Evaluation of Total Isoflavones in Chickpea (Cicer arietinum L.) Sprouts Germinated under Precursors (p-Coumaric Acid and L-Phenylalanine) Supplementation
Abstract
1. Introduction
2. Result and Discussion
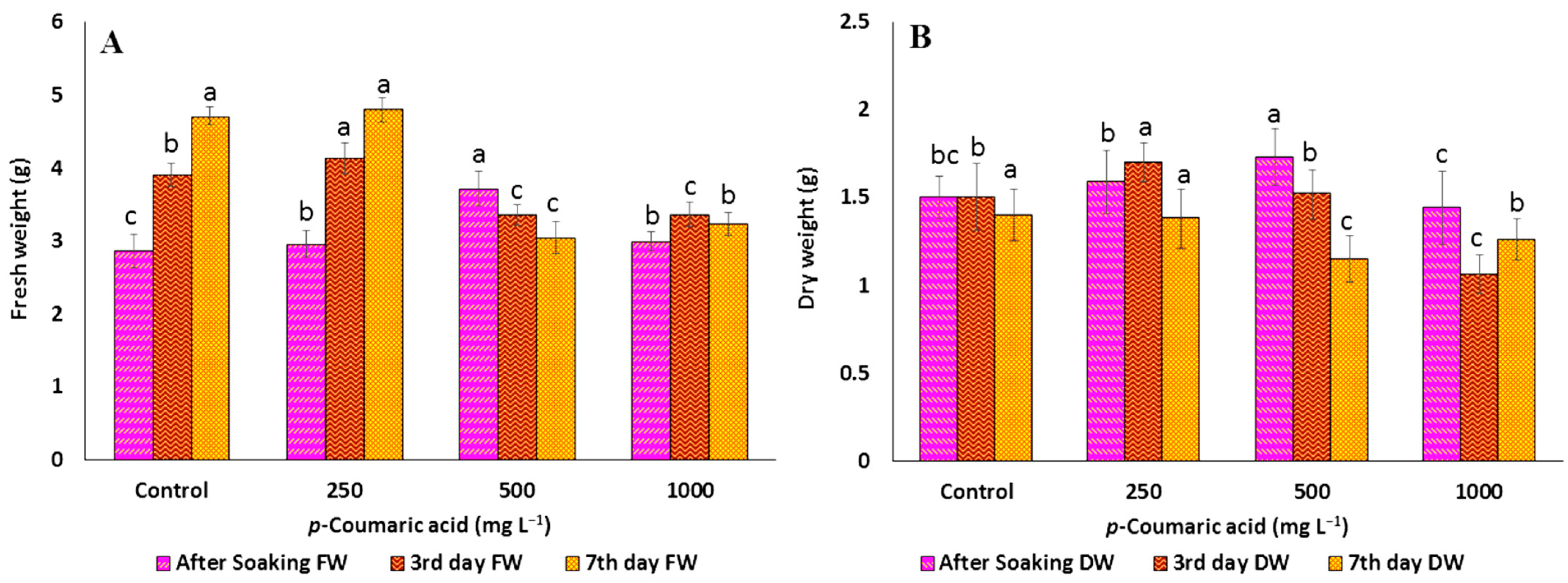
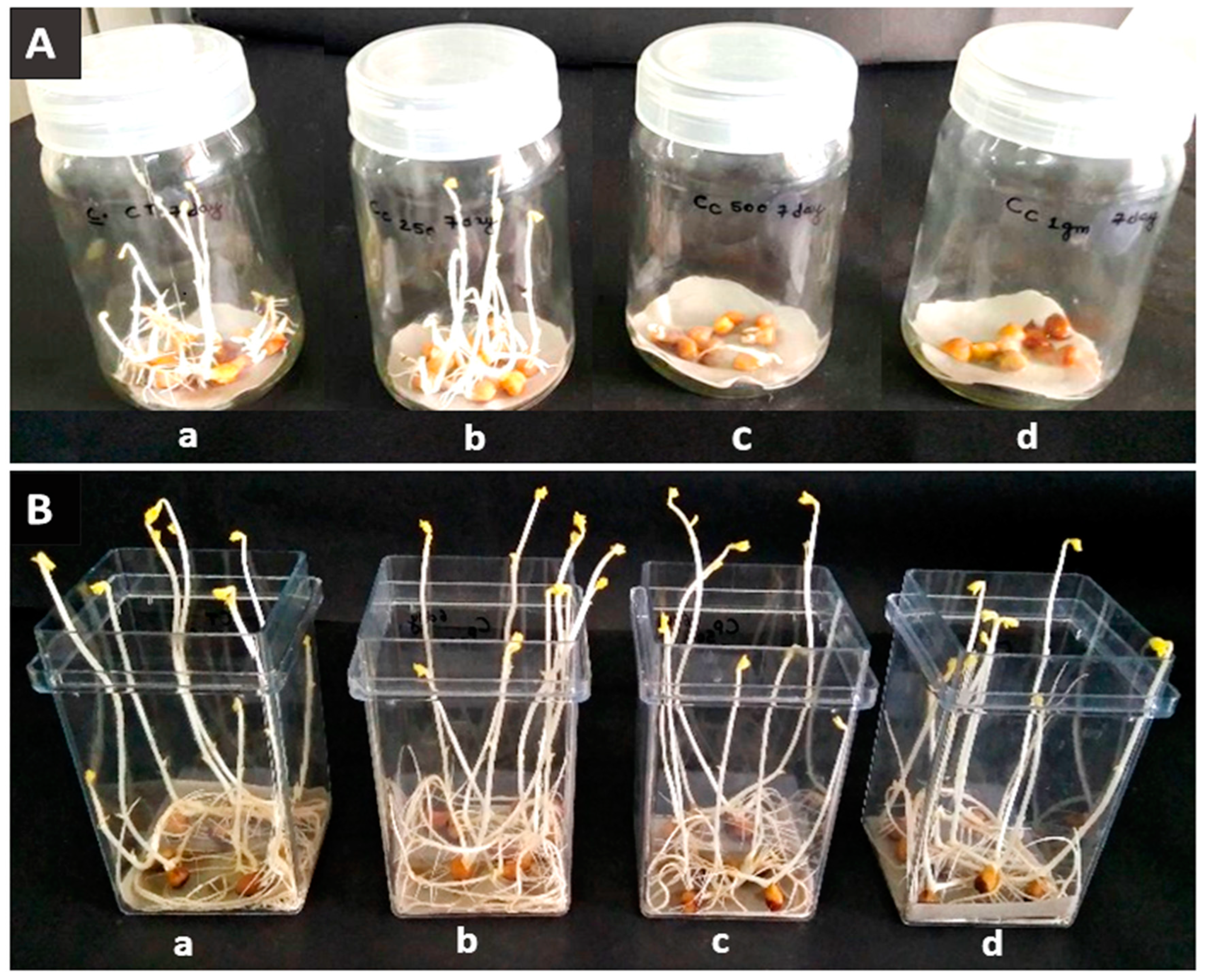
2.1. Effect of Precursors on the Morphological Parameters of C. arietinum Sprouts
2.2. Effect of Precursors on Total Phenolic Contents of C. arietinum Sprouts
2.3. Effect of Precursors on Antioxidant Activities of C. arietinum Sprouts
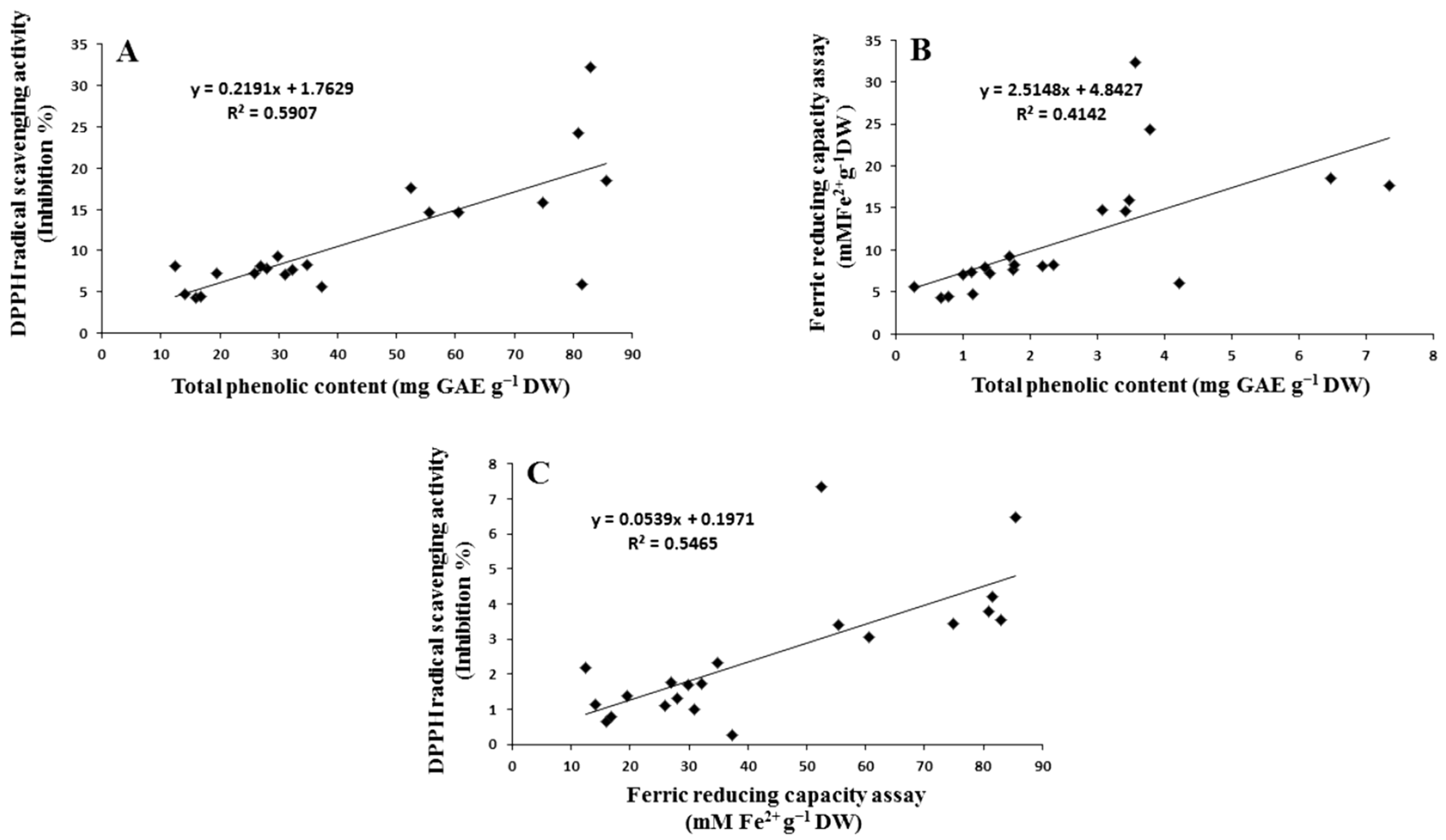
2.4. Correlation between Assays
2.5. High-Performance Liquid Chromatography Analysis of Phenolic Content in Control and Precursor-Treated C. arietinum Sprouts
3. Materials and Methods
3.1. Plant Genotype
3.2. Reagents
3.3. Sprouting Procedure
3.4. Study of Morphological Parameters
3.5. Preparation of the Extract
3.6. Determination of Total Phenolic Content
3.7. Determination of Antioxidant Activities
3.7.1. DPPH Radical Scavenging Activity
3.7.2. Ferric-Reducing Antioxidant Potential (FRAP) Assay
3.8. Sample Preparation for HPLC
High-Performance Liquid Chromatography (HPLC)
3.9. Experimental Design and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Benouis, K. Phytochemicals and bioactive compounds of pulses and their impact on health. Chem. Int. 2017, 3, 224–229. [Google Scholar]
- Bessada, S.M.; Barreira, J.C.; Oliveira, M.B.P. Pulses and food security: Dietary protein, digestibility, bioactive and functional properties. Trends Food Sci. Technol. 2019, 93, 53–68. [Google Scholar] [CrossRef]
- Gupta, S.; Nawaz, K.; Parween, S.; Roy, R.; Sahu, K.; Kumar Pole, A.; Khandal, H.; Srivastava, R.; Kumar Parida, S.; Chattopadhyay, D. Draft genome sequence of Cicer reticulatum L., the wild progenitor of chickpea pro ides a resource for agronomic trait improvement. DNA Res. 2017, 24, 1–10. [Google Scholar]
- Yagiz, Y.; Gu, L. Potential Health Promoting Properties of Isoflavones, Saponins, Proanthocyanidins, and Other Phytonutrients in Pulses. In Health Benefits of Pulses; Dahl, W., Ed.; Springer: Cham, Switzerland; Singapore, 2019; pp. 109–127. [Google Scholar]
- Hsiao, Y.H.; Ho, C.T.; Pan, M.H. Bioavailability and health benefits of major isoflavone aglycones and their metabolites. J. Funct. Foods 2020, 74, 104164. [Google Scholar] [CrossRef]
- Sajid, M.; Stone, S.R.; Kaur, P. Recent advances in heterologous synthesis paving way for future green-modular bioindustries: A review with special reference to isoflavonoids. Front. Bioeng. Biotechnol. 2021, 9, 673270. [Google Scholar] [CrossRef]
- Laddha, A.P.; Kulkarni, Y.A. Pharmacokinetics, pharmacodynamics, toxicity, and formulations of daidzein: An important isoflavone. Phytother. Res. 2023, 37, 2578–2604. [Google Scholar] [CrossRef]
- Pabich, M.; Materska, M. Biological effect of soy isoflavones in the prevention of civilization diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted grains: A comprehensive review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.E.; Rizwan, M.; Tourky, S.M. Assessment of early physiological and biochemical responses in chia (Salvia hispanica L.) sprouts under salt stress. Acta Physiol. Plant. 2021, 43, 121. [Google Scholar] [CrossRef]
- Ohanenye, I.C.; Ekezie, F.G.C.; Sarteshnizi, R.A.; Boachie, R.T.; Emenike, C.U.; Sun, X.; Nwachukwu, I.D.; Udenigwe, C.C. Legume seed protein digestibility as influenced by traditional and emerging physical processing technologies. Foods 2022, 11, 2299. [Google Scholar] [CrossRef]
- Ribeiro, I.C.; Leclercq, C.C.; Simões, N.; Toureiro, A.; Duarte, I.; Freire, J.B.; Chaves, M.M.; Renaut, J.; Pinheiro, C. Identification of chickpea seed proteins resistant to simulated in vitro human digestion. J. Proteom. 2017, 169, 143–152. [Google Scholar] [CrossRef]
- Bera, I.; O’Sullivan, M.; Flynn, D.; Shields, D.C. Relationship between protein digestibility and the proteolysis of legume proteins during seed germination. Molecules 2023, 28, 3204. [Google Scholar] [CrossRef] [PubMed]
- Guleria, P.; Kumar, V. Understanding the phenylpropanoid pathway for agronomical and nutritional improvement of mungbean. J. Hortic. Sci. Biotechnol. 2017, 92, 335–348. [Google Scholar] [CrossRef]
- Mehta, T.; Meena, M.; Nagda, A. Bioactive compounds of Curvularia species as a source of various biological activities and biotechnological applications. Front. Microbiol. 2022, 13, 1069095. [Google Scholar] [CrossRef]
- Meena, S.; Kanthaliya, B.; Joshi, A.; Khan, F.; Choudhary, S.; Arora, J. In vitro Production of Alkaloids. In Nutraceuticals Production from Plant Cell Factory; Belwal, T., Georgiev, M.I., Al-Khayri, J.M., Eds.; Springer Nature: Singapore, 2022; pp. 143–168. [Google Scholar]
- Meena, M.; Prasad, V.; Upadhyay, R.S. Evaluation of Alternaria alternata isolates for metabolite production isolated from different sites of Varanasi, India. J. Agric. Res. 2017, 2, 000124. [Google Scholar]
- Meena, M.; Gupta, S.K.; Swapnil, P.; Zehra, A.; Dubey, M.K.; Upadhyay, R.S. Alternaria toxins: Potential virulence factors and genes related to pathogenesis. Front. Microbiol. 2017, 8, 1451. [Google Scholar] [CrossRef]
- Kumari, P.; Meena, M.; Upadhyay, R.S. Characterization of plant growth promoting rhizobacteria (PGPR) isolated from the rhizosphere of Vigna radiata (mung bean). Biocatal. Agric. Biotechnol. 2018, 16, 155–162. [Google Scholar] [CrossRef]
- Arora, J.; Ramawat, K.G. Bioactive Molecules, Nutraceuticals, and Functional Foods in Indian Vegetarian Diet and During Postpartum Healthcare. In Bioactive Molecules in Food. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland; Singapore, 2018; pp. 1–30. [Google Scholar]
- Aksenova, M.A.; Nechaeva, T.L.; Zubova, M.Y.; Goncharuk, E.A.; Kazantseva, V.V.; Katanskaya, V.M.; Lapshin, P.V.; Zagoskina, N.V. Influence of different precursors on content of polyphenols in Camellia sinensis in vitro callus culture. Plants 2023, 12, 796. [Google Scholar] [CrossRef]
- Dulce-María, D.A.; Adrián, C.R.; Cuauhtémoc, R.M.; Ada-Keila, M.N.; Jorge, M.C.; Erika, A.S.; Edith-Oliva, C.R. Isoflavones from black chickpea (Cicer arietinum L.) sprouts with antioxidant and antiproliferative activity. Saudi J. Biol. Sci. 2021, 28, 1141–1146. [Google Scholar] [CrossRef]
- Arora, J.; Joshi, A.; Kanthaliya, B.; Khan, F. Effect of biotic elicitors on polyphenol production in Cayratia trifolia cell suspension cultures analyzed by HPLC. BioTechnologia J. Biotechnol. Comput. Biol. Bionanotechnol. 2020, 101, 35–43. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhang, Y.J.; Zhu, G.Y.; Shi, X.C.; Chen, X.; Herrera-Balandrano, D.D.; Liu, F.Q.; Laborda, P. Occurrence of isoflavones in soybean sprouts and strategies to enhance their content: A review. J. Food Sci. 2022, 87, 1961–1982. [Google Scholar] [CrossRef] [PubMed]
- Zehra, A.; Meena, M.; Dubey, M.K.; Aamir, M.; Upadhyay, R.S. Synergistic effects of plant defense elicitors and Trichoderma harzianum on enhanced induction of antioxidant defense system in tomato against Fusarium wilt disease. Bot. Stud. 2017, 58, 44. [Google Scholar] [CrossRef] [PubMed]
- Kanthaliya, B.; Joshi, A.; Arora, J.; Alqahtani, M.D.; Abd_Allah, E.F. Effect of biotic elicitors on the growth, antioxidant activity and metabolites accumulation in in vitro propagated shoots of Pueraria tuberosa. Plants 2023, 12, 1300. [Google Scholar] [CrossRef] [PubMed]
- Zehra, A.; Raytekar, N.A.; Meena, M.; Swapnil, P. Efficiency of microbial bio-agents as elicitors in plant defense mechanism under biotic stress: A review. Curr. Res. Microb. Sci. 2021, 2, 100054. [Google Scholar] [CrossRef]
- Świeca, M.; Sęczyk, Ł.; Gawlik-Dziki, U. Elicitation and precursor feeding as tools for the improvement of the phenolic content and antioxidant activity of lentil sprouts. Food Chem. 2014, 161, 288–295. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB light, and methyl jasmonate, alone or combined, redirect the biosynthesis of glucosinolates, phenolics, carotenoids, and chlorophylls in broccoli sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef]
- Li, L.I.; Dong, Y.; Ren, H.; Xue, Y.; Meng, H.; Li, M. Increased antioxidant activity and polyphenol metabolites in methyl jasmonate treated mung bean (Vigna radiata) sprouts. Food Sci. Technol. 2017, 37, 411–417. [Google Scholar] [CrossRef][Green Version]
- Kumari, P.; Meena, M.; Gupta, P.; Dubey, M.K.; Nath, G.; Upadhyay, R.S. Plant growth promoting rhizobacteria and their biopriming for growth promotion in mung bean (Vigna radiata (L.) R. Wilczek). Biocatal. Agric. Biotechnol. 2018, 16, 163–171. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, P.; Gu, Z.; Ma, M.; Yang, R. Spermidine improves antioxidant activity and energy metabolism in mung bean sprouts. Food Chem. 2020, 309, 125759. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Gao, F.; Liu, Y.; Lang, S.; Wang, C.; Zhang, D. Effect of ultrasound combined with exogenous GABA treatment on polyphenolic metabolites and antioxidant activity of mung bean during germination. Ultrason. Sonochem. 2023, 94, 106311. [Google Scholar] [CrossRef]
- Nontasan, S.; Chottanom, P.; Moongngarm, A. Melatonin, its precursors, total phenolic content and antioxidant activity in legumes germinated under normal and saline conditions. J. Sustain. Sci. Manag. 2021, 16, 53–66. [Google Scholar]
- Deng, B.; Zhao, J.; He, M.; Tian, S. Curcumin treatment enhances bioactive metabolite accumulation and reduces enzymatic browning in soybean sprouts during storage. Food Chem. 2023, 17, 100607. [Google Scholar] [CrossRef] [PubMed]
- Meenu, M.; Chen, P.; Mradula, M.; Chang, S.K.; Xu, B. New insights into chemical compositions and health—Promoting effects of black beans (Phaseolus vulgaris L.). Food Front. 2023, 2023, 1–20. [Google Scholar] [CrossRef]
- Yin, Y.; Tian, X.; Yang, J.; Yang, Z.; Tao, J.; Fang, W. Melatonin mediates isoflavone accumulation in germinated soybeans (Glycine max L.) under ultraviolet-B stress. Plant Physiol. Biochem. 2022, 175, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Qian, B.; Cheng, L.; Xu, S.; Wang, R. De novo biosynthesis of p-coumaric acid in E. coli with a trans-cinnamic acid 4-hydroxylase from the Amaryllidaceae plant Lycoris aurea. Molecules 2018, 23, 3185. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ji, D.; Cui, X.; Zhang, Z.; Li, B.; Xu, Y.; Chen, T.; Tian, S. p-Coumaric acid induces antioxidant capacity and defense responses of sweet cherry fruit to fungal pathogens. Postharvest. Biol. Technol. 2020, 169, 111297. [Google Scholar] [CrossRef]
- Feduraev, P.; Skrypnik, L.; Riabova, A.; Pungin, A.; Tokupova, E.; Maslennikov, P.; Chupakhina, G. Phenylalanine and tyrosine as exogenous precursors of wheat (Triticum aestivum L.) secondary metabolism through PAL-associated pathways. Plants 2020, 9, 476. [Google Scholar] [CrossRef]
- Meena, M.; Yadav, G.; Sonigra, P.; Nagda, A.; Mehta, T.; Swapnil, P.; Harish; Marwal, A. Role of elicitors to initiate the induction of systemic resistance in plants to biotic stress. Plant Stress 2022, 5, 100103. [Google Scholar] [CrossRef]
- Lupini, A.; Araniti, F.; Mauceri, A.; Princi, M.P.; Sorgonà, A.; Sunseri, F.; Varanini, Z.; Abenavoli, M.R. Coumarin enhances nitrate uptake in maize roots through modulation of plasma membrane H+—ATPase activity. Plant Biol. 2018, 20, 390–398. [Google Scholar] [CrossRef]
- Koleva-Valkova, L.; Harizanova, A. Deranged Physiology of Peach. In Co-Evolution of Secondary Metabolites: Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland; Singapore, 2020; pp. 377–401. [Google Scholar]
- Tu, Y.; Shen, J.; Peng, Z.; Xu, Y.; Li, Z.; Liang, J.; Wei, Q.; Zhao, H.; Huang, J. Biochar-dual oxidant composite particles alleviate the oxidative stress of phenolic acid on tomato seed germination. Antioxidants 2023, 12, 910. [Google Scholar] [CrossRef]
- Blum, U.; Gerig, T.M. Interrelationships between p-coumaric acid, evapotranspiration, soil water content, and leaf expansion. J. Chem. Ecol. 2006, 32, 1817–1834. [Google Scholar] [CrossRef] [PubMed]
- Buricova, L.; Andjelkovic, M.; Cermakova, A.; Reblova, Z.; Jurcek, O.; Kolehmainen, E.; Verhe, R.; Kvasnicka, F. Antioxidant capacities and antioxidants of strawberry, blackberry and raspberry leaves. Czech J. Food Sci. 2011, 29, 181–189. [Google Scholar] [CrossRef]
- Kheiry, M.; Dianat, M.; Badavi, M.; Mard, S.A.; Bayati, V. p-Coumaric acid protects cardiac function against lipopolysaccharide-induced acute lung injury by attenuation of oxidative stress. Iran. J. Basic Med. Sci. 2019, 22, 949–955. [Google Scholar]
- Duan, C.; Mao, T.; Sun, S.; Guo, X.; Guo, L.; Huang, L.; Yi, Y. Constitutive expression of GmF6′ H1 from soybean improves salt tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2019, 141, 446–455. [Google Scholar] [CrossRef]
- Kowalczyk, K.; Zielony, T.; Gajewski, M. Effect of Aminoplant and Asahi on Yield and Quality of Lettuce Grown on Rockwool. In Vegetable Crops: Biostimulators in Modren Agriculture; Dąbrowski, Z.T., Ed.; Warsaw University of Life Sciences—SGGW: Warsaw, Poland, 2008; pp. 35–43. [Google Scholar]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [PubMed]
- Forde, B.G.; Roberts, M.R. Glutamate receptor-like channels in plants: A role as amino acid sensors in plant defence? F1000Prime Rep. 2014, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Fan, X.; Shen, Q.; Smith, S.J. Amino acids and nitrate as signals for the regulation of nitrogen acquisition. J. Exp. Bot. 2007, 59, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Swapnil, P.; Meena, M.; Rai, A.K. Molecular interaction of nitrate transporter proteins with recombinant glycinebetaine results in efficient nitrate uptake in the cyanobacterium Anabaena PCC 7120. PloS ONE 2021, 16, e0257870. [Google Scholar] [CrossRef]
- Weiland, M.; Mancuso, S.; Baluska, F. Signalling via glutamate and GLRs in Arabidopsis thaliana. Funct. Plant Biol. 2016, 43, 1–25. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Abenavoli, M.R.; Cacco, G.; Sorgonà, A.; Marabottini, R.; Paolacci, A.R.; Ciaffi, M.; Badiani, M. The inhibitory effects of coumarin on the germination of durum wheat (Triticum turgidum ssp. durum, cv. Simeto) seeds. J. Chem. Ecol. 2006, 32, 489. [Google Scholar] [CrossRef]
- Tůmová, L.; Gallová, K.; Rimáková, J. Silybum marianum in vitro. Ceska. Slov. Farm. 2004, 53, 135–140. [Google Scholar]
- Joshi, A.; Kanthaliya, B.; Arora, J. Evaluation of growth and antioxidant activity in Suaeda monoica and Suaeda nudiflora callus cultures under sequential exposure to saline conditions. Curr. Biotechnol. 2019, 8, 42–52. [Google Scholar] [CrossRef]
- Kumar, R.; Swapnil, P.; Meena, M.; Selpair, S.; Yadav, B.G. Plant growth-promoting rhizobacteria (PGPR): Approaches to alleviate abiotic stresses for enhancement of growth and development of medicinal plants. Sustainability 2022, 14, 15514. [Google Scholar] [CrossRef]
- Rajashekar, C.B. Dual role of plant phenolic compounds as antioxidants and prooxidants. Am. J. Plant Sci. 2023, 14, 15–28. [Google Scholar] [CrossRef]
- Kamran, M.; Cheema, Z.A.; Farooq, M.; Ali, Q.; Anjum, M.Z.; Raza, A. Allelopathic influence of sorghum aqueous extract on growth, physiology and photosynthetic activity of maize (Zea mays L.) seedling. Philipp. Agric. Sci. 2019, 102, 33–41. [Google Scholar]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Umburanas, R.C.; Reichardt, K.; Neto, D.D. Foliar and seed application of amino acids affects the antioxidant metabolism of the soybean crop. Front. Plant Sci. 2017, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Świeca, M. Potentially bioaccessible phenolics, antioxidant activity and nutritional quality of young buckwheat sprouts affected by elicitation and elicitation supported by phenylpropanoid pathway precursor feeding. Food Chem. 2016, 192, 625–632. [Google Scholar] [CrossRef]
- Bemani, E.; Ghanati, F.; Rezaei, A.; Jamshidi, M. Effect of phenylalanine on Taxol production and antioxidant activity of extracts of suspension-cultured hazel (Corylus avellana L.) cells. J. Nat. Med. 2013, 67, 446–451. [Google Scholar] [CrossRef]
- Ren, S.; Sun, J. Changes in phenolic content, phenylalanine ammonia-lyase (PAL) activity, and antioxidant capacity of two buckwheat sprouts in relation to germination. J. Funct. Foods 2014, 7, 298–304. [Google Scholar] [CrossRef]
- Sulaiman, S.F.; Yusoff, N.A.M.; Eldeen, I.M.; Seow, E.M.; Sajak, A.A.B.; Ooi, K.L. Correlation between total phenolic and mineral contents with antioxidant activity of eight Malaysian bananas (Musa sp.). J. Food Compost. Anal. 2011, 24, 1–10. [Google Scholar] [CrossRef]
- Ismail, A. Correlation analysis between antihypertensive effect with total phenolic content and antioxidant activity of syzygium polyanthum (serai kayu) leaves fractions. Int. J. Allied Health Sci. 2019, 3, 753. [Google Scholar]
- Arora, J.; Kanthaliya, B.; Joshi, A. Evaluation of genistein content in chickpea (Cicer arietinum L.) and mungbean (Vigna radiata L.) sprouts germinated under different conditions. Curr. Perspect. Med. Aroma. Plant. (CUPMAP) 2019, 2, 1–10. [Google Scholar]
- Kanthaliya, B.; Joshi, A.; Arora, J. Evaluation of isoflavonoid content in context to tuber size and seed biology study of Pueraria tuberosa (Roxb.ex.Willd.) DC: A vulnerable medicinal plant. Vegetos 2019, 32, 247–253. [Google Scholar] [CrossRef]
- Yu, O.; Jung, W.; Shi, J.; Croes, R.A.; Fader, G.M.; McGonigle, B.; Odell, J.T. Production of the isoflavones genistein and daidzein in non-legume dicot and monocot tissues. Plant Physiol. 2000, 124, 781–794. [Google Scholar] [CrossRef]
- Meena, M.; Yadav, G.; Sonigra, P.; Nagda, A.; Mehta, T.; Zehra, A.; Swapnil, P. Role of Microbial Bioagents as Elicitors in Plant Defense Regulation. In Transcription Factors for Biotic Stress Tolerance in Plants; Springer Nature: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Durango, D.; Murillo, J.; Echeverri, F.; Escobar, G.; Quinones, W. Isoflavonoid composition and biological activity of extracts from soybean seedlings treated by different elicitors. An. Acad. Bras. Ciências 2018, 90, 1955–1971. [Google Scholar] [CrossRef]
- Baber, R.J. Phytoestrogens in health: The role of isoflavones. In Isoflavones: Chemistry, Analysis, Function and Effects. Food and Nutritional Components in Focus; Preedy, V.R., Ed.; RCS Publishing: Cambridge, UK, 2013; pp. 3–13. [Google Scholar]
- Sohn, S.I.; Pandian, S.; Oh, Y.J.; Kang, H.J.; Cho, W.S.; Cho, Y.S. Metabolic engineering of isoflavones: An updated overview. Front. Plant Sci. 2021, 12, 670103. [Google Scholar] [CrossRef]
- Farkas, G.L.; Kiraly, Z. Role of phenolic compound in the physiology of plant diseases and disease resistance. J. Phytopathol. 1962, 44, 105–150. [Google Scholar] [CrossRef]
- Hatano, T.; Kagawa, H.; Yasahara, H.T.; Okuda, T. The effect of extracts on DPPH radical was estimated according to the methanol. Food Chem. 1988, 78, 347–354. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Goyal, S.; Sharma, V.; Ramawat, K.G. Marked effect of Cuscuta on puerarin accumulation in cell culture of Pueraria tuberosa grown in shake flasks and a bioreactor. Plant Biotechnol. Rep. 2011, 5, 121–126. [Google Scholar] [CrossRef]
- Krisa, S.; Larronde, F.; Budzinski, H.; Decendit, A.; Deffieux, G.; Merillon, J.M. Stilbenes production by Vitis vinifera cell suspension cultures: Methyl jasmonate induction and 13C biolabeling. J. Nat. Prod. 1999, 62, 1688–1690. [Google Scholar] [CrossRef]


| Concentration (mg L−1) | Third Day | Seventh Day | ||||
|---|---|---|---|---|---|---|
| Germination (%) | Length of Hypocotyle (cm) | Length of Radical (cm) | Germination (%) | Length of Hypocotyle (cm) | Length of Radical (cm) | |
| Control | 100 | 0.82 ± 0.60 a | 2.24 ± 0.35 a | 100 | 9.82 ± 2.37 a | 10.73 ± 3.34 a |
| 250 | 80 | 0.25 ± 0.21 b | 1.53 ± 0.51 b | 75 | 0.48 ± 0.30 b | 0.94 ± 0.19 b |
| 500 | 00 | 0.00 ± 0.00 c | 00 ± 00 c | 00 | 00 ± 00 c | 00 ± 00 c |
| 1000 | 00 | 0.00 ± 0.00 c | 00 ± 00 c | 00 | 00 ± 00 c | 00 ± 00 c |
| Concentration (mg L−1) | Third Day | Seventh Day | ||
|---|---|---|---|---|
| Length of Hypocotyle (cm) | Length of Radical (cm) | Length of Hypocotyle (cm) | Length of Radical (cm) | |
| Control | 0.82 ± 0.60 ab | 2.24 ± 0.35 b | 9.82 ± 2.37 a | 10.73 ± 3.34 b |
| 250 | 0.84 ± 0.46 ab | 3.10 ± 0.84 a | 10.08 ± 4.06 a | 12.72 ± 3.96 a |
| 500 | 0.78 ± 0.54 b | 2.98 ± 1.43 a | 9.20 ± 2.60 a | 7.11 ± 4.03 c |
| 1000 | 0.87 ± 0.36 a | 2.35 ± 0.47 b | 9.28 ± 1.90 a | 12.11 ± 2.09 a |
| Day | Concentration (mg L−1) | TPC (mg GAE g−1 DW) | DPPH Redical Scavenging Activity (%) | FRAP (mM Fe+2 g−1 DW) |
|---|---|---|---|---|
| After soaking | Control | 4.3 ± 0.53 d | 15.99 ± 0.54 d | 0.66 ± 0.61 d |
| 250 | 7.9 ± 0.36 b | 27.96 ± 0.41 c | 1.31 ± 0.25 b | |
| 500 | 9.3 ± 0.91 a | 29.90 ± 0.84 b | 1.69 ± 0.53 a | |
| 1000 | 7.1 ± 0.56 c | 31.02 ± 0.63 a | 0.99 ± 0.39 c | |
| Third day | Control | 5.6 ± 0.66 d | 37.27 ± 0.25 a | 0.27 ± 0.47 c |
| 250 | 7.7 ± 0.42 b | 32.27 ± 0.69 b | 1.74 ± 0.42 a | |
| 500 | 7.2 ± 0.52 c | 19.47 ± 0.36 d | 1.39 ± 0.63 b | |
| 1000 | 8.2 ± 0.99 a | 26.98 ± 0.96 c | 1.76 ± 0.96 a | |
| Seventh day | Control | 18.5 ± 0.36 a | 85.54 ± 0.43 a | 6.47 ± 0.52 b |
| 250 | 17.6 ± 0.58 b | 52.45 ± 0.52 b | 7.34 ± 0.41 a | |
| 500 | 8.3 ± 0.42 c | 34.91 ± 0.94 c | 2.34 ± 0.54 c | |
| 1000 | 7.3 ± 0.91 d | 25.91 ± 0.69 d | 1.11 ± 0.11 d |
| Day | Concentration (mg L−1) | TPC (mg GAE g−1 DW) | DPPH Redical Scavenging Activity (%) | FRAP (mM Fe+2 g−1 DW) |
|---|---|---|---|---|
| After soaking | Control | 4.3 ± 0.53 d | 15.99 ± 0.54 b | 0.66 ± 0.61 d |
| 250 | 8.1 ± 0.61 a | 12.38 ± 0.47 d | 2.17 ± 0.36 a | |
| 500 | 4.7 ± 0.29 b | 14.19 ± 0.35 c | 1.14 ± 0.35 b | |
| 1000 | 4.5 ± 0.72 c | 16.83 ± 0.27 a | 0.78 ± 0.66 c | |
| Third day | Control | 5.6 ± 0.66 c | 37.27 ± 0.47 d | 0.27 ± 0.65 c |
| 250 | 14.9 ± 0.22 b | 55.49 ± 0.92 c | 3.41 ± 0.99 a | |
| 500 | 14.7 ± 0.59 b | 60.64 ± 0.28 b | 3.07 ± 0.44 b | |
| 1000 | 15.9 ± 0.61 a | 74.83 ± 0.53 a | 3.46 ± 0.66 a | |
| Seventh day | Control | 18.5 ± 0.36 c | 83.17 ± 0.35 a | 5.79 ± 0.25 a |
| 250 | 24.3 ± 0.58 b | 80.95 ± 0.36 c | 3.78 ± 0.69 c | |
| 500 | 32.3 ± 0.42 a | 83.03 ± 0.58 a | 3.56 ± 0.49 d | |
| 1000 | 6.0 ± 0.91 d | 81.50 ± 0.85 b | 4.21 ± 0.53 b |
| Sprouts | Daidzein (mg g−1) | Daidzin (mg g−1) | Genistein (mg g−1) | Genistin (mg g−1) | Biochain A (mg g−1) | Total Isoflavanoid (mg g−1) |
|---|---|---|---|---|---|---|
| Control, third day | 0.076 ± 0.08 d | 0.29 ± 0.44 e | 1.05 ± 0.54 d | 0.411 ± 0.31 e | 0.219 ± 0.28 b | 2.046 ± 0.99 d |
| p-CA 250 mg L−1, third day | * | 0.73 ± 0.87 d | * | 1.057 ± 0.63 d | * | 1.787 ± 0.83 e |
| Control, seventh day | 0.507 ± 0.19 b | 5.8 ± 0.25 b | 2.3 ± 0.87 a | 3.945 ± 0.76 c | 0.267 ± 0.24 a | 12.819 ± 0.93 b |
| Phe 250 mg L−1, seventh day | 0.276 ± 0.11 c | 6.33 ± 0.27 a | 2.21 ± 0.59 b | 4.171 ± 0.88 b | * | 12.987 ± 0.86 a |
| Phe 500 mg L−1, seventh day | 0.534 ± 0.36 a | 2.75 ± 0.65 c | 1.99 ± 0.43 c | 6.842 ± 0.71 a | * | 12.116 ± 0.63 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arora, J.; Kanthaliya, B.; Joshi, A.; Meena, M.; Meena, S.; Siddiqui, M.H.; Alamri, S.; Devkota, H.P. Evaluation of Total Isoflavones in Chickpea (Cicer arietinum L.) Sprouts Germinated under Precursors (p-Coumaric Acid and L-Phenylalanine) Supplementation. Plants 2023, 12, 2823. https://doi.org/10.3390/plants12152823
Arora J, Kanthaliya B, Joshi A, Meena M, Meena S, Siddiqui MH, Alamri S, Devkota HP. Evaluation of Total Isoflavones in Chickpea (Cicer arietinum L.) Sprouts Germinated under Precursors (p-Coumaric Acid and L-Phenylalanine) Supplementation. Plants. 2023; 12(15):2823. https://doi.org/10.3390/plants12152823
Chicago/Turabian StyleArora, Jaya, Bhanupriya Kanthaliya, Abhishek Joshi, Mukesh Meena, Supriya Meena, Manzer H. Siddiqui, Saud Alamri, and Hari Prasad Devkota. 2023. "Evaluation of Total Isoflavones in Chickpea (Cicer arietinum L.) Sprouts Germinated under Precursors (p-Coumaric Acid and L-Phenylalanine) Supplementation" Plants 12, no. 15: 2823. https://doi.org/10.3390/plants12152823
APA StyleArora, J., Kanthaliya, B., Joshi, A., Meena, M., Meena, S., Siddiqui, M. H., Alamri, S., & Devkota, H. P. (2023). Evaluation of Total Isoflavones in Chickpea (Cicer arietinum L.) Sprouts Germinated under Precursors (p-Coumaric Acid and L-Phenylalanine) Supplementation. Plants, 12(15), 2823. https://doi.org/10.3390/plants12152823









