Influence of Intensive and Super-Intensive Olive Grove Management on Soil Quality—Nutrients Content and Enzyme Activities
Abstract
:1. Introduction
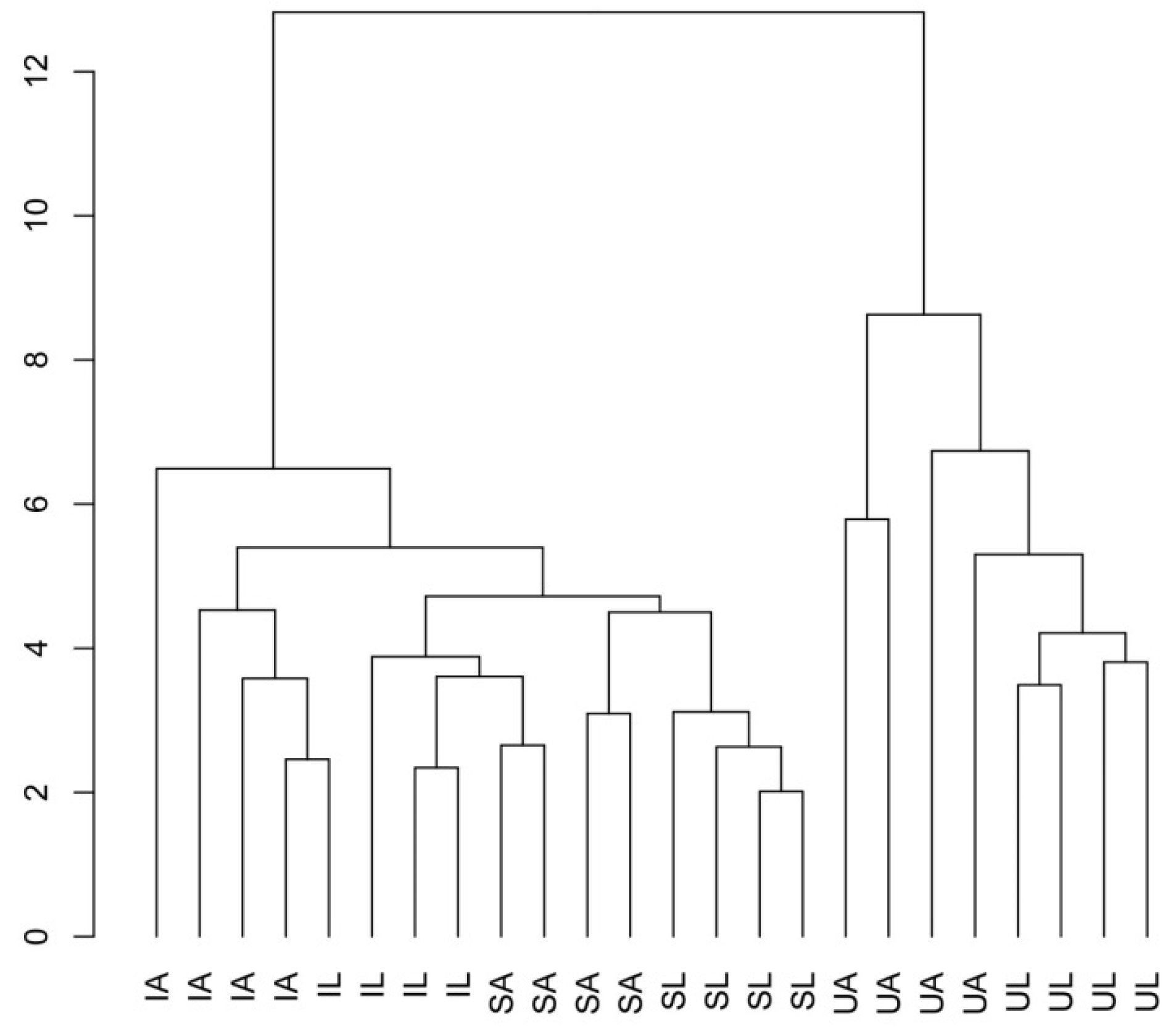
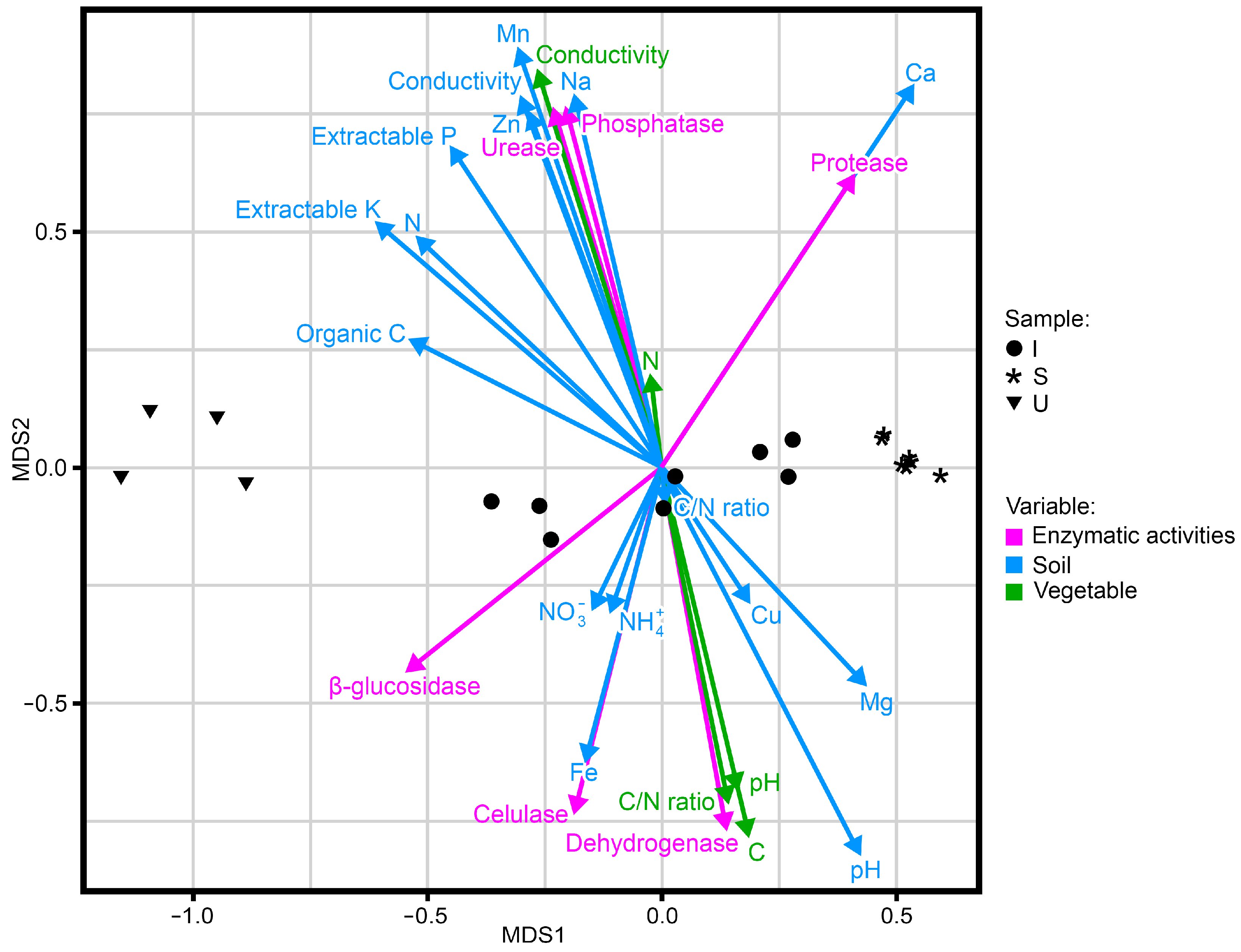
2. Results
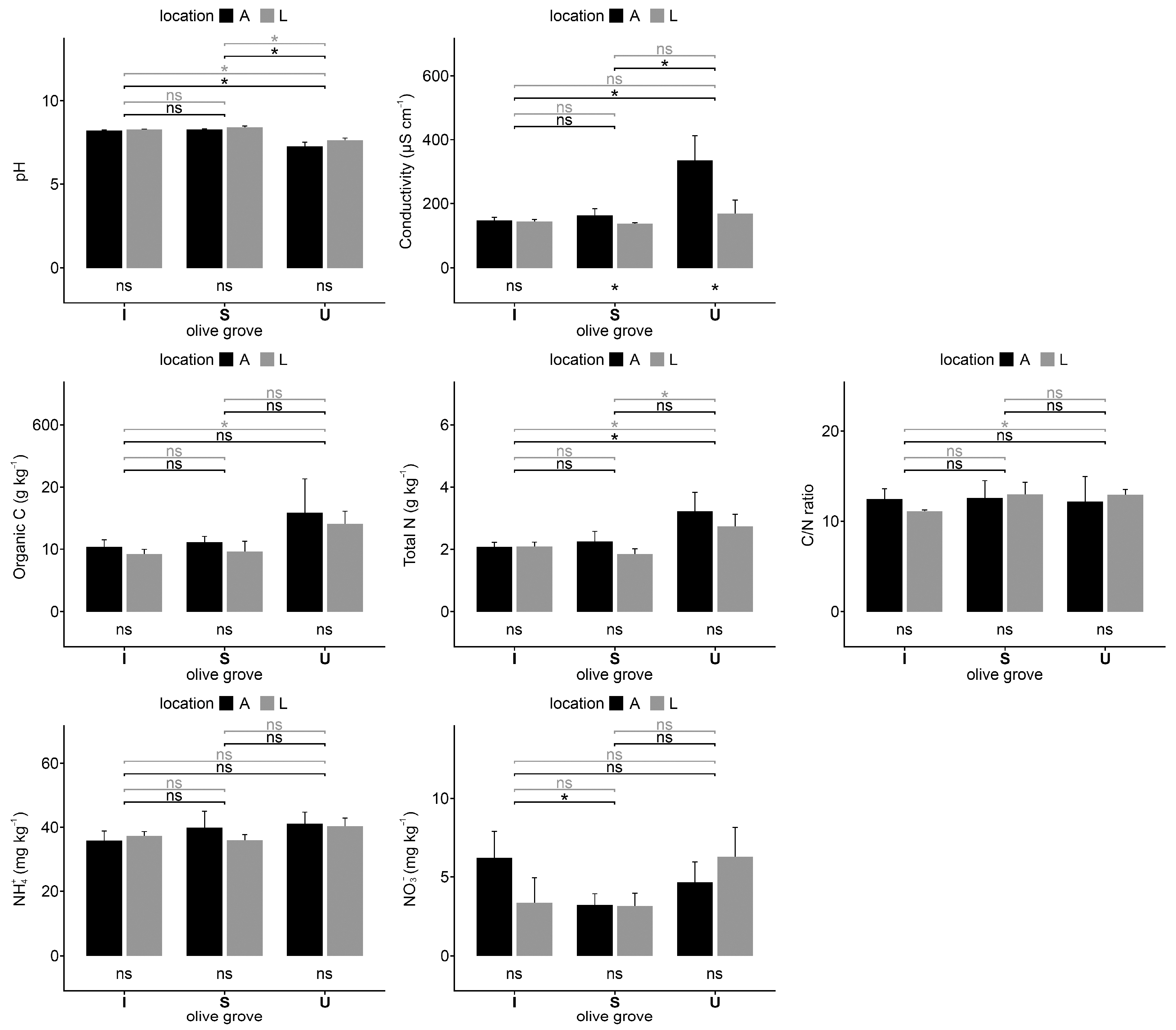
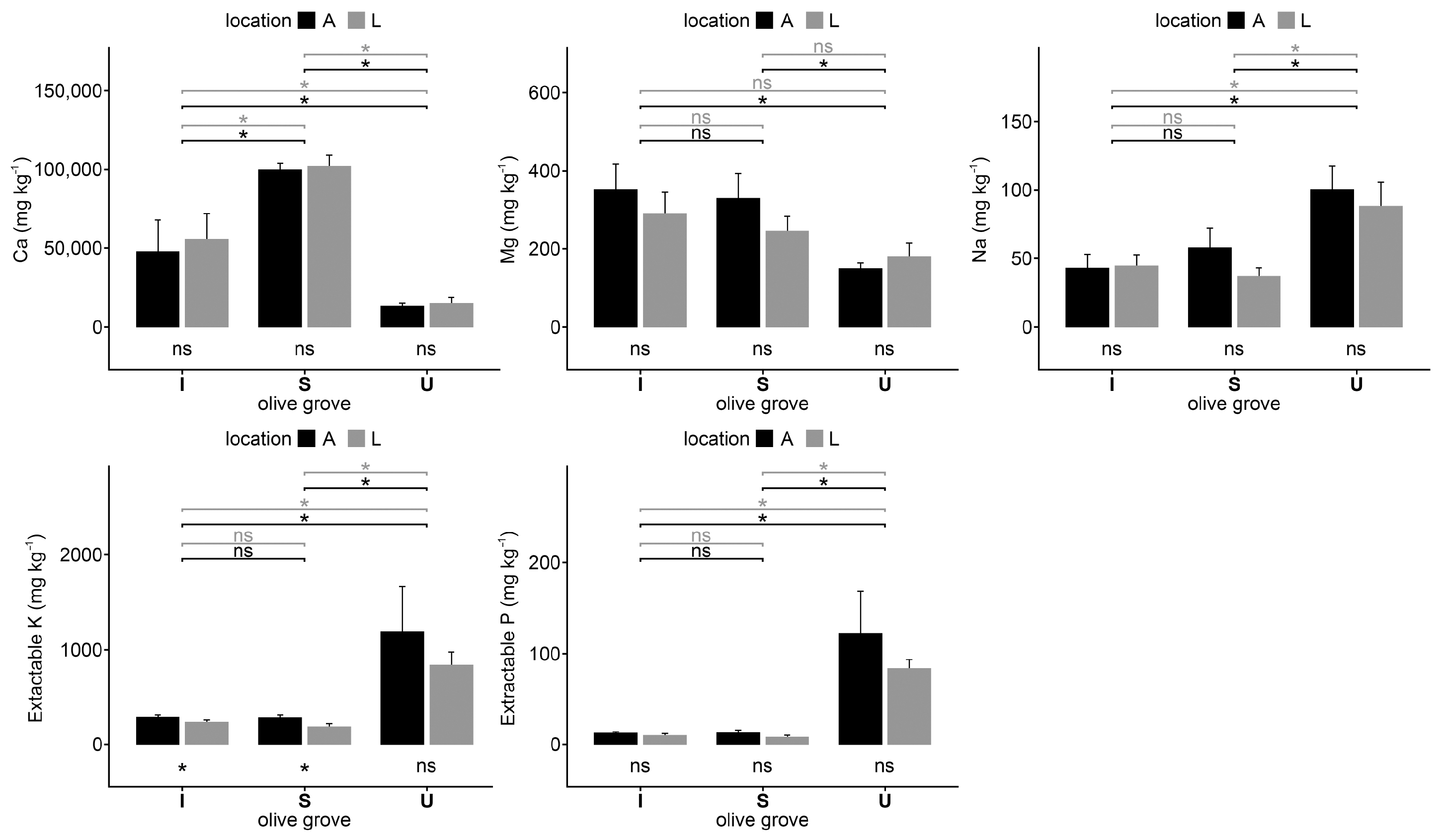
2.1. Soil Analysis
2.2. Litter and Mulch Analysis
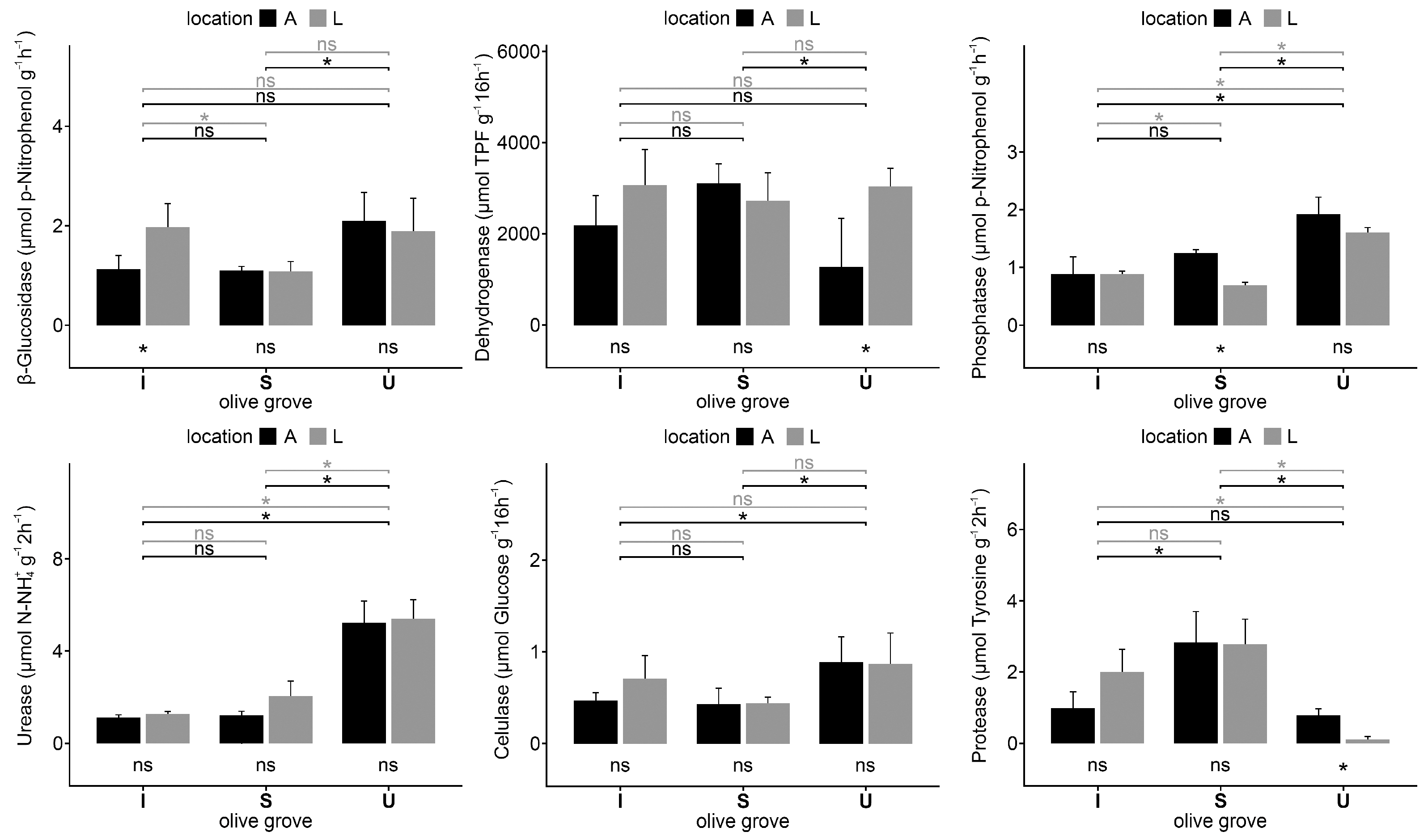
2.3. Soil Enzyme Activities
3. Discussion
4. Materials and Methods
4.1. Study Area Location and Sampling
4.2. Sampling Design
4.3. Soil Samples Analysis
4.4. Litter and Mulch Analysis
4.5. Analysis of Soil Enzymatic Activities
4.6. Statistic Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mairech, H.; López-Bernal, Á.; Moriondo, M.; Dibari, C.; Regni, L.; Proietti, P.; Villalobos, F.J.; Testi, L. Is new olive farming sustainable? A spatial comparison of productive and environmental performances between traditional and new olive orchards with the model OliveCan. Agric. Syst. 2020, 181, 102816. [Google Scholar] [CrossRef]
- Fernandez Escobar, R.; de la Rosa, R.; Leon, L.; Gomez, J.A.; Testi, F.; Orgaz, M.; Gil-Ribes, J.A.; Quesada Moraga, E.; Trapero, A. Evolution and sustainability of the olive production systems. In Present and Future of the Mediterranean Olive Sector; Arcas, N., Arroyo López, F.N., Caballero, J., D’Andria, R., Fernández, M., Fernandez Escobar, R., Garrido, A., López-Miranda, J., Msallem, M., Parras, M., et al., Eds.; CIHEAM: Zaragoza, Spain, 2013; Volume 106, pp. 11–42. [Google Scholar]
- Thoumazeau, A.; Bessou, C.; Renevier, M.S.; Trap, J.; Marichal, R.; Mareschal, L.; Decaëns, T.; Bottinelli, N.; Jaillard, B.; Che-vallier, T.; et al. Biofunctool®: A new framework to assess the impact of land management on soil quality. Part A: Concept and validation of the set of indicators. Ecol. Indic. 2019, 97, 100–110. [Google Scholar] [CrossRef]
- Loumou, A.; Giourga, C. Olive groves: “The life and identity of the Mediterranean”. Agric. Hum. Values 2003, 20, 87–95. [Google Scholar] [CrossRef]
- Nieto, O.M.; Castro, J.; Fernández, E.; Smith, P. Simulation of soil organic carbon stocks in a Mediterranean olive grove under different soil-management systems using the RothC model. Soil Use Manag. 2010, 26, 118–125. [Google Scholar] [CrossRef]
- Sofo, A.; Nuzzo, V.; Palese, A.M.; Xiloyannis, C.; Celano, G.; Zukowskyj, P.; Dichio, B. Net CO2 storage in mediterranean olive and peach orchards. Sci. Hortic. 2005, 107, 17–24. [Google Scholar] [CrossRef]
- Sofo, A.; Mininni, A.N.; Ricciuti, P. Comparing the effects of soil fauna on litter decomposition and organic matter turnover in sustainably and conventionally managed olive orchards. Geoderma 2020, 372, 114393. [Google Scholar] [CrossRef]
- Badagliacca, G.; Petrovičovà, B.; Pathan, S.I.; Roccotelli, A.; Romeo, M.; Monti, M.; Gelsomino, A. Use of solid anaerobic digestate and no-tillage practice for restoring the fertility status of two Mediterranean orchard soils with contrasting properties. Agric. Ecosyst. Environ. 2020, 300, 107010. [Google Scholar] [CrossRef]
- Yang, T.; Siddique, K.H.; Liu, K. Cropping systems in agriculture and their impact on soil health-A review. Glob. Ecol. Conserv. 2020, 23, e01118. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Ncube, B.; Meyer, A.H.; Mulidzi, R.; Lewu, F.B. Soil β-glucosidase activity, organic carbon and nutrients in plant tissue in response to cover crop species and management practices. S. Afr. J. Plant Soil 2020, 37, 202–210. [Google Scholar] [CrossRef]
- Cueff, S.; Alletto, L.; Bourdat-Deschamps, M.; Benoit, P.; Pot, V. Water and pesticide transfers in undisturbed soil columns sampled from a Stagnic Luvisol and a Vermic Umbrisol both cultivated under conventional and conservation agriculture. Geoderma 2020, 377, 114590. [Google Scholar] [CrossRef]
- Zipori, I.; Erel, R.; Yermiyahu, U.; Ben-Gal, A.; Dag, A. Sustainable Management of Olive Orchard Nutrition: A Review. Agriculture 2020, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Blevins, R.L. Idoneidad del Suelo Para el Laboreo Nulo; Agricultura sin laboreo, Ed.; Bellaterra. SA: Barcelona, Spain, 1986; pp. 44–68. [Google Scholar]
- Gómez-Muñoz, B.; Hatch, D.J.; Bol, R.; García-Ruiz, R. Nutrient dynamics during decomposition of the residues from a sown legume or ruderal plant cover in an olive oil orchard. Agric. Ecosyst. Environ. 2014, 184, 115–123. [Google Scholar] [CrossRef]
- Boselli, R.; Fiorini, A.; Santelli, S.; Ardenti, F.; Capra, F.; Maris, S.C.; Tabaglio, V. Cover crops during transition to no-till maintain yield and enhance soil fertility in intensive agro-ecosystems. Field Crops Res. 2020, 255, 107871. [Google Scholar] [CrossRef]
- Fernández-Hernández, A.; Roig, A.; Serramiá, N.; Civantos, C.G.-O.; Sánchez-Monedero, M.A. Application of compost of two-phase olive mill waste on olive grove: Effects on soil, olive fruit and olive oil quality. Waste Manag. 2014, 34, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Tejada, M.; Benítez, C. Effects of different organic wastes on soil biochemical properties and yield in an olive grove. Appl. Soil Ecol. 2020, 146, 103371. [Google Scholar] [CrossRef]
- Hernández, A.J.; Lacasta, C.; Pastor, J. Effects of different management practices on soil conservation and soil water in a rainfed olive orchard. Agric. Water Manag. 2005, 77, 232–248. [Google Scholar] [CrossRef]
- Hedo, J.; Lucas-Borja, M.E.; Wic, C.; Andrés-Abellán, M.; Heras, J.d.L. Soil microbiological properties and enzymatic activities of long-term post-fire recovery in dry and semiarid Aleppo pine (Pinus halepensis M.) forest stands. Solid Earth 2015, 6, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Alkorta, I.; Aizpurua, A.; Riga, P.; Albizu, I.; Amézaga, I.; Garbisu, C. Soil Enzyme Activities as Biological Indicators of Soil Health. Rev. Environ. Health 2003, 18, 65–73. [Google Scholar] [CrossRef]
- Gianfreda, L.; Ruggiero, P. Enzyme activities in soil. In Nucleic Acids and Proteins in Soil; Springer: Berlin/Heidelberg, Germany, 2006; pp. 257–311. [Google Scholar] [CrossRef]
- Cañizares, R.; Moreno, B.; Benitez, E. Biochemical characterization with detection and expression of bacterial β-glucosidase encoding genes of a Mediterranean soil under different long-term management practices. Biol. Fertil. Soils 2012, 48, 651–663. [Google Scholar] [CrossRef]
- Micuți, M.M.; Bădulescu, L.; Israel-Roming, F. A review on the enzymatic indicators for monitoring soil quality. Sci. Bull. Ser. F Biotechnol. Buchar. 2017, 21, 223–228. [Google Scholar]
- Lee, S.-H.; Kim, M.-S.; Kim, J.-G.; Kim, S.-O. Use of Soil Enzymes as Indicators for Contaminated Soil Monitoring and Sustainable Management. Sustainability 2020, 12, 8209. [Google Scholar] [CrossRef]
- Fließbach, A.; Oberholzer, H.-R.; Gunst, L.; Mäder, P. Soil organic matter and biological soil quality indicators after 21 years of organic and conventional farming. Agric. Ecosyst. Environ. 2007, 118, 273–284. [Google Scholar] [CrossRef]
- López, R.; Burgos, P.; Hermoso, J.M.; Hormaza, J.I.; González-Fernández, J.J. Long term changes in soil properties and enzyme activities after almond shell mulching in avocado organic production. Soil Tillage Res. 2014, 143, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Bonanomi, G.; De Filippis, F.; Zotti, M.; Idbella, M.; Cesarano, G.; Al-Rowaily, S.; Abd-ElGawad, A. Repeated applications of organic amendments promote beneficial microbiota, improve soil fertility and increase crop yield. Appl. Soil Ecol. 2020, 156, 103714. [Google Scholar] [CrossRef]
- Feng, H.; Sekaran, U.; Wang, T.; Kumar, S. On-farm assessment of cover cropping effects on soil C and N pools, enzyme activities, and microbial community structure. J. Agric. Sci. 2021, 159, 216–226. [Google Scholar] [CrossRef]
- Samuel, A.D.; Bungau, S.; Tit, D.M.; Melinte, C.E.; Purza, L.; Badea, G.E. Effects of Long Term Application of Organic and Mineral Fertilizers on Soil Enzymes. Rev. Chim. 2018, 69, 2608–2612. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xu, Y.; Jin, M.; Ye, X.; Gao, H.; Chu, W.; Mao, J.; Thompson, M.L. Soil labile organic carbon fractions and soil enzyme activities after 10 years of continuous fertilization and wheat residue incorporation. Sci. Rep. 2020, 10, 11318. [Google Scholar] [CrossRef]
- Coyne, M. Microbiología del Suelo: Un Enfoque Exploratorio (No. 631.46 C881m); Edit. Paraninfo: Madrid, Spain, 2000. [Google Scholar]
- Henríquez, C.; Uribe, L.; Valenciano, A.; Nogales, R. Actividad enzimática del suelo-Deshidrogenasa, ß-Glucosidasa, Fosfatasa y Ureasa-bajo diferentes cultivos. Agron. Costarric. 2014, 38, 43–54. [Google Scholar]
- García, C.; Gil, F.; Hernández, T.; Trasar, C. Técnicas de Análisis de Parámetros Bioquímicos en Suelos: Medida de Actividades Enzimáticas y Biomasa Microbiana; Mundi-Prensa: Madrid, Spain, 2003. [Google Scholar]
- Deng, S.P.; Parham, J.A.; Hattey, J.A.; Babu, D. Animal manure and anhydrous ammonia amendment alter microbial carbon use efficiency, microbial biomass, and activities of dehydrogenase and amidohydrolases in semiarid agroecosystems. Appl. Soil Ecol. 2006, 33, 258–268. [Google Scholar] [CrossRef]
- Siczek, A.; Frąc, M.; Gryta, A.; Kalembasa, S.; Kalembasa, D. Variation in soil microbial population and enzyme activities under faba bean as affected by pentachlorophenol. Appl. Soil Ecol. 2019, 150, 103466. [Google Scholar] [CrossRef]
- Jastrzębska, E.; Kucharski, J. Dehydrogenases, urease and phosphatases activities of soil contaminated with fungicides. Plant Soil Environ. 2007, 53, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Pang, P.C.K.; Kolenko, H. Phosphomonoesterase activity in forest soils. Soil Biol. Biochem. 1986, 18, 35–39. [Google Scholar] [CrossRef]
- Miralles, I. Calidad de Suelos en Ambientes Calizos Mediterráneos: Parque Natural de Sierra MaríA-Los Vélez. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 2006. [Google Scholar]
- Brzezińska, M.; Lipiec, J.; Frąc, M.; Oszust, K.; Szarlip, P.; Turski, M. Quantitative interactions between total and specific enzyme activities and C and N contents in earthworm-affected pear orchard soil. Land Degrad. Dev. 2018, 29, 3379–3389. [Google Scholar] [CrossRef]
- Emran, M.; Doni, S.; Macci, C.; Masciandaro, G.; Rashad, M.; Gispert, M. Susceptible soil organic matter, SOM, fractions to agricultural management practices in salt-affected soils. Geoderma 2020, 366, 114257. [Google Scholar] [CrossRef]
- Dabney, S.M.; Delgado, J.A.; Reeves, D.W. Using Winter Cover Crops to Improve Soil and Water Quality. Commun. Soil Sci. Plant Anal. 2001, 32, 1221–1250. [Google Scholar] [CrossRef]
- Bahnmann, B.; Mašínová, T.; Halvorsen, R.; Davey, M.L.; Sedlák, P.; Tomšovský, M.; Baldrian, P. Effects of oak, beech and spruce on the distribution and community structure of fungi in litter and soils across a temperate forest. Soil Biol. Biochem. 2018, 119, 162–173. [Google Scholar] [CrossRef]
- Niu, G.; Wang, R.; Hasi, M.; Wang, Y.; Geng, Q.; Wang, C.; Jiang, Y.; Huang, J. Availability of soil base cations and micronutrients along soil profile after 13-year nitrogen and water addition in a semi-arid grassland. Biogeochemistry 2021, 152, 223–236. [Google Scholar] [CrossRef]
- Samarajeewa, A.D.; Velicogna, J.R.; Schwertfeger, D.M.; Princz, J.I.; Subasinghe, R.M.; Scroggins, R.P.; Beaudette, L. Ecotoxicological effects of copper oxide nanoparticles (nCuO) on the soil microbial community in a biosolids-amended soil. Sci. Total Environ. 2021, 763, 143037. [Google Scholar] [CrossRef]
- Rivas, S.; Gandullo, J.M. Memoria del Mapa de Series de Vegetación de España; ICONA; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1987.
- Instituto Português do Mar e da Atmosfera. Available online: https://www.ipma.pt/pt/index.html (accessed on 15 June 2020).
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; p. 192. [Google Scholar]
- Lemanowicz, J.; Bartkowiak, A. Changes in the Activity of Phosphatase and the Content of Phosphorus in Salt-Affected Soils Grassland Habitat Natura 2000. Pol. J. Soil Sci. 2017, 49, 149. [Google Scholar] [CrossRef] [Green Version]
- González-Rosado, M.; Lozano-García, B.; Aguilera-Huertas, J.; Parras-Alcántara, L. Short-term effects of land management change linked to cover crop on soil organic carbon in Mediterranean olive grove hillsides. Sci. Total Environ. 2020, 744, 140683. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Trasar-Cepeda, C.; Leirós, M.C.; Seoane, S.; Gil-Sotres, F. Biochemical properties of acid soils under native grassland in a temperate humid zone. N. Z. J. Agric. Res. 2007, 50, 537–548. [Google Scholar] [CrossRef]
- Baldrian, P.; Kolařík, M.; Štursová, M.; Kopecký, J.; Valášková, V.; Větrovský, T.; Žifčáková, L.; Šnajdr, J.; Rídl, J.; Vlček, Č.; et al. Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. ISME J. 2012, 6, 248–258. [Google Scholar] [CrossRef] [Green Version]
- Hesterberg, D. Macroscale Chemical Properties and X-Ray Absorption Spectroscopy of Soil Phosphorus. In Developments in Soil Science; Elsevier: Sydney, Australia, 2010; Volume 34, pp. 313–356. [Google Scholar] [CrossRef]
- Castellano, M.J.; Mueller, K.E.; Olk, D.C.; Sawyer, J.E.; Six, J. Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob. Chang. Biol. 2015, 21, 3200–3209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.; Jiang, M.; Li, X. Adsorption and Properties of Urease Immobilized on Several Iron and Aluminum Oxides (Hydroxides) and Kaolinite. In Effect of Mineral-Organic-Microorganism Interactions on Soil and Freshwater Environments; Springer: Boston, MA, USA, 1999; pp. 167–174. [Google Scholar] [CrossRef]
- Gianfreda, L.; Rao, M.A. Stabilizing enzymes as synthetic complexes. Methods Soil Enzymol. 2011, 9, 319–369. [Google Scholar] [CrossRef]
- Jackson, M.L. Análisis Químicos de Suelos, 2nd ed.; Omega: Barcelona, Spain, 1964; p. 784. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Total nitrogen. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, R.D., Eds.; Agronomy series no. 9; American Society of Agronomy: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis. Part 2, 2nd ed.; Agronomy series no. 9; American Society of Agronomy: Madison, Wisconsin, USA, 1982; pp. 403–430. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Lakanen, E.; Erviö, R. A comparison of eight extractants for the determination of plant available micronutrients in soils. Acta Agric. Fenn. 1971, 123, 223–232. [Google Scholar]
- Tabatabai, M.A. Methods of soil analysis, part 2. Chemical and Microbiological Properties. In Agronomy Monography; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties. In Agronomy Monographs; American Society of Agronomy: Madison, WI, USA, 1994; Volume 5, pp. 775–833. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Hope, C.F.A.; Burns, R.G. Activity, origins and location of cellulases in a silt loam soil. Biol. Fertil. Soils 1987, 5, 164–170. [Google Scholar] [CrossRef]
- Ladd, J.N.; Butler, J.H. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- IBM SPSS. IBM SPSS Statistics for Windows, Version 20.0; IBM Corp.: New York, NY, USA, 2011.
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio Team: Boston, MA, USA, 2015. [Google Scholar]




| Olive Groves | Santarém Intensive (I) | Satarém Super-Intensive (S) | University Intensive (U) |
|---|---|---|---|
| Coordinates | 39°16′40.1″ N 8°39′40.7″ W | 39°17′36.4″ N 8°41′24.0″ W | 38°42′52.8″ N 9°11′05.6″ W |
| Original material | marly limestones | marly limestones | Basalt |
| Height | 35 m s.n.m. | 56 m s.n.m. | 123 m s.n.m. |
| Bioclimatic stage * | Mesomediterranean | mesomediterranean | thermomediterranean |
| Annual average minimum temperature ** | 11.22 °C | 11.22 °C | 13.57 °C |
| Annual average maximum temperature ** | 22.86 °C | 22.86 °C | 21.86 °C |
| Annual average accumulated precipitation ** | 609.33 mm | 609.33 mm | 815.23 mm |
| Soil classification *** | Calcium Cambisol | Calcium Cambisol | Chromic Vertisol |
| Area | 22 ha | 80 ha | 0.9 ha |
| Age of the crop | ≈35 years | 20 years | 35 years |
| Management (framework of the soil) | Intensive (7 × 5 m) | Super-intensive (1.35 × 3.75 m) | Intensive (7.5 × 5 m) |
| Variety | Picual | Arbequina | Picual |
| Application of pruning waste (mulch) on the lines | left on the soil | left on the soil | exported |
| Fertilizer | potassium nitrate, liquid potassium with EDTA | granulated ammonium sulfate | potassium sulfate with magnesium, ammonium sulfate, ammonium nitrate |
| Cover crop management | brush cutter | brush cutter | brush cutter |
| Foliar fertilizer | phosphorus pentoxide, potassium oxide, magnesium oxide | B, K nitrate, Mg nitrate, Fe | sodium borate, dimethoate, copper phosphite |
| Fungicide and pesticide | 20% copper (copper calcium sulfate) | tebuconazole | difenoconazole, trifloxystrobin, copper and calcium sulfate |
| pH regulator | 16% nitrogen and 41% sulfur trioxide | 16% nitrogen and 41% sulfur trioxide | does not need |
| Adjuvants | metoxi poli(etoxi)-propil heptametiltrisiloxano | metoxi poli(etoxi)-propil heptametiltrisiloxano | does not need |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Martín, M.P.; Fernández-Ondoño, E.; Ortiz-Bernad, I.; Abreu, M.M. Influence of Intensive and Super-Intensive Olive Grove Management on Soil Quality—Nutrients Content and Enzyme Activities. Plants 2023, 12, 2779. https://doi.org/10.3390/plants12152779
Reyes-Martín MP, Fernández-Ondoño E, Ortiz-Bernad I, Abreu MM. Influence of Intensive and Super-Intensive Olive Grove Management on Soil Quality—Nutrients Content and Enzyme Activities. Plants. 2023; 12(15):2779. https://doi.org/10.3390/plants12152779
Chicago/Turabian StyleReyes-Martín, Marino Pedro, Emilia Fernández-Ondoño, Irene Ortiz-Bernad, and Maria Manuela Abreu. 2023. "Influence of Intensive and Super-Intensive Olive Grove Management on Soil Quality—Nutrients Content and Enzyme Activities" Plants 12, no. 15: 2779. https://doi.org/10.3390/plants12152779
APA StyleReyes-Martín, M. P., Fernández-Ondoño, E., Ortiz-Bernad, I., & Abreu, M. M. (2023). Influence of Intensive and Super-Intensive Olive Grove Management on Soil Quality—Nutrients Content and Enzyme Activities. Plants, 12(15), 2779. https://doi.org/10.3390/plants12152779







