Storage on Maternal Plants Affects Temperature Requirements during Germination in Rumex obtusifolius
Abstract
:1. Introduction
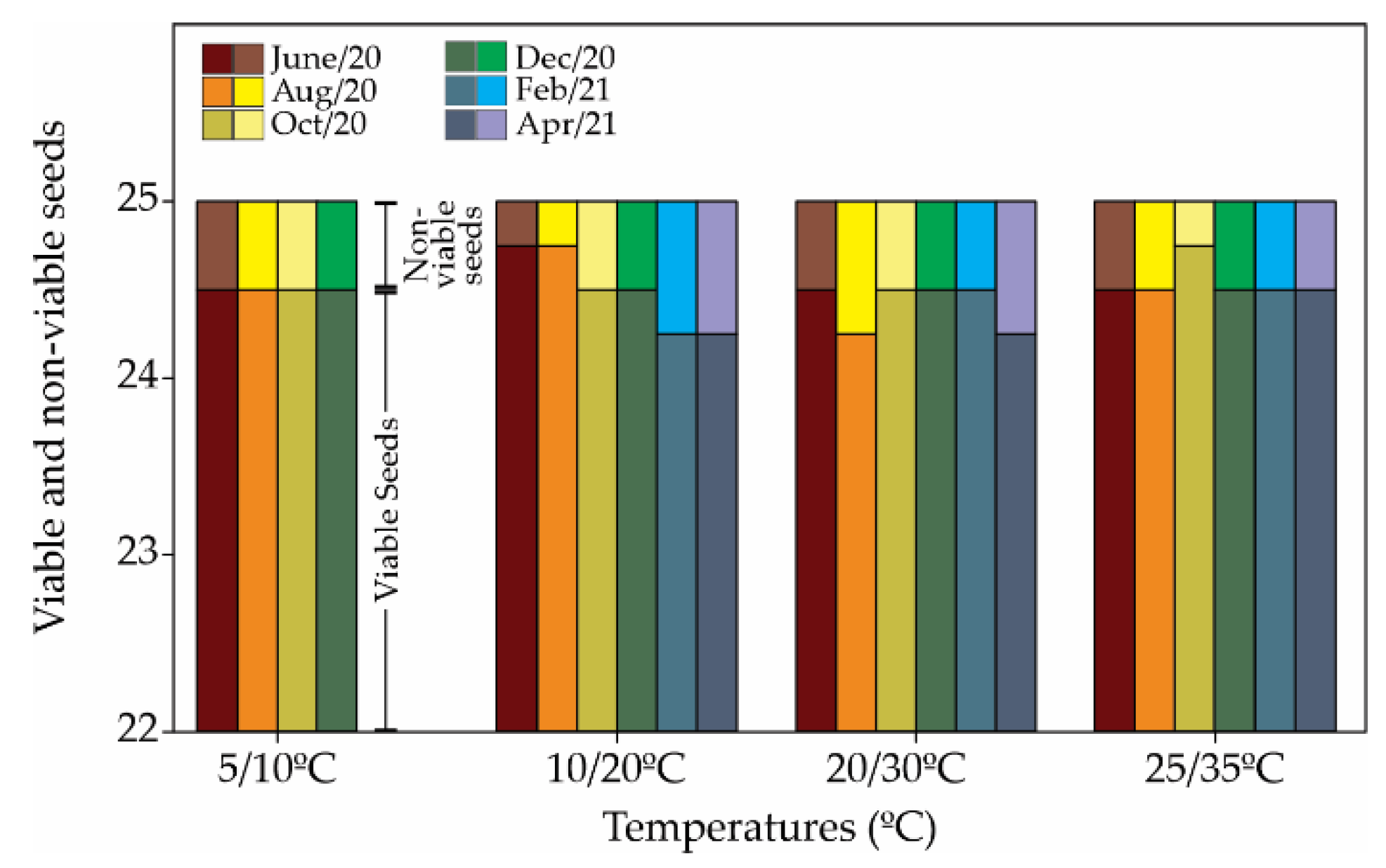
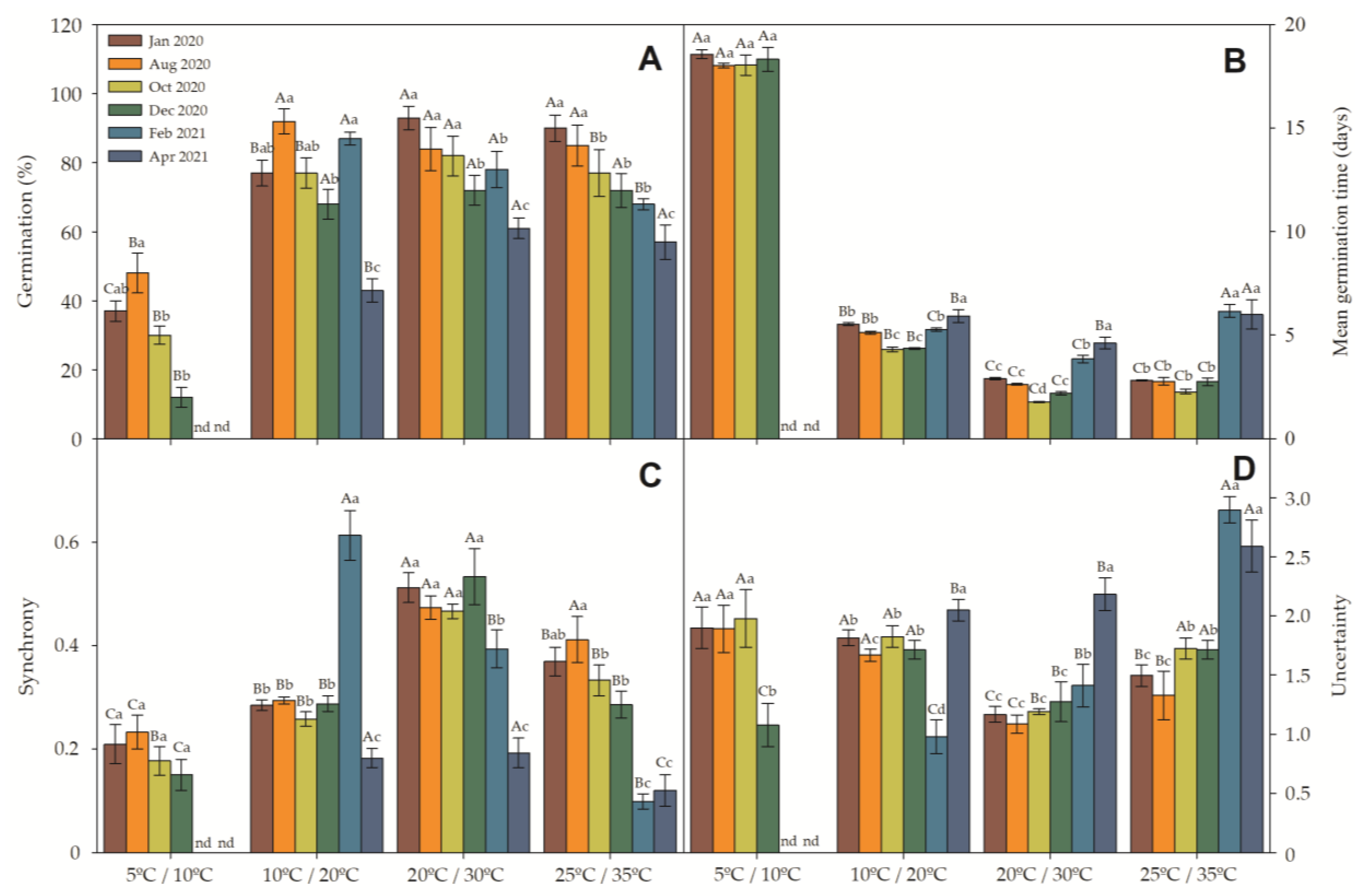
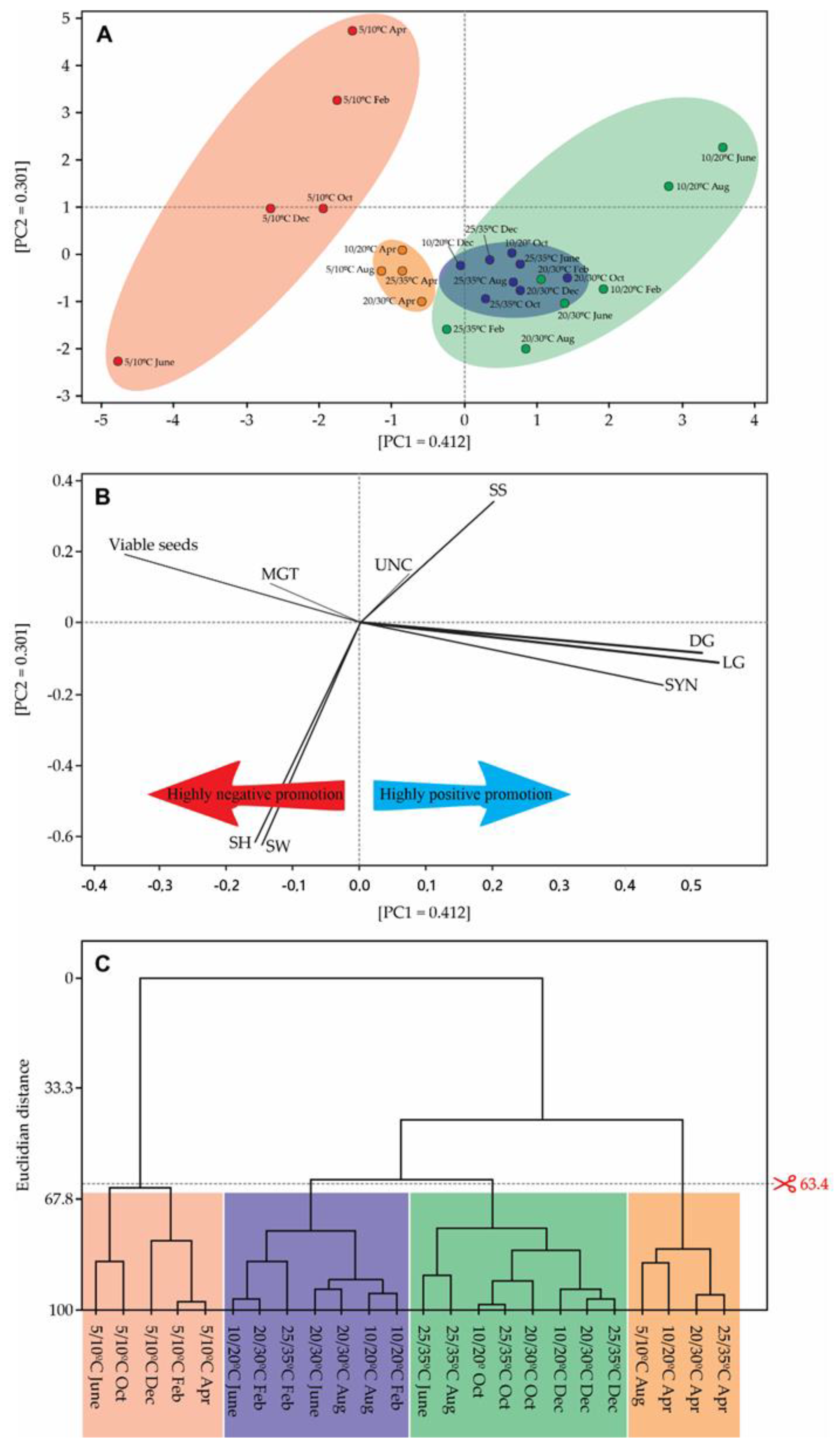
2. Results
3. Materials and Methods
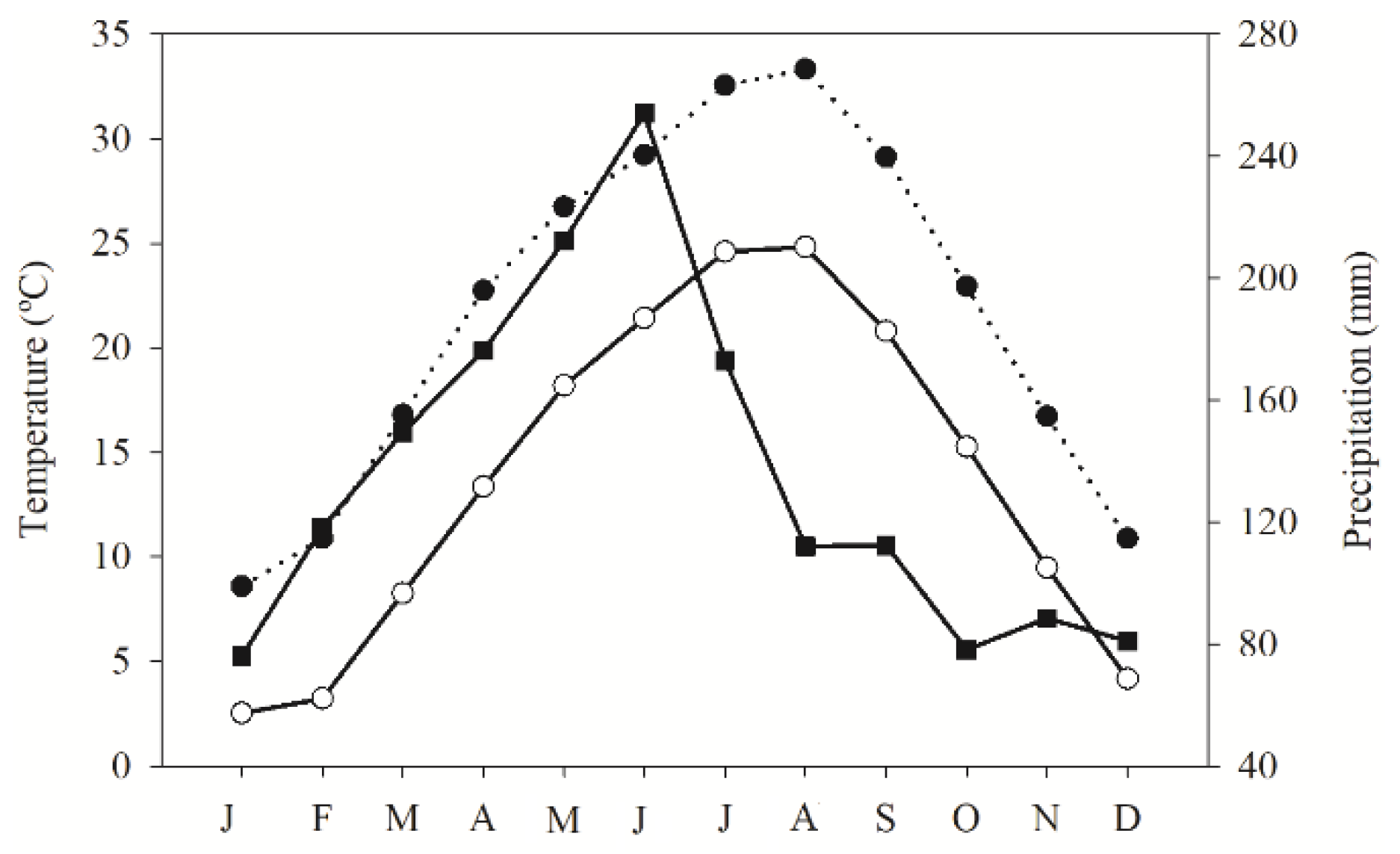
3.1. Collection
3.2. Morphology
3.3. Water Imbibition
3.4. Temperature and Light
3.5. Data Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamont, B.B. Canopy Seed Storage and Release: What’s in a Name? Oikos 1991, 60, 266–268. [Google Scholar] [CrossRef]
- Thanos, C.A. Bradychory—The coining of a new term. In Proceedings of the 10th MEDECOS Conference, Rhodes, Greece, 25 April–1 May 2004; Arianoutsou, M., Papanastasis, V.P., Eds.; Millpress: Rotterdam, The Netherlands, 2004; pp. 1–6. [Google Scholar]
- Gutterman, Y.; Ginott, S. Long-term protected “seed bank” in dry inflorescences of Asteriscus pygmaeus; Achene dispersal mechanism and germination. J. Arid. Environ. 1994, 26, 149–163. [Google Scholar] [CrossRef]
- Bhatt, A.; Phondani, P.C.; Phartyal, S.S.; Santo, A.; Gallacher, D. Influence of aerial seed banks on germination response in three desert plant species. J. Plant Ecol. 2017, 10, 994–1000. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, A.; Bhat, N.R.; Lozano-Isla, F.; Gallacher, D.; Santo, A.; Batista-Silva, W.; Fernandes, D.; Pompelli, M.F. Germination asynchrony is increased by dual seed bank presence in two desert perennial halophytes. Botany 2019, 97, 639–649. [Google Scholar] [CrossRef]
- El-Keblawy, A.A.; Bhatt, A. Aerial seed bank affects germination in two small-seeded halophytes in Arab Gulf desert. J. Arid. Environ. 2015, 117, 10–17. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Gairola, S.; Bhatt, A.; Mahmoud, T. Effects of maternal salinity on salt tolerance during germination of Suaeda aegyptiaca, a facultative halophyte in the Arab Gulf desert. Plant Species Biol. 2017, 32, 45–53. [Google Scholar] [CrossRef]
- Moya, D.; De las Heras, J.; Salvatore, R.; Valero, E.; Leone, V. Fire intensity and serotiny: Response of germination and enzymatic activity in seeds of Pinus halepensis Mill. from southern Italy. Ann. For. Sci. 2013, 70, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Günster, A. Aerial Seed Banks in the Central Namib: Distribution of Serotinous Plants in Relation to Climate and Habitat. J. Biogeogr. 1992, 19, 563–572. [Google Scholar] [CrossRef]
- Cabra-Rivas, I.; Castro-Díez, P. Potential Germination Success of Exotic and Native Trees Coexisting in Central Spain Riparian Forests. Int. J. Ecol. 2016, 2016, 7614683. [Google Scholar] [CrossRef] [Green Version]
- Meusel, H.; Jäger, E.; Weinert, E. Vergleichende Chorologie der Zentraleuropäischen Flora; Karten Gustav Fischer Verlag: Jena, Germany, 1965. [Google Scholar]
- Zaller, J.G. Ecology and non-chemical control of Rumex crispus and R. obtusifolius (Polygonaceae): A review. Weed Res. 2004, 44, 414–432. [Google Scholar] [CrossRef]
- Toole, E.H.; Brown, E. Final results of the Duval buried seed experiment. J. Agric. Res. 1946, 72, 201–210. [Google Scholar]
- Cavers, P.B.; Harper, J.L. Biological flora of the British Isles. Rumex obtusifolius L. and R. crispus L. J. Ecol. 1964, 52, 737–766. [Google Scholar] [CrossRef]
- Totterdell, S.; Roberts, E.H. Characteristics of alternating temperatures which stimulate loss of dormancy in seeds of Rumex obtusifolius L. and Rumex crispus L. Plant Cell Environ. 1980, 3, 3–12. [Google Scholar] [CrossRef]
- Honěk, A.; Martinková, Z. Effects of individual plant phenology on dormancy of Rumex obtusifolius seeds at dispersal. Weed Res. 2002, 42, 148–155. [Google Scholar] [CrossRef]
- Grossrieder, M.; Keary, I.P. The potential for the biological control of Rumex obtusifolius and Rumex crispus using insects in organic farming, with particular reference to Switzerland. Biocontrol News Inf. 2004, 25, 65N–79N. [Google Scholar]
- Hand, D.J.; Craig, G.; Takaki, M.; Kendrick, R.E. Interaction of light and temperature on seed germination of Rumex obtusifolius L. Planta 1982, 156, 457–460. [Google Scholar] [CrossRef]
- Pino, J.; Haggar, R.J.; Sans, F.X.; Masalles, R.M.; Hamilton, R.N.S.; Sackville-Hamilton, R.N. Clonal growth and fragment regeneration of Rumex obtusifolius L. Weed Res. 1995, 35, 141–148. [Google Scholar] [CrossRef]
- Hong, D.Y.; Blackmore, S. Plants of China: A Companion to the Flora of China; Cambridge University Press: London, UK, 2015. [Google Scholar]
- Zhu, J.; Wang, J.; DiTommaso, A.; Zhang, C.; Zheng, G.; Liang, W.; Islam, F.; Yang, C.; Chen, X.; Zhou, W. Weed research status, challenges, and opportunities in China. Crop Prot. 2020, 134, 104449. [Google Scholar] [CrossRef]
- Takaki, M.; Kendrick, R.E.; Dietrich, S.M.C. Interaction of light and temperature on the germination of Rumex obtusifolius L. Planta 1981, 152, 209–214. [Google Scholar] [CrossRef]
- Cloudsley-Thompson, J.L. Seed Germination in Desert Plants; Springer: Berlin/Heidelberg, Germany, 1993; p. 261. [Google Scholar]
- Weaver, S.E.; Cavers, P.B. The effects of date of emergence and emergence order on seedling survival rates in Rumex crispus and R. obtusifolius. Can. J. Bot. 1979, 57, 730–738. [Google Scholar] [CrossRef]
- Van Assche, J.A.; Vanlerberghe, K.A. The Role of Temperature on the Dormancy Cycle of Seeds of Rumex obtusifolius L. Funct. Ecol. 1989, 3, 107–115. [Google Scholar] [CrossRef]
- Benvenuti, S.; Macchia, M.; Miele, S. Quantitative analysis of emergence of seedlings from buried weed seeds with increasing soil depth. Weed Sci. 2001, 49, 528–535. [Google Scholar] [CrossRef]
- Tsuyuzaki, S. Survival and Changes in Germination Response of Rumex obtusifolius, Polygonum longisetum and Oenothera biennis during Burial at Three Soil Depths. Am. J. Environ. Sci. 2006, 2, 74–78. [Google Scholar] [CrossRef]
- Tsuyuzaki, S. Survival characteristics of buried seeds 10 years after the eruption of the Usu volcano in northen Japan. Can. J. Bot. 1991, 69, 2251–2256. [Google Scholar] [CrossRef]
- Tsuyuzaki, S. Seed survival for three decades under thick tephra. Seed Sci. Res. 2010, 20, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Kramer, N.B.; Johnson, F.D. Mature forest seed banks of three habitat types in central Idaho. Can. J. Bot. 1987, 65, 1961–1966. [Google Scholar] [CrossRef]
- Ishikawa-Goto, M.; Tsuyuzaki, S. Methods of estimating seed banks with reference to long-term seed burial. J. Plant Res. 2004, 117, 245–248. [Google Scholar] [CrossRef]
- Traba, J.; Azcárate, F.M.; Peco, B. From what depth do seeds emerge? A soil seed bank experiment with Mediterranean grassland species. Seed Sci. Res. 2004, 14, 297–303. [Google Scholar] [CrossRef]
- Tsuyuzaki, S.; Masaki, G. Persistence of seed bank under thick volcanic deposits twenty years after eruptions of Mount Usu, Hokkaido Island, Japan. Am. J. Bot. 2001, 88, 1813–1817. [Google Scholar] [CrossRef]
- Bhatt, A.; Daibes, L.F.; Gallacher, D.J.; Jarma-Orozco, A.; Pompelli, M.F. Water Stress Inhibits Germination While Maintaining Embryo Viability of Subtropical Wetland Seeds: A Functional Approach with Phylogenetic Contrasts. Front. Plant Sci. 2022, 13, 906771. [Google Scholar] [CrossRef]
- Thompson, K.; Band, S.R.; Hodgson, J.G. Seed Size and Shape Predict Persistence in Soil. Funct. Ecol. 1993, 7, 236–241. [Google Scholar] [CrossRef] [Green Version]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: London, UK, 2014. [Google Scholar]
- Allen, E.; Alvarez, S. International Rules for Seed Testing 2020; The International Seed Testing Association: Zürich, Switzerland, 2020. [Google Scholar]
- Lozano-Isla, F.; Benites-Alfaro, O.E.; Pompelli, M.F. GerminaR: An R package for germination analysis with the interactive web application “GerminaQuant for R”. Ecol. Res. 2019, 34, 339–346. [Google Scholar] [CrossRef]
- Krishnan, P.; Nagarajan, S.; Moharir, A.V. Thermodynamic Characterisation of Seed Deterioration during Storage under Accelerated Ageing Conditions. Biosyst. Eng. 2004, 89, 425–433. [Google Scholar] [CrossRef]
- Yan, H.; Feng, L.; Zhao, Y.; Zhu, C.; Qu, Y.; Wang, H. Predicting the potential distribution of an invasive species, Erigeron canadensis L., in China with a maximum entropy model. Glob. Ecol. Conserv. 2020, 21, e00822. [Google Scholar] [CrossRef]
- Klupczyńska, E.A.; Pawłowski, T.A. Regulation of Seed Dormancy and Germination Mechanisms in a Changing Environment. Int. J. Mol. Sci. 2021, 22, 1357. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.S.A.; Attanda, M.L. Factors that cause seed dormancy. Seed Biol. Updates 2022. [Google Scholar] [CrossRef]
- Hageseth, G.T. Kinetic and Thermodynamic Parameters That Describe Isothermal Seed Germination. J. Exp. Bot. 1978, 29, 281–293. [Google Scholar] [CrossRef]
- Chidananda, K.; Chelladurai, V.; Jayas, D.; Alagusundaram, K.; White, N.; Fields, P. Respiration of pulses stored under different storage conditions. J. Stored Prod. Res. 2014, 59, 42–47. [Google Scholar] [CrossRef]
- Santini, B.A.; Martorell, C. Does retained-seed priming drive the evolution of serotiny in drylands? An assessment using the cactus Mammillaria hernandezii. Am. J. Bot. 2013, 100, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Yang, X.; Yang, F.; Wei, L.; Huang, Z.; Walck, J.L. Aerial and soil seed banks enable populations of an annual species to cope with an unpredictable dune ecosystem. Ann. Bot. 2014, 114, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Throneberry, G.O.; Smith, F.G. Relation of Respiratory and Enzymatic Activity to Corn Seed Viability. Plant Physiol. 1955, 30, 337–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moncaleano-Escandon, J.; Silva, B.C.; Silva, S.R.; Granja, J.A.; Alves, M.C.J.; Pompelli, M.F. Germination responses of Jatropha curcas L. seeds to storage and aging. Ind. Crops Prod. 2012, 44, 684–690. [Google Scholar] [CrossRef]
- Lozano-Isla, F.; Campos, M.L.; Endres, L.; Bezerra-Neto, E.; Pompelli, M.F. Effects of seed storage time and salt stress on the germination of Jatropha curcas L. Ind. Crops Prod. 2018, 118, 214–224. [Google Scholar] [CrossRef]
- Patanè, C.; Cavallaro, V.; Avola, G.; D’Agosta, G. Seed respiration of sorghum [Sorghum bicolor (L.) Moench] during germination as affected by temperature and osmoconditioning. Seed Sci. Res. 2006, 16, 251–260. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Cavers, P.B. Interpopulation and interyear variation in germination in Scotch thistle, Onopordum acanthium L., grown in a common garden: Genetics vs environment. Plant Ecol. 2002, 162, 1–8. [Google Scholar] [CrossRef]
- Zhang, R.; Baskin, J.M.; Baskin, C.C.; Mo, Q.; Chen, L.; Hu, X.; Wang, Y. Effect of population, collection year, after-ripening and incubation condition on seed germination of Stipa bungeana. Sci. Rep. 2017, 7, 13893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, A.; Carón, M.M.; Souza-Filho, P.R.D.M.; Gallacher, D.J. Maternal source affects seed germination of a rare Arabian desert species (Astragalus sieberi). Botany 2021, 99, 293–301. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef] [Green Version]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Maleki, K.; Maleki, K.; Soltani, E.; Oveisi, M.; Gonzalez-Andujar, J.L. A Model for Changes in Germination Synchrony and Its Implements to Study Weed Population Dynamics: A Case Study of Brassicaceae. Plants 2023, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Chen, X.; Pompelli, M.F.; Jamal, A.; Mancinelli, R.; Radicetti, E. Characterization of Invasiveness, Thermotolerance and Light Requirement of Nine Invasive Species in China. Plants 2023, 12, 1192. [Google Scholar] [CrossRef] [PubMed]
- Le Stradic, S.; Silveira, F.A.O.; Buisson, E.; Cazelles, K.; Carvalho, V.; Fernandes, G.W. Diversity of germination strategies and seed dormancy in herbaceous species of campo rupestre grasslands. Austral. Ecol. 2015, 40, 537–546. [Google Scholar] [CrossRef]
- Lara, T.S.; Lira, J.M.S.; Rodrigues, A.C.; Rackocevic, M.; Alvarenga, A.A. Potassium Nitrate Priming Affects the Activity of Nitrate Reductase and Antioxidant Enzymes in Tomato Germination. J. Agric. Sci. 2014, 6, 72–80. [Google Scholar] [CrossRef]
| Feature | Mean (±SE) |
|---|---|
| Non-imbibed seed weight (g) | 0.027 ± 0.003 |
| 24 h-imbibed seed weight (g) | 0.031 ± 0.002 |
| Seed length (mm) | 2.193 ± 0.033 |
| Seed width (mm) | 1.351 ± 0.020 |
| Seed height (mm) | 1.343 ± 0.018 |
| Seed shape index | 0.033 ± 0.002 |
| 1000-seeds weight (g) | 1.090 ± 0.104 |
| Source of Variation | Degrees of Freedom Residuals | Sum of Squares | Mean Squares | Significance (p) |
|---|---|---|---|---|
| Date | 5 | 19,091.42 | 3818.28 | <0.001 |
| Temperature | 3 | 110,324.92 | 36,774.97 | <0.001 |
| Light | 1 | 18,174.08 | 18,174.08 | <0.001 |
| Date × Temperature | 15 | 6929.58 | 461,97 | <0.001 |
| Date × Light | 5 | 3745.42 | 749.08 | <0.001 |
| Temperature × Light | 3 | 1742.92 | 580.97 | <0.001 |
| Date × Temperature × Light | 15 | 4667.58 | 311.17 | <0.001 |
| Residuals | 144 | 3051.50 | 21.19 | --- |
| Temperature | 12 h Light/12 h Darkness | Full Darkness (0 h) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | June/20 | Aug/20 | Oct/20 | Dec/20 | Feb/21 | Apr/21 | June/20 | Aug/20 | Oct/20 | Dec/20 | Feb/21 | Apr/21 |
| 5 °C/10 °C | 37 ± 3.0 *** | 48.0 ± 11.8 ** | 30.0 ± 2.6 *** | 12.0 ± 2.8 *** | nd | nd | 1.0 ± 1.0 | 7.0 ± 1.9 | 0 | 0 | nd | nd |
| 10 °C/20 °C | 77.0 ± 3.8 nd | 92.0 ± 3.7 ns | 77.0 ± 4.4 ** | 68.0 ± 4.3 ** | 87.0 ± 1.9 * | 43.0 ± 3.4 ns | 77.0 ± 2.5 | 85.0 ± 3.0 | 52.0 ± 3.3 | 45.0 ± 2.5 | 78.0 ± 2.6 | 37.0 ± 3.4 |
| 20 °C/30 °C | 93.0 ± 3.4 *** | 84.0 ± 6.3 ns | 82.0 ± 5.8 * | 72.0 ± 4.3 ** | 78.0 ± 5.3 ns | 61.0 ± 3.0 ** | 70.0 ± 2.6 | 68.0 ± 5.2 | 61.0 ± 1.9 | 51.0 ± 3.4 | 75.0 ± 3.4 | 38.0 ± 4.8 |
| 25 °C/35 °C | 90.0 ± 3.8 *** | 85.0 ± 6.0 *** | 77.0 ± 6.8 * | 72.0 ± 4.9 ** | 68.0 ± 1.6 ns | 57.0 ± 5.0 ** | 33.0 ± 1.9 | 44.0 ± 3.4 | 53.0 ± 6.6 | 47.0 ± 3.4 | 66.0 ± 2.6 | 35.0 ± 1.9 |
| Germination in light | |||||||||||
| Germination in darkness | 0.83 ** | ||||||||||
| Mean germination time (light) | −0.2 ns | −0.3 ns | |||||||||
| Uncertainty (light) | −0.2 * | −0.2 ns | 0.36 ** | ||||||||
| Synchrony (light) | 0.68 * | 0.37 ns | −0.1 ns | −0.3 ns | |||||||
| Seed length | 0.34 ns | 0.09 ns | −0.2 ns | 0.01 ns | 0.11 ns | ||||||
| Seed width | −0.2 ns | −0.7 ns | 0.14 ns | 0.13 ns | 0.19 ns | 0.03 ns | |||||
| Seed height | 0.56 ns | −0.1 ns | 0.12 ns | 0.08 ns | −0.1 ns | 0.16 ns | 0.95 ** | ||||
| Seed weight (0 h) | 0.79 ns | −0.1 ns | 0.03 ns | 0.9 ns | −0.9 ** | −0.4 ns | 0.61 ns | 0.51 ns | |||
| Seed weight (24 h) | 0.79 ns | 0.19 ns | 0.33 ns | 0.99 ns | 0.7 * | 0.26 ns | 0.82 ns | 0.75 ns | 0.95 ns | ||
| 1000-seed weight | 0.535 ns | 0.562 ns | 0.03 ns | 0.9 ns | −0.3 ns | −0.3 ns | 0.61 ns | 0.51 ns | 0.998 ** | 0.95 ns | |
| Germination in light | Germination in darkness | Mean germination time (light) | Uncertainty (light) | Synchrony (light) | Seed length | Seed width | Seed height | Seed weight (0 h) | Seed weight (24 h) | 1000-seed weight |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatt, A.; Chen, X.; Gallacher, D.J.; Phartyal, S.S.; Rodriguez-Paez, L.A.; Pineda-Rodriguez, Y.Y.; Pompelli, M.F.; Jamal, A.; Mancinelli, R.; Radicetti, E. Storage on Maternal Plants Affects Temperature Requirements during Germination in Rumex obtusifolius. Plants 2023, 12, 2403. https://doi.org/10.3390/plants12132403
Bhatt A, Chen X, Gallacher DJ, Phartyal SS, Rodriguez-Paez LA, Pineda-Rodriguez YY, Pompelli MF, Jamal A, Mancinelli R, Radicetti E. Storage on Maternal Plants Affects Temperature Requirements during Germination in Rumex obtusifolius. Plants. 2023; 12(13):2403. https://doi.org/10.3390/plants12132403
Chicago/Turabian StyleBhatt, Arvind, Xingxing Chen, David J. Gallacher, Shyam S. Phartyal, Luis Alfonso Rodriguez-Paez, Yirlis Yadeth Pineda-Rodriguez, Marcelo F. Pompelli, Aftab Jamal, Roberto Mancinelli, and Emanuele Radicetti. 2023. "Storage on Maternal Plants Affects Temperature Requirements during Germination in Rumex obtusifolius" Plants 12, no. 13: 2403. https://doi.org/10.3390/plants12132403
APA StyleBhatt, A., Chen, X., Gallacher, D. J., Phartyal, S. S., Rodriguez-Paez, L. A., Pineda-Rodriguez, Y. Y., Pompelli, M. F., Jamal, A., Mancinelli, R., & Radicetti, E. (2023). Storage on Maternal Plants Affects Temperature Requirements during Germination in Rumex obtusifolius. Plants, 12(13), 2403. https://doi.org/10.3390/plants12132403











