Phosphorus Plays Key Roles in Regulating Plants’ Physiological Responses to Abiotic Stresses
Abstract
:1. Introduction
2. Physiological Adaptations to Low P and Regulatory Networks of Phosphorus in Plant Growth
2.1. Growth Retardation and Yield Loss under Low P
2.2. Root Architecture and Exudation
2.3. Molecular Responses to Low P
2.4. Hormonal Responses to Low P
2.5. Phosphorus Homeostasis
3. Stomatal Activity and Photosynthetic Efficiency in Response to P Deficiency
3.1. Stomatal Responses to Low P
3.2. Biochemical Responses to Low P
3.3. Mesophyll Resistance under Low P
4. Linking P Availability with Plant Tolerance to Environmental Stresses
4.1. Phosphorus and Drought Stress Tolerance
4.2. Phosphorus and Salinity Stress Tolerance
4.3. Phosphorus and Temperature Stress Tolerance
4.4. Phosphorus and Heavy Metal Stress Tolerance
4.5. Phosphorus, Waterlogging and Elevated CO2 Stress Tolerance
5. Future Prospects
- ▪
- It is essential to delve deeper into the relationship between phosphorus and abiotic stresses considering the bidirectional impact they have on each other. While phosphorus aids plants in adapting to stress conditions, it is crucial to recognize that stresses can also affect a plant’s ability to acquire phosphorus, forming a negative feedback loop. To overcome this challenge, it is necessary to focus on identifying and understanding the mechanisms underlying phosphorus tolerance in plants, particularly in the context of abiotic stresses. Investigating the genetic and physiological basis of phosphorus tolerance and exploring the molecular pathways involved will pave the way for developing crop varieties with enhanced phosphorus tolerance and stress resilience.
- ▪
- Phosphorus use efficiency (PUE) is a critical factor that can be influenced by abiotic stresses. By improving PUE, we can not only address phosphorus-related issues in agriculture but also enhance plant resilience to abiotic stresses. Therefore, it is crucial to investigate and interpret the mechanisms governing phosphorus use efficiency in plants under stress conditions. Understanding the molecular and physiological processes that contribute to efficient phosphorus utilization will provide insights into how to develop innovative strategies for improving PUE in crops. Moreover, identifying and characterizing crop varieties with high phosphorus use efficiency that can withstand abiotic stresses should be a focal point for future breeding programs.
- ▪
- Given the close relationship between phosphorus, stomata and abiotic stresses, it is imperative to explore the specific interactions and mechanisms involved in the “P-stomata-Abiotic stresses” nexus. The stomata, as key regulators of water loss and gas exchange, have a profound impact on plant tolerance to abiotic stresses such as drought, salinity and high temperatures. Integrating the role of phosphorus in stomatal regulation under stress conditions can provide valuable insights into how to improve plant resilience and stress tolerance.
- ▪
- Excessive phosphorus (P) can also elicit diverse and complex responses in plants, impacting both molecular and physiological modifications. At the molecular level, it alters gene expression, signaling pathways and epigenetic modifications. Physiologically, P promotes enhanced growth, increased photosynthesis and changes in root architecture. However, excess P can also disrupt nutrient balances, trigger oxidative stress and affect mycorrhizal associations. Understanding these responses is crucial for optimizing phosphorus management in agriculture and ensuring sustainable plant growth under high P use efficiency.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sarfraz, S.; Ali, F.; Hameed, A.; Ahmad, Z.; Riaz, K. Sustainable Agriculture Through Technological Innovations. In Sustainable Agriculture in the Era of the OMICs Revolution; Springer: Cham, Switzerland, 2023; pp. 223–239. [Google Scholar] [CrossRef]
- Heuer, S.; Gaxiola, R.; Schilling, R.; Herrera-Estrella, L.; López-Arredondo, D.; Wissuwa, M.; Delhaize, E.; Rouached, H. Improving Phosphorus Use Efficiency: A Complex Trait with Emerging Opportunities. Plant J. 2017, 90, 868–885. [Google Scholar] [CrossRef] [Green Version]
- Paz-Ares, J.; Puga, M.I.; Rojas-Triana, M.; Martinez-Hevia, I.; Diaz, S.; Poza-Carrión, C.; Miñambres, M.; Leyva, A. Plant Adaptation to Low Phosphorus Availability: Core Signaling, Crosstalks, and Applied Implications. Mol. Plant 2022, 15, 104–124. [Google Scholar] [CrossRef] [PubMed]
- López-Arredondo, D.L.; Leyva-González, M.A.; González-Morales, S.I.; López-Bucio, J.; Herrera-Estrella, L. Phosphate Nutrition: Improving Low-Phosphate Tolerance in Crops. Annu. Rev. Plant Biol. 2014, 65, 95–123. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Ecology-Controlling Eutrophication: Nitrogen and Phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef]
- Johnston, A.E.; Poulton, P.R.; Fixen, P.E.; Curtin, D. Phosphorus. Its Efficient Use in Agriculture. Adv. Agron. 2014, 123, 177–228. [Google Scholar] [CrossRef]
- Baker, A.; Ceasar, S.A.; Palmer, A.J.; Paterson, J.B.; Qi, W.; Muench, S.P.; Baldwin, S.A. Replace, Reuse, Recycle: Improving the Sustainable Use of Phosphorus by Plants. J. Exp. Bot. 2015, 66, 3523–3540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkesford, M.J.; Cakmak, I.; Coskun, D.; De Kok, L.J.; Lambers, H.; Schjoerring, J.K.; White, P.J. Functions of Macronutrients. Marschner’s Mineral Nutrition of Plants; Academic Press: Cambridge, MA, USA, 2023; pp. 201–281. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus Acquisition and Utilization in Plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef] [PubMed]
- George, T.S.; Hinsinger, P.; Turner, B.L. Phosphorus in Soils and Plants – Facing Phosphorus Scarcity. Plant Soil 2016, 401, 1–6. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus Dynamics: From Soil to Plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [Green Version]
- Baroowa, B.; Paul, S.; Gogoi, N. Role of Phosphorus in Imparting Abiotic Stress Tolerance to Plants. In Climate Change and Agriculture: Perspectives, Sustainability and Resilience; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 239–262. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2018; pp. 171–190. [Google Scholar] [CrossRef]
- Jin, J.; Lauricella, D.; Armstrong, R.; Sale, P.; Tang, C. Phosphorus Application and Elevated CO2 Enhance Drought Tolerance in Field Pea Grown in a Phosphorus-Deficient Vertisol. Ann. Bot. 2015, 116, 975–985. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Wang, C.; Qi, L.; Zhang, X.; Tang, G.; Li, L.; Guo, J.; Jia, Y.; Dou, X.; Lu, M. Phosphorus Is More Effective than Nitrogen in Restoring Plant Communities of Heavy Metals Polluted Soils. Environ. Pollut. 2020, 266, 115259. [Google Scholar] [CrossRef] [PubMed]
- Isidra-Arellano, M.C.; Delaux, P.M.; Valds-Lpez, O. The Phosphate Starvation Response System: Its Role in the Regulation of Plant-Microbe Interactions. Plant Cell Physiol. 2021, 62, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Jezek, M.; Allan, A.C.; Jones, J.J.; Geilfus, C.-M. Why Do Plants Blush When They Are Hungry? New Phytol. 2023, 239, 494–505. [Google Scholar] [CrossRef]
- Lopez, G.; Ahmadi, S.H.; Amelung, W.; Athmann, M.; Ewert, F.; Gaiser, T.; Gocke, M.I.; Kautz, T.; Postma, J.; Rachmilevitch, S.; et al. Nutrient Deficiency Effects on Root Architecture and Root-to-Shoot Ratio in Arable Crops. Front Plant Sci. 2023, 13, 5385. [Google Scholar] [CrossRef] [PubMed]
- Abobatta, W.; Abd Alla, M. Role of Phosphates Fertilizers in Sustain Horticulture Production: Growth and Productivity of Vegetable Crops. Asian J. Agric. Res. 2023, 17, 1–7. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring Phosphorus Fertilizers and Fertilization Strategies for Improved Human and Environmental Health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar] [CrossRef] [Green Version]
- Menezes-Blackburn, D.; Giles, C.; Darch, T.; George, T.S.; Blackwell, M.; Stutter, M.; Shand, C.; Lumsdon, D.; Cooper, P.; Wendler, R.; et al. Opportunities for Mobilizing Recalcitrant Phosphorus from Agricultural Soils: A Review. Plant Soil 2017, 427, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Ludemann, C.I.; Gruere, A.; Heffer, P.; Dobermann, A. Global Data on Fertilizer Use by Crop and by Country. Sci. Data 2022, 9, 1–8. [Google Scholar] [CrossRef]
- Teng, Z.; Zhu, J.; Shao, W.; Zhang, K.; Li, M.; Whelan, M.J. Increasing Plant Availability of Legacy Phosphorus in Calcareous Soils Using Some Phosphorus Activators. J. Environ. Manag. 2020, 256, 109952. [Google Scholar] [CrossRef]
- Hu, M.; Le, Y.; Sardans, J.; Yan, R.; Zhong, Y.; Sun, D.; Tong, C.; Peñuelas, J. Moderate Salinity Improves the Availability of Soil P by Regulating P-Cycling Microbial Communities in Coastal Wetlands. Glob. Chang. Biol. 2023, 29, 276–288. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Z.; Tian, F.; Liu, X.; Li, T.; He, Y.; Li, B.; Zhang, Z.; Yu, B. Phosphate-Solubilizing Microorganisms Regulate the Release and Transformation of Phosphorus in Biochar-Based Slow-Release Fertilizer. Sci. Total Environ. 2023, 869, 161622. [Google Scholar] [CrossRef]
- Fang, N.; Chen, Z.; Liu, Z.; Dai, H.; Yang, X.; Wang, W. Effects of Mechanochemically Activated Phosphate Rock on Maize Growth and Phosphorus Use. Plant Soil Environ. 2022, 68, 155–161. [Google Scholar] [CrossRef]
- Teixeira, R.d.S.; Ribeiro da Silva, I.; Nogueira de Sousa, R.; Márcio Mattiello, E.; Barros Soares, E.M. Organic Acid Coated-Slow-Release Phosphorus Fertilizers Improve P Availability and Maize Growth in a Tropical Soil. J. Soil Sci. Plant Nutr. 2016, 16, 1097–1112. [Google Scholar] [CrossRef] [Green Version]
- Fertahi, S.; Bertrand, I.; Ilsouk, M.; Oukarroum, A.; Amjoud, M.B.; Zeroual, Y.; Barakat, A. New Generation of Controlled Release Phosphorus Fertilizers Based on Biological Macromolecules: Effect of Formulation Properties on Phosphorus Release. Int. J. Biol. Macromol. 2020, 143, 153–162. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.H. Current and Future Perspectives on the Use of Nanofertilizers for Sustainable Agriculture: The Case of Phosphorus Nanofertilizer. 3 Biotech 2021, 11, 357. [Google Scholar] [CrossRef]
- McBeath, T.M.; Facelli, E.; Peirce, C.A.E.; Arachchige, V.K.; McLaughlin, M.J.; McBeath, T.M.; Facelli, E.; Peirce, C.A.E.; Arachchige, V.K.; McLaughlin, M.J. Assessment of Foliar-Applied Phosphorus Fertiliser Formulations to Enhance Phosphorus Nutrition and Grain Production in Wheat. Crop Pasture Sci. 2020, 71, 795–806. [Google Scholar] [CrossRef]
- Scholz, R.W.; Wellmer, F.W. Approaching a Dynamic View on the Availability of Mineral Resources: What We May Learn from the Case of Phosphorus? Glob. Environ. Chang. 2013, 23, 11–27. [Google Scholar] [CrossRef]
- Faucon, M.P.; Houben, D.; Reynoird, J.P.; Mercadal-Dulaurent, A.M.; Armand, R.; Lambers, H. Advances and Perspectives to Improve the Phosphorus Availability in Cropping Systems for Agroecological Phosphorus Management. Adv. Agron. 2015, 134, 51–79. [Google Scholar] [CrossRef]
- Ibrahim, M.; Iqbal, M.; Tang, Y.T.; Khan, S.; Guan, D.X.; Li, G. Phosphorus Mobilization in Plant-Soil Environments and Inspired Strategies for Managing Phosphorus: A Review. Agronomy 2022, 12, 2539. [Google Scholar] [CrossRef]
- Plaxton, W.C.; Lambers, H. Phosphorus Metabolism in Plants. Phosphorus metab. Plants 2015, 48, 1–449. [Google Scholar] [CrossRef]
- Tariq, A.; Zeng, F.; Graciano, C.; Ullah, A.; Sadia, S.; Ahmed, Z.; Murtaza, G.; Ismoilov, K.; Zhang, Z. Regulation of Metabolites by Nutrients in Plants. Plant Ionomics 2023, 1–18. [Google Scholar] [CrossRef]
- Wen, Z.; Li, H.; Shen, J.; Rengel, Z. Maize Responds to Low Shoot P Concentration by Altering Root Morphology Rather than Increasing Root Exudation. Plant Soil 2017, 416, 377–389. [Google Scholar] [CrossRef]
- Veronica, N.; Subrahmanyam, D.; Vishnu Kiran, T.; Yugandhar, P.; Bhadana, V.P.; Padma, V.; Jayasree, G.; Voleti, S.R. Influence of Low Phosphorus Concentration on Leaf Photosynthetic Characteristics and Antioxidant Response of Rice Genotypes. Photosynthetica 2017, 55, 285–293. [Google Scholar] [CrossRef]
- Chea, L.; Meijide, A.; Meinen, C.; Pawelzik, E.; Naumann, M. Cultivar-Dependent Responses in Plant Growth, Leaf Physiology, Phosphorus Use Efficiency, and Tuber Quality of Potatoes Under Limited Phosphorus Availability Conditions. Front Plant Sci. 2021, 12, 1728. [Google Scholar] [CrossRef]
- Nord, E.A.; Lynch, J.P. Delayed Reproduction in Arabidopsis thaliana Improves Fitness in Soil with Suboptimal Phosphorus Availability. Plant Cell Environ. 2008, 31, 1432–1441. [Google Scholar] [CrossRef]
- Taliman, N.A.; Dong, Q.; Echigo, K.; Raboy, V.; Saneoka, H. Effect of Phosphorus Fertilization on the Growth, Photosynthesis, Nitrogen Fixation, Mineral Accumulation, Seed Yield, and Seed Quality of a Soybean Low-Phytate Line. Plants 2019, 8, 119. [Google Scholar] [CrossRef] [Green Version]
- Sandaña, P.; Pinochet, D. Ecophysiological Determinants of Biomass and Grain Yield of Wheat under P Deficiency. Field Crops Res. 2011, 120, 311–319. [Google Scholar] [CrossRef]
- Gutiérrez-Boem, F.H.; Thomas, G.W. Phosphorus Nutrition Affects Wheat Response to Water Deficit. Agron. J. 1998, 90, 166–171. [Google Scholar] [CrossRef]
- Zhang, C.; Simpson, R.J.; Kim, C.M.; Warthmann, N.; Delhaize, E.; Dolan, L.; Byrne, M.E.; Wu, Y.; Ryan, P.R. Do Longer Root Hairs Improve Phosphorus Uptake? Testing the Hypothesis with Transgenic Brachypodium Distachyon Lines Overexpressing Endogenous RSL Genes. New Phytol. 2018, 217, 1654–1666. [Google Scholar] [CrossRef] [Green Version]
- Mollier, A.; Pellerin, S. Maize Root System Growth and Development as Influenced by Phosphorus Deficiency. J. Exp. Bot. 1999, 50, 487–497. [Google Scholar] [CrossRef]
- Nacry, P.; Canivenc, G.; Muller, B.; Azmi, A.; Van Onckelen, H.; Rossignol, M.; Doumas, P. A Role for Auxin Redistribution in the Responses of the Root System Architecture to Phosphate Starvation in Arabidopsis. Plant Physiol. 2005, 138, 2061–2074. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Zhang, L.; Niu, D.; Nan, S.; Wu, S.; Gao, H.; Fu, H. Transcriptome Analysis of Zygophyllum Xanthoxylum Adaptation Strategies to Phosphate Stress. Front Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- López-Bucio, J.; Hernández-Abreu, E.; Sánchez-Calderón, L.; Nieto-Jacobo, M.F.; Simpson, J.; Herrera-Estrella, L. Phosphate Availability Alters Architecture and Causes Changes in Hormone Sensitivity in the Arabidopsis Root System. Plant Physiol. 2002, 129, 244–256. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Andújar, C.; Ruiz-Lozano, J.M.; Dodd, I.C.; Albacete, A.; Pérez-Alfocea, F. Hormonal and Nutritional Features in Contrasting Rootstock-Mediated Tomato Growth under Low-Phosphorus Nutrition. Front Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, L.; Müller, R.; Nielsen, T.H. Dissecting the Plant Transcriptome and the Regulatory Responses to Phosphate Deprivation. Physiol. Plant 2010, 139, 129–143. [Google Scholar] [CrossRef]
- Niu, Y.F.; Chai, R.S.; Jin, G.L.; Wang, H.; Tang, C.X.; Zhang, Y.S. Responses of Root Architecture Development to Low Phosphorus Availability: A Review. Ann. Bot. 2013, 112, 391–408. [Google Scholar] [CrossRef]
- Nguyen, V.L.; Stangoulis, J. Variation in Root System Architecture and Morphology of Two Wheat Genotypes Is a Predictor of Their Tolerance to Phosphorus Deficiency. Acta Physiol. Plant 2019, 41, 109. [Google Scholar] [CrossRef] [Green Version]
- Ligaba, A.; Yamaguchi, M.; Shen, H.; Sasaki, T.; Yamamoto, Y.; Matsumoto, H. Phosphorus Deficiency Enhances Plasma Membrane H+-ATPase Activity and Citrate Exudation in Greater Purple Lupin (Lupinus pilosus). Funct. Plant Biol. 2004, 31, 1075–1083. [Google Scholar] [CrossRef]
- Cosse, M.; Seidel, T. Plant Proton Pumps and Cytosolic PH-Homeostasis. Front. Plant Sci. 2021, 12, 846. [Google Scholar] [CrossRef]
- Irfan, M.; Aziz, T.; Maqsood, M.A.; Bilal, H.M.; Siddique, K.H.M.; Xu, M. Phosphorus (P) Use Efficiency in Rice Is Linked to Tissue-Specific Biomass and P Allocation Patterns. Sci. Rep. 2020, 10, 4278. [Google Scholar] [CrossRef] [Green Version]
- Dong, D.; Peng, X.; Yan, X. Organic Acid Exudation Induced by Phosphorus Deficiency and/or Aluminium Toxicity in Two Contrasting Soybean Genotypes. Physiol. Plant 2004, 122, 190–199. [Google Scholar] [CrossRef]
- Brisson, V.L.; Richardy, J.; Kosina, S.M.; Northen, T.R.; Vogel, J.P.; Gaudin, A.C.M. Phosphate Availability Modulates Root Exudate Composition and Rhizosphere Microbial Community in a Teosinte and a Modern Maize Cultivar. Phytobiomes J. 2022, 6, 83–94. [Google Scholar] [CrossRef]
- Shi, J.; Wang, X.; Wang, E. Mycorrhizal symbiosis in Plant Growth and Stress Adaptation: From Genes to Ecosystems. Annu. Rev. Plant Biol. 2023, 74. [Google Scholar] [CrossRef]
- Pantigoso, H.A.; Manter, D.K.; Fonte, S.J.; Vivanco, J.M. Root Exudate-Derived Compounds Stimulate the Phosphorus Solubilizing Ability of Bacteria. Sci. Rep. 2023, 13, 1–12. [Google Scholar] [CrossRef]
- Wang, G.; Jin, Z.; Wang, X.; George, T.S.; Feng, G.; Zhang, L. Simulated Root Exudates Stimulate the Abundance of Saccharimonadales to Improve the Alkaline Phosphatase Activity in Maize Rhizosphere. Appl. Soil Ecol. 2022, 170, 104274. [Google Scholar] [CrossRef]
- Ticconi, C.A.; Lucero, R.D.; Sakhonwasee, S.; Adamson, A.W.; Creff, A.; Nussaume, L.; Desnos, T.; Abel, S. ER-Resident Proteins PDR2 and LPR1 Mediate the Developmental Response of Root Meristems to Phosphate Availability. Proc. Natl. Acad. Sci. USA 2009, 106, 14174–14179. [Google Scholar] [CrossRef]
- Rubio, V.; Linhares, F.; Solano, R.; Martín, A.C.; Iglesias, J.; Leyva, A.; Paz-Ares, J. A Conserved MYB Transcription Factor Involved in Phosphate Starvation Signaling Both in Vascular Plants and in Unicellular Algae. Genes Dev. 2001, 15, 2122–2133. [Google Scholar] [CrossRef] [Green Version]
- Duan, K.; Yi, K.; Dang, L.; Huang, H.; Wu, W.; Wu, P. Characterization of a Sub-Family of Arabidopsis Genes with the SPX Domain Reveals Their Diverse Functions in Plant Tolerance to Phosphorus Starvation. Plant J. 2008, 54, 965–975. [Google Scholar] [CrossRef]
- Zhang, D.; Song, H.; Cheng, H.; Hao, D.; Wang, H.; Kan, G.; Jin, H.; Yu, D. The Acid Phosphatase-Encoding Gene GmACP1 Contributes to Soybean Tolerance to Low-Phosphorus Stress. PLoS Genet. 2014, 10, e100406. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Yin, Z.; Chao, M.; Ning, L.; Zhang, D.; Yu, D. Functional Properties and Expression Quantitative Trait Loci for Phosphate Transporter GmPT1 in Soybean. Plant Cell Environ. 2014, 37, 462–472. [Google Scholar] [CrossRef]
- Fan, C.; Wang, X.; Hu, R.; Wang, Y.; Xiao, C.; Jiang, Y.; Zhang, X.; Zheng, C.; Fu, Y.F. The Pattern of Phosphate Transporter 1 Genes Evolutionary Divergence in Glycine Max L. BMC Plant Biol. 2013, 13. [Google Scholar] [CrossRef] [Green Version]
- Bayuelo-Jiménez, J.S.; Gallardo-Valdéz, M.; Pérez-Decelis, V.A.; Magdaleno-Armas, L.; Ochoa, I.; Lynch, J.P. Genotypic Variation for Root Traits of Maize (Zea mays L.) from the Purhepecha Plateau under Contrasting Phosphorus Availability. Field Crops Res. 2011, 121, 350–362. [Google Scholar] [CrossRef]
- Gahoonia, T.S.; Nielsen, N.E. Phosphorus (P) Uptake and Growth of a Root Hairless Barley Mutant (Bald Root Barley, Brb) and Wild Type in Low- and High-P Soils. Plant Cell Environ. 2003, 26, 1759–1766. [Google Scholar] [CrossRef] [Green Version]
- Bates, T.R.; Lynch, J.P. Plant Growth and Phosphorus Accumulation of Wild Type and Two Root Hair Mutants of Arabidopsis thaliana (Brassicaceae). Am. J. Bot. 2000, 87, 958–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Wang, Y.; Yang, A.; Zhang, W.H. OsMYB2P-1, an R2R3 MYB Transcription Factor, Is Involved in the Regulation of Phosphate-Starvation Responses and Root Architecture in Rice. Plant Physiol. 2012, 159, 169–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, K.E.; Lauter, N.; Lin, S.F.; Scott, M.P.; Shoemaker, R.C. Evaluation and QTL Mapping of Phosphorus Concentration in Soybean Seed. Euphytica 2013, 189, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Liang, Q.; Cheng, X.; Mei, M.; Yan, X.; Liao, H. QTL Analysis of Root Traits as Related to Phosphorus Efficiency in Soybean. Ann. Bot. 2010, 106, 223. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Liu, C.; Cheng, H.; Kan, G.; Cui, S.; Meng, Q.; Gai, J.; Yu, D. Quantitative Trait Loci Associated with Soybean Tolerance to Low Phosphorus Stress Based on Flower and Pod Abscission. Plant Breed. 2010, 129, 243–249. [Google Scholar] [CrossRef]
- Misson, J.; Raghothama, K.G.; Jain, A.; Jouhet, J.; Block, M.A.; Bligny, R.; Ortet, P.; Creff, A.; Somerville, S.; Rolland, N.; et al. A Genome-Wide Transcriptional Analysis Using Arabidopsis thaliana Affymetrix Gene Chips Determined Plant Responses to Phosphate Deprivation. Proc. Natl. Acad. Sci. USA 2005, 102, 11934–11939. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Vazquez, C.; Ibarra-Laclette, E.; Caballero-Perez, J.; Herrera-Estrella, L. Transcript Profiling of Zea mays Roots Reveals Gene Responses to Phosphate Deficiency at the Plant- and Species-Specific Levels. J. Exp. Bot. 2008, 59, 2479–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Liu, C.; Lian, X. Gene Expression Profiles in Rice Roots under Low Phosphorus Stress. Plant Mol. Biol. 2010, 72, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Wang, G.; Zhang, Y.; Hu, X.; Pi, E.; Zhu, Y.; Wang, H.; Du, L.; Hammond, J.; Zeng, H.; et al. Genome-Wide Identification of Phosphate-Deficiency-Responsive Genes in Soybean Roots by High-Throughput Sequencing. Plant Soil 2015, 398, 207–227. [Google Scholar] [CrossRef]
- Martín, A.C.; Del Pozo, J.C.; Iglesias, J.; Rubio, V.; Solano, R.; De La Peña, A.; Leyva, A.; Paz-Ares, J. Influence of Cytokinins on the Expression of Phosphate Starvation Responsive Genes in Arabidopsis. Plant J. 2000, 24, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Vysotskaya, L.; Akhiyarova, G.; Feoktistova, A.; Akhtyamova, Z.; Korobova, A.; Ivanov, I.; Dodd, I.; Kuluev, B.; Kudoyarova, G. Effects of Phosphate Shortage on Root Growth and Hormone Content of Barley Depend on Capacity of the Roots to Accumulate ABA. Plants 2020, 9, 1722. [Google Scholar] [CrossRef]
- Ha, S.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S.P. Cytokinins: Metabolism and Function in Plant Adaptation to Environmental Stresses. Trends Plant Sci. 2012, 17, 172–179. [Google Scholar] [CrossRef]
- Srivastava, S.; Ranjan, M.; Bano, N.; Asif, M.H.; Srivastava, S. Comparative Transcriptome Analysis Reveals the Phosphate Starvation Alleviation Mechanism of Phosphate Accumulating Pseudomonas putida in Arabidopsis thaliana. Sci. Rep. 2023, 13, 4918. [Google Scholar] [CrossRef]
- Nagarajan, V.K.; Smith, A.P. Ethylene’s Role in Phosphate Starvation Signaling: More than Just a Root Growth Regulator. Plant Cell Physiol. 2012, 53, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Santoro, V.; Schiavon, M.; Visentin, I.; Constán-Aguilar, C.; Cardinale, F.; Celi, L. Strigolactones Affect Phosphorus Acquisition Strategies in Tomato Plants. Plant Cell Environ. 2021, 44, 3628–3642. [Google Scholar] [CrossRef]
- Satheesh, V.; Tahir, A.; Li, J.; Lei, M. Plant Phosphate Nutrition: Sensing the Stress. Stress Biol. 2022, 2, 1–14. [Google Scholar] [CrossRef]
- Wang, F.; Deng, M.; Xu, J.; Zhu, X.; Mao, C. Molecular Mechanisms of Phosphate Transport and Signaling in Higher Plants. Semin. Cell Dev. Biol. 2018, 74, 114–122. [Google Scholar] [CrossRef]
- Ham, B.K.; Chen, J.; Yan, Y.; Lucas, W.J. Insights into Plant Phosphate Sensing and Signaling. Curr. Opin. Biotechnol. 2018, 49, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nussaume, L.; Kanno, S.; Javot, H.; Marin, E.; Pochon, N.; Ayadi, A.; Nakanishi, T.M.; Thibaud, M.C. Phosphate Import in Plants: Focus on the PHT1 Transporters. Front. Plant Sci. 2011, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.Y.; Shirley, N.; Genc, Y.; Shi, B.; Langridge, P. Phosphate Utilization Efficiency Correlates with Expression of Low-Affinity Phosphate Transporters and Noncoding RNA, IPS1, in Barley. Plant Physiol. 2011, 156, 1217–1229. [Google Scholar] [CrossRef] [Green Version]
- Bhadouria, J.; Giri, J. Purple Acid Phosphatases: Roles in Phosphate Utilization and New Emerging Functions. Plant Cell Rep. 2022, 41, 33–51. [Google Scholar] [CrossRef]
- Lapis-Gaza, H.R.; Jost, R.; Finnegan, P.M. Arabidopsis PHOSPHATE TRANSPORTER1 Genes PHT1;8 and PHT1;9 Are Involved in Root-to-Shoot Translocation of Orthophosphate. BMC Plant Biol. 2014, 14. [Google Scholar] [CrossRef] [Green Version]
- González, E.; Solano, R.; Rubio, V.; Leyva, A.; Paz-Ares, J. PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 Is a Plant-Specific SEC12-Related Protein That Enables the Endoplasmic Reticulum Exit of a High-Affinity Phosphate Transporter in Arabidopsis. Plant Cell 2005, 17, 3500–3512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustos, R.; Castrillo, G.; Linhares, F.; Puga, M.I.; Rubio, V.; Pérez-Pérez, J.; Solano, R.; Leyva, A.; Paz-Ares, J. A Central Regulatory System Largely Controls Transcriptional Activation and Repression Responses to Phosphate Starvation in Arabidopsis. PLoS Genet. 2010, 6, e1001102. [Google Scholar] [CrossRef] [Green Version]
- Prathap, V.; Kumar, A.; Maheshwari, C.; Tyagi, A. Phosphorus Homeostasis: Acquisition, Sensing, and Long-Distance Signaling in Plants. Mol. Biol. Rep. 2022, 49, 8071–8086. [Google Scholar] [CrossRef]
- Devaiah, B.N.; Karthikeyan, A.S.; Raghothama, K.G. WRKY75 Transcription Factor Is a Modulator of Phosphate Acquisition and Root Development in Arabidopsis. Plant Physiol. 2007, 143, 1789–1801. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Wang, C.; Wang, H.; Li, L.; Wang, C. The Function of MAPK Cascades in Response to Various Stresses in Horticultural Plants. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Ludwig, A.A.; Romeis, T.; Jones, J.D.G. CDPK-Mediated Signalling Pathways: Specificity and Cross-Talk. J. Exp. Bot. 2004, 55, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Ingargiola, C.; Duarte, G.T.; Robaglia, C.; Leprince, A.S.; Meyer, C. The Plant Target of Rapamycin: A Conductor of Nutrition and Metabolism in Photosynthetic Organisms. Genes 2020, 11, 1285. [Google Scholar] [CrossRef] [PubMed]
- Burkart, G.M.; Brandizzi, F. A Tour of TOR Complex Signaling in Plants. Trends Biochem. Sci. 2021, 46, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; White, P.J.; Cheng, L. Mechanisms for Improving Phosphorus Utilization Efficiency in Plants. Ann. Bot. 2022, 129, 247. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Huang, T.K.; Kuo, H.F.; Chiou, T.J. Role of Vacuoles in Phosphorus Storage and Remobilization. J. Exp. Bot. 2017, 68, 3045–3055. [Google Scholar] [CrossRef] [Green Version]
- Jeong, K.; Baten, A.; Waters, D.L.E.; Pantoja, O.; Julia, C.C.; Wissuwa, M.; Heuer, S.; Kretzschmar, T.; Rose, T.J. Phosphorus Remobilization from Rice Flag Leaves during Grain Filling: An RNA-Seq Study. Plant Biotechnol. J. 2017, 15, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Paul, P.; Sharma, S.; Pandey, R. Phosphorus Scavenging and Remobilization from Root Cell Walls Under Combined Nitrogen and Phosphorus Stress Is Regulated by Phytohormones and Nitric Oxide Cross-Talk in Wheat. J. Plant Growth Regul. 2022, 42, 1614–1630. [Google Scholar] [CrossRef]
- Jacob, J.; Lawlor, D.W. Stomatal and Mesophyll Limitations of Photosynthesis in Phosphate Deficient Sunflower, Maize and Wheat Plants. J. Exp. Bot. 1991, 42, 1003–1011. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.T.; Wang, L.F.; Guo, J.X.; Lu, K.K.; Song, R.F.; Zuo, J.X.; Chen, H.H.; Liu, W.C. Abscisic Acid Facilitates Phosphate Acquisition through the Transcription Factor ABA INSENSITIVE5 in Arabidopsis. Plant J. 2022, 111, 269–281. [Google Scholar] [CrossRef]
- Iqbal, A.; Huiping, G.; Qiang, D.; Xiangru, W.; Hengheng, Z.; Xiling, Z.; Meizhen, S. Differential Responses of Contrasting Low Phosphorus Tolerant Cotton Genotypes under Low Phosphorus and Drought Stress. BMC Plant Biol. 2023, 23, 168. [Google Scholar] [CrossRef]
- Chtouki, M.; Laaziz, F.; Naciri, R.; Garré, S.; Nguyen, F.; Oukarroum, A. Interactive Effect of Soil Moisture Content and Phosphorus Fertilizer Form on Chickpea Growth, Photosynthesis, and Nutrient Uptake. Sci. Rep. 2022, 12, 6671. [Google Scholar] [CrossRef] [PubMed]
- Talbi Zribi, O.; Abdelly, C.; Debez, A. Interactive Effects of Salinity and Phosphorus Availability on Growth, Water Relations, Nutritional Status and Photosynthetic Activity of Barley (Hordeum Vulgare L.). Plant Biol. 2011, 13, 872–880. [Google Scholar] [CrossRef]
- Nagarajah, S.; Ratnasuriya, G.B. The Effect of Phosphorus and Potassium Deficiencies on Transpiration in Tea (Camellia sinensis). Physiol. Plant 1978, 42, 103–108. [Google Scholar] [CrossRef]
- Radin, J.W. Stomatal Responses to Water Stress and to Abscisic Acid in Phosphorus-Deficient Cotton Plants. Plant Physiol. 1984, 76, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Castro-Valdecantos, P.; Martínez-Melgarejo, P.A.; Pérez-Alfocea, F.; Tian, J.; Dodd, I.C. Stem Girdling Enhances ABA-Induced Stomatal Closure of Phosphorus-Deprived Soybean Plants. Environ. Exp. Bot. 2023, 208, 105266. [Google Scholar] [CrossRef]
- Li, Y.-F.; Luo, A.-C.; Muhammad, J.H.; Wei, X.-H. Effect of Phosphorus Deficiency on Leaf Photosynthesis and Carbohydrates Partitioning in Two Rice Genotypes with Contrasting Low Phosphorus Susceptibility. Rice Sci. 2006, 13, 283. [Google Scholar]
- Starck, Z.; Niemyska, B.; Bogdan, J.; Akour Tawalbeh, R.N. Response of Tomato Plants to Chilling Stress in Association with Nutrient or Phosphorus Starvation. Plant Soil 2000, 226, 99–106. [Google Scholar] [CrossRef]
- Carstensen, A.; Herdean, A.; Schmidt, S.B.; Sharma, A.; Spetea, C.; Pribil, M.; Husted, S. The Impacts of Phosphorus Deficiency on the Photosynthetic Electron Transport Chain. Plant Physiol. 2018, 177, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Garcia, A.; Gaju, O.; Bowerman, A.F.; Buck, S.A.; Evans, J.R.; Furbank, R.T.; Gilliham, M.; Millar, A.H.; Pogson, B.J.; Reynolds, M.P.; et al. Enhancing Crop Yields through Improvements in the Efficiency of Photosynthesis and Respiration. New Phytol. 2023, 237, 60–77. [Google Scholar] [CrossRef]
- Saengwilai, P.J.; Bootti, P.; Klinnawee, L. Responses of Rubber Tree Seedlings (Hevea brasiliensis) to Phosphorus Deficient Soils. Soil Sci. Plant Nutr. 2023, 69, 78–87. [Google Scholar] [CrossRef]
- Iqbal, A.; Qiang, D.; Xiangru, W.; Huiping, G.; Hengheng, Z.; Xiling, Z.; Meizhen, S. Genotypic Variation in Cotton Genotypes for Low Phosphorus Tolerance and Efficiency Under Different Growth Conditions. Gesunde Pflanzen 2023, 75, 1–19. [Google Scholar] [CrossRef]
- Kayoumu, M.; Iqbal, A.; Muhammad, N.; Li, X.; Li, L.; Wang, X.; Gui, H.; Qi, Q.; Ruan, S.; Guo, R.; et al. Phosphorus Availability Affects the Photosynthesis and Antioxidant System of Contrasting Low-P-Tolerant Cotton Genotypes. Antioxidants 2023, 12, 466. [Google Scholar] [CrossRef] [PubMed]
- Terry, N.; Ulrich, A. Effects of Phosphorus Deficiency on the Photosynthesis and Respiration of Leaves of Sugar Beet. Plant Physiol. 1973, 51, 43–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieters, A.J.; Paul, M.J.; Lawlor, D.W. Low Sink Demand Limits Photosynthesis under Pi Deficiency. J. Exp. Bot. 2001, 52, 1083–1091. [Google Scholar] [CrossRef] [Green Version]
- Ben Hamed, S.; Lefi, E.; Chaieb, M. Effect of Phosphorus Concentration on the Photochemical Stability of PSII and CO2 Assimilation in Pistacia Vera L. and Pistacia Atlantica Desf. Plant Physiol. Biochem. 2019, 142, 283–291. [Google Scholar] [CrossRef]
- Lin, Z.H.; Chen, L.S.; Chen, R.B.; Zhang, F.Z.; Jiang, H.X.; Tang, N. CO2 Assimilation, Ribulose-1,5-Bisphosphate Carboxylase/ Oxygenase, Carbohydrates and Photosynthetic Electron Transport Probed by the JIP-Test, of Tea Leaves in Response to Phosphorus Supply. BMC Plant Biol. 2009, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Chen, W.W.; Wang, Y.Y.; Huang, Z.R.; Ye, X.; Chen, L.S.; Yang, L.T. Effects of Phosphorus Deficiency on the Absorption of Mineral Nutrients, Photosynthetic System Performance and Antioxidant Metabolism in Citrus Grandis. PLoS ONE 2021, 16, e0246944. [Google Scholar] [CrossRef]
- Thuynsma, R.; Kleinert, A.; Kossmann, J.; Valentine, A.J.; Hills, P.N. The Effects of Limiting Phosphate on Photosynthesis and Growth of Lotus Japonicus. S. Afr. J. Bot. 2016, 104, 244–248. [Google Scholar] [CrossRef]
- Nazir, M.M.; Li, Q.; Noman, M.; Ulhassan, Z.; Ali, S.; Ahmed, T.; Zeng, F.; Zhang, G. Calcium Oxide Nanoparticles Have the Role of Alleviating Arsenic Toxicity of Barley. Front Plant Sci. 2022, 13, 843795. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Liang, Y.; Lu, T.; Liu, Z.; Jin, X.; Hou, L.; Xu, J.; Zhao, H.; Shi, Y.; et al. Comparative Transcriptomic and Metabolomic Analyses Reveal the Protective Effects of Silicon against Low Phosphorus Stress in Tomato Plants. Plant Physiol. Biochem. 2021, 166, 78–87. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; He, L.; Zhu, W.; Yan, L.; Chen, Q.; He, C. The PHOSPHATE1 Genes Participate in Salt and Pi Signaling Pathways and Play Adaptive Roles during Soybean Evolution. BMC Plant Biol. 2019, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Yang, Y.; Sun, C.; Liu, X.; Lv, L.; Hu, Z.; Yu, D.; Zhang, D. Up-Regulating GmETO1 Improves Phosphorus Uptake and Use Efficiency by Promoting Root Growth in Soybean. Plant Cell Environ. 2020, 43, 2080–2094. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cai, C.; He, J.; Gu, J.; Zhu, G.; Zhang, W.; Zhu, J.; Liu, G. Yield, Dry Matter Distribution and Photosynthetic Characteristics of Rice under Elevated CO2 and Increased Temperature Conditions. Field Crops Res. 2020, 248. [Google Scholar] [CrossRef]
- Du, W.; Ning, L.; Liu, Y.; Zhang, S.; Yang, Y.; Wang, Q.; Chao, S.; Yang, H.; Huang, F.; Cheng, H.; et al. Identification of Loci and Candidate Gene GmSPX-RING1 Responsible for Phosphorus Efficiency in Soybean via Genome-Wide Association Analysis. BMC Genom. 2020, 21. [Google Scholar] [CrossRef]
- Xue, Y.B.; Xiao, B.X.; Zhu, S.N.; Mo, X.H.; Liang, C.Y.; Tian, J.; Liao, H. GmPHR25, a GmPHR Member up-Regulated by Phosphate Starvation, Controls Phosphate Homeostasis in Soybean. J. Exp. Bot. 2017, 68, 4951–4967. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Song, L.; Zhang, Y.; Zheng, Z.; Liu, D. Arabidopsis PHL2 and PHR1 Act Redundantly as the Key Components of the Central Regulatory System Controlling Transcriptional Responses to Phosphate Starvation. Plant Physiol. 2016, 170, 499–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Q.; Li, X.; Qiu, Z.; Hong, Y.; Tian, T.; Li, S.; Ran, J.; Qiao, G. Chinese Cherry (Cerasus pseudocerasus Lindl.) ARF7 Participates in Root Development and Responds to Drought and Low Phosphorus. Horticulturae 2022, 8, 158. [Google Scholar] [CrossRef]
- Li, L.; Pan, S.; Melzer, R.; Fricke, W. Apoplastic Barriers, Aquaporin Gene Expression and Root and Cell Hydraulic Conductivity in Phosphate-Limited Sheepgrass Plants. Physiol. Plant 2020, 168, 118–132. [Google Scholar] [CrossRef]
- Armand, T.; Cullen, M.; Boiziot, F.; Li, L.; Fricke, W. Cortex Cell Hydraulic Conductivity, Endodermal Apoplastic Barriers and Root Hydraulics Change in Barley (Hordeum vulgare L.) in Response to a Low Supply of N and P. Ann. Bot. 2019, 124, 1091–1107. [Google Scholar] [CrossRef]
- Lian, W.; Yu, Q.; Zhang, P.; Cui, Y.; Yin, Z.; Cui, H.; Chen, L.; Jia, H. NtPIN3 Positively Regulates Low Phosphorus Tolerance by Changing Root Elongation, Pi Concentration and Antioxidant Capacity in Tobacco. Environ. Exp. Bot. 2023, 208. [Google Scholar] [CrossRef]
- Du, K.; Yang, Y.; Li, J.; Wang, M.; Jiang, J.; Wu, J.; Fang, Y.; Xiang, Y.; Wang, Y. Functional Analysis of Bna-MiR399c-PHO2 Regulatory Module Involved in Phosphorus Stress in Brassica napus. Life 2023, 13, 310. [Google Scholar] [CrossRef] [PubMed]
- Attarzadeh, M.; Balouchi, H.; Rajaie, M.; Movahhedi Dehnavi, M.; Salehi, A. Growth and Nutrient Content of Echinacea Purpurea as Affected by the Combination of Phosphorus with Arbuscular Mycorrhizal Fungus and Pseudomonas Florescent Bacterium under Different Irrigation Regimes. J. Environ. Manag. 2019, 231, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Mamnabi, S.; Nasrollahzadeh, S.; Ghassemi-Golezani, K.; Raei, Y. Improving Yield-Related Physiological Characteristics of Spring Rapeseed by Integrated Fertilizer Management under Water Deficit Conditions. Saudi J. Biol. Sci. 2020, 27, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Sun, F.; Zhang, L.; Wu, X.; Chen, W.; Song, D.; et al. Phosphorous Fertilization Alleviates Drought Effects on Alnus cremastogyne by Regulating Its Antioxidant and Osmotic Potential. Sci. Rep. 2018, 8, 5644. [Google Scholar] [CrossRef]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Li, N.; Song, D.; Sun, F.; Wu, X.; Dakhil, M.A.; et al. Impact of Phosphorus Application on Drought Resistant Responses of Eucalyptus Grandis Seedlings. Physiol. Plant 2019, 166, 894–908. [Google Scholar] [CrossRef]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Sun, F.; Sun, X.; Song, D.; Chen, W.; Zhang, A.; et al. Phosphorous Application Improves Drought Tolerance of Phoebe zhennan. Front. Plant Sci. 2017, 8, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Wang, G.; Liu, X.; Pan, X.; Herbert, S.J.; Tang, C. Interaction between Phosphorus Nutrition and Drought on Grain Yield, and Assimilation of Phosphorus and Nitrogen in Two Soybean Cultivars Differing in Protein Concentration in Grains. J. Plant Nutr. 2007, 29, 1433–1449. [Google Scholar] [CrossRef]
- Loudari, A.; Mayane, A.; Zeroual, Y.; Colinet, G.; Oukarroum, A. Photosynthetic Performance and Nutrient Uptake under Salt Stress: Differential Responses of Wheat Plants to Contrasting Phosphorus Forms and Rates. Front. Plant Sci. 2022, 13, 1038672. [Google Scholar] [CrossRef]
- Bouras, H.; Choukr-Allah, R.; Amouaouch, Y.; Bouaziz, A.; Devkota, K.P.; El Mouttaqi, A.; Bouazzama, B.; Hirich, A. How Does Quinoa (Chenopodium quinoa Willd.) Respond to Phosphorus Fertilization and Irrigation Water Salinity? Plants 2022, 11, 216. [Google Scholar] [CrossRef]
- Bouras, H.; Bouaziz, A.; Bouazzama, B.; Hirich, A.; Choukr-Allah, R. How Phosphorus Fertilization Alleviates the Effect of Salinity on Sugar Beet (Beta vulgaris L.) Productivity and Quality. Agronomy 2021, 11, 1491. [Google Scholar] [CrossRef]
- Talbi Zribi, O.; Mbarki, S.; Metoui, O.; Trabelsi, N.; Zribi, F.; Ksouri, R.; Abdelly, C. Salinity and Phosphorus Availability Differentially Affect Plant Growth, Leaf Morphology, Water Relations, Solutes Accumulation and Antioxidant Capacity in Aeluropus littoralis. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2020, 155, 935–943. [Google Scholar] [CrossRef]
- Tang, H.; Niu, L.; Wei, J.; Chen, X.; Chen, Y. Phosphorus Limitation Improved Salt Tolerance in Maize Through Tissue Mass Density Increase, Osmolytes Accumulation, and Na+ Uptake Inhibition. Front. Plant Sci. 2019, 10, 856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frosi, G.; Barros, V.A.; Oliveira, M.T.; Santos, M.; Ramos, D.G.; Maia, L.C.; Santos, M.G. Arbuscular Mycorrhizal Fungi and Foliar Phosphorus Inorganic Supply Alleviate Salt Stress Effects in Physiological Attributes, but Only Arbuscular Mycorrhizal Fungi Increase Biomass in Woody Species of a Semiarid Environment. Tree Physiol. 2018, 38, 25–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bargaz, A.; Nassar, R.M.A.; Rady, M.M.; Gaballah, M.S.; Thompson, S.M.; Brestic, M.; Schmidhalter, U.; Abdelhamid, M.T. Improved Salinity Tolerance by Phosphorus Fertilizer in Two Phaseolus Vulgaris Recombinant Inbred Lines Contrasting in Their P-Efficiency. J. Agron. Crop Sci. 2016, 202, 497–507. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, M.; Avdhesh, C.; Singh, P. Effect of Phosphorus and Zinc on Yield and Nutrient Uptake by Okra (Abelmoschus esculentus L.) under Different Salinity Conditions. Int. J. Chem. Stud. 2017, 5, 284–288. [Google Scholar]
- Sadji-Ait Kaci, H.; Chaker-Haddadj, A.; Aid, F. Interactive Effects of Salinity and Two Phosphorus Fertilizers on Growth and Grain Yield of Cicer Arietinum L. Acta Agric. Scand. B Soil Plant Sci 2017, 67, 208–216. [Google Scholar] [CrossRef]
- Xu, H.; Hassan, M.A.; Sun, D.; Wu, Z.; Jiang, G.; Liu, B.; Ni, Q.; Yang, W.; Fang, H.; Li, J.; et al. Effects of Low Temperature Stress on Source-Sink Organs in Wheat and Phosphorus Mitigation Strategies. Front. Plant Sci. 2022, 13, 807844. [Google Scholar] [CrossRef]
- Singh, S.K.; Reddy, V.R.; Fleisher, D.H.; Timlin, D.J. Phosphorus Nutrition Affects Temperature Response of Soybean Growth and Canopy Photosynthesis. Front. Plant Sci. 2018, 9, 1116. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Ihsan, Z.; Shah, A.N.; Wu, C.; Yousaf, M.; Nasim, W.; Alharby, H.; et al. Exogenously Applied Plant Growth Regulators Enhance the Morpho-Physiological Growth and Yield of Rice under High Temperature. Front. Plant Sci. 2016, 7, 1250. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Wu, J.; Liu, X.; Chen, X.; Wu, Y.; Yu, S. Inorganic Phosphorus Fertilizer Ameliorates Maize Growth by Reducing Metal Uptake, Improving Soil Enzyme Activity and Microbial Community Structure. Ecotoxicol. Environ. Saf. 2017, 143, 322–329. [Google Scholar] [CrossRef]
- Sun, Q.B.; Shen, R.F.; Zhao, X.Q.; Chen, R.F.; Dong, X.Y. Phosphorus Enhances Al Resistance in Al-Resistant Lespedeza Bicolor but Not in Al-Sensitive L. cuneata under Relatively High Al Stress. Ann. Bot. 2008, 102, 795–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, J.d.l.C.; Cardoso, J.A.; Arango-Londoño, D.; Fischer, G.; Rao, I. Influence of Soil Fertility on Waterlogging Tolerance of Two Brachiaria Grasses. Agron. Colomb. 2015, 33, 20–28. [Google Scholar] [CrossRef]
- Zhao, C.; Haigh, A.M.; Holford, P.; Chen, Z.H. Roles of Chloroplast Retrograde Signals and Ion Transport in Plant Drought Tolerance. Int. J. Mol. Sci. 2018, 19, 963. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Zia ur Rehman, M.; Saleem, M.H.; Adrees, M.; Rizwan, M.; Javed, A.; Rafique, M.; Qayyum, M.F.; Ali, S. Effect of Phosphorus Sources on Growth and Cadmium Accumulation in Wheat under Different Soil Moisture Levels. Environ. Pollut. 2022, 311, 119977. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.K.; George, T.S.; Thompson, J.A.; Wright, G.; Lyon, J.; Dupuy, L.; Hubbard, S.F.; White, P.J. What Are the Implications of Variation in Root Hair Length on Tolerance to Phosphorus Deficiency in Combination with Water Stress in Barley (Hordeum vulgare)? Ann. Bot. 2012, 110, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Jarzyniak, K.M.; Jasiński, M. Membrane Transporters and Drought Resistance - A Complex Issue. Front. Plant Sci. 2014, 5, 687. [Google Scholar] [CrossRef] [Green Version]
- Saito, S.; Uozumi, N. Calcium-Regulated Phosphorylation Systems Controlling Uptake and Balance of Plant Nutrients. Front. Plant Sci. 2020, 11, 510159. [Google Scholar] [CrossRef] [Green Version]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- Bechtaoui, N.; Rabiu, M.K.; Raklami, A.; Oufdou, K.; Hafidi, M.; Jemo, M. Phosphate-Dependent Regulation of Growth and Stresses Management in Plants. Front. Plant Sci. 2021, 12, 2357. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Zhang, L. AMF Inoculation and Phosphorus Supplementation Alleviates Drought Induced Growth and Photosynthetic Decline in Nicotiana Tabacum by Up-Regulating Antioxidant Metabolism and Osmolyte Accumulation. Environ. Exp. Bot. 2020, 176, 104088. [Google Scholar] [CrossRef]
- Bouras, H.; Choukr-Allah, R.; Mosseddaq, F.; Bouaziz, A.; Devkota, K.P.; el Mouttaqi, A.; Bouazzama, B.; Hirich, A. Does Phosphorus Fertilization Increase Biomass Production and Salinity Tolerance of Blue Panicum (Panicum antidotale Retz.) in the Salt-Affected Soils of Arid Regions? Agronomy 2022, 12, 791. [Google Scholar] [CrossRef]
- Mohamed, H.I.; El-Sayed, A.A.; Rady, M.M.; Caruso, G.; Sekara, A.; Abdelhamid, M.T. Coupling Effects of Phosphorus Fertilization Source and Rate on Growth and Ion Accumulation of Common Bean under Salinity Stress. PeerJ 2021, 9, e11463. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Mu, C.; Zheng, H.; Lu, S.; Zhang, H.; Zhang, X.; Liu, X. Exogenous Pi Supplementation Improved the Salt Tolerance of Maize (Zea mays L.) by Promoting Na+ Exclusion. Sci. Rep. 2018, 8, 16203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zangani, E.; Afsahi, K.; Shekari, F.; Sweeney, E.M.; Mastinu, A. Nitrogen and Phosphorus Addition to Soil Improves Seed Yield, Foliar Stomatal Conductance, and the Photosynthetic Response of Rapeseed (Brassica Napus L.). Agriculture 2021, 11, 483. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Tanveer, M.; Ihsan, M.Z.; Shah, A.N.; Ullah, A.; Nasrullah; Khan, F.; et al. A Combined Application of Biochar and Phosphorus Alleviates Heat-Induced Adversities on Physiological, Agronomical and Quality Attributes of Rice. Plant Physiol. Biochem. 2016, 103, 191–198. [Google Scholar] [CrossRef]
- Xu, H.; Wu, Z.; Xu, B.; Sun, D.; Hassan, M.A.; Cai, H.; Wu, Y.; Yu, M.; Chen, A.; Li, J.; et al. Optimized Phosphorus Application Alleviated Adverse Effects of Short-Term Low-Temperature Stress in Winter Wheat by Enhancing Photosynthesis and Improved Accumulation and Partitioning of Dry Matter. Agronomy 2022, 12, 1700. [Google Scholar] [CrossRef]
- Huang, Q.; An, H.; Chen, J.; Li, X.; Shao, G. Phosphorus Application Interferes Expression of Fe Uptake-Associated Genes to Feedback Regulate Cd Accumulation in Rice (Oryza sativa L.) and Relieves Cd Toxicity via Antioxidant Defense. Plant Growth Regul. 2023, 1, 1–14. [Google Scholar] [CrossRef]
- Liu, W.; Gao, J.; Wan, X.; Li, Q.; Fu, Q.; Zhu, J.; Hu, H. Effect of Phosphorus Fertilizer on Phytoextraction Using Ricinus Communis L. in Cu and Cd Co-Contaminated Soil. Int. J. Phytoremediation 2022, 25, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Monroy-Licht, A. Effect of Phosphate on Arsenic Species Uptake in Plants under Hydroponic Conditions. J. Plant Res. 2022, 1, 1–14. [Google Scholar] [CrossRef]
- Cao, X.; Dermatas, D.; Xu, X.; Shen, G. Immobilization of Lead in Shooting Range Soils by Means of Cement, Quicklime, and Phosphate Amendments. Environ. Sci. Pollut. Res. Int. 2008, 15, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Xing, W.; Ma, C.; Zhang, Y.; Wang, G.; Yang, L. Phosphorus Amendment of a Lead-Spiked Soil with Low Phosphorus Availability: Roles of Phosphorus on Soil and Plant Lead. Commun. Soil Sci. Plant Anal. 2012, 43, 1053–1064. [Google Scholar] [CrossRef]
- Ayub, M.; Nadeem, M.A.; Sharar, M.S.; Mahmood, N. Response of Maize (Zea mays L.) Fodder to Different Levels of Nitrogen and Phosphorus. Asian J. Plant Sci. 2002, 1, 352–354. [Google Scholar] [CrossRef] [Green Version]
- Barton-Johnson, R. Waterlogging in the Temperate Plantation Species Eucalyptus globulus and E. nitens. Ph.D. Thesis, University of Tasmania, Hobart, Australia, 2006. [Google Scholar]
- Singh, D.; Singh, V.; Sale, P.; Pallaghy, C.K.; Routley, R. Enhanced Tolerance of High-p Plants to Environmental Stresses Is Related to Primary Root Diameter and Potential Root Hydraulic Conductivity for Water and Nutrient Uptake. In Proceedings of the 11th Australian Agronomy Conference, Geelong, Australia, 2–6 February 2003; Available online: http://www.regional.org.au/index.htm (accessed on 23 April 2023).
- Jin, J.; Tang, C.; Sale, P. The Impact of Elevated Carbon Dioxide on the Phosphorus Nutrition of Plants: A Review. Ann. Bot. 2015, 116, 987–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
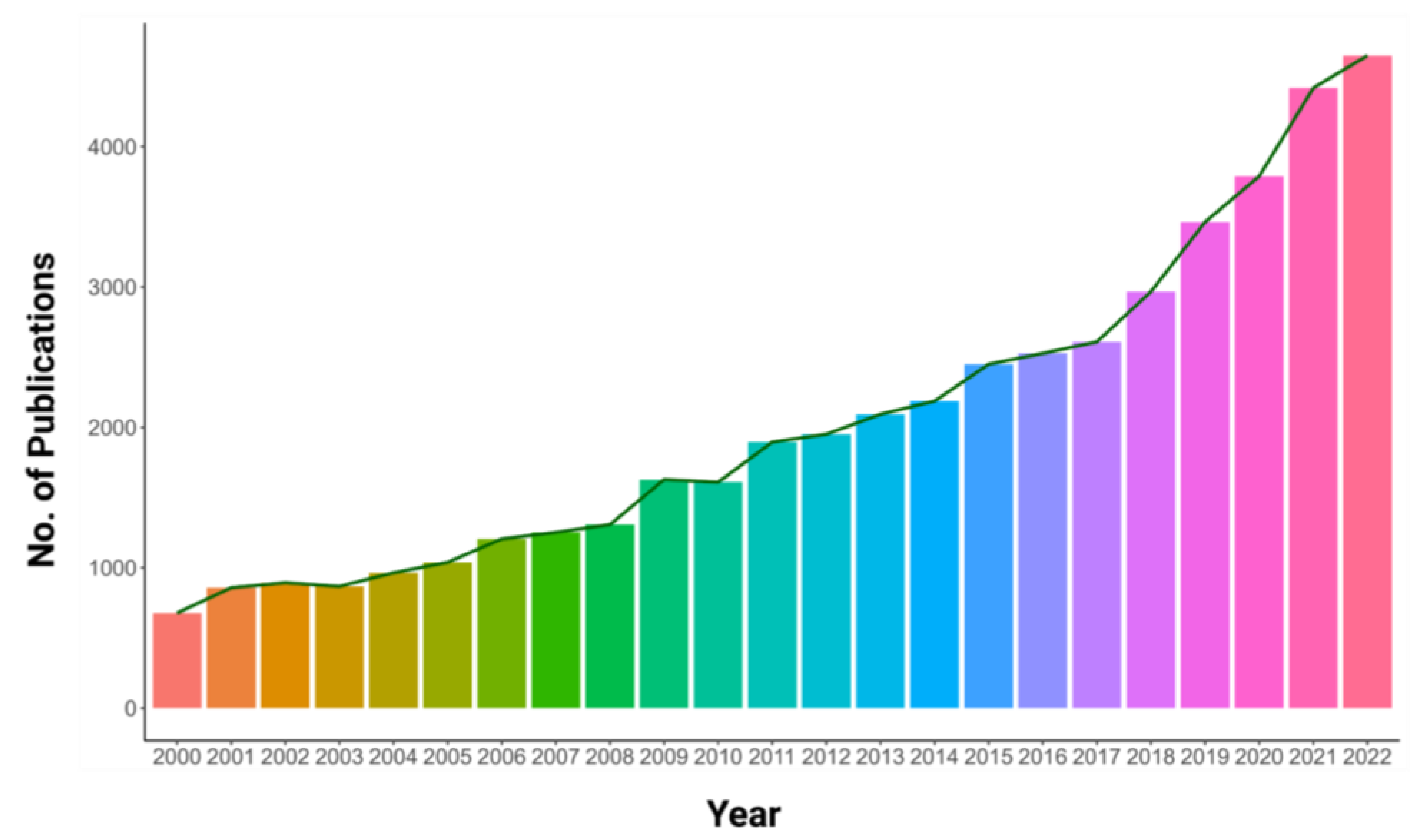
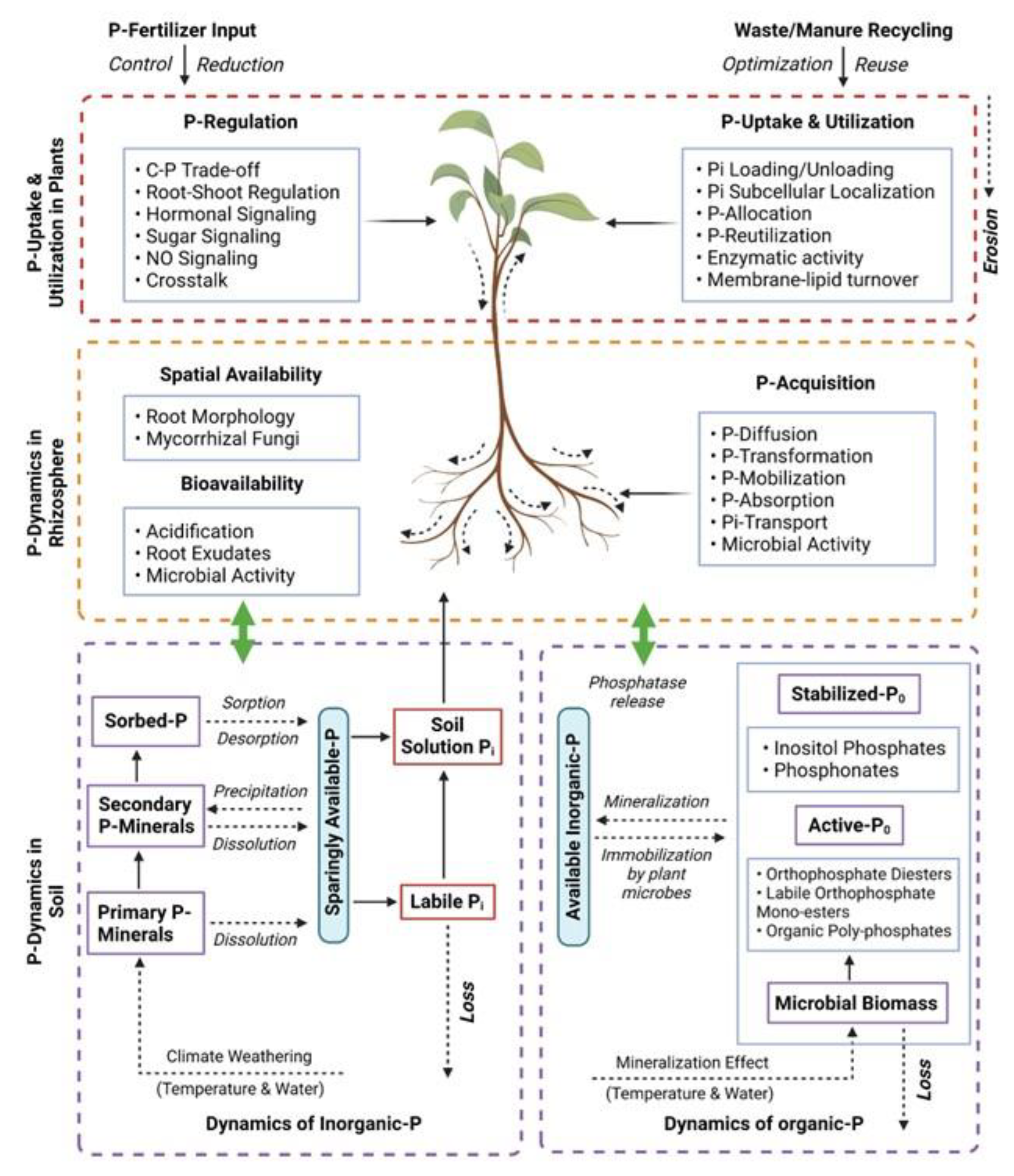
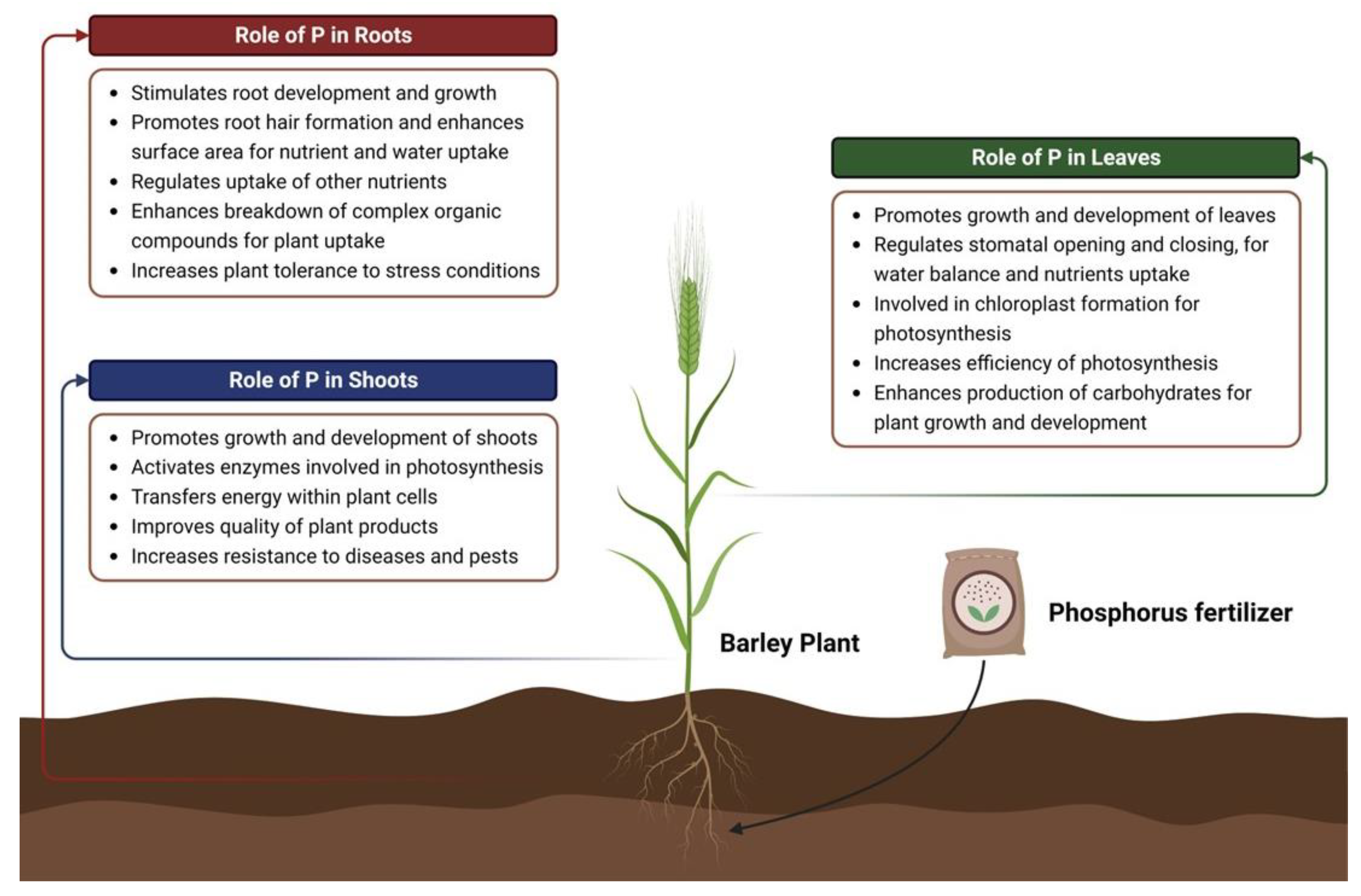
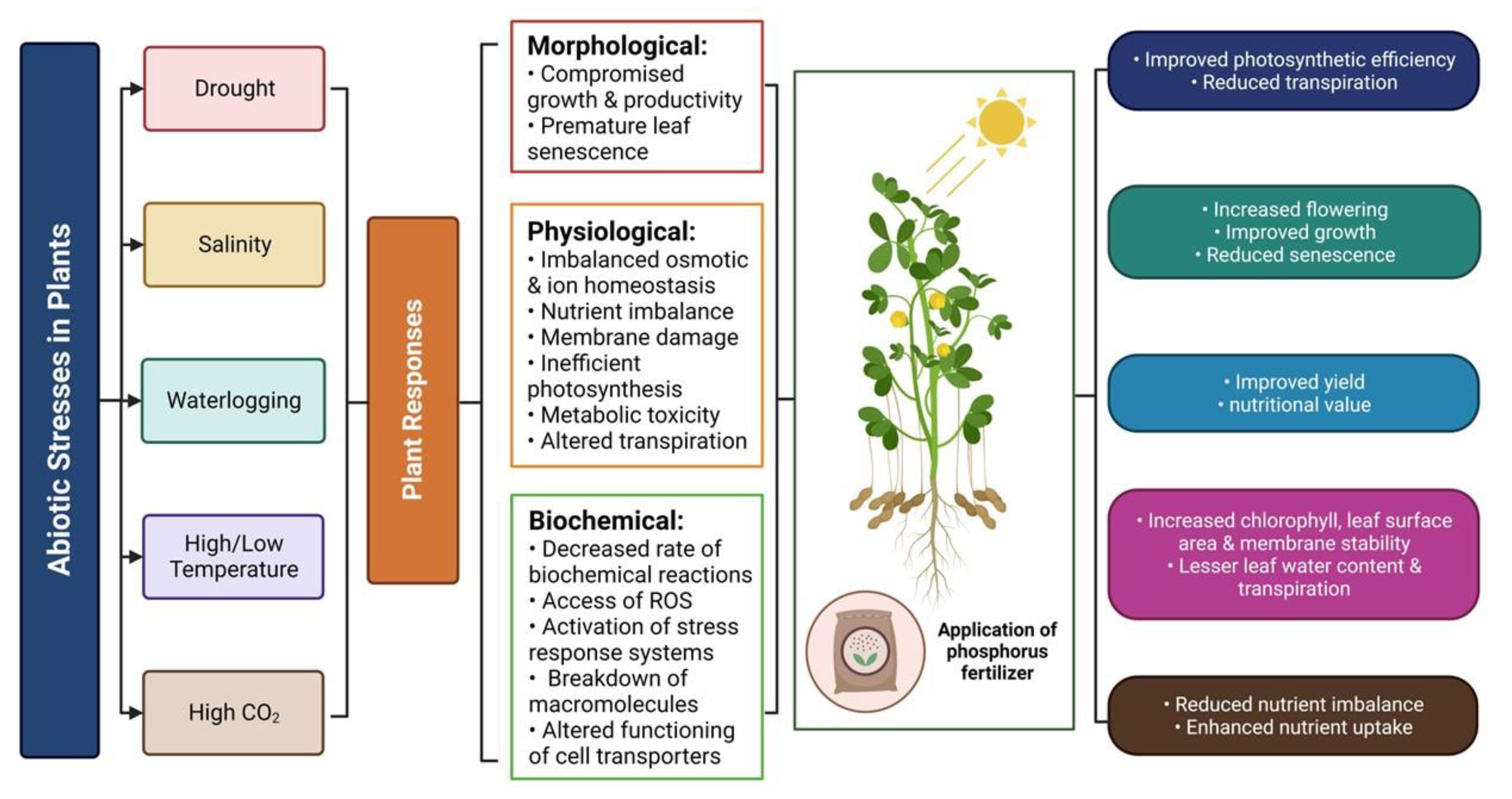
| Plants | Potential Gene | Family | Abiotic Stress Tolerance | Functions | Reference |
|---|---|---|---|---|---|
| Tomato | Lept1 and lept2 | Lept | Low Pi | Regulation of P homeostasis | [124] |
| Glycine max | H12/14 H1/4, PHO1; H5 and H8 | PHOSPHATE1 (PHO1) | Low Pi and salt stress | Tolerance against salt stress Morphological adaptation and divergence | [125] |
| GmETO1 | Ethylene Overproduction Protein | Low Pi | Development of root under Pi starvation and expressed notably for ethylene biosynthesis | [126] | |
| GmPAP12 | GmPAP | Low Pi | Stimulate the purple acid phosphatases synthesis for nodules to absorb more Pi | [127] | |
| GmSPX-RING1 | SPX-RING | Low Pi | Control the efficiency of Pi in different soybean cultivars | [128] | |
| GmPHR25 | GmPT Pi transporter | Low Pi | Upregulated in root hairs to facilitate the absorption of Pi | [129] | |
| Arabidopsis thaliana | microRNA399 and PHR1 | PHR1 | Low Pi | Regulation of P homeostasis | [130] |
| Cerasus pseudocerasus | CpERF7 | ERF | Drought and low Pi | Stimulate the auxin-mediated stress-responsive genes to increase tolerance against drought and low P | [131] |
| Hordeum vulgare | HvPIP2;1; HvPIP2;2, HvPIP1;3, HvPIP2;4 and HvPIP2;5 | Plasma membrane intrinsic proteins | Drought and low Pi | Encode water channels that facilitate the transit of small solutes and water through cell membrane | [132,133] |
| HvPHT1;1, HvPHT1;3, HvPHT1;4, HvPHT1;6, | PHT | Arsenic stress | Reduced the toxicity of arsenic stress | [123] | |
| Tobacco | NtPIN3 | Cytoplasmic membrane transporter | Low Pi | Increased tolerance against low Pi by modifying root elongation | [134] |
| Brassica napus | PHO2 | PHO | Low Pi | Enhance the uptake and transportation of Pi in soil | [135] |
| Leymus chinensis | LcPIP1;1 LcPIP2;1, LcPIP2;4; | Plasma membrane intrinsic proteins | Low Pi and drought stress | PIP genes are overexpressed in drought or Pi starvation, which resulted in alleviation of abiotic stresses | [132] |
| Abiotic Stresses | Plant | P application | Alleviation Mechanisms | References |
|---|---|---|---|---|
| Drought | Echinacea purpurea | Split plot experiment was performed with drought stress in term of available water depletion (25, 50 and 75%) to retain the filed capacity. P fertilizer was used as control without P fertilizer, 100% plant required P from triple super-phosphate, sole application of mycorrhizal arbuscular fungi (AMF) and Pseudomonas fluorescens bacteria (PFB) and AMF or PFB combination with 50% of the plant’s P requirement. | Application of P increased root biomass and yield by 57% and 47%, respectively, under drought conditions. In 25% of AWD, the highest root cynarin (0.583 mg/g dry matter) was observed in the joint application of phosphorus + AMF. Together with the AMF, P improved the drought tolerance traits. | [136] |
| Water deficit | Rapeseed | Plants were grown under different irrigation levels (70-, 100-, 130- and 160-mm evaporation from class A pan, respectively), which were treated with five fertilizers (control, chemical fertilizer including N and P (about 300 and 150 kg ha−1, respectively, based on soil analysis)). | P fertilizers especially in combination with other fertilizers decreased proline content and leaf temperature, with an increase in antioxidants and enzymatic activities including chlorophyll content, leaf water content, membrane stability index and stomatal conductance with improved yield. | [137] |
| Drought | Alnus cremastogyne | P fertilizer was applied in the form of NaH2PO4 (25.5% P) to each of the pots in each 30-day interval 3 times in the entire crop duration. | Application of P improved the relative water content, photosynthesis rate, increase in antioxidative enzymes including SOD, CAT, POD, osmolytes accumulation, soluble proteins and decrease in lipid peroxidation levels. | [138] |
| Drought | Eucalyptus grandis | P fertilizer was applied in the form of NaH2PO4 (25.5% P) to each of the pots in each 30-day interval 3 times in the entire crop duration. | P application alleviated the drought stress effect by improving the leaf relative water content, net photosynthesis, quantum efficiency of photosystem II and amelioration in some other physiological traits related to drought stress tolerance. | [139] |
| Drought | Phoebe zhennan | P fertilizer was applied in the form of NaH2PO4 (25.5% P) to each of the pots in each 30-day interval. | P alleviated the drought effect by increasing the relative water content, net photosynthesis rate, higher quantum efficiency, higher rooting systems, enhanced root biomass, decrease in MDA and upregulations of photosynthetic pigments, osmolytes and nitrogenous compounds. | [140] |
| Drought | Pisum sativum | P was applied in the form of KH2PO4 at two different rates: 15 (P15) and 60 mg P kg−1 (P60), mixed evenly throughout the soil. | Optimal P application enhanced the water use efficiency, soluble sugars and relative water content. Application of P increased the salt tolerance index with higher root length, nodule number, stomatal conductance and uptake of nitrogen. | [14] |
| Drought | Soybean | The P levels (added as KH2PO4) were 0, 15 and 30 mg P kg−1 soil. | Addition of P enhanced the concentration and accumulation of nitrogen in shoots and seeds. P application mitigated the drought effects on plant by increasing the protein concentration. | [141] |
| Salinity | Wheat | P was used in the form of Ortho P fertilizer, phosphoric acid-based fertilizers with K and N containing 52% and 62% of P2O5, respectively, with 100% orthoP for each one. | Sufficient P application alleviated the salinity stress by promoting growth and reducing salt toxicity. Fertilized plants have higher shoot and root dry weights under salinity stress. | [142] |
| Salinity | Quinoa | Filed experiment was set up with different EC levels of irrigation water (5, 12 and 17 dS·m−1). P was added in the form of P2O5 at the rate of 0, 60 and 70 kg of P2O5 ha−1. | P application under saline conditions minimized the effect of salinity and improved the yield with higher water and nutrient uptake. The results suggested that P application minimizes the adverse effects of high soil salinity and can be adopted as a coping strategy under saline conditions. | [143] |
| Salinity | Sugar beet | Plants were grown under salinity water with different EC values of 0.7, 4, 8 and 12 dS·m−1). P was applied in the form of P2O5 with a rate of 100, 120 and 140 kg P2O5·ha−1. | P improved the rate of yield and sugar content of sugar beets under the tested salinity levels. | [144] |
| Salinity | Aeluropus littoralis | Plants were grown at moderate salinity (100 mM NaCl). P fertilizers were applied at different doses: low, moderate and high (5 mM, 60 mM and 180 mM KH2PO4). | P fertilization improved the salinity tolerant characteristics including increase in leaf hair and trichome densities, total polyphenol content and total antioxidant capacity in plants cultivated. | [145] |
| Salinity | Maize | P was introduced to the nutrient medium in the form of KH2PO4, with concentrations of 5 μM considered as low P and 200 μM as high P. | Higher P application prevented leaf chlorosis under salinity stress with improved | [146] |
| Salinity | Woody species | Plants were grown in a factorial 2 × 2 × 2 (presence or absence of salt, AMF, or Pi), totaling eight treatments: control, Pi, AMF, AMF ∗ Pi, salt, salt ∗ Pi, salt ∗ AMF and salt ∗ AMF ∗ Pi, with eight replicates each and one plant per pot, totaling 64 experimental units. | P fertilization increased biomass and photosynthetic pigments under salinity conditions. Metabolites were also positively impacted due to fertilization. | [147] |
| Salinity | Phaseolus vulgaris | Plants were grown under different salinity conditions (1.56, 4.78 and 8.83 dS·m−1) and P fertilizer at the rate of 0, 30, 60 and 90 kg ha−1. | P application significantly increased the total chlorophyll content, total soluble sugars, carotenoids, total free amino acid and proline with higher accumulation of K+, Ca2+, Mg2+ and higher tolerance and yield. | [148] |
| Salinity | Okra | Plants were grown under two different salinity conditions (4 and 8 dS·m−1) and combined with different rates of P fertilizers (0, 30, 60 and 90 kg ha−1 from triple super phosphate). | Application of P increased the green and dry pod yield under salinity stress. | [149] |
| Salinity | Cicer arietinum L. | Plants were grown under two salinity conditions (0 and 150 mM NaCl) and treated with three P fertilizers (0, 90, 200 kg h−1 of P2O5 in the form of super triple phosphate). | P application improved plant growth. P fertilization increased the leghemoglobin (92%), reduced proline content (−69%) and protected membranes against peroxidation compared to saline conditions. Also, yield was increased due to the P fertilizers. | [150] |
| Low temperatures | Wheat | Pot experiment was conducted with tolerant and sensitive cultivar and was treated under chilling (T1 at 4 °C) and freezing treatment (T2 at −4 °C) as well as ambient temperature (CK at 11 °C) during the anther differentiation treated with P fertilizers. | Application of P alleviated low-temperature stress by increasing the stomatal conductance, dry matter accumulation, transportation of assimilates, grain number per spike, 1000 grain weights, yield per plant. | [151] |
| Low and high temperatures | Soybean | Plants were grown at different temperatures (22, 26, 30 and 34 °C corresponds to moderately low, optimum, moderately high and high temperature) with combination of 0.5 mM and 0.08 mM P nutrition. | Sufficient P fertilization improved the temperature stress tolerance by increasing the plant efficiency in utilization and biomass partitioning to pods. | [152] |
| High-temperature stress | Rice | Plants were grown in controlled growth conditions with high day and night temperatures (35 °C ± 2 and 32 °C ± 2, respectively). P fertilizers were added singly or combined with biochar. | P fertilization ameliorated the adverse impact of high temperatures with higher grain formation and grain quality. | [153] |
| Heavy metals | - | Five different types of treatments were added: control (C), heavy metal pollution (H), heavy metal pollution + nitrogen (HN), heavy metal pollution + phosphorus (HP), heavy metal pollution + nitrogen and phosphorus (HNP) | P addition of HP and HNP treatments restored plant species richness and increased plant diversity under heavy metal pollution. P addition had a better performance in restoring the species composition and relative dominance of plant communities. | [15] |
| Heavy metals | Maize | Maize plants were grown in soil collected from paddy field located near recycle area and fertilizers were applied as two amendments (calcium–magnesium phosphate fertilizer, PF; commercial organic fertilizer, OF) tested in single and in combination. | Application of P increased the maize shoot and root biomass and enzymatic activities such as urease and catalase. Additionally, microbial community structure was improved. | [154] |
| Heavy metals | Lespedeza bicolor; Lespedeza cuneata | Two species, Lespedeza bicolor and L. cuneata, were grown for 30 d with alternate Al and P treatments in a hydroponics system. | P application improved root growth and heavy metal tolerance by lowering the Al uptake and accumulation. Enhancement of Al resistance by P in the resistant species might be associated with its more efficient P accumulation and translocation to shoots and greater Al exclusion from root tips after P application. | [155] |
| Waterlogging | Brachlaria grass | Tolerant and sensitive cultivars were grown under control and waterlogged conditions with two different fertilizer levels (low and high). | Higher availability of P under waterlogged soil imparted the tolerance. | [156] |
| Drought and elevated CO2 | Field pea (Pisum sativum) | Plants were grown in P-deficient vertisol, supplied with two doses of P (15 mg and 60 mg Kg−1) and treated with ambient and elevated CO2 (380–400 ppm and 550–580 ppm). | P improved water use efficiency under elevated CO2 levels. | [14] |
| Strategies | Description | End Products | Mode of Action | Benefits | Limitations |
|---|---|---|---|---|---|
| Chemical fertilizers | These are synthetic fertilizers that contain a high concentration of P in the form of water-soluble salts. They are widely used in agriculture to provide quick and efficient nutrition to plants | Biomass, increased yield | Direct supply of P to plants, quick response | Quick and efficient nutrient supply, high P concentration | Expensive, environmental pollution, soil degradation, reduced microbial activity |
| Organic amendments | These are natural sources of P such as animal manure, compost and bone meal. They provide a slow and steady release of P through the breakdown of organic matter in the soil | Biomass, improved soil structure, microbial activity | Slow release of P through mineralization, increased soil fertility | Slow and steady release of P, improved soil structure and health, increased microbial activity | Requires large quantities for adequate P supply, potential for nutrient imbalances, may contain pathogens or weed seeds |
| Biofertilizers | These are microbial inoculants that contain P-solubilizing microorganisms such as bacteria, fungi and algae. They enhance the availability of P in the soil and improve nutrient uptake by plants | Biomass, improved soil health | Biological fixation of atmospheric P into plant-available form, symbiotic relationship with plants | Improved nutrient uptake and stress tolerance, reduced environmental pollution, improved soil health | Limited availability and variability, potential for ineffective inoculants |
| Nano P fertilizers | These are engineered nanoparticles that contain P and are designed to improve the solubility and bioavailability of P in the soil. They are considered to be highly efficient and cost-effective | Improved nutrient uptake, increased yield | Enhanced solubility and bioavailability, reduced leaching and environmental impact | Highly efficient and cost-effective, improved solubility and bioavailability of P, reduced environmental pollution | Limited research on potential environmental and health impacts |
| Mycorrhizal inoculants | These are symbiotic associations between plants and fungi that enhance the absorption of P from the soil. They also improve plant growth and nutrient uptake under stress conditions | Improved nutrient uptake, increased resistance to stress | Enhanced surface area and absorption, improved soil structure and nutrient cycling | Improved P uptake and nutrient use efficiency, enhanced plant growth and stress tolerance | Limited host range, potential for ineffective inoculants |
| Genetic engineering | This involves the manipulation of genes involved in P transport, signaling and metabolism in plants to enhance their efficiency in utilizing P | Improved nutrient uptake and utilization | Manipulation of genes involved in P transport, signaling and metabolism | Improved P use efficiency and stress tolerance, enhanced nutrient uptake, potential for long-term sustainability | Limited research on environmental and health impacts, potential for unintended consequences |
| Plant growth-promoting rhizobacteria | These are soil bacteria that colonize the root zone of plants and improve their growth and nutrient uptake by producing phytohormones and metabolites | Increased growth, improved nutrient uptake and tolerance to abiotic stress | Production of phytohormones and metabolites, biocontrol of plant pathogens | Improved nutrient uptake and stress tolerance, enhanced plant growth, reduced environmental pollution | Limited effectiveness on some plant species, potential for ineffective inoculants |
| Biostimulants | These are natural or synthetic substances that enhance plant growth and nutrient uptake by stimulating physiological processes in plants | Increased growth, improved stress tolerance | Activation of physiological and biochemical pathways, enhanced nutrient uptake and utilization | Improved plant growth and nutrient uptake, enhanced stress tolerance, reduced environmental pollution | Limited research on effectiveness and potential environmental impacts |
| P-solubilizing microorganisms | These are microorganisms that enhance the solubility and availability of P in the soil by releasing organic acids and enzymes that break down P-containing minerals | Increased P availability, improved soil health | Solubilization and mineralization of soil P, biological fixation of atmospheric P | Improved solubility and bioavailability of P, reduced environmental pollution, improved soil health | Limited research on effectiveness and potential environmental impacts |
| Soil acidification | This involves the application of acidic substances such as sulfur or ammonium sulfate to lower the pH of the soil, thereby increasing the solubility and bioavailability of P | Improved P availability, increased yield | Lowered pH increases solubility and bioavailability of soil P | Improved solubility and bioavailability of P, reduced environmental pollution | Potential for soil degradation, increased leaching of other nutrients, potential for negative impacts on soil microorganisms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus Plays Key Roles in Regulating Plants’ Physiological Responses to Abiotic Stresses. Plants 2023, 12, 2861. https://doi.org/10.3390/plants12152861
Khan F, Siddique AB, Shabala S, Zhou M, Zhao C. Phosphorus Plays Key Roles in Regulating Plants’ Physiological Responses to Abiotic Stresses. Plants. 2023; 12(15):2861. https://doi.org/10.3390/plants12152861
Chicago/Turabian StyleKhan, Fahad, Abu Bakar Siddique, Sergey Shabala, Meixue Zhou, and Chenchen Zhao. 2023. "Phosphorus Plays Key Roles in Regulating Plants’ Physiological Responses to Abiotic Stresses" Plants 12, no. 15: 2861. https://doi.org/10.3390/plants12152861
APA StyleKhan, F., Siddique, A. B., Shabala, S., Zhou, M., & Zhao, C. (2023). Phosphorus Plays Key Roles in Regulating Plants’ Physiological Responses to Abiotic Stresses. Plants, 12(15), 2861. https://doi.org/10.3390/plants12152861










