Comparative Transcriptome Analysis Points to the Biological Processes of Hybrid Incompatibility between Brassica napus and B. oleracea
Abstract
1. Introduction
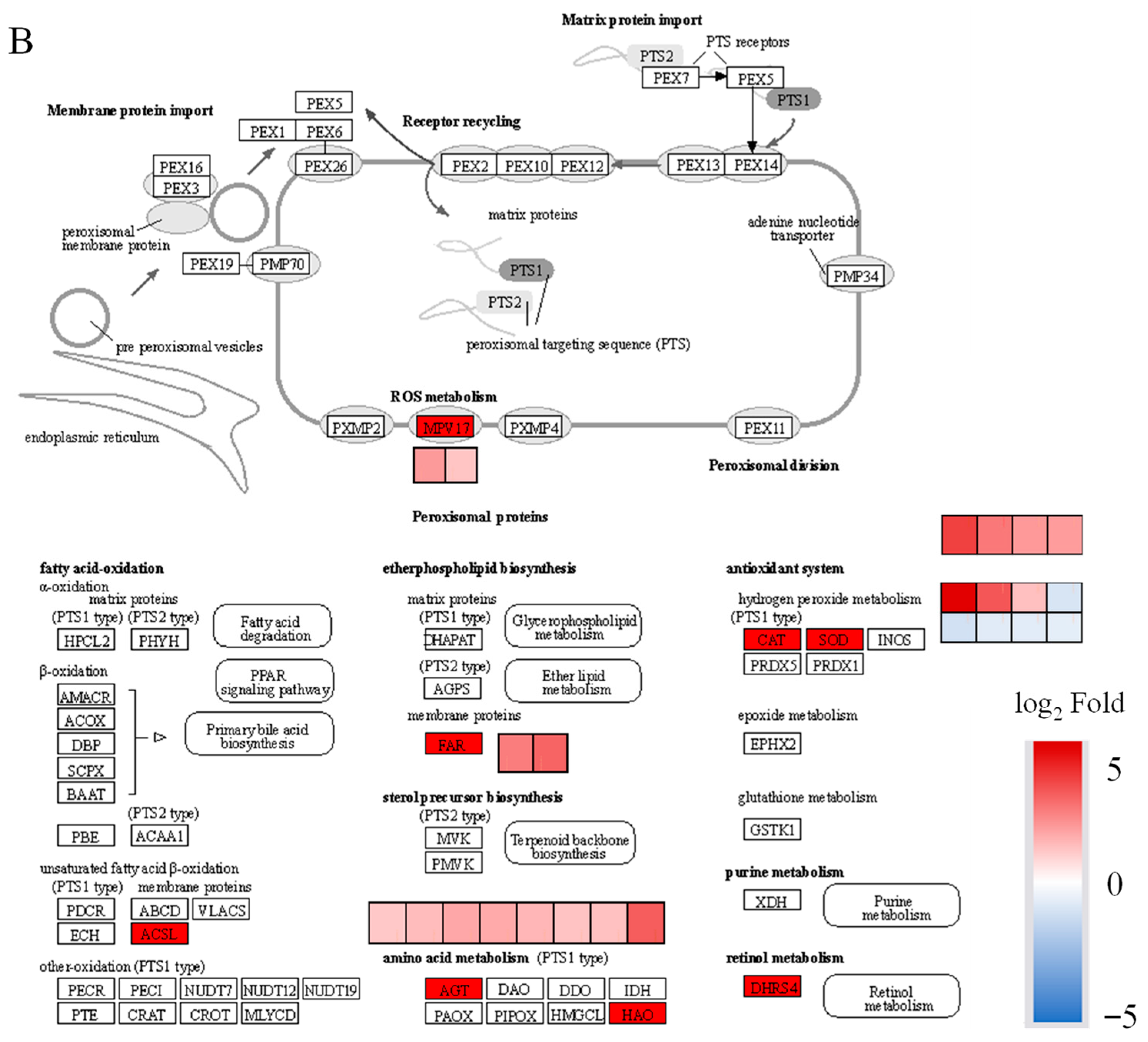
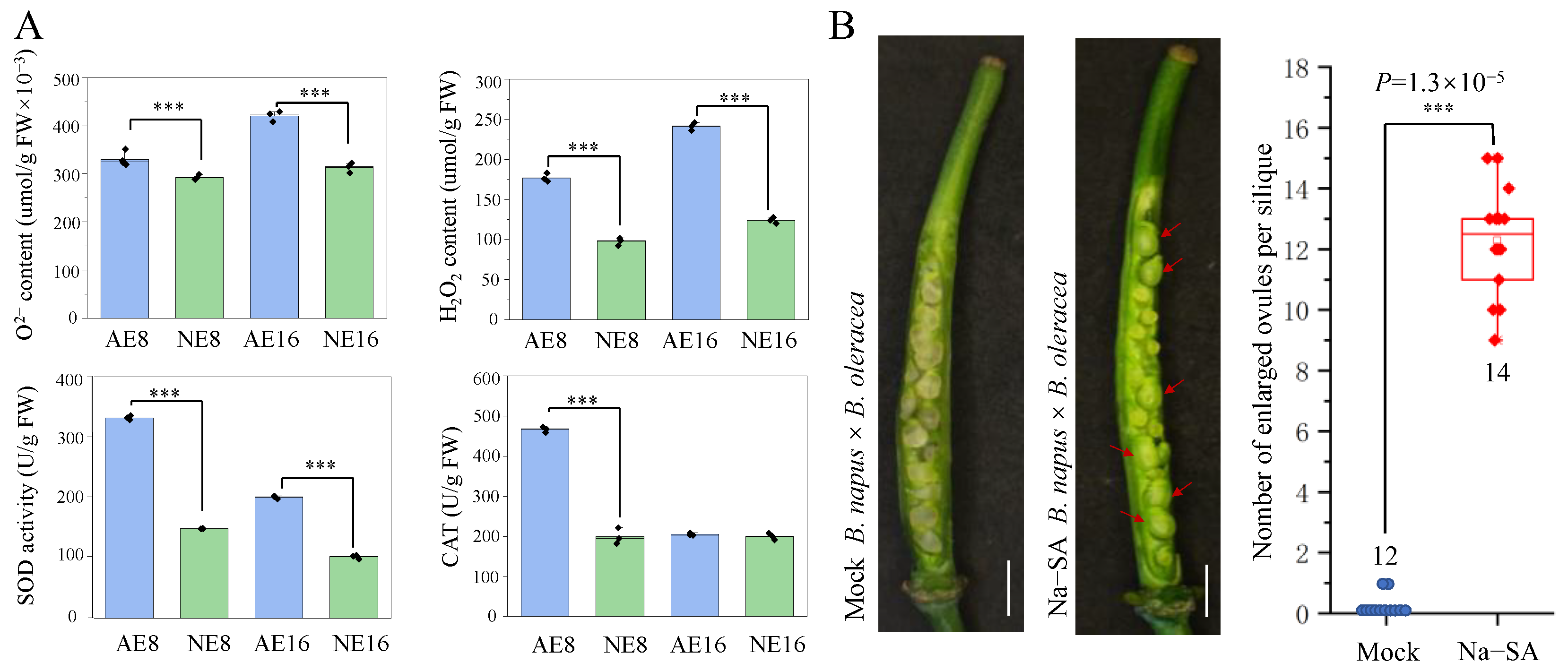
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Morphological Observation
4.3. Transcriptome Sequencing and Differentially Expressed Gene Analysis
4.4. ROS content and Antioxidant Enzyme Activity Assay
4.5. Quantitative RT-PCR
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katche, E.; Quezada-Martinez, D.; Ihien Katche, E.; Vasquez-Teuber, P.; Mason, A.S. Interspecific Hybridization for Brassica Crop Improvement. Crop Breed. Genet. Genom. 2019, 1, e190007. [Google Scholar]
- Lu, K.; Wei, L.; Li, X.; Wang, Y.; Wu, J.; Liu, M.; Zhang, C.; Chen, Z.; Xiao, Z.; Jian, H.; et al. Whole-genome resequencing reveals Brassica napus origin and genetic loci involved in its improvement. Nat. Commun. 2019, 10, 1154. [Google Scholar] [CrossRef]
- Nagaharu, U. Genome-analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Jpn. J. Bot. 1935, 7, 389–452. [Google Scholar]
- Friedt, W.; Tu, J.; Fu, T. Academic and Economic Importance of Brassica napus Rapeseed. In The Brassica napus Genome; Springer: Cham, Switzerland, 2018; Chapter 1; pp. 1–20. [Google Scholar]
- Sharma, B.B.; Kalia, P.; Singh, D.; Sharma, T.R. Introgression of black rot resistance from Brassica carinata to cauliflower (Brassica oleracea botrytis group) through embryo rescue. Front. Plant Sci. 2017, 8, 1255. [Google Scholar] [CrossRef] [PubMed]
- Attri, R.; Rahman, H. Introgression of allelic diversity from genetically distinct variants of Brassica rapa into Brassica napus canola and inheritance of the B. rapa alleles. Crop Pasture Sci. 2018, 69, 94–106. [Google Scholar] [CrossRef]
- Das, G.G.; Malek, M.A.; Shamsuddin, A.K.M.; Sagor, G.H.M. Development of high yielding early matured and shattering tolerant Brassica napus L. through interspecific hybridization between B. rapa L. and B. oleracea L. Genet. Resour. Crop Evol. 2022, 69, 1009–1018. [Google Scholar] [CrossRef]
- Thakur, A.K.; Singh, K.H.; Singh, L.; Nanjundan, J.; Khan, Y.J.; Singh, D. SSR marker variations in Brassica species provide insight into the origin and evolution of Brassica amphidiploids. Hereditas 2018, 155, 6. [Google Scholar] [CrossRef]
- McAlvay, A.C.; Ragsdale, A.P.; Mabry, M.E.; Qi, X.; Bird, K.A.; Velasco, P.; An, H.; Pires, J.C.; Emshwiller, E. Brassica rapa Domestication: Untangling Wild and Feral Forms and Convergence of Crop Morphotypes. Mol. Biol. Evol. 2021, 38, 3358–3372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, H.; Channa, S.A.; Zhang, Y.; Guo, Y.; Klima, M.; Yu, F.; Hu, S. Genetic diversity in Chinese and exotic Brassica rapa L. accessions revealed by SSR and SRAP markers. Rev. Bras. Bot. 2017, 40, 973–982. [Google Scholar] [CrossRef]
- Mabry, M.E.; Turner-Hissong, S.D.; Gallagher, E.Y.; McAlvay, A.C.; An, H.; Edger, P.P.; Moore, J.D.; Pink, D.A.C.; Teakle, G.R.; Stevens, C.J.; et al. The Evolutionary History of Wild, Domesticated, and Feral Brassica oleracea (Brassicaceae). Mol. Biol. Evol. 2021, 38, 4419–4434. [Google Scholar] [CrossRef]
- Mei, J.; Qian, L.; Disi, J.O.; Yang, X.; Qian, W. Identification of resistant sources against Sclerotinia sclerotiorum in Brassica species with emphasis on B. oleracea. Euphytica 2010, 177, 393–399. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, K.; Li, H.; Han, S.; Meng, Q.; Khan, S.U.; Fan, C.; Xie, K.; Zhou, Y. Precise editing of CLAVATA genes in Brassica napus L. regulates multilocular silique development. Plant Biotechnol. J. 2018, 16, 1322–1335. [Google Scholar] [CrossRef]
- Amosova, A.V.; Zemtsova, L.V.; Yurkevich, O.Y.; Zhidkova, E.N.; Książczyk, T.; Shostak, N.G.; Muravlev, A.A.; Artemyeva, A.M.; Samatadze, T.E.; Zoshchuk, S.A.; et al. Genomic changes in generations of synthetic rapeseed-like allopolyploid grown under selection. Euphytica 2017, 213, 217. [Google Scholar] [CrossRef]
- Wen, J.; Zhu, L.; Qi, L.; Ke, H.; Yi, B.; Shen, J.; Tu, J.; Ma, C.; Fu, T.D. Characterization of interploid hybrids from crosses between Brassica juncea and B. oleracea and the production of yellow-seeded B. napus. Theor. Appl. Genet. 2012, 125, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Fan, G.; Hu, Q.; Zhou, Y.; Guan, M.; Tong, C.; Li, J.; Du, D.; Qi, C.; Jiang, L.; et al. The high-quality genome of Brassica napus cultivar ‘ZS11’ reveals the introgression history in semi-winter morphotype. Plant J. 2017, 92, 452–468. [Google Scholar] [CrossRef]
- Zou, J.; Mao, L.; Qiu, J.; Wang, M.; Jia, L.; Wu, D.; He, Z.; Chen, M.; Shen, Y.; Shen, E.; et al. Genome-wide selection footprints and deleterious variations in young Asian allotetraploid rapeseed. Plant Biotechnol. J. 2019, 17, 1998–2010. [Google Scholar] [CrossRef]
- Bhatia, R.; Dey, S.S.; Sharma, K.; Singh, S.; Kumar, S.; Pramanik, A.; Parkash, C.; Kumar, R. Back-cross introgression of ‘Tour’ cytoplasm from Brassica napus through in vitro embryo rescue reveals partial restoration of sterility in B. oleracea. Sci. Hortic. 2021, 282, 110014. [Google Scholar] [CrossRef]
- Hasan, M.J.; Shaikh, R.; Basu, U.; Rahman, H. Mapping clubroot resistance of Brassica rapa introgressed into Brassica napus and development of molecular markers for the resistance. Crop Sci. 2021, 61, 4112–4127. [Google Scholar] [CrossRef]
- Mei, J.; Shao, C.; Yang, R.; Feng, Y.; Gao, Y.; Ding, Y.; Li, J.; Qian, W. Introgression and pyramiding of genetic loci from wild Brassica oleracea into B. napus for improving Sclerotinia resistance of rapeseed. Theor. Appl. Genet. 2020, 133, 1313–1319. [Google Scholar] [CrossRef]
- Hu, D.; Zhao, Y.; Shen, J.; He, X.; Zhang, Y.; Jiang, Y.; Snowdon, R.; Meng, J.; Reif, J.C.; Zou, J. Genome-wide prediction for hybrids between parents with distinguished difference on exotic introgressions in Brassica napus. Crop J. 2021, 9, 1169–1178. [Google Scholar] [CrossRef]
- Kaminski, P.; Marasek-Ciolakowska, A.; Podwyszynska, M.; Starzycki, M.; Starzycka-Korbas, E.; Nowak, K. Development and Characteristics of Interspecific Hybrids between Brassica oleracea L. and B. napus L. Agronomy 2020, 10, 1339. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Q.; Mei, J.; Zhang, Y.; Li, J.; Li, Z.; Ge, X.; Xiong, Z.; Huang, Y.; Qian, W. Improvement of Brassica napus via interspecific hybridization between B. napus and B. oleracea. Mol. Breed. 2014, 34, 1955–1963. [Google Scholar] [CrossRef]
- Kushwah, S.; Banasiak, A.; Nishikubo, N.; Derba-Maceluch, M.; Majda, M.; Endo, S.; Kumar, V.; Gomez, L.; Gorzsas, A.; Mcqueen-Mason, S. Arabidopsis XTH4 and XTH9 contribute to wood cell expansion and secondary wall formation. Cold Spring Harb. Lab. 2019, 182, 1946–1965. [Google Scholar] [CrossRef]
- Pitaksaringkarn, W.; Matsuoka, K.; Asahina, M.; Miura, K.; Sage-Ono, K.; Ono, M.; Yokoyama, R.; Nishitani, K.; Ishii, T.; Iwai, H.; et al. XTH20 and XTH19 regulated by ANAC071 under auxin flow are involved in cell proliferation in incised Arabidopsis inflorescence stems. Plant J. 2014, 80, 604–614. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, D.; Held, M.A.; Cannon, M.C.; Ray, T.; Saha, P.; Frye, A.N.; Mort, A.J.; Kieliszewski, M.J. Identification of the abundant hydroxyproline-rich glycoproteins in the root walls of wild-type Arabidopsis, an EXT3 mutant line, and its phenotypic revertant. Plants 2015, 4, 85–111. [Google Scholar] [CrossRef]
- Di Marzo, M.; Herrera-Ubaldo, H.; Caporali, E.; Novák, O.; Strnad, M.; Balanzà, V.; Ezquer, I.; Mendes, M.A.; de Folter, S.; Colombo, L. SEEDSTICK Controls Arabidopsis Fruit Size by Regulating Cytokinin Levels and FRUITFULL. Cell Rep. 2020, 30, 2846–2857.e3. [Google Scholar] [CrossRef] [PubMed]
- Mizzotti, C.; Mendes, M.A.; Caporali, E.; Schnittger, A.; Kater, M.M.; Battaglia, R.; Colombo, L. The MADS box genes SEEDSTICK and ARABIDOPSIS Bsister play a maternal role in fertilization and seed development. Plant J. 2012, 70, 409–420. [Google Scholar] [CrossRef]
- Anfang, M.; Shani, E. Transport mechanisms of plant hormones. Curr. Opin. Plant Biol. 2021, 63, 102055. [Google Scholar] [CrossRef]
- Pernisova, M.; Grochova, M.; Konecny, T.; Plackova, L.; Harustiakova, D.; Kakimoto, T.; Heisler, M.G.; Novak, O.; Hejatko, J. Cytokinin signalling regulates organ identity via the AHK4 receptor in arabidopsis. Development 2018, 145, 556–566. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Robertson-Hoyt, J.; Yun, J.; Benavente, L.M.; Xie, D.Y.; Doležal, K.; Schlereth, A.; Jürgens, G.; Alonso, J.M. TAA1-Mediated Auxin Biosynthesis Is Essential for Hormone Crosstalk and Plant Development. Cell 2008, 133, 177–191. [Google Scholar] [CrossRef]
- Ye, L.; Liu, L.; Xing, A.; Kang, D. Characterization of a dwarf mutant allele of Arabidopsis MDR-like ABC transporter AtPGP1 gene. Biochem. Biophys. Res. Commun. 2013, 441, 782–786. [Google Scholar] [CrossRef]
- Buzduga, I.M.; Volkov, R.A.; Panchuk, I.I. Loss of catalase 2 activity affects the ascorbate metabolism in Arabidopsis upon heavy metal stress. Plant Physiol. Genet. 2020, 52, 306–319. [Google Scholar] [CrossRef]
- Moreno, J.I.; Martín, R.; Castresana, C. Arabidopsis SHMT1, a serine hydroxymethyltransferase that functions in the photorespiratory pathway influences resistance to biotic and abiotic stress. Plant J. 2005, 41, 451–463. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, L.; Yang, L.; Wu, X.; Cui, X.; Zhang, L.; Hui, J.; Zhao, Y.; Yang, H.; Liu, S.; et al. Stigma receptors control intraspecies and interspecies barriers in Brassicaceae. Nature 2023, 614, 303–308. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Datta, S. Antioxidant enzyme activities during in vitro morphogenesis of gladiolus and the effect of application of antioxidants on plant regeneration. Biol. Plant. 2004, 47, 179–183. [Google Scholar] [CrossRef]
- Lampl, N.; Alkan, N.; Davydov, O.; Fluhr, R. Set-point control of RD21 protease activity by AtSerpin1 controls cell death in Arabidopsis. Plant J. 2013, 74, 498–510. [Google Scholar] [CrossRef]
- Miao, Y.; Zentgraf, U. The antagonist function of Arabidopsis WRKY53 and ESR/ESP in leaf senescence is modulated by the jasmonic and salicylic acid equilibrium. Plant Cell 2007, 19, 819–830. [Google Scholar] [CrossRef]
- Murray, S.L.; Ingle, R.A.; Petersen, L.N.; Denby, K.J. Basal resistance against Pseudomonas syringae in Arabidopsis involves WRKY53 and a protein with homology to a nematode resistance protein. Mol. Plant-Microbe Interact. 2007, 20, 1431–1438. [Google Scholar] [CrossRef]
- Park, D.S.; Graham, M.Y.; Graham, T.L. Identification of soybean elicitation competency factor, CF-1, as the soybean Kunitz trypsin inhibitor. Physiol. Mol. Plant Pathol. 2001, 59, 265–273. [Google Scholar] [CrossRef]
- Ren, C.M.; Zhu, Q.; Gao, B.D.; Ke, S.Y.; Yu, W.C.; Xie, D.X.; Peng, W. Transcription factor WRKY70 displays important but no indispensable roles in jasmonate and salicylic acid signaling. J. Integr. Plant Biol. 2008, 50, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.T.; Wang, F.; Ma, Y.P.; Jia, L.J.; Liu, N.; Wang, H.Y.; Zhao, P.; Xia, G.X.; Zhong, N.Q. Ectopic expression of SsPETE2, a plastocyanin from Suaeda salsa, improves plant tolerance to oxidative stress. Plant Sci. 2018, 268, 1–10. [Google Scholar] [CrossRef]
- Iftikhar, R.; Wang, X.; Rahman, H. Broadening the genetic base of Brassica napus canola by interspecific crosses with different variants of B. oleracea. Euphytica 2018, 214, 133. [Google Scholar] [CrossRef]
- Kozaki, A.; Takeba, G. Photorespiration protects C3 plants from photooxidation. Nature 1996, 384, 557–560. [Google Scholar] [CrossRef]
- Guyomarc’h, S.; Boutté, Y.; Laplaze, L. AP2/ERF transcription factors orchestrate very long chain fatty acid biosynthesis during Arabidopsis lateral root development. Mol. Plant 2021, 14, 205–207. [Google Scholar] [CrossRef]
- Girón-Ramírez, A.; Peña-Rodríguez, L.M.; Escalante-Erosa, F.; Fuentes, G.; Santamaría, J.M. Identification of the SHINE clade of AP2/ERF domain transcription factors genes in Carica papaya; Their gene expression and their possible role in wax accumulation and water deficit stress tolerance in a wild and a commercial papaya genotypes. Environ. Exp. Bot. 2021, 83, 104341. [Google Scholar] [CrossRef]
- Krizek, B.A.; Blakley, I.C.; Ho, Y.Y.; Freese, N.; Loraine, A.E. The Arabidopsis transcription factor AINTEGUMENTA orchestrates patterning genes and auxin signaling in the establishment of floral growth and form. Plant J. 2020, 103, 752–768. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hou, H.; Wu, Z.; Zhao, J.; Zhang, F.; Teng, R.; Chen, F.; Teng, N. Chrysanthemum embryo development is negatively affected by a novel ERF transcription factor, CmERF12. J. Exp. Bot. 2021, 73, 197–212. [Google Scholar] [CrossRef]
- Denness, L.; McKenna, J.F.; Segonzac, C.; Wormit, A.; Madhou, P.; Bennett, M.; Mansfield, J.; Zipfel, C.; Hamann, T. Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Arabidopsis. Plant Physiol. 2011, 156, 1364–1374. [Google Scholar] [CrossRef]
- Tognetti, V.B.; Bielach, A.; Hrtyan, M. Redox regulation at the site of primary growth: Auxin, cytokinin and ROS crosstalk. Plant Cell Environ. 2017, 40, 2586–2605. [Google Scholar] [CrossRef]
- Bouquin, T.; Meier, C.; Foster, R.; Nielsen, M.E.; Mundy, J. Control of specific gene expression by gibberellin and brassinosteroid. Plant Physiol. 2001, 127, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Wang, H. Two homologous ATP-binding cassette transporter proteins, AtMDR1 and AtPGP1, regulate Arabidopsis photomorphogenesis and root development by mediating polar auxin transport. Plant Physiol. 2005, 138, 949–964. [Google Scholar] [CrossRef]
- Xu, W.; Campbell, P.; Vargheese, A.K.; Braam, J. The Arabidopsis XET-related gene family: Environmental and hormonal regulation of expression. Plant J. 1996, 9, 879–889. [Google Scholar] [CrossRef]
- Ma, K.; Song, Y.; Huang, Z.; Lin, L.; Zhang, Z.; Zhang, D. The low fertility of Chinese white poplar: Dynamic changes in anatomical structure, endogenous hormone concentrations, and key gene expression in the reproduction of a naturally occurring hybrid. Plant Cell Rep. 2013, 32, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.; Liu, Z. A model for an early role of auxin in Arabidopsis gynoecium morphogenesis. Front. Plant Sci. 2014, 5, 327. [Google Scholar] [CrossRef] [PubMed]
- Dhooghe, E.; Reheul, D.; Versluys, T.; Van Labeke, M.C. Pollen characteristics and stigma receptivity for Anemone coronaria L. Euphytica 2018, 214, 211–224. [Google Scholar] [CrossRef]
- Robinson Mark, D.; McCarthy Davis, J.; Smyth Gordon, K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Tian, M.; Gu, Q.; Zhu, M. The involvement of hydrogen peroxide and antioxidant enzymes in the process of shoot organogenesis of strawberry callus. Plant Sci. 2003, 165, 701–707. [Google Scholar] [CrossRef]
- Li, S.W.; Xue, L.; Xu, S.; Feng, H.; An, L. Hydrogen peroxide acts as a signal molecule in the adventitious root formation of mung bean seedlings. Environ. Exp. Bot. 2009, 65, 63–71. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, W.; Qin, M.; Li, S.; Qiao, M.; Liu, Z.; Xiang, F. Drought tolerance conferred in soybean (Glycine max. L) by GmMYB84, a novel R2R3-MYB transcription factor. Plant Cell Physiol. 2017, 58, 1764–1776. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, F.; Zheng, F.; Li, Q.; Mei, J.; Shu, C.; Qian, W. Comparative Transcriptome Analysis Points to the Biological Processes of Hybrid Incompatibility between Brassica napus and B. oleracea. Plants 2023, 12, 2622. https://doi.org/10.3390/plants12142622
Yue F, Zheng F, Li Q, Mei J, Shu C, Qian W. Comparative Transcriptome Analysis Points to the Biological Processes of Hybrid Incompatibility between Brassica napus and B. oleracea. Plants. 2023; 12(14):2622. https://doi.org/10.3390/plants12142622
Chicago/Turabian StyleYue, Fang, Fajing Zheng, Qinfei Li, Jiaqin Mei, Chunlei Shu, and Wei Qian. 2023. "Comparative Transcriptome Analysis Points to the Biological Processes of Hybrid Incompatibility between Brassica napus and B. oleracea" Plants 12, no. 14: 2622. https://doi.org/10.3390/plants12142622
APA StyleYue, F., Zheng, F., Li, Q., Mei, J., Shu, C., & Qian, W. (2023). Comparative Transcriptome Analysis Points to the Biological Processes of Hybrid Incompatibility between Brassica napus and B. oleracea. Plants, 12(14), 2622. https://doi.org/10.3390/plants12142622




