Impact of Exogenous Melatonin Application on Photosynthetic Machinery under Abiotic Stress Conditions
Abstract
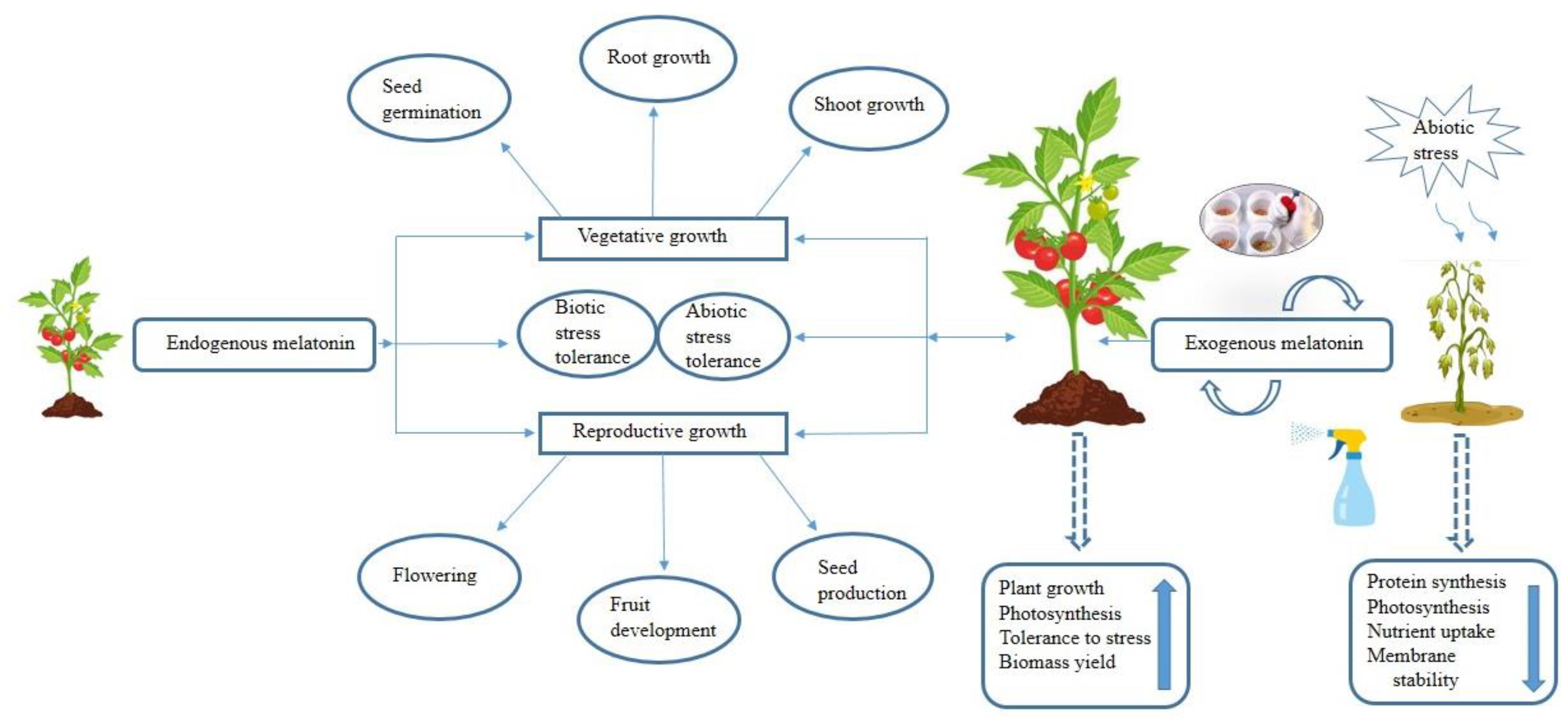
1. Introduction
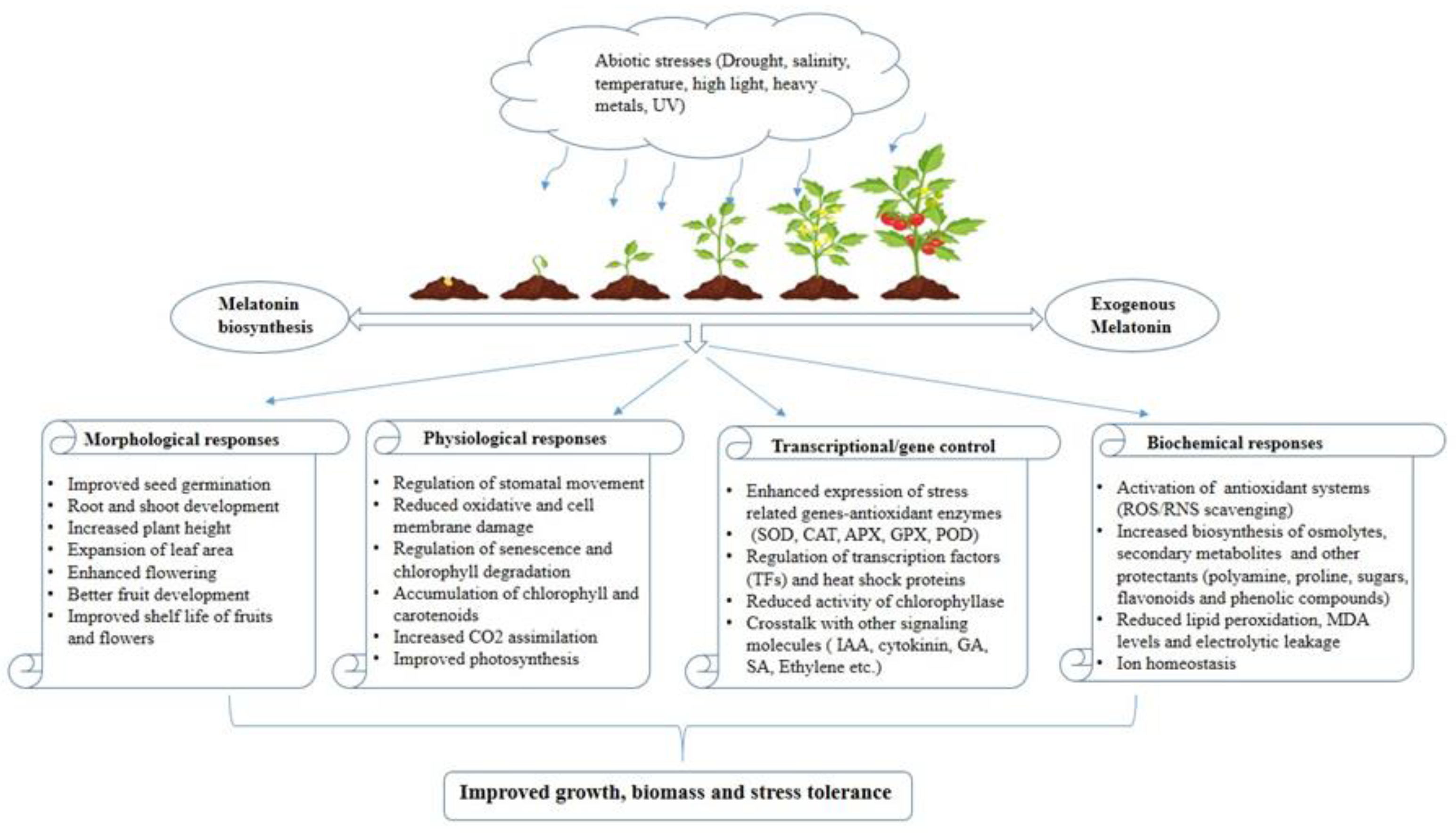
2. Impact of Abiotic Stress on Photosynthetic Components
3. Melatonin and Its Protective Effects on Photosynthetic Machinery under Various Abiotic Stresses
3.1. Drought
3.2. Salinity
3.3. Temperature
3.4. High Light Intensity
3.5. Metal Toxicity
3.6. UV Radiation
4. Conclusion and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a chemical substance or as phytomelatonin rich-extracts for use as plant protector and/or biostimulant in accordance with EC legislation. Agronomy 2019, 9, 570. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Jiao, Y.; Chen, C.; Shireen, F.; Zheng, Z.; Imtiaz, M.; Bie, Z.; Huang, Y. Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J. Plant Physiol. 2018, 220, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.; Hernandez-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef]
- Yu, R.; Zuo, T.; Diao, P.; Fu, J.; Fan, Y.; Wang, Y.; Zhao, Q.; Ma, X.; Lu, W.; Li, A.; et al. Melatonin Enhances Seed Germination and Seedling Growth of Medicago sativa Under Salinity via a Putative Melatonin Receptor MsPMTR1. Front. Plant Sci. 2021, 12, 702875. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Jiang, G.L.; Rutto, L. Melatonin priming enhances symbiotic nitrogen fixation in soybean, Glycine max L. J. Biotech Res. 2019, 10, 136–144. [Google Scholar]
- Yang, S.; Zhao, Y.; Qin, X.; Ding, C.; Chen, Y.; Tang, Z.; Huang, Y.; Reiter, R.J.; Yuan, S.; Yuan, M. New insights into the role of melatonin in photosynthesis. J. Exp. Bot. 2022, 73, 5918–5927. [Google Scholar] [CrossRef]
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Wu, H.; Qian, Y.; Zhu, X.G.; Tan, D.X.; et al. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J. Pineal Res. 2015, 59, 91–101. [Google Scholar] [CrossRef]
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A multifunctional factor in plants. Int. J. Mol. Sci. 2018, 19, 1528. [Google Scholar] [CrossRef]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in flowering, fruit set and fruit ripening. Plant Reprod. 2020, 33, 77–87. [Google Scholar] [CrossRef]
- Zeng, W.; Mostafa, S.; Lu, Z.; Jin, B. Melatonin-Mediated Abiotic Stress Tolerance in Plants. Front. Plant Sci. 2022, 13, 847175. [Google Scholar] [CrossRef]
- Varghese, N.; Alyammahi, O.; Nasreddine, S.; Alhassani, A.; Gururani, M.A. Melatonin positively influences the photosynthetic machinery and antioxidant system of avena sativa during salinity stress. Plants 2019, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Zhang, L.; Chen, S.; Gong, M.; Liu, L.; Hou, X.; Mi, Y.; Wang, X.; Wang, J.; Zhang, Y.; et al. Exogenous Melatonin Enhances the Yield and Secondary Metabolite Contents of Prunella vulgaris by Modulating Antioxidant System, Root Architecture and Photosynthetic Capacity. Plants 2023, 12, 1129. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a potent and inducible endogenous antioxidant: Synthesis and metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a regulatory hub of plant hormone levels and action in stress situations. Plant Biol. 2021, 1, 7–19. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, D.D.; Wang, J.C.; Tian, B.; Li, Y.Y.; Sun, G.Y.; Zhang, H. Exogenous melatonin alleviates NO2 damage in tobacco leaves by promoting antioxidant defense, modulating redox homeostasis, and signal transduction. J. Hazard. Mater. 2022, 424, 127265. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.U.; Ghareeb, R.Y.; Nawaz, M.; Mahmood, A.; Shah, A.N.; Abdel-Megeed, A.; Abdelsalam, N.R.; Hashem, M.; Alamri, S.; Thabit, M.A.; et al. Melatonin: A Vital Pro-Tectant for Crops against Heat Stress: Mechanisms and Prospects. Agronomy 2022, 12, 1116. [Google Scholar] [CrossRef]
- Ahmad, I.; Song, X.; Hussein Ibrahim, M.; Jamal, Y.; Younas, M.U.; Zhu, G.; Zhou, G.; Adam Ali, A.Y. The role of melatonin in plant growth and metabolism, and its interplay with nitric oxide and auxin in plants under different types of abiotic stress. Front. Plant Sci. 2023, 14, 1108507. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, X.; Li, L.; Sun, Q.; Wang, Q.; Huang, H.; Tong, Z.; Zhang, J. Melatonin mediated development and abiotic stress tolerance in plants. Front. Plant Sci. 2023, 14, 1100827. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef]
- Martinez, V.; Nieves-cordones, M.; Lopez-delacalle, M.; Rodenas, R.; Mestre, T.C.; Garcia-sanchez, F.; Rubio, F.; Nortes, P.A.; Mittler, R.; Rivero, R.M. Tolerance to Stress Combination in Tomato Plants: New Insights in the Protective Role of Melatonin. Molecules 2018, 23, 535. [Google Scholar] [CrossRef]
- Li, X.; Brestic, M.; Tan, D.X.; Zivcak, M.; Zhu, X.; Liu, S.; Song, F.; Reiter, R.J.; Liu, F. Melatonin alleviates low PS I-limited carbon assimilation under elevated CO2 and enhances the cold tolerance of offspring in chlorophyll b-deficient mutant wheat. J. Pineal Res. 2018, 64, e12453. [Google Scholar] [CrossRef]
- Gururani, M.A.; Mohanta, T.K.; Bae, H. Current understanding of the interplay between phytohormones and photosynthesis under environmental stress. Int. J. Mol. Sci. 2015, 16, 19055–19085. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Nellaepalli, S.; Zsiros, O.; Tóth, T.; Yadavalli, V.; Garab, G.; Subramanyam, R.; Kovács, L. Heat- and light-induced detachment of the light harvesting complex from isolated photosystem i supercomplexes. J. Photochem. Photobiol. B Biol. 2014, 137, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Sasi, S.; Venkatesh, J.; Daneshi, R.; Gururani, M. Photosystem II Extrinsic Proteins and Their Putative Role in Abiotic Stress Tolerance in Higher Plants. Plants 2018, 7, 100. [Google Scholar] [CrossRef]
- Sasi, S.; Kappachery, S.; Venkatesh, J.; Ghosh, R.; Gururani, M.A. Overexpression of potato StPIP2-7 ameliorates PEG-induced osmotic stress in transgenic Arabidopsis plants. Plant Growth Regul. 2023, 1–15. [Google Scholar] [CrossRef]
- Pinto, M.; Soares, C.; Martins, M.; Sousa, B.; Valente, I.; Pereira, R.; Fidalgo, F. Herbicidal effects and cellular targets of aqueous extracts from young Eucalyptus globulus labill. Leaves. Plants 2021, 10, 1159. [Google Scholar] [CrossRef] [PubMed]
- Rivas, R.; Falcão, H.M.; Ribeiro, R.V.; Machado, E.C.; Pimentel, C.; Santos, M.G. Drought tolerance in cowpea species is driven by less sensitivity of leaf gas exchange to water deficit and rapid recovery of photosynthesis after rehydration. South African J. Bot. 2016, 103, 101–107. [Google Scholar] [CrossRef]
- Chen, Y.E.; Zhang, C.M.; Su, Y.Q.; Ma, J.; Zhang, Z.W.; Yuan, M.; Zhang, H.Y.; Yuan, S. Responses of photosystem II and antioxidative systems to high light and high temperature co-stress in wheat. Environ. Exp. Bot. 2017, 135, 45–55. [Google Scholar] [CrossRef]
- Osuna, J.L.; Baldocchi, D.D.; Kobayashi, H.; Dawson, T.E. Seasonal trends in photosynthesis and electron transport during the Mediterranean summer drought in leaves of deciduous oaks. Tree Physiol. 2015, 35, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, S.; Deng, X.; Yin, L.; Xiong, B.; Wang, X. Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol. Plant. 2016, 38, 48. [Google Scholar] [CrossRef]
- Hameed, A.; Ahmed, M.Z.; Hussain, T.; Aziz, I.; Ahmad, N.; Gul, B.; Nielsen, B.L. Effects of salinity stress on chloroplast structure and function. Cells 2021, 10, 2023. [Google Scholar] [CrossRef]
- Mohamed, I.A.A.; Shalby, N.; Bai, C.; Qin, M.; Agami, R.A.; Jie, K.; Wang, B.; Zhou, G. Stomatal and photosynthetic traits are associated with investigating sodium chloride tolerance of Brassica napus L. Cultivars. Plants 2020, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Maximova, E.; Fuggi, A.; Carillo, P. Durum wheat roots adapt to salinity remodeling the cellular content of nitrogen metabolites and sucrose. Front. Plant Sci. 2017, 7, 2035. [Google Scholar] [CrossRef]
- Bacu, A.; Ibro, V.; Nushi, M. Compared salt tolerance of five local wheat (Triticum aestivum L.) cultivars of Albania based on morphology, pigment synthesis and glutathione content. Eurobiotech J. 2020, 4, 42–52. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, Q.; Ding, C.; Huang, Y.; Liao, J.; Chen, T.; Feng, S.; Zhou, L.; Zhang, Z.; Chen, Y.; et al. Effect of low temperature on chlorophyll biosynthesis and chloroplast biogenesis of rice seedlings during greening. Int. J. Mol. Sci. 2020, 21, 1390. [Google Scholar] [CrossRef]
- Lípová, L.; Krchňák, P.; Komenda, J.; Ilík, P. Heat-induced disassembly and degradation of chlorophyll-containing protein complexes in vivo. Biochim. Biophys. Acta-Bioenerg. 2010, 1797, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Abdulmajeed, A.M.; Derby, S.R.; Strickland, S.K.; Qaderi, M.M. Interactive effects of temperature and UVB radiation on methane emissions from different organs of pea plants grown in hydroponic system. J. Photochem. Photobiol. B Biol. 2017, 166, 193–201. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and Responses of Chloroplasts to Heat Stress in Plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef]
- Pospíšil, P.; Yamamoto, Y. Damage to photosystem II by lipid peroxidation products. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Sun, A.H.; Chen, H.L.; Yang, F.S.; Pan, J.L.; Guan, M.Y. Effects of chilling and high temperatures on photosynthesis and chlorophyll fluorescence in leaves of watermelon seedlings. Biol. Plant. 2016, 60, 148–154. [Google Scholar] [CrossRef]
- Hlaváčová, M.; Klem, K.; Rapantová, B.; Novotná, K.; Urban, O.; Hlavinka, P.; Smutná, P.; Horáková, V.; Škarpa, P.; Pohanková, E.; et al. Interactive effects of high temperature and drought stress during stem elongation, anthesis and early grain filling on the yield formation and photosynthesis of winter wheat. F. Crop. Res. 2018, 221, 182–195. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, M.P.; Kaur, S.; Bhardwaj, R.; Zheng, B.; Sharma, A. Modulation of the Functional Components of Growth, Photosynthesis, and Anti-Oxidant Stress Markers in Cadmium Exposed Brassica juncea L. Plants 2019, 8, 260. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Jasrotia, S.; Zheng, B.; Yuan, H.; Yan, D. Chromium bioaccumulation and its impacts on plants: An overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead uptake, toxicity, and detoxification in plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.H.; Ahmad, H.; Li, F.B. Mechanisms Regulating the Dynamics of Photosynthesis Under Abiotic Stresses. Front. Plant Sci. 2021, 11, 615942. [Google Scholar] [CrossRef]
- Rochaix, J.-D. Regulation and dynamics of the light-harvesting system. Annu. Rev. Plant Biol. 2014, 65, 287–309. [Google Scholar] [CrossRef]
- Fu, Y.; Li, H.Y.; Yu, J.; Liu, H.; Cao, Z.Y.; Manukovsky, N.S.; Liu, H. Interaction effects of light intensity and nitrogen concentration on growth, photosynthetic characteristics and quality of lettuce (Lactuca sativa L. Var. youmaicai). Sci. Hortic. 2017, 214, 51–57. [Google Scholar] [CrossRef]
- Faseela, P.; Puthur, J.T. Chlorophyll a fluorescence changes in response to short and long term high light stress in rice seedlings. Indian J. Plant Physiol. 2017, 22, 30–33. [Google Scholar] [CrossRef]
- Sato, R.; Ito, H.; Tanaka, A. Chlorophyll b degradation by chlorophyll b reductase under high-light conditions. Photosynth. Res. 2015, 126, 249–259. [Google Scholar] [CrossRef]
- Salama, H.M.H.; Al Watban, A.A.; Al-Fughom, A.T. Effect of ultraviolet radiation on chlorophyll, carotenoid, protein and proline contents of some annual desert plants. Saudi J. Biol. Sci. 2011, 18, 79–86. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Sztatelman, O.; Grzyb, J.; Gabryś, H.; Banaś, A.K. The effect of UV-B on Arabidopsis leaves depends on light conditions after treatment. BMC Plant Biol. 2015, 15, 281. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, D.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef]
- Kabiri, R.; Hatami, A.; Oloumi, H.; Naghizadeh, M.; Nasibi, F.; Tahmasebi, Z. Foliar application of melatonin induces tolerance to drought stress in Moldavian balm plants (Dracocephalum moldavica) through regulating the antioxidant system. Folia Hortic. 2018, 30, 155–167. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Zhou, Z.; Cruz, M.H.C.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: Assisting plants to survive and thrive. Molecules 2015, 20, 7396–7437. [Google Scholar] [CrossRef]
- Yang, X.L.; Xu, H.; Li, D.; Gao, X.; Li, T.L.; Wang, R. Effect of melatonin priming on photosynthetic capacity of tomato leaves under low-temperature stress. Photosynthetica 2018, 56, 884–892. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Y.; Zhang, X.; Du, H.; Xu, B.; Huang, B. Melatonin suppression of heat-induced leaf senescence involves changes in abscisic acid and cytokinin biosynthesis and signaling pathways in perennial ryegrass (Lolium perenne L.). Environ. Exp. Bot. 2017, 138, 36–45. [Google Scholar] [CrossRef]
- Ahmad, S.; Muhammad, I.; Wang, G.Y.; Zeeshan, M.; Yang, L.; Ali, I.; Zhou, X.B. Ameliorative effect of melatonin improves drought tolerance by regulating growth, photosynthetic traits and leaf ultrastructure of maize seedlings. BMC Plant Biol. 2021, 21, 368. [Google Scholar] [CrossRef]
- Imran, M.; Mpovo, C.L.; Aaqil Khan, M.; Shaffique, S.; Ninson, D.; Bilal, S.; Khan, M.; Kwon, E.H.; Kang, S.M.; Yun, B.W.; et al. Synergistic Effect of Melatonin and Lysinibacillus fusiformis L. (PLT16) to Mitigate Drought Stress via Regulation of Hormonal, Antioxidants System, and Physio-Molecular Responses in Soybean Plants. Int. J. Mol. Sci. 2023, 24, 8489. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.N.; Ávila, R.G.; de Souza, K.R.D.; Azevedo, L.M.; Alves, J.D. Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica L. plants. Agric. Water Manag. 2019, 211, 37–47. [Google Scholar] [CrossRef]
- Liang, D.; Ni, Z.; Xia, H.; Xie, Y.; Lv, X.; Wang, J.; Lin, L.; Deng, Q.; Luo, X. Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Sci. Hortic. 2019, 246, 34–43. [Google Scholar] [CrossRef]
- Karaca, P.; Cekic, F.Ö. Exogenous melatonin-stimulated defense responses in tomato plants treated with polyethylene glycol. Int. J. Veg. Sci. 2019, 25, 601–609. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Cherono, S.; Ntini, C.; Wassie, M.; Dulal Mollah, M.; Belal, M.A.; Ogutu, C.; Han, Y. Exogenous application of melatonin improves drought tolerance in coffee by regulating photosynthetic efficiency and oxidative damage. J. Am. Soc. Hortic. Sci. 2021, 146, 24–32. [Google Scholar] [CrossRef]
- Alyammahi, O.; Gururani, M.A. Chlorophyll-a fluorescence analysis reveals differential response of photosynthetic machinery in melatonin-treated oat plants exposed to osmotic stress. Agronomy 2020, 10, 1520. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.E.; Zhao, Y.Q.; Ding, C.B.; Liao, J.Q.; Hu, C.; Zhou, L.J.; Zhang, Z.W.; Yuan, S.; Yuan, M. Exogenous melatonin alleviates oxidative damages and protects photosystem ii in maize seedlings under drought stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, D.; Wang, H.; Li, Y.; Li, R. Physiological response of plants to polyethylene glycol (PEG-6000) by exogenous melatonin application in wheat. Zemdirbyste 2017, 104, 219–228. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crops Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Imran, M.; Latif Khan, A.; Shahzad, R.; Aaqil Khan, M.; Bilal, S.; Khan, A.; Kang, S.M.; Lee, I.J. Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. AoB Plants 2021, 13, plab026. [Google Scholar] [CrossRef]
- Luo, Y.; Hu, T.; Huo, Y.; Wang, L.; Zhang, L.; Yan, R. Effects of Exogenous Melatonin on Chrysanthemum Physiological Characteristics and Photosynthesis under Drought Stress. Horticulturae 2023, 9, 106. [Google Scholar] [CrossRef]
- Sadak, M.S.; Abdalla, A.M.; Abd Elhamid, E.M.; Ezzo, M.I. Role of melatonin in improving growth, yield quantity and quality of Moringa oleifera L. plant under drought stress. Bull. Natl. Res. Cent. 2020, 44, 18. [Google Scholar] [CrossRef]
- Eisa, E.A.; Honfi, P.; Tilly-Mándy, A.; Gururani, M.A. Exogenous Application of Melatonin Alleviates Drought Stress in Ranunculus asiaticus by Improving Its Morphophysiological and Biochemical Attributes. Horticulturae 2023, 9, 262. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Zhu, Y.; Zheng, W.; Li, M.; Hu, J.; Fei, Y.; Zhu, S. Exogenous application of melatonin to mitigate drought stress-induced oxidative damage in Phoebe sheareri seedlings. PeerJ 2023, 11, e15159. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Liu, J.L.; Wang, W.X.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27. [Google Scholar] [CrossRef]
- Yin, Z.; Lu, J.; Meng, S.; Liu, Y.; Mostafa, I.; Qi, M.; Li, T. Exogenous melatonin improves salt tolerance in tomato by regulating photosynthetic electron flux and the ascorbate–glutathione cycle. J. Plant Interact. 2019, 14, 453–463. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Alsubaie, Q.D.; Ali, H.M. Melatonin and Gibberellic Acid Promote Growth and Chlorophyll Biosynthesis by Regulating Antioxidant and Methylglyoxal Detoxification System in Tomato Seedlings Under Salinity. J. Plant Growth Regul. 2020, 39, 1488–1502. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, H.; Cao, K.; Hu, L.; Du, T.; Baluška, F.; Zou, Z. Beneficial roles of melatonin on redox regulation of photosynthetic electron transport and synthesis of D1 protein in tomato seedlings under salt stress. Front. Plant Sci. 2016, 7, 1823. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, L.; Wang, X.; Wang, Z.; Zhang, H.; Chen, J.; Liu, X.; Wang, Y.; Li, C. Beneficial Effects of Exogenous Melatonin on Overcoming Salt Stress in Sugar Beets (Beta vulgaris L.). Plants 2021, 10, 886. [Google Scholar] [CrossRef]
- ElSayed, A.; Rafudeen, M.; Gomaa, A.; Hasanuzzaman, M. Exogenous melatonin enhances the reactive oxygen species metabolism, antioxidant defense-related gene expression, and photosynthetic capacity of Phaseolus vulgaris L. to confer salt stress tolerance. Physiol. Plant. 2021, 173, 1369–1381. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, J.; Li, W.; Ding, Y.; Zhong, Q.; Xu, X.; Wei, H.; Li, G. Exogenous melatonin alleviates salt stress by improving leaf photosynthesis in rice seedlings. Plant Physiol. Biochem. 2021, 163, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Meng, Y.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Exogenous melatonin improves the salt tolerance of cotton by removing active oxygen and protecting photosynthetic organs. BMC Plant Biol. 2021, 21, 331. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Ye, J.; Wang, B.; Ren, J.; Yin, L.; Deng, X.; Wang, S. Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Front. Plant Sci. 2018, 9, 914. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Ma, X.; Ouyang, Z.; Deng, L.; Shen, S.; Dong, X.; Du, N.; Dong, H.; Guo, Z.; et al. Melatonin Alleviates Copper Toxicity via Improving ROS Metabolism and Antioxidant Defense Response in Tomato Seedlings. Antioxidants 2022, 11, 758. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Xu, W.; Liu, A.; Chen, S. COMT1 silencing aggravates heat stress-induced reduction in photosynthesis by decreasing chlorophyll content, photosystem II activity, and electron transport efficiency in tomato. Front. Plant Sci. 2018, 9, 998. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shu, H.; Hao, Y.; Mumtaz, M.A.; Lu, X.; Wang, Z. Melatonin Affects the Photosynthetic Performance of Pepper (Capsicum annuum L.) Seedlings under Cold Stress. Antioxidants 2022, 11, 2414. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.F.; Yu, Y.; Shi, T.C.; Fu, Y.S.; Zhao, T.; Zhang, Z.W. Melatonin treatment of pre-veraison grape berries modifies phenolic components and antioxidant activity of grapes and wine. Food Sci. Technol. 2019, 39, 35–42. [Google Scholar] [CrossRef]
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin Suppressed the Heat Stress-Induced Damage in Wheat Seedlings by Modulating the Antioxidant Machinery. Plants 2020, 9, 809. [Google Scholar] [CrossRef]
- Iqbal, N.; Fatma, M.; Gautam, H.; Umar, S.; Sofo, A.; D’ippolito, I.; Khan, N.A. The crosstalk of melatonin and hydrogen sulfide determines photosynthetic performance by regulation of carbohydrate metabolism in wheat under heat stress. Plants 2021, 10, 1778. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, J.; Sun, X.; Zhu, Y.; Li, Q.; Zhang, L.; Zhao, D.; Huang, L.; Zhang, C.; Liu, Q. Exogenous Melatonin Improves the Quality Performance of Rice under High Temperature during Grain Filling. Agronomy 2022, 12, 949. [Google Scholar] [CrossRef]
- Xu, W.; Cai, S.Y.; Zhang, Y.; Wang, Y.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Reiter, R.J.; et al. Melatonin enhances thermotolerance by promoting cellular protein protection in tomato plants. J. Pineal Res. 2016, 61, 457–469. [Google Scholar] [CrossRef]
- Jahan, M.S.; Shu, S.; Wang, Y.; Hasan, M.M.; El-Yazied, A.A.; Alabdallah, N.M.; Hajjar, D.; Altaf, M.A.; Sun, J.; Guo, S. Melatonin Pretreatment Confers Heat Tolerance and Repression of Heat-Induced Senescence in Tomato Through the Modulation of ABA- and GA-Mediated Pathways. Front. Plant Sci. 2021, 12, 650955. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Meng, S.; Wang, B.; Zhao, S.; Liu, Y.; Qi, M.; Wang, Z.; Yin, Z.; Li, T. Exogenous melatonin enhances tomato heat resistance by regulating photosynthetic electron flux and maintaining ROS homeostasis. Plant Physiol. Biochem. 2023, 196, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Yang, S.J.; Chen, Y.Y. Effects of melatonin on photosynthetic performance and antioxidants in melon during cold and recovery. Biol. Plant. 2017, 61, 571–578. [Google Scholar] [CrossRef]
- Han, Q.H.; Huang, B.; Ding, C.B.; Zhang, Z.W.; Chen, Y.E.; Hu, C.; Zhou, L.J.; Huang, Y.; Liao, J.Q.; Yuan, S.; et al. Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef]
- Zhou, R.; Wan, H.; Jiang, F.; Li, X.; Yu, X.; Rosenqvist, E.; Ottosen, C.O. The alleviation of photosynthetic damage in tomato under drought and cold stress by high CO2 and melatonin. Int. J. Mol. Sci. 2020, 21, 5587. [Google Scholar] [CrossRef]
- Chang, J.; Guo, Y.; Zhang, Z.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X.; Li, H. CBF-responsive pathway and phytohormones are involved in melatonin-improved photosynthesis and redox homeostasis under aerial cold stress in watermelon. Acta Physiol. Plant. 2020, 42, 159. [Google Scholar] [CrossRef]
- Yang, S.J.; Huang, B.; Zhao, Y.Q.; Hu, D.; Chen, T.; Ding, C.B.; Chen, Y.E.; Yuan, S.; Yuan, M. Melatonin Enhanced the Tolerance of Arabidopsis thaliana to High Light Through Improving Anti-oxidative System and Photosynthesis. Front. Plant Sci. 2021, 12, 752584. [Google Scholar] [CrossRef]
- Ksas, B.; Becuwe, N.; Chevalier, A.; Havaux, M. Plant tolerance to excess light energy and photooxidative damage relies on plastoquinone biosynthesis. Sci. Rep. 2015, 5, 10919. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Ahammed, G.J.; Yin, L.; Shi, K.; Xia, X.; Zhou, Y.; Yu, J.; Zhou, J. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 2015, 6, 601. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Zhang, J.; Gong, X.; Zhang, Z.; Sun, J.; Chen, X.; Wang, Y. Exogenous Melatonin Improves Physiological Characteristics and Promotes Growth of Strawberry Seedlings Under Cadmium Stress. Hortic. Plant J. 2021, 7, 13–22. [Google Scholar] [CrossRef]
- Zuo, Z.; Sun, L.; Wang, T.; Miao, P.; Zhu, X.; Liu, S.; Song, F.; Mao, H.; Li, X. Melatonin improves the photosynthetic carbon assimilation and antioxidant capacity in wheat exposed to nano-zno stress. Molecules 2017, 22, 1727. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Guo, S.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y.; Ahammed, G.J.; Kabir, K.; Roy, R. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol. Environ. Saf. 2020, 197, 110593. [Google Scholar] [CrossRef]
- Ayyaz, A.; Amir, M.; Umer, S.; Iqbal, M.; Bano, H.; Gul, H.S.; Noor, Y.; Kanwal, A.; Khalid, A.; Javed, M.; et al. Melatonin induced changes in photosynthetic efficiency as probed by OJIP associated with improved chromium stress tolerance in canola (Brassica napus L.). Heliyon 2020, 6, e04364. [Google Scholar] [CrossRef]
- Yang, X.; Ren, J.; Lin, X.; Yang, Z.; Deng, X.; Ke, Q. Melatonin Alleviates Chromium Toxicity in Maize by Modulation of Cell Wall Polysaccharides Biosynthesis, Glutathione Metabolism, and Antioxidant Capacity. Int. J. Mol. Sci. 2023, 24, 3816. [Google Scholar] [CrossRef]
- Wei, Z.; Li, C.; Gao, T.; Zhang, Z.; Liang, B.; Lv, Z.; Zou, Y.; Ma, F. Melatonin increases the performance of Malus hupehensis after UV-B exposure. Plant Physiol. Biochem. 2019, 139, 630–641. [Google Scholar] [CrossRef]
- Haskirli, H.; Yilmaz, O.; Ozgur, R.; Uzilday, B.; Turkan, I. Melatonin mitigates UV-B stress via regulating oxidative stress response, cellular redox and alternative electron sinks in Arabidopsis thaliana. Phytochemistry 2021, 182, 112592. [Google Scholar] [CrossRef]
- Zhao, Z.; Yun, C.; Gu, L.; Liu, J.; Yao, L.; Wang, W.; Wang, H. Melatonin enhances biomass, phenolic accumulation, and bioactivities of rosemary (Rosmarinus officinalis) in vitro shoots under UV-B stress. Physiol. Plant. 2023, 175, e13956. [Google Scholar] [CrossRef]
- Zuo, B.; Zheng, X.; He, P.; Wang, L.; Lei, Q.; Feng, C.; Zhou, J.; Li, Q.; Han, Z.; Kong, J. Overexpression of MzASMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis thaliana plants. J. Pineal Res. 2014, 57, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Fan, X.; Zhang, Y.; Jiang, J.; Sun, L.; Rahman, F.U.; Liu, C. VvSNAT1 overexpression enhances melatonin production and salt tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2021, 166, 485–494. [Google Scholar] [CrossRef]
- Huangfu, L.; Chen, R.; Lu, Y.; Zhang, E.; Miao, J.; Zuo, Z.; Zhao, Y.; Zhu, M.; Zhang, Z.; Li, P.; et al. OsCOMT, encoding a caffeic acid O-methyltransferase in melatonin biosynthesis, increases rice grain yield through dual regulation of leaf senescence and vascular development. Plant Biotechnol. J. 2022, 20, 1122–1139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zheng, S.; Fan, W.; Zhang, M.; Xia, Z.; Chen, X.; Zhao, A. Ectopic overexpression of mulberry MnT5H2 enhances melatonin production and salt tolerance in tobacco. Front. Plant Sci. 2022, 13, 1061141. [Google Scholar] [CrossRef] [PubMed]

| Stress | Crop | Effect on Photosynthesis | Reference |
|---|---|---|---|
| Drought | Maize | Improvement of the number, length, and width of stomata, and stabilization of the chloroplast structure, increasing photosynthesis. | [62] |
| Ranunculus asiaticus | Accumulation of chlorophyll and carotenoids. | [75] | |
| Phoebe | Increase photosynthetic efficiency by enhancing the concentration of photosynthetic pigment and regulation of phytohormones such as jasmonic acid and ABA, scavenging ROS. | [76] | |
| Chrysanthemum | Improvement of photosynthetic efficiency reduces the loss of relative water content and chlorophyll in leaves, regulation of osmotic metabolism by increasing the concentration of soluble proteins and sugars. | [73] | |
| Maize | Enhances the stomatal length, width, area, and number of pores. Mitigates ROS-induced oxidative damages by increasing the photosynthetic pigments, antioxidant enzyme activities, relative water content, and osmoprotectants. | [61] | |
| Moringa | Enhances the photosynthetic pigments, phenolic contents, and IAA accumulation. Reduction of ROS accumulation by increasing the activities of APX, CAT, and SOD. | [74] | |
| Coffee | Enhances gas exchange, improves carboxylation efficiency, and chlorophyll content, and reduces damage to the photosynthetic machinery. | [63] | |
| Triticale | Increases stomatal conductance, net photosynthetic rate, transpiration rate and chlorophyll concentration. | [71] |
| Stress | Crop | Effects on Photosynthesis | Reference |
|---|---|---|---|
| Salinity | Wheat | High water status, low level of H2O2 content, and balanced [K+]/[Na+] ratio in leaves, high content of proline, protein, soluble sugars essential amino acids and Ca2+ in leaves resulting in a high photosynthetic rate. | [86] |
| Sugarbeet | Increases the level of photosynthetic net rate, chlorophyll fluorescence, and chlorophyll content. | [81] | |
| Cotton | Reduction of ROS production, increases plant biomass and chlorophylls level, preservation of mitochondria and grana lamella of chloroplasts. | [84] | |
| Rice | Upregulation of gene expression associated with antioxidant system, photosynthetic parameters, and ROS scavenging enzymes. Reduction of electrolyte leakage. | [83] | |
| Tomato | Reduction of chlorophyll degradation; alleviated PSII inhibition and OEC damage. | [78] | |
| Oats | Upregulation of genes encoding ROS scavenging enzymes, stress-responsive, NAC transcription factors, and PSII core proteins. Increases stomatal conductance and chlorophyll content. Reduced the levels of MDA and electrolyte leakage. | [13] | |
| Cucumber | Improvement of photosynthetic efficiency, inhibition of chlorophyll degradation, reduction of MDA and ROS contents. Increased the expression of antioxidant-associated genes. | [77] |
| Stress | Crop | Effects on Photosynthesis | Reference |
|---|---|---|---|
| Heat stress | Tomato | Improves photosynthetic parameters, such as net photosynthetic rate and chlorophyll fluorescence. Reduces oxidative stress to PSII. | [95] |
| Rice | Enhances the photosynthetic capacity. Promotes the fresh green appearance of leaves and higher values of photosynthesis-related parameters. | [92] | |
| Wheat | Reduces oxidative damage by lowering the TBARS and H2O2 content. Improves photosynthetic efficacy through enhancement of antioxidants. Improves photosynthetic efficacy through enhancement of antioxidants, accumulation of proline, chlorophyll and carotenoids. | [90,91] | |
| Tomato | Increases Fv/Fm ratio and chlorophyll levels. Decreases ROS generation. Reduces expression of RBOHS and chlorophyll degradation-associated genes. | [94] | |
| Cold stress | Melon | Increases concentration of antioxidant enzymes, chlorophyll content, photosynthetic rate, stomatal conductance, and maximal quantum yield of PS II. | [96] |
| Rice | Enhances net photosynthetic rate, stomatal conductance, intercellular CO2 and water use efficiency. | [97] | |
| Tomato | Improves pigment content, gas exchange elements, and chlorophyll fluorescence metrics. | [98] | |
| Pepper | Improves photochemical activity of PSII and PSI and photosynthetic enzymes. Increases the levels of chlorophyll a, chlorophyll b, and carotenoids. Enhances photosynthesis under cold stress conditions. | [88] |
| Stress | Crop | Effects on Photosynthesis | Reference |
|---|---|---|---|
| High light | Arabidopsis | Increases photosynthetic rate, chlorophyll content and reduces ROS levels. | [100] |
| Stress | Crop | Effects on Photosynthesis | Reference |
|---|---|---|---|
| Cr toxicity | Maize | Improves photosynthetic rate, chlorophyll content, and antioxidant enzyme synthesis. | [107] |
| Cr toxicity | Canola | Prevents photo-inhibition of PSII from oxidative damage. | [106] |
| Zn toxicity | Wheat | Increases photosynthetic carbon assimilation, RUBISCO and ATPase activity, and chlorophyll concentration. | [104] |
| Cd toxicity | Tomato | Increases in Fv/Fm ratio and net photosynthetic rate increase activity of H+-ATPase activity, and reduces ROS accumulation. | [102] |
| Cu toxicity | Tomato | Upregulate antioxidant-associated gene expression. | [86] |
| Ni toxicity | Tomato | Improves photosynthetic and transpiration rate, intercellular CO2 concentration, and stomatal conductance. | [105] |
| Cd toxicity | Strawberry | Increases activity of antioxidant enzymes. | [103] |
| Cr toxicity | Maize | Improves photosynthetic rate, chlorophyll content, and antioxidant enzyme synthesis. | [107] |
| Stress | Crop | Effects on Photosynthesis | Reference |
|---|---|---|---|
| UV radiation | Arabidopsis | Upregulated the genes encoding for ascorbate peroxidase and catalase, lowered lipid peroxidation and increased the Fv/Fm value | [108,109] |
| Rosemay | Increased the activity of SOD, CAT and POD, and the accumulation of total phenols | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karumannil, S.; Khan, T.A.; Kappachery, S.; Gururani, M.A. Impact of Exogenous Melatonin Application on Photosynthetic Machinery under Abiotic Stress Conditions. Plants 2023, 12, 2948. https://doi.org/10.3390/plants12162948
Karumannil S, Khan TA, Kappachery S, Gururani MA. Impact of Exogenous Melatonin Application on Photosynthetic Machinery under Abiotic Stress Conditions. Plants. 2023; 12(16):2948. https://doi.org/10.3390/plants12162948
Chicago/Turabian StyleKarumannil, Sameera, Tanveer Alam Khan, Sajeesh Kappachery, and Mayank Anand Gururani. 2023. "Impact of Exogenous Melatonin Application on Photosynthetic Machinery under Abiotic Stress Conditions" Plants 12, no. 16: 2948. https://doi.org/10.3390/plants12162948
APA StyleKarumannil, S., Khan, T. A., Kappachery, S., & Gururani, M. A. (2023). Impact of Exogenous Melatonin Application on Photosynthetic Machinery under Abiotic Stress Conditions. Plants, 12(16), 2948. https://doi.org/10.3390/plants12162948







