The Key Role of Cyclic Electron Flow in the Recovery of Photosynthesis in the Photobiont during Rehydration of the Lichen Cladonia stellaris
Abstract
:1. Introduction
2. Results
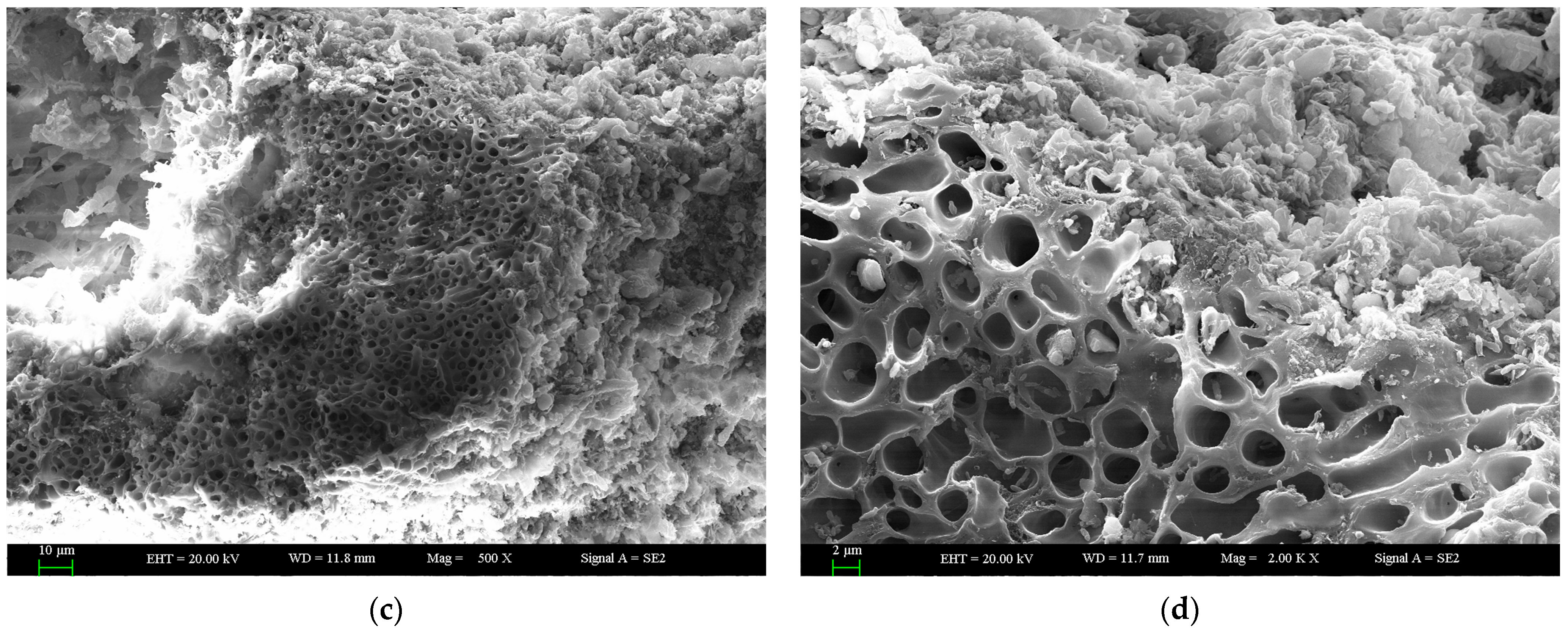
2.1. Scanning Electron Microscopy (SEM) Observation
2.2. Maximal Photochemical Efficiency of PSII (Fv/Fm)
2.3. Quantum Yields of Two Photosystems and CEF during Rehydration
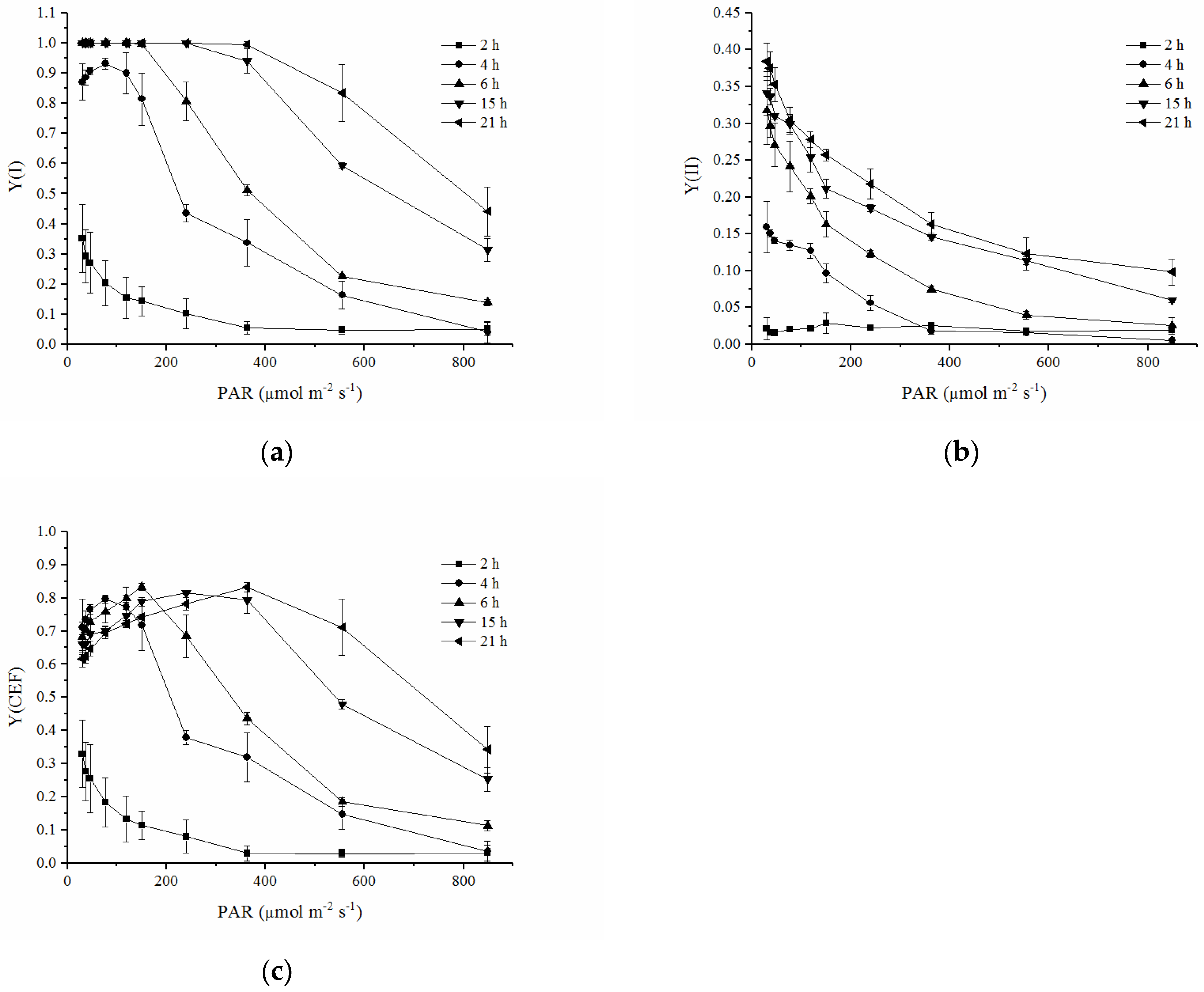
2.4. RLCs of Y(I), Y(II) and Y(CEF)
2.5. RLCs of rETR(I) and rETR(II)
2.6. RLCs of Non-Photochemical Quenching
3. Discussion
4. Materials and Methods
4.1. Rehydration of Cladonia stellaris
4.2. Scanning Electron Microscopy (SEM)
4.3. Application of the Dual-PAM-100 System
4.4. Measurement of the Rapid Light Response Curves (RLCs)
4.5. Quantum Yields of the Photosystems and the CEF
4.6. RLCs of Relative Electron Transport Rates in PSI and PSII
4.7. Energy Dissipation by Non-Photochemical Quenching
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Clancy, J.; Jensen, J.; McMullin, R.T.; Wang, L.; Leavitt, S.D. Providing scale to a known taxonomic unknown-at least a 70-fold increase in species diversity in a cosmopolitan nominal taxon of lichen-forming fungi. J. Fungi 2022, 8, 490. [Google Scholar] [CrossRef]
- Gasulla, F.; del Campo, E.M.; Casano, L.M.; Guéra, A. Advances in understanding of desiccation tolerance of lichens and lichen-forming algae. Plants 2021, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Bowker, M.A.; Cantón, Y.; Castillo-Monroy, A.P.; Cortina, J.; Escolar, C.; Escudero, A.; Lázaro, R.; Martínez, I. Ecology and functional roles of biological soil crusts in semi-arid ecosystems of Spain. J. Arid Environ. 2011, 75, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Zaady, E.; Arbel, S.; Barkai, D.; Sarig, S. Long-term impact of agricultural practices on biological soil crusts and their hydrological processes in a semiarid landscape. J. Arid Environ. 2013, 90, 5–11. [Google Scholar] [CrossRef]
- Fitton, N.; Alexander, P.; Arnell, N.; Bajzelj, B.; Calvin, K.; Doelman, J.; Gerber, J.S.; Havlik, P.; Hasegawa, T.; Herrero, M.; et al. The vulnerabilities of agricultural land and food production to future water scarcity. Glob. Environ. Chang. 2019, 58, 101944. [Google Scholar] [CrossRef]
- del Campo, E.M.; Gasulla, F.; Hell, A.F.; González-Hourcade, M.; Casano, L.M. Comparative Transcriptomic and Proteomic Analyses Provide New Insights into the Tolerance to Cyclic Dehydration in a Lichen Phycobiont. Microb. Ecol. 2023, 86, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Kranner, I.; Beckett, R.; Hochman, A.; Nash, T.H., III. Desiccation-tolerance in lichens: A review. Bryologist 2008, 111, 576–593. [Google Scholar] [CrossRef]
- Maphangwa, K.W.; Musil, C.F.; Raitt, L.; Zedda, L. Differential interception and evaporation of fog, dew and water vapour and elemental accumulation by lichens explain their relative abundance in a coastal desert. J. Arid Environ. 2012, 82, 71–80. [Google Scholar] [CrossRef]
- Expósito, J.R.; Martín San Román, S.; Barreno, E.; Reig-Armiñana, J.; García-Breijo, F.J.; Catalá, M. Inhibition of NO biosynthetic activities during rehydration of Ramalina farinacea lichen thalli provokes increases in lipid peroxidation. Plants 2019, 8, 189. [Google Scholar] [CrossRef]
- Ručová, D.; Đorđević, T.; Baláž, M.; Weidinger, M.; Lang, I.; Gajdoš, A.; Goga, M. Investigation of calcium forms in lichens from travertine sites. Plants 2022, 11, 620. [Google Scholar] [CrossRef]
- Green, T.G.A.; Sancho, L.G.; Pintado, A. Ecophysiology of desiccation/rehydration cycles in mosses and lichens. In Plant Desiccation Tolerance; Lüttge, U., Beck, E., Bartels, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 215, pp. 89–120. [Google Scholar]
- Beckett, R.P.; Alyabyev, A.J.; Minibayeva, F.V. Patterns of heat production during desiccation and rehydration in lichens differing in desiccation tolerance. Lichenologist 2011, 43, 178–183. [Google Scholar] [CrossRef]
- Hell, A.F.; Gasulla, F.; González-Houcarde, M.; Pelegrino, M.T.; Seabra, A.B.; del Campo, E.M.; Casano, L.M.; Centeno, D.C. Polyols-related gene expression is affected by cyclic desiccation in lichen microalgae. Environ. Exp. Bot. 2021, 185, 104397. [Google Scholar] [CrossRef]
- Oukarroum, A.; Strasser, R.J.; Schansker, G. Heat stress and the photosynthetic electron transport chain of the lichen Parmelina tiliacea (Hoffm.) Ach. in the dry and the wet state: Differences and similarities with the heat stress response of higher plants. Photosynth. Res. 2012, 111, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Hell, A.F.; Gasulla, F.; González-Hourcade, M.; del Campo, E.M.; Centeno, D.C.; Casano, L.M. Tolerance to cyclic desiccation in lichen microalgae is related to habitat preference and involves specific priming of the antioxidant system. Plant Cell Physiol. 2019, 60, 1880–1891. [Google Scholar] [CrossRef] [PubMed]
- Green, T.G.A.; Schlensog, M.; Sancho, L.G.; Winkler, B.J.; Broom, F.D.; Schroeter, B. The photobiont determines the pattern of photosynthetic activity within a single lichen thallus containing cyanobacterial and green algal sectors (photosymbiodeme). Oecologia 2002, 130, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Sass, L.; Csintalan, Z.; Tuba, Z.; Vass, I. Thermoluminescence studies on the function of photosystem II in the desiccation tolerant lichen Cladonia convoluta. Photosynth. Res. 1996, 48, 205–212. [Google Scholar] [CrossRef]
- Wieners, P.C.; Mudimu, O.; Bilger, W. Desiccation-induced non-radiative dissipation in isolated green lichen algae. Photosynth. Res. 2012, 113, 239–247. [Google Scholar] [CrossRef]
- Calatayud, A.; Deltoro, V.I.; Barreno, E.; del Valle-Tascon, S. Changes in in vivo chlorophyll fluorescence quenching in lichen thalli as a function of water content and suggestion of zeaxanthin-associated photoprotection. Physiol. Plant. 1997, 101, 93–102. [Google Scholar] [CrossRef]
- Calatayud, A.; Temple, P.J.; Barreno, E. Chlorophyll a fluorescence emission, xanthophyll cycle activity, and net photosynthetic rate responses to ozone in some foliose and fruticose lichen species. Photosynthetica 2000, 38, 281–286. [Google Scholar] [CrossRef]
- Gasulla, F.; de Nova, P.G.; Esteban-Carrasco, A.; Zapata, J.M.; Barreno, E.; Guéra, A. Dehydration rate and time of desiccation affect recovery of the lichenic algae Trebouxia erici: Alternative and classical protective mechanisms. Planta 2009, 231, 195–208. [Google Scholar] [CrossRef]
- Shi, Q.; Sun, H.; Timm, S.; Zhang, S.; Huang, W. Photorespiration alleviates photoinhibition of Photosystem I under fluctuating light in tomato. Plants 2022, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Zhao, J.P.; Xu, P.L.; Meng, J.J.; He, Q.W. Effects of cyclic electron flow inhibitor (antimycin A) on photosystem photoinhibition of sweet pepper leaves upon exposure to chilling stress under low irradiance. Agric. Sci. China 2006, 5, 506–511. [Google Scholar] [CrossRef]
- Gao, S.; Wang, G.C. The enhancement of cyclic electron flow around photosystem I improves the recovery of severely desiccated Porphyra yezoensis (Bangiales, Rhodophyta). J. Exp. Bot. 2012, 63, 4349–4358. [Google Scholar] [CrossRef] [PubMed]
- Beckett, R.P.; Roach, T.; Minibayeva, F.; Werth, S. Alternative electron transport pathways contribute to tolerance to high light stress in lichenized algae. Physiol. Plant. 2023, 175, e13904. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, U.; Connan, S.; Stengel, D.B. Chlorophyll a fluorescence responses of temperate Phaeophyceae under submersion and emersion regimes: A comparison of rapid and steady-state light curves. Photosynth. Res. 2012, 114, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Saroussi, S.; Beer, S. Acclimations of macroalgae as reflected in photosynthetic parameters derived from PAM fluorometry, and possible implications for abundance patterns. Mar. Ecol. 2007, 28, 377–383. [Google Scholar] [CrossRef]
- Platt, T.; Gallegos, C.L.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Cho, S.M.; Lee, H.; Hong, S.G.; Lee, J. Study of ecophysiological responses of the Antarctic fruticose lichen Cladonia borealis using the PAM fluorescence system under natural and laboratory conditions. Plants 2020, 9, 85. [Google Scholar] [CrossRef]
- Barron-Gafford, G.A.; Angert, A.L.; Venable, D.L.; Tyler, A.P.; Gerst, K.L.; Huxman, T.E. Photosynthetic temperature responses of co-occurring desert winter annuals with contrasting resource-use efficiencies and different temporal patterns of resource utilization may allow for species coexistence. J. Arid Environ. 2013, 91, 95–103. [Google Scholar] [CrossRef]
- Ye, C.P.; Zhang, M.C.; Yang, Y.F.; Thirumaran, G. Photosynthetic performance in aquatic and terrestrial colonies of Nostoc flagelliforme (Cyanophyceae) under aquatic and aerial conditions. J. Arid Environ. 2012, 85, 56–61. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700+-absorbance changes at 830 nm. Planta 1994, 192, 261–268. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Saturation Pulse method for assessment of energy conversion in PS I. PAM Appl. Notes 2008, 1, 11–14. [Google Scholar]
- Determeyer-Wiedmann, N.; Sadowsky, A.; Convey, P.; Ott, S. Physiological life history strategies of photobionts of lichen species from Antarctic and moderate European habitats in response to stressful conditions. Polar Biol. 2019, 42, 395–405. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, S.B.; Cao, K.F. The different effects of chilling stress under moderate light intensity on photosystem II compared with photosystem I and subsequent recovery in tropical tree species. Photosynth. Res. 2010, 103, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wufuer, R.; Duo, J.; Li, W.; Pan, X. Cadmium caused different toxicity to photosystem I and photosystem II of freshwater unicellular algae Chlorella pyrenoidosa (Chlorophyta). Toxics 2022, 10, 352. [Google Scholar] [CrossRef]
- Radeka, M.; Ranogajec, J.; Kiurski, J.; Markov, S.; Marinković-Nedučin, R. Influence of lichen biocorrosion on the quality of ceramic roofing tiles. J. Eur. Ceram. Soc. 2007, 27, 1763–1766. [Google Scholar] [CrossRef]
- Daminova, A.G.; Rogov, A.M.; Rassabina, A.E.; Beckett, R.P.; Minibayeva, F.V. Effect of melanization on thallus microstructure in the lichen Lobaria pulmonaria. J. Fungi 2022, 8, 791. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Solhaug, K.A. High-light-intensity damage to the foliose lichen Lobaria pulmonaria within a natural forest: The applicability of chlorophyll fluorescence methods. Lichenologist 2000, 32, 271–289. [Google Scholar] [CrossRef]
- Beckett, R.P.; Mayaba, N.; Minibayeva, F.V.; Alyabyev, A.J. Hardening by partial dehydration and ABA increase desiccation tolerance in the cyanobacterial lichen Peltigera polydactylon. Ann. Bot. 2005, 96, 109–115. [Google Scholar] [CrossRef]
- Chowaniec, K.; Rola, K. Evaluation of the importance of ionic and osmotic components of salt stress on the photosynthetic efficiency of epiphytic lichens. Physiol. Mol. Biol. Plants 2022, 28, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Bukhov, N.G.; Govindachary, S.; Egorova, E.A.; Carpentier, R. Recovery of photosystem I and II activities during re-hydration of lichen Hypogymnia physodes thalli. Planta 2004, 219, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.L.; Zhang, D.Y.; Chen, X.; Li, L.; Mu, G.J.; Li, L.H.; Bao, A.M.; Liu, J.; Zhu, H.S.; Song, W.J.; et al. Effects of short-term low temperatures on photosystem II function of samara and leaf of Siberian maple (Acer ginnala) and subsequent recovery. J. Arid Land 2009, 1, 57–63. [Google Scholar]
- Wang, S.Z.; Zhang, D.Y.; Pan, X.L. Effects of arsenic on growth and photosystem II (PSII) activity of Microcystis aeruginosa. Ecotoxicol. Environ. Saf. 2012, 84, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Ilík, P.; Pavlovič, A.; Kouřil, R.; Alboresi, A.; Morosinotto, T.; Allahverdiyeva, Y.; Aro, E.-M.; Yamamoto, H.; Shikanai, T. Alternative electron transport mediated by flavodiiron proteins is operational in organisms from cyanobacteria up to gymnosperms. New Phytol. 2017, 214, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, S.-B.; Cao, K.-F. Stimulation of cyclic electron flow during recovery after chilling-induced photoinhibition of PSII. Plant Cell Physiol. 2010, 51, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.N. Physiology of PSI cyclic electron transport in higher plants. Biochim. Biophys. Acta 2011, 1807, 384–389. [Google Scholar] [CrossRef]
- Weissman, L.; Garty, J.; Hochman, A. Rehydration of the lichen Ramalina lacera results in production of reactive oxygen species and nitric oxide and a decrease in antioxidants. Appl. Environ. Microbiol. 2005, 71, 2121–2129. [Google Scholar] [CrossRef]
- Huang, W.; Yang, S.J.; Zhang, S.B.; Zhang, J.L.; Cao, K.F. Cyclic electron flow plays an important role in photoprotection for the resurrection plant Paraboea rufescens under drought stress. Planta 2012, 235, 819–828. [Google Scholar] [CrossRef]
- Gasulla, F.; Herrero, J.; Esteban-Carrasco, A.; Ros-Barceló, A.; Barreno, E.; Zapata, J.M.; Guéra, A. Photosynthesis in lichen: Light reactions and protective mechanisms. In Advances in Photosynthesis—Fundamental Aspects; Najafpour, M.M., Ed.; InTech: Rijeka, Croatia, 2012; pp. 149–174. [Google Scholar]
- Pintado, A.; Sancho, L.G. Ecological significance of net photosynthesis activation by water vapour uptake in Ramalina capitata from rain protected habitats in central Spain. Lichenologist 2002, 34, 403–413. [Google Scholar] [CrossRef]
- Pfündel, E.; Klughammer, C.; Schreiber, U. Monitoring the effects of reduced PS II antenna size on quantum yields of photosystems I and II using the Dual-PAM-100 measuring system. PAM Appl. Notes 2008, 1, 21–24. [Google Scholar]
- White, A.J.; Critchley, C. Rapid light curves: A new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth. Res. 1999, 59, 63–72. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Saroussi, S.; Beer, S. Alpha and quantum yield of aquatic plants derived from PAM fluorometry: Uses and misuses. Aquat. Bot. 2007, 86, 89–92. [Google Scholar] [CrossRef]
- Kühl, M.; Glud, R.N.; Borum, J.; Roberts, R.; Rysgaard, S. Photosynthetic performance of surface-associated algae below sea ice as measured with a pulse-amplitude-modulated (PAM) fluorometer and O2 microsensors. Mar. Ecol. Prog. Ser. 2001, 223, 1–14. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]





| Rehydration Time (h) | Parameters of the Rapid Light Curves of rETR(I) | Parameters of the Rapid Light Curves of rETR(II) | ||||
|---|---|---|---|---|---|---|
| α (e− Photon−1) | rETRmax (μmol e− m−2 s−1) | rEk (μmol Photon m−2 s−1) | α (e− Photon−1) | rETRmax (μmol e− m−2 s−1) | rEk (μmol Photon m−2 s−1) | |
| 2 | 0.25 ± 0.14 a | 33.86 ± 15.10 a | 169.26 ± 110.66 a | 0.02 ± 0.01 a | 7.37 ± 1.17 a | 301.87 ± 52.87 a |
| 4 | 1.32 ± 0.06 b | 234.86 ± 43.03 b | 177.19 ± 24.35 a | 0.24 ± 0.01 b | 26.32 ± 10.31 a | 112.31 ± 45.72 a |
| 6 | 1.47 ± 0.04 c | 526.87 ± 54.75 c | 358.55 ± 45.50 b | 0.37 ± 0.03 d | 41.09 ± 19.96 a | 112.41 ± 60.75 a |
| 15 | 1.49 ± 0.07 c | 1169.89 ± 135.04 d | 787.25 ± 86.01 c | 0.32 ± 0.01 c | 185.79 ± 75.6 b | 584.30 ± 253.63 b |
| 21 | 1.44 ± 0.06 c | 1726.43 ± 175.50 e | 1197.14 ± 137.53 d | 0.35 ± 0.01 cd | 206.51 ± 54.94 b | 601.41 ± 179.58 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Li, W.; Wufuer, R.; Duo, J.; Pei, L.; Pan, X. The Key Role of Cyclic Electron Flow in the Recovery of Photosynthesis in the Photobiont during Rehydration of the Lichen Cladonia stellaris. Plants 2023, 12, 4011. https://doi.org/10.3390/plants12234011
Wang S, Li W, Wufuer R, Duo J, Pei L, Pan X. The Key Role of Cyclic Electron Flow in the Recovery of Photosynthesis in the Photobiont during Rehydration of the Lichen Cladonia stellaris. Plants. 2023; 12(23):4011. https://doi.org/10.3390/plants12234011
Chicago/Turabian StyleWang, Shuzhi, Wenfeng Li, Rehemanjiang Wufuer, Jia Duo, Liang Pei, and Xiangliang Pan. 2023. "The Key Role of Cyclic Electron Flow in the Recovery of Photosynthesis in the Photobiont during Rehydration of the Lichen Cladonia stellaris" Plants 12, no. 23: 4011. https://doi.org/10.3390/plants12234011
APA StyleWang, S., Li, W., Wufuer, R., Duo, J., Pei, L., & Pan, X. (2023). The Key Role of Cyclic Electron Flow in the Recovery of Photosynthesis in the Photobiont during Rehydration of the Lichen Cladonia stellaris. Plants, 12(23), 4011. https://doi.org/10.3390/plants12234011







