Solanum aethiopicum L. from the Basilicata Region Prevents Lipid Absorption, Fat Accumulation, Oxidative Stress, and Inflammation in OA-Treated HepG2 and Caco-2 Cell Lines
Abstract
1. Introduction
2. Results and Discussion
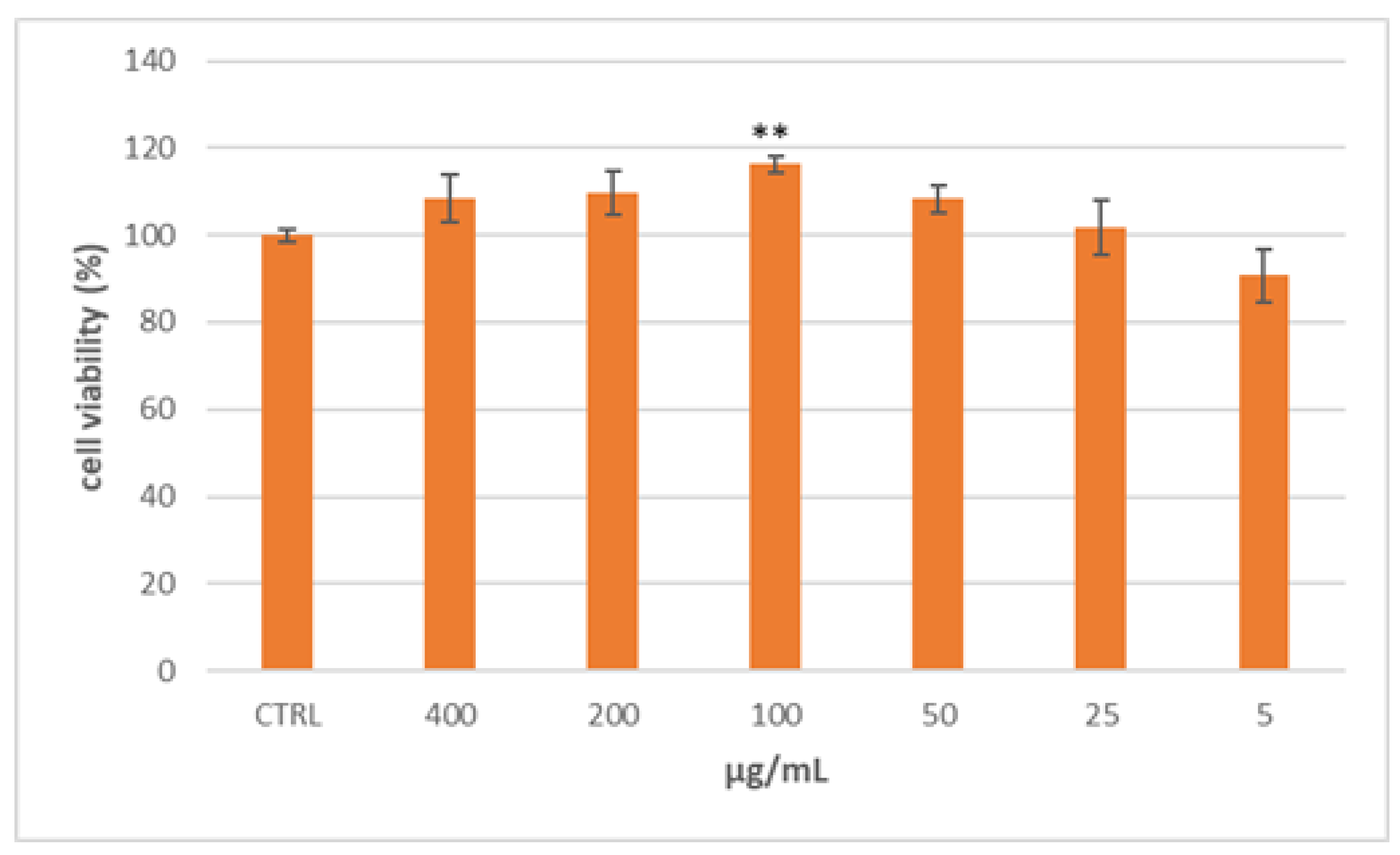
2.1. S. aethiopicum Improved Lipid Absorption in Caco-2 Cells
2.2. S. aethiopicum Reduced Lipid Accumulation and Fatty Acid Metabolism in HepG2 Cells
2.3. Antioxidant Activity of S. aethiopicum in OA-Treated HepG2 Cells
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Cell Culture
3.3. Cell Viability
3.4. Determination of Total Lipid Accumulation in HepG2 Cell Line by Oil Red O Staining
3.5. Intracellular ROS Determination
3.6. Quantitative qRT-PCR
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miah, P.; Mohona, S.B.S.; Rahman, M.M.; Subhan, N.; Khan, F.; Hossain, H.; Sharker, S.M.; Alam, M.A. Supplementation of cumin seed powder prevents oxidative stress, hyperlipidemia and non-alcoholic fatty liver in high fat diet fed rats. Biomed. Pharmacother. 2021, 141, 111908. [Google Scholar] [CrossRef]
- Ponticelli, M.; Russo, D.; Faraone, I.; Sinisgalli, C.; Labanca, F.; Lela, L.; Milella, L. The promising ability of Humulus lupulus L. Iso-α-acids vs. diabetes, inflammation, and metabolic syndrome: A systematic review. Molecules 2021, 26, 954. [Google Scholar] [CrossRef]
- Bona, M.D.; Torres, C.H.d.M.; Lima, S.C.V.C.; Morais, A.H.d.A.; Lima, A.Â.M.; Maciel, B.L.L. Intestinal Barrier Permeability in Obese Individuals with or without Metabolic Syndrome: A Systematic Review. Nutrients 2022, 14, 3649. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. S1), S81–S90. [Google Scholar] [CrossRef]
- Nwanna, E.E.; Ibukun, E.O.; Oboh, G. Effect of some tropical eggplant fruits (Solanum spp.) supplemented diet on diabetic neuropathy in experimental male Wistar rats in-vivo. Funct. Foods Health Dis. 2016, 6, 661–676. [Google Scholar] [CrossRef]
- Gürbüz, N.; Uluişik, S.; Frary, A.; Frary, A.; Doğanlar, S. Health benefits and bioactive compounds of eggplant. Food Chem. 2018, 268, 602–610. [Google Scholar] [CrossRef]
- Mbondo, N.N.; Owino, W.O.; Ambuko, J.; Sila, D.N. Effect of drying methods on the retention of bioactive compounds in African eggplant. Food Sci. Nutr. 2018, 6, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Mata, M.-C.; Yokoyama, W.E.; Hong, Y.-J.; Prohens, J. α-Solasonine and α-solamargine contents of gboma (Solanum macrocarpon L.) and scarlet (Solanum aethiopicum L.) eggplants. J. Agric. Food Chem. 2010, 58, 5502–5508. [Google Scholar] [CrossRef]
- Han, M.; Opoku, K.N.; Bissah, N.A.; Su, T. Solanum aethiopicum: The Nutrient-Rich Vegetable Crop with Great Economic, Genetic Biodiversity and Pharmaceutical Potential. Horticulturae 2021, 7, 126. [Google Scholar] [CrossRef]
- Sunseri, F.; Polignano, G.; Alba, V.; Lotti, C.; Bisignano, V.; Mennella, G.; Drsquo, A.; Bacchi, M.; Riccardi, P.; Fiore, M. Genetic diversity and characterization of African eggplant germplasm collection. Afr. J. Plant Sci. 2010, 4, 231–241. [Google Scholar]
- Faraone, I.; Lela, L.; Ponticelli, M.; Gorgoglione, D.; De Biasio, F.; Valentão, P.; Andrade, P.B.; Vassallo, A.; Caddeo, C.; Falabella, R.; et al. New Insight on the Bioactivity of Solanum aethiopicum Linn. Growing in Basilicata Region (Italy): Phytochemical Characterization, Liposomal Incorporation, and Antioxidant Effects. Pharmaceutics 2022, 14, 1168. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; Segura-Carretero, A.; del Mar Contreras, M. Phenolic compounds as natural and multifunctional anti-obesity agents: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1212–1229. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.L.; Mujawar, Z.; Tamehiro, N. ABC transporters, atherosclerosis and inflammation. Atherosclerosis 2010, 211, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Jochems, P.G.; Garssen, J.; Van Keulen, A.M.; Masereeuw, R.; Jeurink, P.V. Evaluating human intestinal cell lines for studying dietary protein absorption. Nutrients 2018, 10, 322. [Google Scholar] [CrossRef]
- Bateman, P.A.; Jackson, K.G.; Maitin, V.; Yaqoob, P.; Williams, C.M. Differences in cell morphology, lipid and apo B secretory capacity in Caco-2 cells following long term treatment with saturated and monounsaturated fatty acids. Biochim. Biophys. Acta—Mol. Cell Biol. 2007, 1771, 475–485. [Google Scholar] [CrossRef]
- Chen, G.; Wang, H.; Zhang, X.; Yang, S.-T. Nutraceuticals and functional foods in the management of hyperlipidemia. Crit. Rev. Food Sci. Nutr. 2014, 54, 1180–1201. [Google Scholar] [CrossRef]
- Savel, J.; Lafitte, M.; Pucheu, Y.; Pradeau, V.; Tabarin, A.; Couffinhal, T. Very low levels of HDL cholesterol and atherosclerosis, a variable relationship–a review of LCAT deficiency. Vasc. Health Risk Manag. 2012, 8, 357. [Google Scholar]
- Shao, F.; Ford, D.A. Differential regulation of ABCA1 and macrophage cholesterol efflux by elaidic and oleic acids. Lipids 2013, 48, 757–767. [Google Scholar] [CrossRef]
- Ogunka-Nnoka, C.; Bm, O.O.; Omeje, H.C. Effects of Ethanol Extracts of S. aethiopicumStalks on Lipid Profile and Haematological Parameters of Wistar Albino Rats. Int. J. Sci. Res. Methodol. 2018, 10, 215–229. [Google Scholar]
- Field, F.J.; Born, E.; Murthy, S.; Mathur, S.N. Gene expression of sterol regulatory element-binding proteins in hamster small intestine. J. Lipid Res. 2001, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, R.; Jideonwo, V.; Ahn, M.; Surendran, S.; Tagliabracci, V.S.; Hou, Y.; Gamble, A.; Kerner, J.; Irimia-Dominguez, J.M.; Puchowicz, M.A. Sterol regulatory element-binding protein-1 (SREBP-1) is required to regulate glycogen synthesis and gluconeogenic gene expression in mouse liver. J. Biol. Chem. 2014, 289, 5510–5517. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.A.; Colak, N.; Topuz, M.; Tarkowski, P.; Jaworek, P.; Seiler, G.; Inceer, H. Comparison of nutrient content in fruit of commercial cultivars of eggplant (Solanum melongena L.). Pol. J. Food Nutr. Sci. 2015, 65, 251–259. [Google Scholar] [CrossRef]
- Adeyeye, A.; Ayodele, O.D.; Akinnuoye, G.A.; Sulaiman, W. Proximate composition and fatty acid profiles of two edible leafy vegetables in Nigeria. Am. J. Food Nutr. Health 2018, 3, 51–55. [Google Scholar]
- Susilowati, R.; Jannah, J.; Maghfuroh, Z.; Kusuma, M.T. Antihyperlipidemic effects of apple peel extract in high-fat diet-induced hyperlipidemic rats. J. Adv. Pharm. Technol. Res. 2020, 11, 128. [Google Scholar] [CrossRef]
- Hsieh, J.; Hayashi, A.A.; Webb, J.; Adeli, K. Postprandial dyslipidemia in insulin resistance: Mechanisms and role of intestinal insulin sensitivity. Atheroscler. Suppl. 2008, 9, 7–13. [Google Scholar] [CrossRef]
- Gil-Cardoso, K.; Ginés, I.; Pinent, M.; Ardévol, A.; Blay, M.; Terra, X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr. Res. Rev. 2016, 29, 234–248. [Google Scholar] [CrossRef]
- Dou, W.; Zhang, J.; Sun, A.; Zhang, E.; Ding, L.; Mukherjee, S.; Wei, X.; Chou, G.; Wang, Z.-T.; Mani, S. Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κB signalling. Br. J. Nutr. 2013, 110, 599–608. [Google Scholar] [CrossRef]
- Hwang, D.; Kang, M.J.; Kang, C.W.; Kim, G.D. Kaempferol-3-O-β-rutinoside suppresses the inflammatory responses in lipopolysaccharide-stimulated RAW264. 7 cells via the NF-κB and MAPK pathways. Int. J. Mol. Med. 2019, 44, 2321–2328. [Google Scholar] [CrossRef]
- Tveden-Nyborg, P.; Lykkesfeldt, J. Normal weight dyslipidemia: Is it all about the liver? Obesity 2016, 24, 556–567. [Google Scholar] [CrossRef]
- Angin, Y.; Beauloye, C.; Horman, S.; Bertrand, L. Regulation of carbohydrate metabolism, lipid metabolism, and protein metabolism by AMPK. In AMP-Activated Protein Kinase; Springer: Berlin/Heidelberg, Germany, 2016; pp. 23–43. [Google Scholar] [CrossRef]
- Huang, K.; Liang, X.c.; Zhong, Y.l.; He, W.y.; Wang, Z. 5-Caffeoylquinic acid decreases diet-induced obesity in rats by modulating PPARα and LXRα transcription. J. Sci. Food Agric. 2015, 95, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, I.R.; Joshi, M. CPT1A-mediated fat oxidation, mechanisms, and therapeutic potential. Endocrinology 2020, 161, bqz046. [Google Scholar] [CrossRef] [PubMed]
- Tutunchi, H.; Ostadrahimi, A.; Saghafi-Asl, M.; Hosseinzadeh-Attar, M.-J.; Shakeri, A.; Asghari-Jafarabadi, M.; Roshanravan, N.; Farrin, N.; Naemi, M.; Hasankhani, M. Oleoylethanolamide supplementation in obese patients newly diagnosed with non-alcoholic fatty liver disease: Effects on metabolic parameters, anthropometric indices, and expression of PPAR-α, UCP1, and UCP2 genes. Pharmacol. Res. 2020, 156, 104770. [Google Scholar] [CrossRef]
- Ajiboye, T.; Ajala-Lawal, R.; Abdullahi, R. Metabolic syndrome: Protective potentials of dietary phenolic acids. In Molecular Nutrition: Carbohydrates; Elsevier: Amsterdam, The Netherlands, 2019; pp. 225–235. [Google Scholar]
- Zeng, Z.; He, W.; Jia, Z.; Hao, S. Lycopene improves insulin sensitivity through inhibition of STAT3/Srebp-1c-mediated lipid accumulation and inflammation in mice fed a high-fat diet. Exp. Clin. Endocrinol. 2017, 125, 610–617. [Google Scholar] [CrossRef]
- Min, H.-K.; Kapoor, A.; Fuchs, M.; Mirshahi, F.; Zhou, H.; Maher, J.; Kellum, J.; Warnick, R.; Contos, M.J.; Sanyal, A.J. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012, 15, 665–674. [Google Scholar] [CrossRef]
- Liang, N.; Li, Y.-M.; He, Z.; Hao, W.; Zhao, Y.; Liu, J.; Zhu, H.; Kwek, E.; Ma, K.-Y.; He, W.-S. Rutin and Quercetin Decrease Cholesterol in HepG2 Cells but Not Plasma Cholesterol in Hamsters by Oral Administration. Molecules 2021, 26, 3766. [Google Scholar] [CrossRef]
- de Vries, H.E.; Witte, M.; Hondius, D.; Rozemuller, A.J.; Drukarch, B.; Hoozemans, J.; van Horssen, J. Nrf2-induced antioxidant protection: A promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic. Biol. Med. 2008, 45, 1375–1383. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Wu, J.-X.; Shen, T.-T.; Chai, H.-S.; Chen, H.-F.; Zhang, Q. Quzhi Formula Alleviates Nonalcoholic Steatohepatitis by Impairing Hepatocyte Lipid Accumulation and Inflammation via Bip/eIF2α Signaling. J. Clin. Transl. Hepatol. 2022, 10, 1050–1058. [Google Scholar] [CrossRef]
- Esposito, V.; Grosjean, F.; Tan, J.; Huang, L.; Zhu, L.; Chen, J.; Xiong, H.; Striker, G.E.; Zheng, F. CHOP deficiency results in elevated lipopolysaccharide-induced inflammation and kidney injury. Am. J. Physiol. Renal Physiol. 2013, 304, F440–F450. [Google Scholar] [CrossRef]
- Nennig, S.; Schank, J. The role of NFkB in drug addiction: Beyond inflammation. Alcohol Alcohol. 2017, 52, 172–179. [Google Scholar] [CrossRef]
- Kaneko, M.; Niinuma, Y.; Nomura, Y. Activation signal of nuclear factor-κB in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol. Pharm. Bull. 2003, 26, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Anosike, C.A.; Obidoa, O.; Ezeanyika, L.U. The anti—Inflammatory activity of garden egg (Solanum aethiopicum) on egg albumin—Induced oedema and granuloma tissue formation in rats. Asian Pac. J. Trop. Med. 2012, 5, 62–66. [Google Scholar] [CrossRef]
- Hwang, Y.-J.; Wi, H.-R.; Kim, H.-R.; Park, K.W.; Hwang, K.-A. Pinus densiflora Sieb. et Zucc. alleviates lipogenesis and oxidative stress during oleic acid-induced steatosis in HepG2 cells. Nutrients 2014, 6, 2956–2972. [Google Scholar] [CrossRef] [PubMed]
- Sinisgalli, C.; Faraone, I.; Vassallo, A.; Caddeo, C.; Bisaccia, F.; Armentano, M.F.; Milella, L.; Ostuni, A. Phytochemical profile of Capsicum annuum L. cv Senise, incorporation into liposomes, and evaluation of cellular antioxidant activity. Antioxidants 2020, 9, 428. [Google Scholar] [CrossRef]
- Armentano, M.F.; Caterino, M.; Miglionico, R.; Ostuni, A.; Pace, M.C.; Cozzolino, F.; Monti, M.; Milella, L.; Carmosino, M.; Pucci, P. New insights on the functional role of URG7 in the cellular response to ER stress. Biol. Cell 2018, 110, 147–158. [Google Scholar] [CrossRef]







| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| β-actin | 5′-CCTGGCACCCAGCACAAT-3′ | 5′-GCCGATCCACACGGAGTACT-3′ |
| ABCG5 | 5′-GCTCCAGGATCCTAAGG-3′ | 5′-GAAAAAGCTCAGAACGG-3′ |
| ABCA1 | 5′-GGACATGCACAAGGTCCTGA-3′ | 5′-CAGAAAATCCTGGAGCTTCAAA-3′ |
| BIP | 5’-GTTTGCTGATAATTGGTTGAACA-3’ | 5’-GAATCGCCTGACACCTGAAGA-3’ |
| CAT | 5′-ATACCTGTGAACTGTCCCTACCG-3′ | 5′-GTTGAATCTCCGCACTTCTCCAG-3′ |
| CHOP | 5’-TCT CCT TCA TGC GCT GCT TTC-3′ | 5’-GTA CCT ATG TTT CAC CTC CTG-3′ |
| NQO1 | 5′-GGTGGTGGAGTCGGACCTCTA-3′ | 5′-AGGGTCCTTCAGTTTACCTGTGAT-3′ |
| Nrf2 | 5′-AACTACTCCCAGGTTGCCCA-3′ | 5′-CATTGTCATCTACAAACGGGAA-3′ |
| SOD2 | 5′-CCGACCTGCCCTACGACTAC-3′ | 5′-AACGCCTCCTGGTACTTCTCC-3′ |
| AMPK | 5’-TTCAAAAGGCTAATCACAGAAG-3’ | 5’-TTCAGGAAGATTGTATGCAGG-3’ |
| CPT1A | 5’-TTTGCAGTGCCCATCCTCCG-3′ | 5’-ACAGGTGGTTTGACAAGTCG-3’ |
| HMGCoA (HMGCR) | 5’-GCACCTTTCTCTGCATTC C-3’ | 5’-CGAGAAAGAAAAGTTGAGGT-3’ |
| PPARα | 5’-GAATCGCGTTGTGTGACATG-3’ | 5’-AGG CTGCAAGGGCTTCTTTC-3’ |
| SREBP-1c | 5’-TCCTTCAGAGATTTGCTTTTG-3’ | 5’-TGAGGCAAAGCTGAATAAATC-3’ |
| UCP2 | 5’-TTACGAGCAACATTGGGAGAG-3′ | 5’-GCTGGAGGTGGTCGGAGATA-3’ |
| LDLr | 5′-AGTGCGGGACCAACGAA-3′ | 5′-ATGGAGCCCACAGCCTT-3′ |
| TNFα | 5’-CACGATCAGGAAGGAGAAGA-3’ | 5’-TCTGGCCCAGGCAGTCAGAT-3’ |
| IL-6 | 5’-CTACTCTCAAATCTGTTCTGG-3’ | 5’-GGATTCAATGAGGAGACTTG-3’ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lela, L.; Russo, D.; De Biasio, F.; Gorgoglione, D.; Ostuni, A.; Ponticelli, M.; Milella, L. Solanum aethiopicum L. from the Basilicata Region Prevents Lipid Absorption, Fat Accumulation, Oxidative Stress, and Inflammation in OA-Treated HepG2 and Caco-2 Cell Lines. Plants 2023, 12, 2859. https://doi.org/10.3390/plants12152859
Lela L, Russo D, De Biasio F, Gorgoglione D, Ostuni A, Ponticelli M, Milella L. Solanum aethiopicum L. from the Basilicata Region Prevents Lipid Absorption, Fat Accumulation, Oxidative Stress, and Inflammation in OA-Treated HepG2 and Caco-2 Cell Lines. Plants. 2023; 12(15):2859. https://doi.org/10.3390/plants12152859
Chicago/Turabian StyleLela, Ludovica, Daniela Russo, Filomena De Biasio, Domenico Gorgoglione, Angela Ostuni, Maria Ponticelli, and Luigi Milella. 2023. "Solanum aethiopicum L. from the Basilicata Region Prevents Lipid Absorption, Fat Accumulation, Oxidative Stress, and Inflammation in OA-Treated HepG2 and Caco-2 Cell Lines" Plants 12, no. 15: 2859. https://doi.org/10.3390/plants12152859
APA StyleLela, L., Russo, D., De Biasio, F., Gorgoglione, D., Ostuni, A., Ponticelli, M., & Milella, L. (2023). Solanum aethiopicum L. from the Basilicata Region Prevents Lipid Absorption, Fat Accumulation, Oxidative Stress, and Inflammation in OA-Treated HepG2 and Caco-2 Cell Lines. Plants, 12(15), 2859. https://doi.org/10.3390/plants12152859






