Effects of Seasonal Changes on Chlorophyll Fluorescence and Physiological Characteristics in the Two Taxus Species
Abstract
:1. Introduction
2. Results
2.1. Seasonal Dynamics of Chlorophyll Fluorescence Parameters and Images in T. media and T. mairei
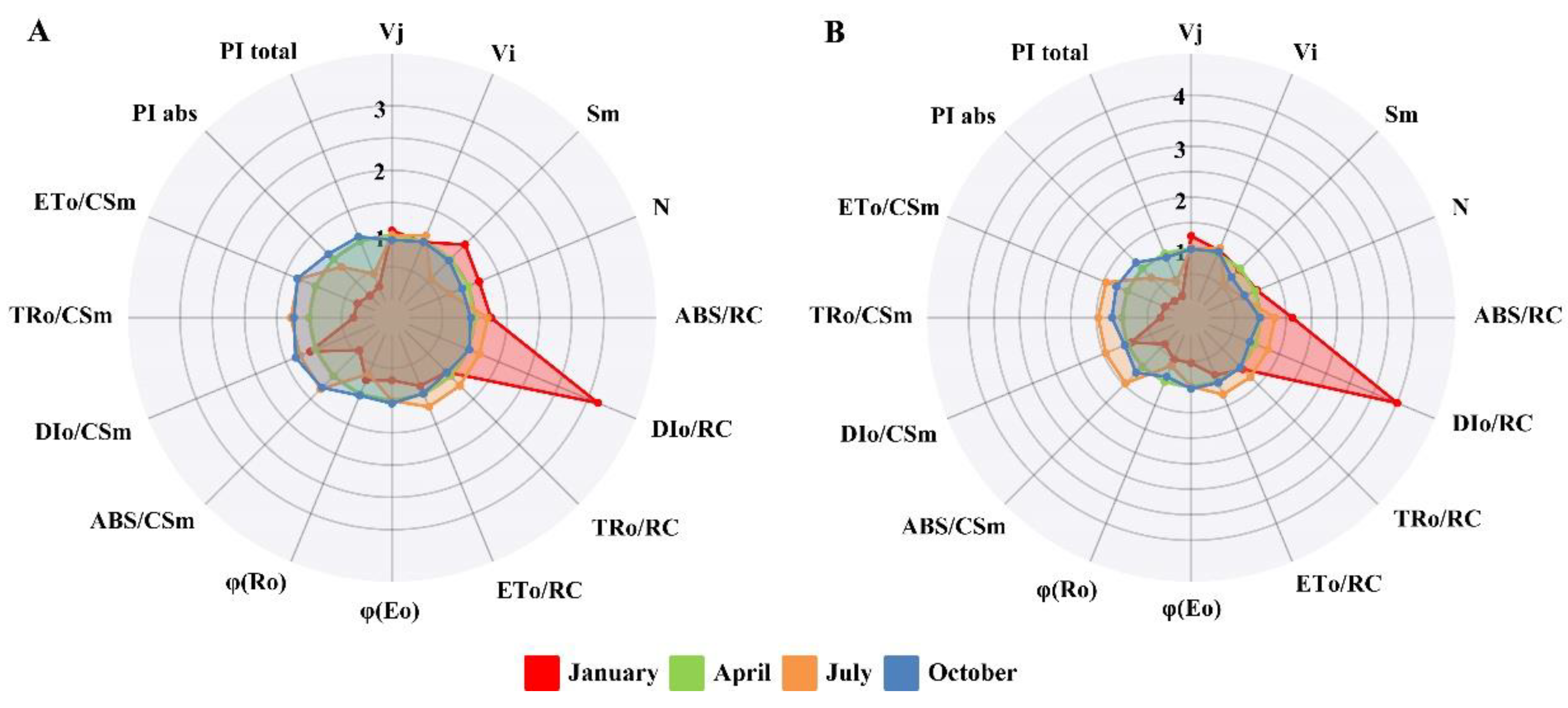
2.2. Changes of Chlorophyll a Fluorescence Transient and JIP-Test Parameters in T. media and T. mairei
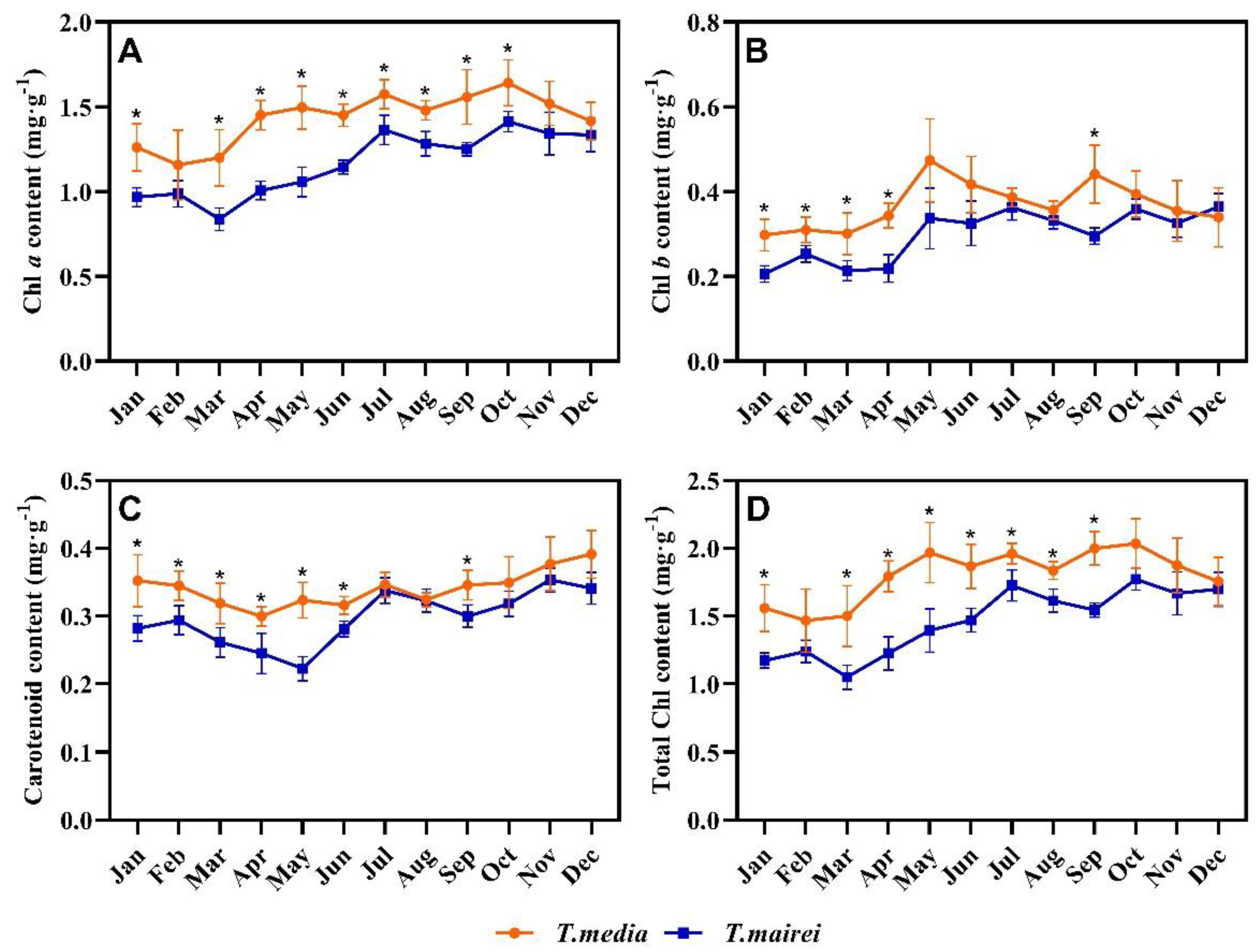
2.3. Seasonal Dynamics of Chlorophyll and Carotenoid Contents in T. media and T. mairei
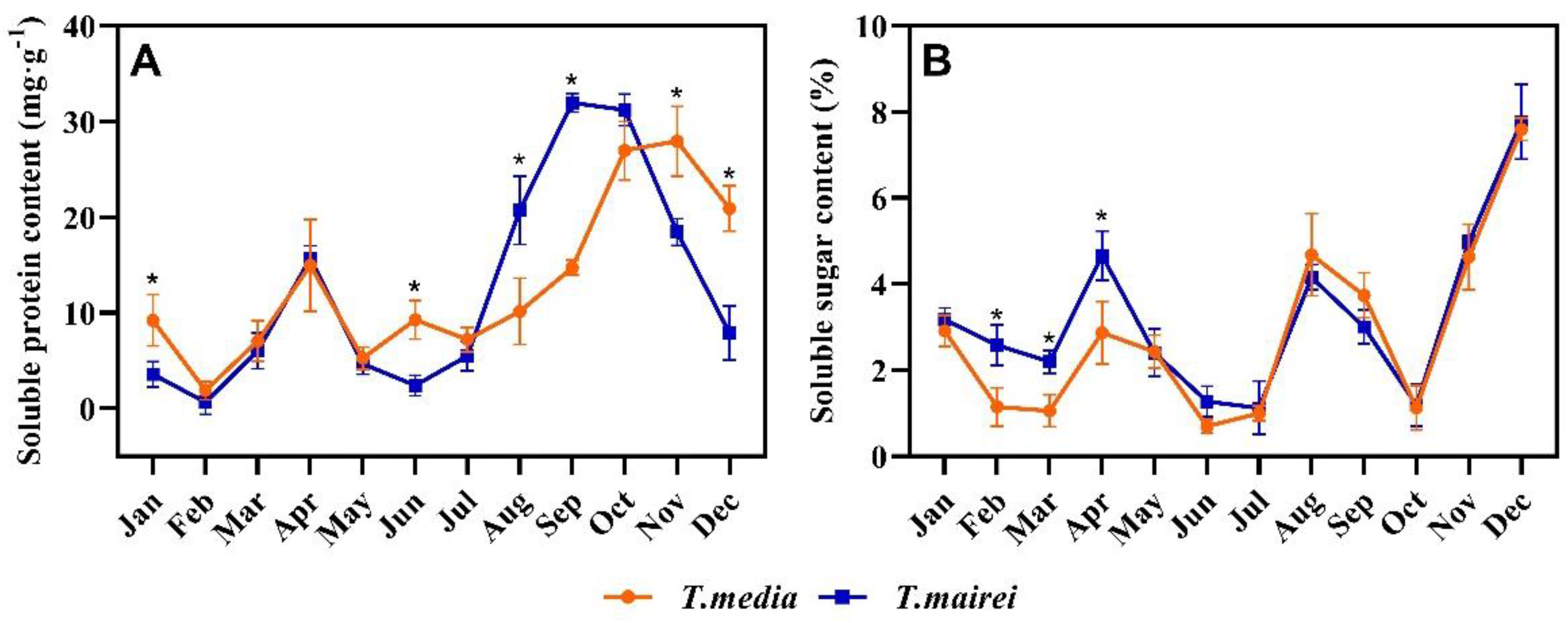
2.4. Seasonal Dynamics of Soluble Protein and Soluble Sugar Content in T. media and T. mairei
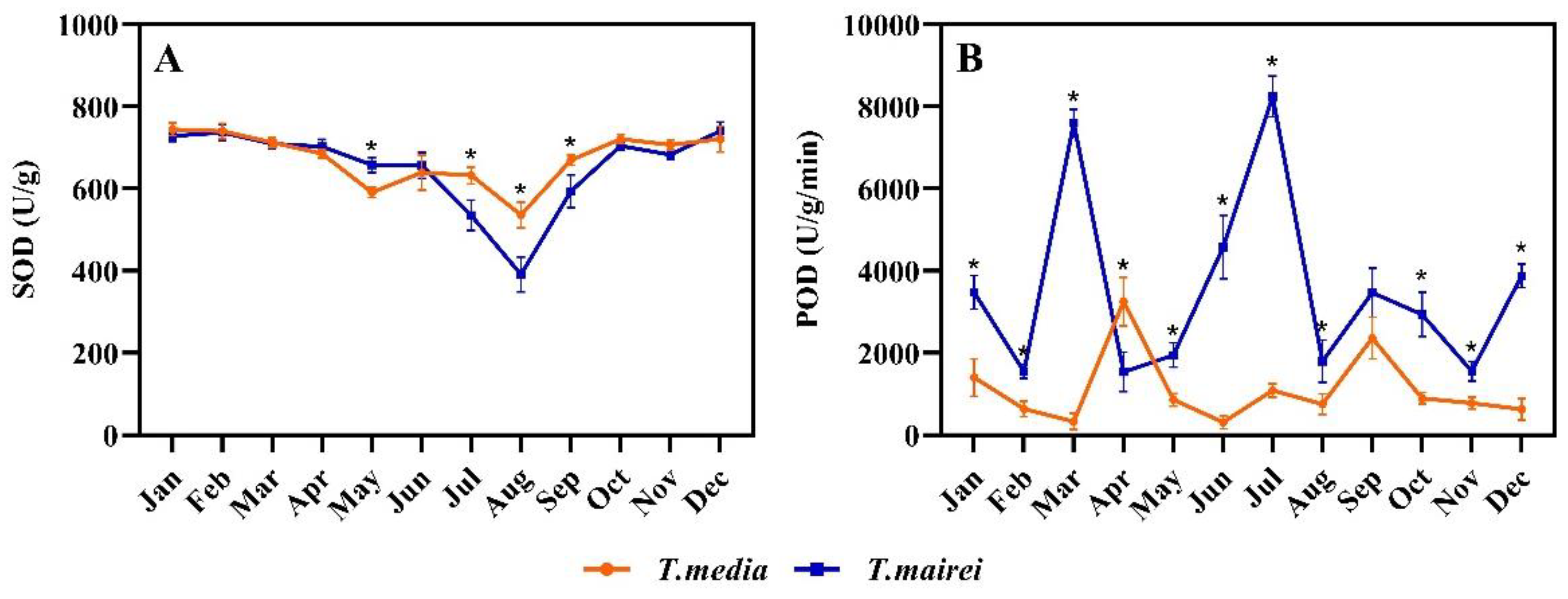
2.5. Seasonal Dynamics of SOD and POD Activity in T. media and T. mairei
2.6. Pearson Correlation Analysis of Chl Fluorescence and Physiological Indicators in T. media and T. mairei
2.7. Redundancy Discriminant Analysis between Environmental Factors and Chlorophyll Fluorescence and Physiological Indicators in T. media and T. mairei
3. Discussions
4. Materials and Methods
4.1. Experimental Site and Plant Material
4.2. Measurement of Chlorophyll a Fluorescence Transient
4.3. Measurement of Chlorophyll Fluorescence-Imaging
4.4. Measurement of Photosynthetic Pigment Content
4.5. Measurement of Soluble Protein and Soluble Sugar
4.6. Measurement of SOD and POD Activity
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hao, D.C.; Ge, G.; Xiao, P.; Zhang, Y.; Yang, L. The first insight into the tissue specific taxus transcriptome via Illumina second generation sequencing. PLoS ONE 2011, 6, e21220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croteau, R.; Ketchum, R.E.; Long, R.M.; Kaspera, R.; Wildung, M.R. Taxol biosynthesis and molecular genetics. Phytochem. Rev. 2006, 5, 75–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Dong, Y.; Nie, L.; Lu, M.; Fu, C.; Yu, L. High-throughput sequencing reveals miRNA effects on the primary and secondary production properties in long-term subcultured Taxus cells. Front. Plant Sci. 2015, 6, 604. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y. The national key protected wild plants in China. Plants 1999, 151, 3–11. [Google Scholar]
- Möller, M.; Liu, J.; Li, Y.; Li, J.-H.; Ye, L.-J.; Mill, R.; Thomas, P.; Li, D.-Z.; Gao, L.-M. Repeated intercontinental migrations and recurring hybridizations characterise the evolutionary history of yew (Taxus L.). Mol. Phylogenetics Evol. 2020, 153, 106952. [Google Scholar] [CrossRef]
- Liu, J.; Milne, R.I.; Möller, M.; Zhu, G.F.; Ye, L.J.; Luo, Y.H.; Yang, J.B.; Wambulwa, M.C.; Wang, C.N.; Li, D.Z. Integrating a comprehensive DNA barcode reference library with a global map of yews (Taxus L.) for forensic identification. Mol. Ecol. Resour. 2018, 18, 1115–1131. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, A.S.; Swanberg, A.; Thao, M.; Whiteman, J. Seasonal changes in leaf antioxidant systems and xanthophyll cycle characteristics in Taxus x media growing in sun and shade environments. Physiol. Plant. 2005, 123, 428–434. [Google Scholar] [CrossRef]
- Yu, X.; Liu, X.; Liu, X. Response of rhizosphere bacterial community of Taxus chinensis var. mairei to temperature changes. PLoS ONE 2019, 14, e0226500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousef, A.F.; Ali, M.M.; Rizwan, H.M.; Tadda, S.A.; Kalaji, H.M.; Yang, H.; Ahmed, M.A.; Wróbel, J.; Xu, Y.; Chen, F. Photosynthetic apparatus performance of tomato seedlings grown under various combinations of LED illumination. PLoS ONE 2021, 16, e0249373. [Google Scholar] [CrossRef]
- Guiamba, H.D.S.S.; Zhang, X.; Sierka, E.; Lin, K.; Ali, M.M.; Ali, W.M.; Lamlom, S.F.; Kalaji, H.M.; Telesiński, A.; Yousef, A.F. Enhancement of photosynthesis efficiency and yield of strawberry (Fragaria ananassa Duch.) plants via LED systems. Front. Plant Sci. 2022, 13, 918038. [Google Scholar] [CrossRef]
- Masarmi, A.G.; Solouki, M.; Fakheri, B.; Kalaji, H.M.; Mahgdingad, N.; Golkari, S.; Telesiński, A.; Lamlom, S.F.; Kociel, H.; Yousef, A.F. Comparing the salinity tolerance of twenty different wheat genotypes on the basis of their physiological and biochemical parameters under NaCl stress. PLoS ONE 2023, 18, e0282606. [Google Scholar] [CrossRef]
- Tanaka, A. Photosynthetic activity in winter needles of the evergreen tree Taxus cuspidata at low temperatures. Tree Physiol. 2007, 27, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhou, Z.; Li, W.; Liu, T. Physiological and photosynthetic response to different water conditions of Taxus cuspidate seedlings. J. Beijing For. Univ. 2012, 34, 73–78. [Google Scholar]
- Zu, Y.-g.; Pang, H.-H.; Yu, J.-H.; Li, D.-W.; Wei, X.-X.; Gao, Y.-X.; Tong, L. Responses in the morphology, physiology and biochemistry of Taxus chinensis var. mairei grown under supplementary UV-B radiation. J. Photochem. Photobiol. B Biol. 2010, 98, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A. Acclimation of Pacific yew (Taxus brevifolia) foliage to sun and shade. Tree Physiol. 1998, 18, 749–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Jin, H.; Xu, H.; Zhou, Z.; Zhang, Y. Diurnal changes of photosynthetic characteristics of Taxus cuspidata seedlings under different light conditions. J. Beijing For. Univ. 2015, 37, 67–72. [Google Scholar]
- Robakowski, P.; Pers-Kamczyc, E.; Ratajczak, E.; Thomas, P.A.; Ye, Z.-P.; Rabska, M.; Iszkuło, G. Photochemistry and antioxidative capacity of female and male Taxus baccata L. acclimated to different nutritional environments. Front. Plant Sci. 2018, 9, 742. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Li, C.; Li, Q.; Qiao, B.; Shi, S.; Zhao, C. Enhancement of interplanting of Ficus carica L. with Taxus cuspidata Sieb. et Zucc. on growth of two plants. Agriculture 2021, 11, 1276. [Google Scholar] [CrossRef]
- Begovic, L.; Galic, V.; Abicic, I.; Loncaric, Z.; Lalic, A.; Mlinaric, S. Implications of intra-seasonal climate variations on chlorophyll a fluorescence and biomass in winter barley breeding program. Photosynthetica 2020, 58, 995–1008. [Google Scholar] [CrossRef]
- Wang, T.; Li, L.; Cheng, G.; Shu, X.; Wang, N.; Zhang, F.; Zhuang, W.; Wang, Z. Physiological and Molecular Analysis Reveals the Differences of Photosynthesis between Colored and Green Leaf Poplars. Int. J. Mol. Sci. 2021, 22, 8982. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence; Springer: Berlin/Heidelberg, Germany, 2004; pp. 321–362. [Google Scholar]
- Li, P.-M.; Gao, H.-Y.; Strasser, R.J. Application of the fast chlorophyll fluorescence induction dynamics analysis in photosynthesis study. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao = J. Plant Physiol. Mol. Biol. 2005, 31, 559–566. [Google Scholar]
- Gray, G.R.; Hope, B.J.; Qin, X.; Taylor, B.G.; Whitehead, C.L. The characterization of photoinhibition and recovery during cold acclimation in Arabidopsis thaliana using chlorophyll fluorescence imaging. Physiol. Plant. 2003, 119, 365–375. [Google Scholar] [CrossRef]
- Jedmowski, C.; Brüggemann, W. Imaging of fast chlorophyll fluorescence induction curve (OJIP) parameters, applied in a screening study with wild barley (Hordeum spontaneum) genotypes under heat stress. J. Photochem. Photobiol. B Biol. 2015, 151, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, A.; Roca, D.; Martínez, P.F. Spatial-temporal variations in rose leaves under water stress conditions studied by chlorophyll fluorescence imaging. Plant Physiol. Biochem. 2006, 44, 564–573. [Google Scholar] [CrossRef]
- Yuan, Y.; Shu, S.; Li, S.; He, L.; Li, H.; Du, N.; Sun, J.; Guo, S. Effects of exogenous putrescine on chlorophyll fluorescence imaging and heat dissipation capacity in cucumber (Cucumis sativus L.) under salt stress. J. Plant Growth Regul. 2014, 33, 798–808. [Google Scholar] [CrossRef]
- Takayama, K.; Sakai, Y.; Nishina, H.; Omasa, K. Chlorophyll fluorescence imaging at 77 K for assessing the heterogeneously distributed light stress over a leaf surface. Environ. Control. Biol. 2007, 45, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Liang, D.; Yousef, A.F.; Wei, X.; Ali, M.M.; Yu, W.; Yang, L.; Oelmüller, R.; Chen, F. Increasing the performance of Passion fruit (Passiflora edulis) seedlings by LED light regimes. Sci. Rep. 2021, 11, 20967. [Google Scholar] [CrossRef]
- Dong, Z.; Men, Y.; Liu, Z.; Li, J.; Ji, J. Application of chlorophyll fluorescence imaging technique in analysis and detection of chilling injury of tomato seedlings. Comput. Electron. Agric. 2020, 168, 105109. [Google Scholar] [CrossRef]
- Gorbe, E.; Calatayud, A. Applications of chlorophyll fluorescence imaging technique in horticultural research: A review. Sci. Hortic. 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, Y.; Hao, T.; Jin, H.; Zhang, H.; He, L.; Zhou, Q.; Huang, D.; Hui, D.; Yu, J. Effects of heat shock on photosynthetic properties, antioxidant enzyme activity, and downy mildew of cucumber (Cucumis sativus L.). PLoS ONE 2016, 11, e0152429. [Google Scholar] [CrossRef] [Green Version]
- Meng, D.; Yu, X.; Ma, L.; Hu, J.; Liang, Y.; Liu, X.; Yin, H.; Liu, H.; He, X.; Li, D. Transcriptomic response of Chinese yew (Taxus chinensis) to cold stress. Front. Plant Sci. 2017, 8, 468. [Google Scholar] [CrossRef]
- Zinta, G.; Khan, A.; AbdElgawad, H.; Verma, V.; Srivastava, A.K. Unveiling the redox control of plant reproductive development during abiotic stress. Front. Plant Sci. 2016, 7, 700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Jiang, L.; Wang, X.; Han, H.; Chen, D.; Qiu, D.; Yang, Y. Transcriptome-wide analysis of AP2/ERF transcription factors involved in regulating taxol biosynthesis in Taxus× media. Ind. Crops Prod. 2021, 171, 113972. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.; Zhuang, W.; Zhang, F.; Shu, X.; Wang, Z.; Yang, Q. Transcriptome sequencing reveals regulatory mechanisms of taxol synthesis in Taxus wallichiana var. Mairei. Int. J. Genom. 2019, 2019, 1596895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, R.; Yu, X.; Kjær, K.H.; Rosenqvist, E.; Ottosen, C.-O.; Wu, Z. Screening and validation of tomato genotypes under heat stress using Fv/Fm to reveal the physiological mechanism of heat tolerance. Environ. Exp. Bot. 2015, 118, 1–11. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Epron, D.; Dreyer, E.; Aussenac, G. A comparison of photosynthetic responses to water stress in seedlings from 3 oak species: Quercus petraea (Matt) Liebl, Q rubra L and Q cerris L. In Annales des Sciences Forestières; EDP Sciences: Les Ulis, France, 1993; pp. 48s–60s. [Google Scholar]
- Borek, M.; Bączek-Kwinta, R.; Rapacz, M. Photosynthetic activity of variegated leaves of Coleus× hybridus hort. cultivars characterised by chlorophyll fluorescence techniques. Photosynthetica 2016, 54, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Buschmann, C.; Lichtenthaler, H.K. Principles and characteristics of multi-colour fluorescence imaging of plants. J. Plant Physiol. 1998, 152, 297–314. [Google Scholar] [CrossRef]
- Buschmann, C.; Langsdorf, G.; Lichtenthaler, H.K. 4.2 blue, green, red, and far-red fluorescence signatures of plant tissues, their multicolor fluorescence imaging, and application for agrofood assessment. In Optical Monitoring of Fresh and Processed Agricultural Crops; CRC Press: Boca Raton, FL, USA, 2008; p. 272. [Google Scholar]
- Miyata, K.; Noguchi, K.; Terashima, I. Cost and benefit of the repair of photodamaged photosystem II in spinach leaves: Roles of acclimation to growth light. Photosynth. Res. 2012, 113, 165–180. [Google Scholar] [CrossRef]
- Yokono, M.; Akimoto, S.; Tanaka, A. Seasonal changes of excitation energy transfer and thylakoid stacking in the evergreen tree Taxus cuspidata: How does it divert excess energy from photosynthetic reaction center? Biochim. Biophys. Acta (BBA)-Bioenerg. 2008, 1777, 379–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, H.Y.; Bassi, R. Carotenoids: Localization and function. In Oxygenic Photosynthesis: The Light Reactions; Springer: Berlin/Heidelberg, Germany, 1996; pp. 539–563. [Google Scholar]
- Mortain-Bertrand, A.; Stammitti, L.; Telef, N.; Colardelle, P.; Brouquisse, R.; Rolin, D.; Gallusci, P. Effects of exogenous glucose on carotenoid accumulation in tomato leaves. Physiol. Plant. 2008, 134, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Oukarroum, A.; El Madidi, S.; Strasser, R.J. Differential heat sensitivity index in barley cultivars (Hordeum vulgare L.) monitored by chlorophyll a fluorescence OKJIP. Plant Physiol. Biochem. 2016, 105, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Shan, Y.; Kochian, L.; Strasser, R.J.; Chen, G. Photochemical properties in flag leaves of a super-high-yielding hybrid rice and a traditional hybrid rice (Oryza sativa L.) probed by chlorophyll a fluorescence transient. Photosynth. Res. 2015, 126, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, J.; Zhang, M.; Strasser, R.J.; Qiang, S. Classification and characteristics of heat tolerance in Ageratina adenophora populations using fast chlorophyll a fluorescence rise OJIP. Environ. Exp. Bot. 2016, 122, 126–140. [Google Scholar] [CrossRef]
- Wang, T.; Ma, Y.; Zhou, Y.; Li, L.; Xu, D.; Luo, S.; Zhuang, W.; Zhang, W.; Xie, Y. Physiological and proteomic analyses of Malus crabapples exposed to long-term warming and short-term heat shock treatments reveal the response characteristics of photosynthetic apparatus. Sci. Hortic. 2023, 308, 111565. [Google Scholar] [CrossRef]
- Savitch, L.V.; Leonardos, E.D.; Krol, M.; Jansson, S.; Grodzinski, B.; Huner, N.; Öquist, G. Two different strategies for light utilization in photosynthesis in relation to growth and cold acclimation. Plant Cell Environ. 2002, 25, 761–771. [Google Scholar] [CrossRef]
- Bąba, W.; Kalaji, H.M.; Kompała-Bąba, A.; Goltsev, V. Acclimatization of photosynthetic apparatus of tor grass (Brachypodium pinnatum) during expansion. PLoS ONE 2016, 11, e0156201. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Chen, Z.; Yu, H.; Shang, H. Differential responses in non-structural carbohydrates of Machilus ichangensis Rehd. et Wils. and Taxus wallichiana Zucc. Var. chinensis (Pilg.) florin seedlings to elevated Ozone. Forests 2017, 8, 323. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Wei, Y.; Guo, D.; Sun, B.; Wang, X.; Liu, C. Effects of different breaking dormancy ways on the photosynthetic characteristics and activities of protective enzymes of ‘misty’blueberry leaves. Sci. Agric. Sin. 2015, 48, 4517–4528. [Google Scholar]
- An, M.; Wang, H.; Fan, H.; Ippolito, J.; Meng, C.; Li, Y.; Wang, K.; Wei, C. Effects of modifiers on the growth, photosynthesis, and antioxidant enzymes of cotton under cadmium toxicity. J. Plant Growth Regul. 2019, 38, 1196–1205. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Extraction of phtosynthetic tissues: Chlorophylls and carotenoids. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.2.1–F4.2.6. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Yemm, E.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
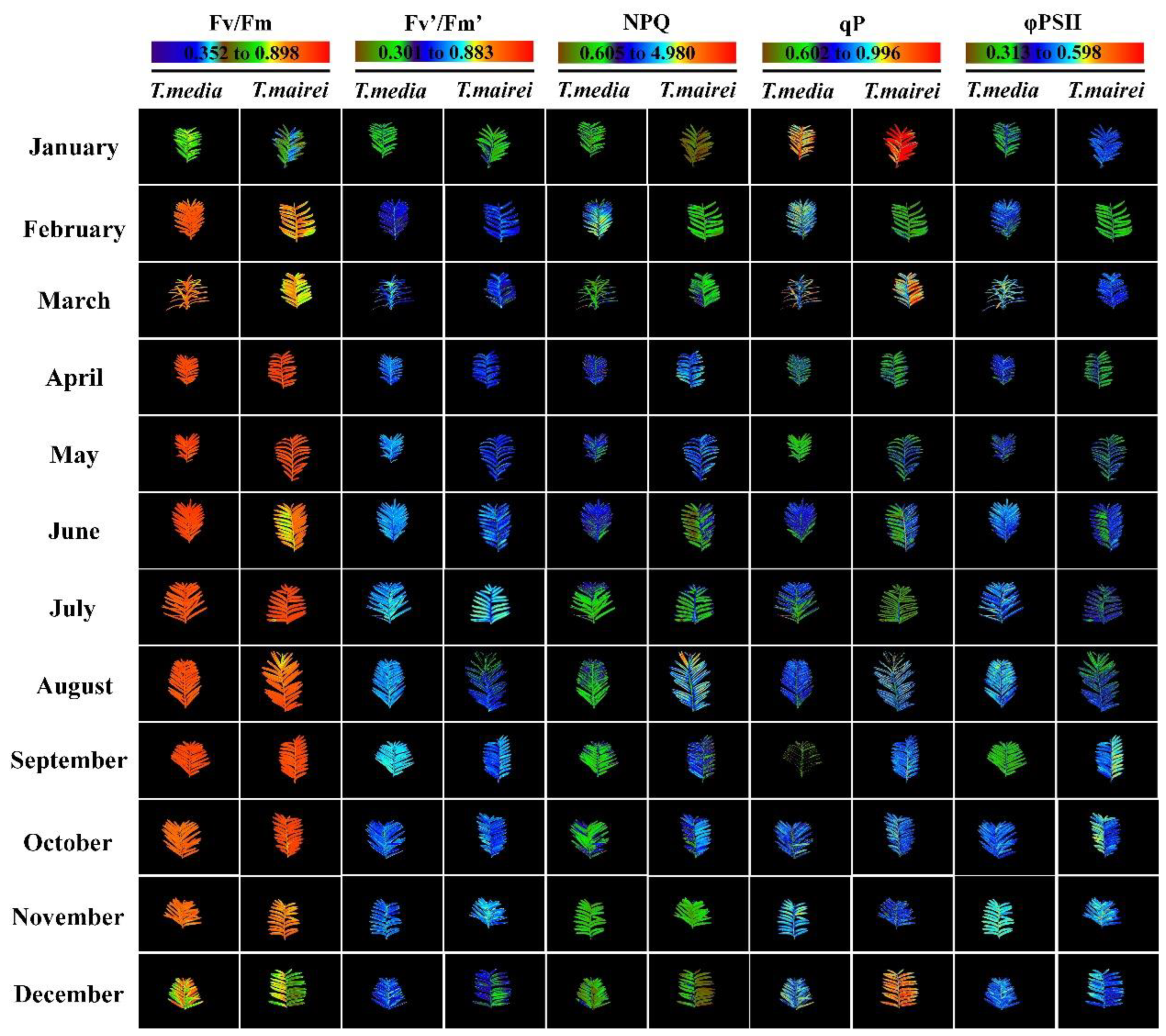



| Fv/Fm | Fv′/Fm′ | NPQ | qP | φPSII | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T. media | T. mairei | T. media | T. mairei | T. media | T. mairei | T. media | T. mairei | T. media | T. mairei | |
| January | 0.67 ± 0.03 * | 0.60 ± 0.02 | 0.50 ± 0.05 | 0.45 ± 0.02 | 1.22 ± 0.12 * | 0.88 ± 0.17 | 0.86 ± 0.05 * | 0.96 ± 0.02 | 0.42 ± 0.03 | 0.45 ± 0.01 |
| February | 0.81 ± 0.02 * | 0.75 ± 0.01 | 0.56 ± 0.03 | 0.57 ± 0.03 | 2.53 ± 0.50 * | 1.40 ± 0.22 | 0.79 ± 0.03 | 0.75 ± 0.06 | 0.44 ± 0.01 | 0.42 ± 0.04 |
| March | 0.78 ± 0.01 * | 0.70 ± 0.03 | 0.59 ± 0.04 * | 0.48 ± 0.02 | 1.79 ± 0.30 | 1.66 ± 0.17 | 0.80 ± 0.04 * | 0.91 ± 0.03 | 0.47 ± 0.01 * | 0.44 ± 0.01 |
| April | 0.83 ± 0.02 | 0.82 ± 0.01 | 0.61 ± 0.03 | 0.57 ± 0.02 | 2.15 ± 0.19 | 2.60 ± 0.29 | 0.70 ± 0.04 | 0.72 ± 0.01 | 0.42 ± 0.01 | 0.41 ± 0.02 |
| May | 0.84 ± 0.01 | 0.82 ± 0.01 | 0.61 ± 0.01 * | 0.57 ± 0.02 | 2.38 ± 0.11 | 2.46 ± 0.27 | 0.69 ± 0.01 | 0.73 ± 0.02 | 0.42 ± 0.01 | 0.41 ± 0.01 |
| June | 0.83 ± 0.01 * | 0.77 ± 0.03 | 0.62 ± 0.01 * | 0.57 ± 0.02 | 2.21 ± 0.28 | 1.80 ± 0.59 | 0.75 ± 0.03 | 0.74 ± 0.06 | 0.46 ± 0.02 | 0.42 ± 0.02 |
| July | 0.81 ± 0.01 | 0.82 ± 0.01 | 0.62 ± 0.01 | 0.58 ± 0.04 | 1.79 ± 0.12 * | 2.41 ± 0.23 | 0.73 ± 0.01 | 0.71 ± 0.05 | 0.45 ± 0.01 * | 0.41 ± 0.01 |
| August | 0.82 ± 0.02 | 0.79 ± 0.02 | 0.61 ± 0.01 * | 0.56 ± 0.02 | 1.97 ± 0.41 | 2.24 ± 0.66 | 0.77 ± 0.01 | 0.78 ± 0.06 | 0.46 ± 0.01 * | 0.43 ± 0.02 |
| September | 0.81 ± 0.03 | 0.83 ± 0.01 | 0.61 ± 0.03 | 0.58 ± 0.01 | 1.89 ± 0.14 * | 2.61 ± 0.47 | 0.65 ± 0.05 * | 0.77 ± 0.02 | 0.39 ± 0.04 | 0.45 ± 0.02 |
| October | 0.76 ± 0.02 | 0.79 ± 0.02 | 0.59 ± 0.02 | 0.58 ± 0.01 | 1.85 ± 0.26 * | 2.46 ± 0.28 | 0.77 ± 0.02 | 0.80 ± 0.06 | 0.46 ± 0.01 | 0.46 ± 0.02 |
| November | 0.75 ± 0.01 | 0.74 ± 0.02 | 0.60 ± 0.02 * | 0.54 ± 0.04 | 1.57 ± 0.09 * | 2.25 ± 0.25 | 0.76 ± 0.02 * | 0.84 ± 0.02 | 0.46 ± 0.01 | 0.46 ± 0.03 |
| December | 0.71 ± 0.03 | 0.70 ± 0.05 | 0.56 ± 0.02 | 0.55 ± 0.05 | 1.24 ± 0.16 | 1.35 ± 0.17 | 0.81 ± 0.03 | 0.83 ± 0.03 | 0.46 ± 0.01 | 0.45 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Li, L.; Qin, Y.; Lu, B.; Xu, D.; Zhuang, W.; Shu, X.; Zhang, F.; Wang, N.; Wang, Z. Effects of Seasonal Changes on Chlorophyll Fluorescence and Physiological Characteristics in the Two Taxus Species. Plants 2023, 12, 2636. https://doi.org/10.3390/plants12142636
Wang T, Li L, Qin Y, Lu B, Xu D, Zhuang W, Shu X, Zhang F, Wang N, Wang Z. Effects of Seasonal Changes on Chlorophyll Fluorescence and Physiological Characteristics in the Two Taxus Species. Plants. 2023; 12(14):2636. https://doi.org/10.3390/plants12142636
Chicago/Turabian StyleWang, Tao, Lingyu Li, Yalong Qin, Bo Lu, Donghuan Xu, Weibing Zhuang, Xiaochun Shu, Fengjiao Zhang, Ning Wang, and Zhong Wang. 2023. "Effects of Seasonal Changes on Chlorophyll Fluorescence and Physiological Characteristics in the Two Taxus Species" Plants 12, no. 14: 2636. https://doi.org/10.3390/plants12142636






