ. 2017, 40, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components—Calculation of qP and Fv′/Fm′; without measuring Fo′. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Eilers, P.H.C.; Peeters, J.C.H. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol. Model. 1988, 42, 199–215. [Google Scholar] [CrossRef]





| Parameter | Meaning | Source |
|---|---|---|
| FV/FM | maximum (potential) yield of photosynthetic processes in PSII | [50] |
| ΦPSII | effective quantum yield of photosynthetic reactions in PSII | [51] |
| NPQ | non-photochemical quenching according to | [52] |
| NPQt | non-photochemical quenching according to | [53] |
| qIt | photoinhibitory quenching | [53] |
| qEt | energy-dependent quenching | [53] |
| Parameter | Meaning |
|---|---|
| α | maximum quantum yield for whole-chain electron transport (“alpha” represents the slope of the ETR curve at low light intensities) |
| IK | PAR value of the point of intersection between a horizontal line ETRmax and the extrapolated initial slope. |
| ETRmax | maximum electron transport capacity found at light saturation |
| FV/FM x ETR factor/2 | maximum yield of electron transport calculated from FV/FM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barták, M.; Hájek, J.; Halıcı, M.G.; Bednaříková, M.; Casanova-Katny, A.; Váczi, P.; Puhovkin, A.; Mishra, K.B.; Giordano, D. Resistance of Primary Photosynthesis to Photoinhibition in Antarctic Lichen Xanthoria elegans: Photoprotective Mechanisms Activated during a Short Period of High Light Stress. Plants 2023, 12, 2259. https://doi.org/10.3390/plants12122259
Barták M, Hájek J, Halıcı MG, Bednaříková M, Casanova-Katny A, Váczi P, Puhovkin A, Mishra KB, Giordano D. Resistance of Primary Photosynthesis to Photoinhibition in Antarctic Lichen Xanthoria elegans: Photoprotective Mechanisms Activated during a Short Period of High Light Stress. Plants. 2023; 12(12):2259. https://doi.org/10.3390/plants12122259
Chicago/Turabian StyleBarták, Miloš, Josef Hájek, Mehmet Gökhan Halıcı, Michaela Bednaříková, Angelica Casanova-Katny, Peter Váczi, Anton Puhovkin, Kumud Bandhu Mishra, and Davide Giordano. 2023. "Resistance of Primary Photosynthesis to Photoinhibition in Antarctic Lichen Xanthoria elegans: Photoprotective Mechanisms Activated during a Short Period of High Light Stress" Plants 12, no. 12: 2259. https://doi.org/10.3390/plants12122259
APA StyleBarták, M., Hájek, J., Halıcı, M. G., Bednaříková, M., Casanova-Katny, A., Váczi, P., Puhovkin, A., Mishra, K. B., & Giordano, D. (2023). Resistance of Primary Photosynthesis to Photoinhibition in Antarctic Lichen Xanthoria elegans: Photoprotective Mechanisms Activated during a Short Period of High Light Stress. Plants, 12(12), 2259. https://doi.org/10.3390/plants12122259






