Sustainable Isolation of Bioactive Compounds and Proteins from Plant-Based Food (and Byproducts)
Abstract
:1. Introduction
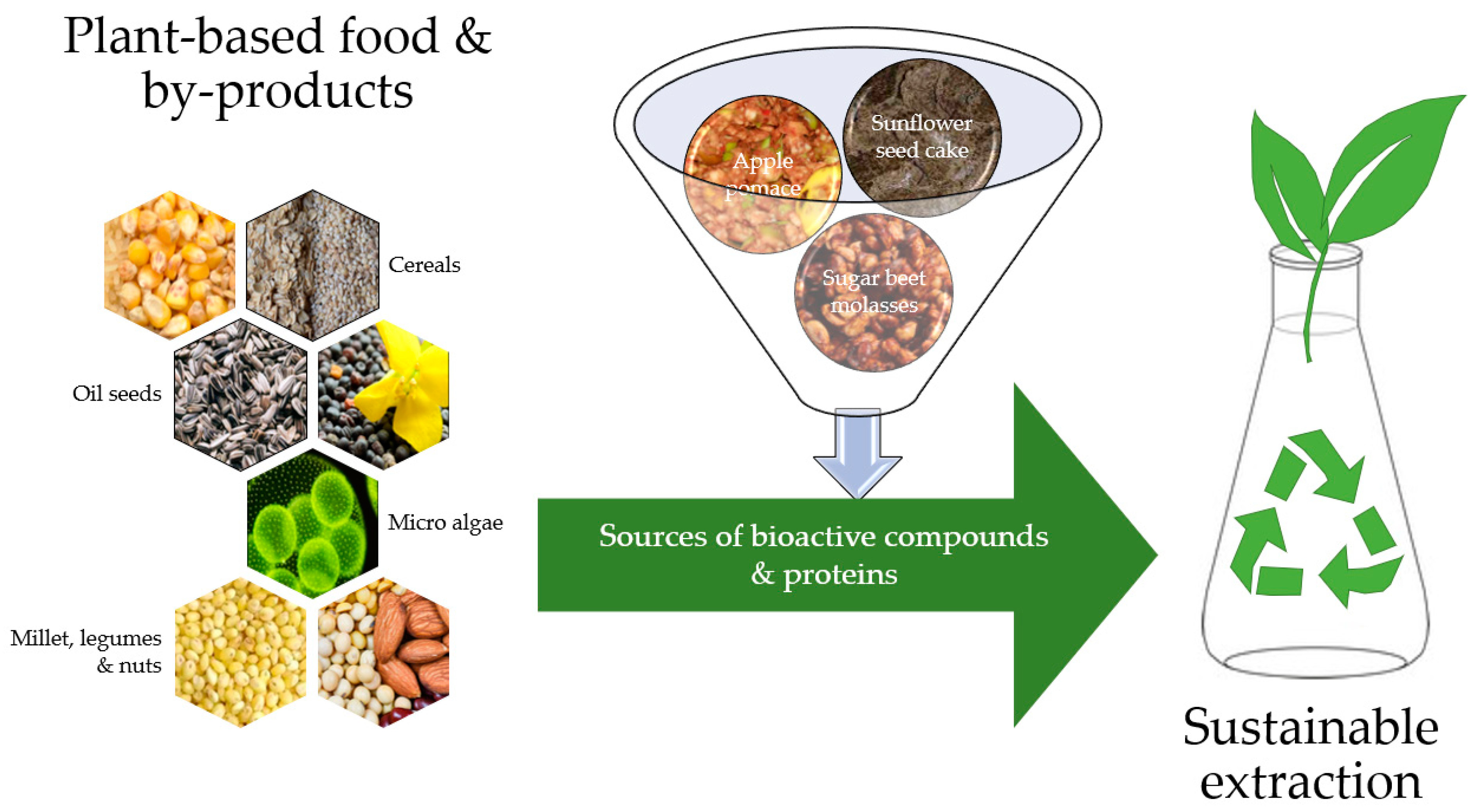
2. Processing of Waste Plant-Food Production
2.1. Sustainable Isolation of Bioactive Compounds from Plant-Based Food Byproducts
2.2. Sustainable Isolation of Plant Proteins
2.2.1. Source of Plant-Based Proteins—Cereals
2.2.2. Source of Plant-Based Proteins—Oilseeds
2.2.3. Source of Plant-Based Proteins—Microalgae
2.2.4. Source of Plant-Based Proteins—Millet Proteins
3. Challenges of Plant Proteins Use
4. Protein Isolation
4.1. Dry Extraction
4.2. Wet Extraction
4.2.1. Enzyme-Based Extraction
4.2.2. Aqueous Two-Phase System
4.2.3. Reverse Micelles Extraction (RM)
4.2.4. Subcritical Water Extraction
4.3. Novel Green Techniques of Extraction
4.3.1. Microwave-Assisted Extraction
4.3.2. High Pressure-Assisted Extraction
4.3.3. Pulsed Electric Field-Assisted Extraction
4.3.4. Ultrasound-Assisted Extraction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ritchie, H.; Roser, M. Environmental Impacts of Food Production. Available online: https://ourworldindata.org/ (accessed on 15 March 2023).
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [Green Version]
- EU Commission. Farm to fork strategy: For a fair, healthy and environmentally-friendly food system. Commun. Comm. Eur. Parliam. Counc. Eur. Econ. Soc. Comm. Comm. Reg. 2020, 381, 1–9. [Google Scholar]
- Lin, T.-Y.; Chiu, Y.; Xu, W.-Z. Environmental efficiency and sustainability of food production and consumption in the EU. Sustain. Prod. Consum. 2022, 34, 440–452. [Google Scholar] [CrossRef]
- Chakrapani, G.; Zare, M.; Ramakrishna, S. Biomaterials from the value-added food wastes. Bioresour. Technol. Rep. 2022, 19, 101181. [Google Scholar] [CrossRef]
- Tsironi, T.; Koutinas, A.; Mandala, I.; Stoforos, N.G. Current and new Green Deal solutions for sustainable food processing. Curr. Opin. Environ. Sci. Health 2021, 21, 100244. [Google Scholar] [CrossRef]
- Spratt, O.; Suri, R.; Deutsch, J. Defining upcycled food products. J. Culin. Sci. Technol. 2021, 19, 485–496. [Google Scholar] [CrossRef]
- Grasso, S.; Fu, R.; Goodman-Smith, F.; Lalor, F.; Crofton, E. Consumer attitudes to upcycled foods in US and China. J. Clean. Prod. 2023, 388, 135919. [Google Scholar] [CrossRef]
- Moshtaghian, H.; Bolton, K.; Rousta, K. Challenges for upcycled foods: Definition, inclusion in the food waste management hierarchy and public acceptability. Foods 2021, 10, 2874. [Google Scholar] [CrossRef]
- Yilmaz, E.; Kahveci, D. Consumers’ purchase intention for upcycled foods: Insights from Turkey. Future Foods 2022, 6, 100172. [Google Scholar] [CrossRef]
- Antunović, Z.; Klir, Ž.; Novoselec, J. Nusproizvodi Prehrambeno-Prerađivačkih Industrija Biljnog Podrijetla u Hranidbi Ovaca. 2018. Available online: https://www.bib.irb.hr/970919 (accessed on 5 June 2023).
- Martins, Z.E.; Pinho, O.; Ferreira, I. Food industry by-products used as functional ingredients of bakery products. Trends Food Sci. Technol. 2017, 67, 106–128. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef]
- Sloan, A.E. What to Watch for as the Plant-based Food Market Grows. Available online: https://www.ift.org/news-and-publications/food-technology-magazine/issues/2021/august/columns/consumer-trends-plant-based-food-market?gclid=Cj0KCQjw2qKmBhCfARIsAFy8buIy0G6vdK_S8Wo5ELIkk7xGZVUPhawT4mfJbMqMR-8OQpvDC8OfxOoaAksVEALw_wcB (accessed on 31 July 2023).
- Food Insight. 2021 Food & Healthy Survey. Available online: https://foodinsight.org/2021-food-health-survey/ (accessed on 31 July 2023).
- Alae-Carew, C.; Green, R.; Stewart, C.; Cook, B.; Dangour, A.D.; Scheelbeek, P.F.D. The role of plant-based alternative foods in sustainable and healthy food systems: Consumption trends in the UK. Sci. Total Environ. 2022, 807, 151041. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Public Attitude Survey 2020—Findings. Available online: https://www.eating-better.org/uploads/Documents/2020/Eating%20Better%202020%20public%20survey%20analysis.docx.pdf (accessed on 30 July 2023).
- Le, T.H.; Disegna, M.; Lloyd, T. National food consumption patterns: Converging trends and the implications for health. EuroChoices 2023, 22, 66–73. [Google Scholar] [CrossRef]
- Li, Y.; Schoufour, J.; Wang, D.D.; Dhana, K.; Pan, A.; Liu, X.; Song, M.; Liu, G.; Shin, H.J.; Sun, Q.; et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: Prospective cohort study. BMJ 2020, 368, l6669. [Google Scholar] [CrossRef] [Green Version]
- Vulić, J.; Cebović, T.; Canadanović-Brunet, J.; Cetković, G.; Čanadanović, V.; Djilas, S.; Šaponjac, V.T. In vivo and in vitro antioxidant effects of beetroot pomace extracts. J. Funct. Foods 2014, 6, 168–175. [Google Scholar] [CrossRef]
- Ogutu, F.; Mu, T. Ultrasonic degradation of sweet potato pectin and its antioxidant activity. Ultrason. Sonochem. 2017, 38, 726–734. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, H.; Ma, Q.; Cao, Y.; Ma, J.; Ma, C. Isolation, identification and quantification of unsaturated fatty acids, amides, phenolic compounds and glycoalkaloids from potato peel. Food Chem. 2012, 135, 2425–2429. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Kozukue, N.; Kim, H.; Choi, S.; Mizuno, M. Glycoalkaloid, phenolic, and flavonoid content and antioxidative activities of conventional nonorganic and organic potato peel powders from commercial gold, red, and Russet potatoes. J. Food Comp. Anal. 2017, 62, 69–75. [Google Scholar] [CrossRef]
- Ares, A.; Bernal, J.; Nozal, M.; Turner, C.; Plaza, M. Fast determination of intact glucosinolates in broccoli leaf by pressurized liquid extraction and ultra-high performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Res. Int. 2015, 76, 498–505. [Google Scholar] [CrossRef]
- Thomas, M.; Badr, A.; Desjardins, Y.; Gosselin, A.; Angers, P. Characterization of industrial broccoli discards (Brassica oleracea var. italica) for their glucosinolate, polyphenol and flavonoid contents using UPLC MS/MS and spectrophotometric methods. Food Chem. 2018, 245, 1204–1211. [Google Scholar] [CrossRef]
- de Andrade Lima, M.; Charalampopoulos, D.; Chatzifragkou, A. Optimisation and modelling of supercritical CO2 extraction process of carotenoids from carrot peels. J. Supercrit. Fluids 2018, 133, 94–102. [Google Scholar] [CrossRef]
- Encalada, A.I.; Pérez, C.; Flores, S.; Rossetti, L.; Fissore, E.; Rojas, A. Antioxidant pectin enriched fractions obtained from discarded carrots (Daucus carota L.) by ultrasound-enzyme assisted extraction. Food Chem. 2019, 289, 453–460. [Google Scholar] [CrossRef]
- Gonzales, G.; Raes, K.; Coelus, S.; Struijs, K.; Smagghe, G.; Van Camp, J. Ultra(high)-pressure liquid chromatography-electrospray ionization-time-of-flight-ion mobility-high definition mass spectrometry for the rapid identification and structural characterization of flavonoid glycosides from cauliflower waste. J. Chromatogr. 2014, 1323, 39–48. [Google Scholar] [CrossRef]
- Amofa-Diatuo, T.; Anang, D.; Barba, F.; Tiwari, B. Development of new apple beverages rich in isothiocyanates by using extracts obtained from ultrasound-treated cauliflower by-products: Evaluation of physical properties and consumer acceptance. J. Food Comp. Anal. 2017, 61, 73–81. [Google Scholar] [CrossRef]
- Xu, Y.; Bao, T.; Han, W.; Chen, W.; Zheng, X.; Wang, J. Purification and identification of an angiotensin I- converting enzyme inhibitory peptide from cauliflower by-products protein hydrolysate. Process Biochem. 2016, 51, 1299–1305. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, B.; Luo, H.; Meng, K.; Wang, Y.; Liu, M.; Bai, Y.; Yao, B.; Tu, T. Production pectin oligosaccharides using Humicola insolens Y1-derived unusual pectate lyase. J. Biosci. Bioeng. 2019, 129, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Barreira, J.; Arraibi, A.; Ferreira, I. Bioactive and functional compounds in apple pomace from juice and cider manufacturing: Potential use in dermal formulations. Trends Food Sci. Technol. 2019, 90, 76–87. [Google Scholar] [CrossRef]
- García, Y.D.; Valles, B.; Lobo, A.P. Phenolic and antioxidant composition of by-products from the cider industry: Apple pomace. Food Chem. 2009, 117, 731–738. [Google Scholar] [CrossRef]
- Lavelli, V.; Corti, S. Phloridzin and other phytochemicals in apple pomace: Stability evaluation upon dehydration and storage of dried product. Food Chem. 2011, 129, 1578–1583. [Google Scholar] [CrossRef]
- Shin, S.-K.; Cho, S.-J.; Jung, U.J.; Ryu, R.; Choi, M.-S. Phlorizin supplementation attenuates obesity, inflammation, and hyperglycemia in diet-induced obese mice fed a high-fat diet. Nutrients 2016, 8, 92. [Google Scholar] [CrossRef]
- Xavier Machado TD, O.; Portugal IB, M.; Padilha CV, D.S.; Ferreira Padilha, F.; dos Santos Lima, M. New trends in the use of enzymes for the recovery of polyphenols in grape byproducts. J. Food Biochem. 2021, 45, e13712. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. The potential of grape pomace varieties as a dietary source of pectic substances. Foods 2021, 10, 867. [Google Scholar] [CrossRef]
- Iqbal, A.; Schulz, P.; Rizvi, S.S. Valorization of bioactive compounds in fruit pomace from agro-fruit industries: Present Insights and future challenges. Food Biosci. 2021, 44, 101384. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Silva, P. The wine industry by-products: Applications for food industry and health benefits. Antioxidants 2022, 11, 2025. [Google Scholar] [CrossRef]
- Averilla, J.N.; Oh, J.; Kim, H.J.; Kim, J.S.; Kim, J.-S. Potential health benefits of phenolic compounds in grape processing by-products. Food Sci. Biotechnol. 2019, 28, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Kontogiannopoulos, K.N.; Patsios, S.I.; Karabelas, A.J. Tartaric acid recovery from winery lees using cation exchange resin: Optimization by response surface methodology. Sep. Purif. Technol. 2016, 165, 32–41. [Google Scholar] [CrossRef]
- Fontana, A.; Antoniolli, A.; D’Amario Fernández, M.A.; Bottini, R. Phenolics profiling of pomace extracts from different grape varieties cultivated in Argentina. RSC Adv. 2017, 7, 29446–29457. [Google Scholar] [CrossRef] [Green Version]
- Sójka, M.; Kołodziejczyk, K.; Milala, J.; Abadias, M.; Viñas, I.; Guyot, S.; Baron, A. Composition and properties of the polyphenolic extracts obtained from industrial plum pomaces. J. Funct. Foods 2015, 12, 168–178. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.; Gutiérrez, L.; Vargas, S.; Martinez-Correa, H.; Parada-Alfonso, F.; Narváez-Cuenca, C. Valorisation of mango peel: Proximate composition, supercritical fluid extraction of carotenoids, and application as an antioxidant additive for an edible oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Ballesteros-Vivas, D.; Álvarez-Rivera, G.; Morantes, S.J.; Sánchez-Camargo, A.d.P.; Ibáñez, E.; Parada-Alfonso, F.; Cifuentes, A. An integrated approach for the valorization of mango seed kernel: Efficient extraction solvent selection, phytochemical profiling and antiproliferative activity assessment. Food Res. Int. 2019, 126, 108616. [Google Scholar] [CrossRef] [PubMed]
- Torres-León, C.; Rojas, R.; Contreras-Esquivel, J.; Serna-Cock, L.; Belmares-Cerda, R.; Aguilar, C. Mango seed: Functional and nutritional properties. Trends Food Sci. Technol. 2016, 55, 109–117. [Google Scholar] [CrossRef]
- Klavins, L.; Kviesis, J.; Nakurte, I.; Klavins, M. Berry press residues as a valuable source of polyphenolics: Extraction optimisation and analysis. LWT 2018, 93, 583–591. [Google Scholar] [CrossRef]
- Vu, H.; Scarlett, C.; Vuong, Q. Phenolic compounds within banana peel and their potential uses: A review. J. Funct. Foods 2018, 40, 238–248. [Google Scholar] [CrossRef]
- Russo, M.; Bonaccorsi, I.; Torre, G.; Saro, M.; Dugo, P.; Mondello, L. Underestimated sources of flavonoids, limonoids and dietary fiber: Availability in lemon’s by-products. J. Funct. Foods 2014, 9, 18–26. [Google Scholar] [CrossRef]
- Russo, M.; Bonaccorsi, I.; Inferrera, V.; Dugo, P.; Mondello, L. Underestimated sources of flavonoids, limonoids and dietary fiber: Availability in orange’s by-products. J. Funct. Foods 2015, 12, 150–157. [Google Scholar] [CrossRef]
- Carle, R.; Keller, P.; Schieber, A.; Rentschler, C.; Katzschner, T.; Rauch, D.; Fox, G.F.; Endress, H.U. Method for obtaining useful materials from the by-products of fruit and vegetable processing. Pat. Appl. WO 2001, 1, A1. [Google Scholar]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Environmental Hotspots in the Argentine Wine Industry Amfori Sustainable Wine Programme Pouring over Key Information: 218,232. (n.d.). Available online: www.amfori.org (accessed on 30 July 2023).
- Rodríguez-Pérez, C.; García-Villanova, B.; Guerra-Hernández, E.; Verardo, V. Grape seeds proanthocyanidins: An overview of in vivo bioactivity in animal models. Nutrients 2019, 11, 2435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milanović, V.; Cardinali, F.; Ferrocino, I.; Boban, A.; Franciosa, I.; Gajdoš Kljusurić, J.; Mucalo, A.; Osimani, A.; Aquilanti, L.; Garofalo, C.; et al. Croatian white grape variety Maraština: First taste of its indigenous mycobiota. Food Res. Int. 2022, 162, 111917. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, H.; Kozlowska, H.; Lewczuk, B. Bioactive compounds in the cereal grains before and after hydrothermal processing. Innov. Food Sci. Emerg. Technol. 2001, 2, 159–169. [Google Scholar] [CrossRef]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef] [Green Version]
- Smuda, S.S.; Mohsen, S.M.; Olsen, K.; Aly, M.H. Bioactive compounds and antioxidant activities of some cereal milling by-products. J. Food Sci. Technol. 2018, 55, 1134–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brewer, L.R.; Kubola, J.; Siriamornpun, S.; Herald, T.J.; Shi, Y.C. Wheat bran particle size influence on phytochemical extractability and antioxidant properties. Food Chem. 2014, 152, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Luthria, D.L.; Lu, Y.; John, K.M. Bioactive phytochemicals in wheat: Extraction, analysis, processing, and functional properties. J. Funct. Foods 2015, 18, 910–925. [Google Scholar] [CrossRef]
- Brouns, F.; Hemery, Y.; Price, R.; Anson, N.M. Wheat aleurone: Separation, composition, health aspects, and potential food use. Crit. Rev. Food Sci. Nutr. 2012, 52, 553–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.-M.; Guo, X.-N.; Zhu, K.-X. Impact of solid state fermentation on nutritional, physical and flavor properties of wheat bran. Food Chem. 2017, 217, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Guo, X.; Liu, Y.; Fang, Z.; Zhang, M.; Zhang, R.; You, L.; Li, T.; Liu, R.H. A full utilization of rice husk to evaluate phytochemical bioactivities and prepare cellulose nanocrystals. Sci. Rep. 2018, 8, 10482. [Google Scholar] [CrossRef] [Green Version]
- Petersen, C. Analyse des phloridzins. Ann. Der Pharm. 1835, 15, 178. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.-L.; Gong, X.; Ji, M.-Y.; Wang, C.-C.; Wang, J.-H.; Li, M.-H. Bioactive Compounds from Plant-Based Functional Foods: A Promising Choice for the Prevention and Management of Hyperuricemia. Foods 2020, 9, 973. [Google Scholar] [CrossRef]
- Gosch, C.; Halbwirth, H.; Stich, K. Phloridzin: Biosynthesis, distribution and physiological relevance in plants. Phytochemistry 2010, 71, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, J.R.; Lewis, N.G.; Kahn, C.R.; Roth, J. Phlorizin: A review. Diabetes Metab. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef]
- Gorjanović, S.; Micić, D.; Pastor, F.; Tosti, T.; Kalušević, A.; Ristić, S.; Zlatanović, S. Evaluation of apple pomace flour obtained industrially by dehydration as a source of biomolecules with antioxidant, antidiabetic and antiobesity effects. Antioxidants 2020, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent Advances in health benefits of bioactive compounds from food wastes and by-products: Biochemical aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef]
- Melo-Silveira, R.F.; Fidelis, G.P.; Viana, R.L.S.; Soeiro, V.C.; da Silva, R.A.; Machado, D.; Costa, L.S.; Ferreira, C.V.; Rocha, H.A.O. Antioxidant and antiproliferative activities of methanolic extract from a neglected agricultural product: Corn cobs. Molecules 2014, 19, 5360–5378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helkar, P.B.; Sahoo, A.K.; Patil, N.J. Review: Food industry by-products used as a functional food ingredients. Int. J. Waste Resour. 2016, 6, 1000248. Available online: https://www.semanticscholar.org/paper/Review%3A-Food-Industry-By-Products-used-as-a-Food-Helkar-Sahoo/35e7a40e663a1407018c9f986e0db40818dfcc99 (accessed on 2 May 2023).
- Soceanu, A.; Dobrinas, S.; Sirbu, A.; Manea, N.; Popescu, V. Economic aspects of waste recovery in the wine industry. A multidisciplinary approach. Sci. Total Environ. 2021, 759, 143643. [Google Scholar] [CrossRef] [PubMed]
- Fresán, U.; Sabaté, J. Vegetarian diets: Planetary health and its alignment with human health. Adv. Nutr. 2019, 10 (Suppl. S4), S380–S388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magkos, F.; Tetens, I.; Bügel, S.G.; Felby, C.; Schacht, S.R.; Hill, J.O.; Ravussin, E.; Astrup, A. A perspective on the transition to plant-based diets: A diet change may attenuate climate change, but can it also attenuate obesity and chronic disease risk? Adv. Nutr. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Aleksandrowicz, L.; Green, R.; Joy, E.J.M.; Smith, P.; Haines, A. The impacts of dietary change on greenhouse gas emissions, land use, water use, and health: A systematic review. PLoS ONE 2016, 11, e0165797. [Google Scholar] [CrossRef] [Green Version]
- da Silva, R.F.; Carneiro, C.N.; de Sousa CB, D.C.; Gomez, F.J.; Espino, M.; Boiteux, J.; Fernández, M.d.l.Á.; Silva, M.F.; Dias, F.D.S. Sustainable extraction bioactive compounds procedures in medicinal plants based on the principles of green analytical chemistry: A review. Microchem. J. 2022, 175, 107184. [Google Scholar] [CrossRef]
- Donno, D.; Turrini, F.; Boggia, R.; Guido, M.; Gamba, G.; Mellano, M.G.; Riondato, I.; Beccaro, G.L. Sustainable extraction and use of natural bioactive compounds from the waste management process of Castanea spp. bud-derivatives: The Finnover project. Sustainability 2020, 12, 10640. [Google Scholar] [CrossRef]
- Kultys, E.; Kurek, M.A. Green extraction of carotenoids from fruit and vegetable byproducts: A review. Molecules 2022, 27, 518. [Google Scholar] [CrossRef]
- More, P.R.; Jambrak, A.R.; Arya, S.S. Green, environment-friendly and sustainable techniques for extraction of food bioactive compounds and waste valorization. Trends Food Sci. Technol. 2022, 128, 296–315. [Google Scholar] [CrossRef]
- Savic, I.M.; Gajic, I.M.S. Development of the sustainable extraction procedures of bioactive compounds from industrial food wastes and their application in the products for human uses. Sustainability 2023, 15, 2102. [Google Scholar] [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A critical comparison of the advanced extraction techniques applied to obtain health-promoting compounds from seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef] [PubMed]
- Dhua, S.; Kumar, K.; Sharanagat, V.S.; Nema, P.K. Bioactive compounds and its optimization from food waste: Review on novel extraction techniques. Nutr. Food Sci. 2022, 52, 1270–1288. [Google Scholar] [CrossRef]
- Jafari, S.M.; Doost, A.S.; Nasrabadi, M.N.; Boostani, S.; Van der Meeren, P. Phytoparticles for the stabilization of pickering emulsions in the formulation of novel food colloidal dispersions. Trends Food Sci. Technol. 2020, 98, 117–128. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Peschel, A.O. Consumer perception of plant-based proteins: The value of source transparency for alternative protein ingredients. Food Hydrocoll. 2019, 96, 20–28. [Google Scholar] [CrossRef]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef]
- Larkins, B.A. Proteins of the Kernel. In Corn; Elsevier: Amsterdam, The Netherlands, 2019; pp. 319–336. [Google Scholar]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Composition and protein profile analysis of rice protein ingredients. J. Food Compos. Anal. 2017, 59, 18–26. [Google Scholar] [CrossRef]
- Schuppan, D.; Gisbert-Schuppan, K.; Schuppan, D.; Gisbert-Schuppan, K. Wheat, gluten and ATI: An overview. In Wheat Syndromes; Springer: Cham, Switzerland, 2019; pp. 5–10. [Google Scholar]
- Yu, W.; Tan, X.; Zou, W.; Hu, Z.; Fox, G.P.; Gidley, M.J.; Gilbert, R.G. Relationships between protein content, starch molecular structure and grain size in barley. Carbohydr. Polym. 2017, 155, 271–279. [Google Scholar] [CrossRef]
- Johnson, J.; Wallace, T.C. Whole Grains and Their Bioactives: Composition and Health; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Wanasundara, J.P.D.; Tan, S.; Alashi, A.M.; Pudel, F.; Blanchard, C. Proteins from canola/rapeseed: Current status. In Sustainable Protein Sources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 285–304. [Google Scholar]
- González-Pérez, S. Sunflower proteins. In Sunflower; Elsevier: Amsterdam, The Netherlands, 2015; pp. 331–393. [Google Scholar]
- Wang, Y.; Tibbetts, S.M.; McGinn, P.J. Microalgae as sources of high-quality protein for human food and protein supplements. Foods 2021, 10, 3002. [Google Scholar] [CrossRef]
- FAO. Sorghum and Millets in Human Nutrition. Available online: https://www.fao.org/3/t0818e/t0818e0d.htm (accessed on 18 July 2023).
- Nikolopoulou, D.; Grigorakis, K.; Stasini, M.; Alexis, M.N.; Iliadis, K. Differences in chemical composition of field pea (Pisum sativum) cultivars: Effects of cultivation area and year. Food Chem. 2007, 103, 847–852. [Google Scholar] [CrossRef]
- Singla, P.; Sharma, S.; Singh, S. Amino acid composition, protein fractions and electrophoretic analysis of seed storage proteins in Lupins. Indian J. Agric. Biochem. 2017, 30, 33–40. [Google Scholar] [CrossRef]
- Ciabotti, S.; Silva, A.C.B.B.; Juhasz, A.C.P.; Mendonça, C.D.; Tavano, O.L.; Mandarino, J.M.G.; Conçalves, C.A.A. Chemical composition, protein profile, and isoflavones content in soybean genotypes with different seed coat colors. Int. Food Res. J. 2016, 23, 621–629. [Google Scholar]
- Khazaei, H.; Subedi, M.; Nickerson, M.; Martínez-Villaluenga, C.; Frias, J.; Vandenberg, A. Seed protein of lentils: Current status, progress, and food applications. Foods 2019, 8, 391. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Peng, Q.; Zhong, J.; Liu, W.; Zhong, Y.; Wang, F. Molecular and functional properties of protein fractions and isolate from cashew nut (Anacardium occidentale L.). Molecules 2018, 23, 393. [Google Scholar] [CrossRef] [Green Version]
- Terzo, S.; Baldassano, S.; Caldara, G.F.; Ferrantelli, V.; Dico, G.L.; Mulè, F.; Amato, A. Health benefits of pistachios consumption. Nat. Prod. Res. 2019, 33, 715–726. [Google Scholar] [CrossRef]
- Yada, S.; Lapsley, K.; Huang, G. A review of composition studies of cultivated almonds: Macronutrients and micronutrients. J. Food Compos. Anal. 2011, 24, 469–480. [Google Scholar] [CrossRef]
- Mao, X.; Hua, Y.; Chen, G. Amino acid composition, molecular weight distribution and gel electrophoresis of walnut (Juglans regia L.) proteins and protein fractionations. Int. J. Mol. Sci. 2014, 15, 2003–2014. [Google Scholar] [CrossRef] [Green Version]
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef]
- Shad, Z.M.; Venkitasamy, C.; Wen, Z. Corn distillers dried grains with solubles: Production, properties, and potential uses. Cereal Chem. 2021, 98, 999–1019. [Google Scholar] [CrossRef]
- Wood, C.; Aubert, P.; Rosentrater, K.A.; Muthukumarappan, K. Techno-Economic Modeling of a Corn Based Ethanol Plant in 2011; American Society of Agricultural and Biological Engineers: Dallas, TX, USA, 2012; p. 1. [Google Scholar]
- Rausch, K.D.; Belyea, R.L. The future of coproducts from corn processing. Appl. Biochem. Biotechnol. 2006, 128, 47–86. [Google Scholar] [CrossRef]
- Apprich, S.; Tirpanalan, Ö.; Hell, J.; Reisinger, M.; Böhmdorfer, S.; Siebenhandl-Ehn, S.; Novalin, S.; Kneifel, W. Wheat bran-based biorefinery 2: Valorization of products. LWT-Food Sci. Technol. 2014, 56, 222–231. [Google Scholar] [CrossRef]
- FAO. Food Outlook Biannual Report on Global Food Markets; FAO: Rome, Italy, 2019. [Google Scholar]
- Mereoru, E. Oilseeds. In Conjunctura Economiei Mondiale/World Economic Studies; Institute for World Economy, Romanian Academy: Bucharest, Romania, 2017; pp. 269–275. [Google Scholar]
- Shewry, P.R.; Napier, J.A.; Tatham, A.S. Seed storage proteins: Structures and biosynthesis. Plant Cell 1995, 7, 945. [Google Scholar]
- Lomascolo, A.; Uzan-Boukhris, E.; Sigoillot, J.-C.; Fine, F. Rapeseed and sunflower meal: A review on biotechnology status and challenges. Appl. Microbiol. Biotechnol. 2012, 95, 1105–1114. [Google Scholar] [CrossRef]
- MacLeod, M.J.; Vellinga, T.; Opio, C.; Falcucci, A.; Tempio, G.; Henderson, B.; Makkar, H.; Mottet, A.; Robinson, T.; Steinfeld, H.; et al. Invited review: A position on the global livestock environmental assessment model (GLEAM). Animal 2018, 12, 383–397. [Google Scholar] [CrossRef] [Green Version]
- Milledge, J.J. Commercial application of microalgae other than as biofuels: A brief review. Rev. Environ. Sci. Biotechnol. 2011, 10, 31–41. [Google Scholar] [CrossRef]
- Archibald, R.E.M. Diversity in some South African diatom associations and its relation to water quality. Water Res. 1972, 6, 1229–1238. [Google Scholar] [CrossRef]
- Chacón-Lee, T.L.; González-Mariño, G.E. Microalgae for ‘healthy’ foods—Possibilities and challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Acquah, C.; Tibbetts, S.M.; Pan, S.; Udenigwe, C. Nutritional quality and bioactive properties of proteins and peptides from microalgae. In Handbook of Microalgae-Based Processes and Products; Elsevier: Amsterdam, The Netherlands, 2020; pp. 493–531. [Google Scholar]
- Shah, P.; Dhir, A.; Joshi, R.; Tripathy, N. Opportunities and challenges in food entrepreneurship: In-depth qualitative investigation of millet entrepreneurs. J. Bus. Res. 2023, 155, 113372. [Google Scholar] [CrossRef]
- Renganathan, V.G.; Vanniarajan, C.; Karthikeyan, A.; Ramalingam, J. Barnyard millet for food and nutritional security: Current status and future research direction. Front. Genet. 2020, 11, 500. [Google Scholar] [CrossRef]
- Sachdev, N.; Goomer, S.; Singh, L.R. Foxtail millet: A potential crop to meet future demand scenario for alternative sustainable protein. J. Sci. Food Agric. 2021, 101, 831–842. [Google Scholar] [CrossRef]
- Sharma, B.; Gujral, H.S. Influence of nutritional and antinutritional components on dough rheology and in vitro protein & starch digestibility of minor millets. Food Chem. 2019, 299, 125115. [Google Scholar]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Plant proteins as high-quality nutritional source for human diet. Trends Food Sci. Technol. 2020, 97, 170–184. [Google Scholar] [CrossRef]
- Marcus, J.B. Chapter 5—Protein basics: Animal and vegetable proteins in food and health: Healthy protein choices, roles and applications in nutrition, food science and the culinary arts. Culin. Nutr. 2013, 189–230. [Google Scholar] [CrossRef]
- Nasrabadi, M.N.; Doost, A.S.; Mezzenga, R. Modification approaches of plant-based proteins to improve their techno-functionality and use in food products. Food Hydrocoll. 2021, 118, 106789. [Google Scholar] [CrossRef]
- Görgüç, A.; Özer, P.; Yılmaz, F.M. Microwave-assisted enzymatic extraction of plant protein with antioxidant compounds from the food waste sesame bran: Comparative optimization study and identification of metabolomics using LC/Q-TOF/MS. J. Food Process. Preserv. 2020, 44, e14304. [Google Scholar] [CrossRef]
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [Green Version]
- Satankar, V.; Singh, M.; Mageshwaran, V.; Jhodkar, D.; Changan, S.; Kumar, M.; Mekhemar, M. Cottonseed kernel powder as a natural health supplement: An approach to reduce the gossypol content and maximize the nutritional benefits. Appl. Sci. 2021, 11, 3901. [Google Scholar] [CrossRef]
- Lundgren, M. Composition of Fractions from Air-Classified Wheat Flour; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2011. [Google Scholar]
- Tabtabaei, S.; Vitelli, M.; Rajabzadeh, A.R.; Legge, R.L. Analysis of protein enrichment during single-and multi-stage tribo-electrostatic bioseparation processes for dry fractionation of legume flour. Sep. Purif. Technol. 2017, 176, 48–58. [Google Scholar] [CrossRef]
- Barakat, A.; Jérôme, F.; Rouau, X. A dry platform for separation of proteins from biomass-containing polysaccharides, lignin, and polyphenols. ChemSusChem 2015, 8, 1161–1166. [Google Scholar] [CrossRef]
- Görgüç, A.; Özer, P.; Yılmaz, F.M. Simultaneous effect of vacuum and ultrasound assisted enzymatic extraction on the recovery of plant protein and bioactive compounds from sesame bran. J. Food Compos. Anal. 2020, 87, 103424. [Google Scholar] [CrossRef]
- Görgüç, A.; Bircan, C.; Yılmaz, F.M. Sesame bran as an unexploited by-product: Effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019, 283, 637–645. [Google Scholar] [CrossRef]
- Tirgar, M.; Silcock, P.; Carne, A.; Birch, E.J. Effect of extraction method on functional properties of flaxseed protein concentrates. Food Chem. 2017, 215, 417–424. [Google Scholar] [CrossRef]
- Rommi, K.; Hakala, T.K.; Holopainen, U.; Nordlund, E.; Poutanen, K.; Lantto, R. Effect of enzyme-aided cell wall disintegration on protein extractability from intact and dehulled rapeseed (Brassica rapa L. and Brassica napus L.) press cakes. J. Agric. Food Chem. 2014, 62, 7989–7997. [Google Scholar] [CrossRef]
- Zhan, F.; Shi, M.; Wang, Y.; Li, B.; Chen, Y. Effect of freeze-drying on interaction and functional properties of pea protein isolate/soy soluble polysaccharides complexes. J. Mol. Liq. 2019, 285, 658–667. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Houde, M.; Khodaei, N.; Benkerroum, N.; Karboune, S. Barley protein concentrates: Extraction, structural and functional properties. Food Chem. 2018, 254, 367–376. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K.; et al. Advances in the plant protein extraction: Mechanism and recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Abelson, J.N.; Simon, M.I. Aqueous Two-Phase Systems; Elsevier: Amsterdam, The Netherlands, 1994; Volume 228. [Google Scholar]
- Varadavenkatesan, T.; Pai, S.; Vinayagam, R.; Pugazhendhi, A.; Selvaraj, R. Recovery of value-added products from wastewater using aqueous two-phase systems—A review. Sci. Total Environ. 2021, 778, 146293. [Google Scholar] [CrossRef]
- Pandian, P.S.; Selvan, S.S.; Subathira, A.; Saravanan, S. Optimization of aqueous two phase extraction of proteins from litopenaeus vannamei waste by response surface methodology coupled multi-objective genetic algorithm. Chem. Prod. Process Model. 2019, 15, 20190034. [Google Scholar] [CrossRef]
- Menegotto, A.L.L.; Fernandes, I.A.; Bucior, D.; Balestieri, B.P.; Colla, L.M.; Abirached, C.; Franceschi, E.; Steffens, J.; Valduga, E. Purification of protein from Arthrospira platensis using aqueous two-phase system associate with membrane separation process and evaluation of functional properties. J. Appl. Phycol. 2021, 33, 2967–2982. [Google Scholar]
- Sun, X.; Bandara, N. Applications of reverse micelles technique in food science: A comprehensive review. Trends Food Sci. Technol. 2019, 91, 106–115. [Google Scholar] [CrossRef]
- Zhu, K.-X.; Sun, X.-H.; Zhou, H.-M. Optimization of ultrasound-assisted extraction of defatted wheat germ proteins by reverse micelles. J. Cereal Sci. 2009, 50, 266–271. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, Y.; Zhang, F.; Luo, G. Protein extraction from grape seeds by reverse micelles: Optimization of the forward extraction. Open Access Libr. J. 2017, 4, 1103376. [Google Scholar]
- Liu, J.-G.; Xing, J.-M.; Shen, R.; Yang, C.-L.; Liu, H.-Z. Reverse micelles extraction of nattokinase from fermentation broth. Biochem. Eng. J. 2004, 21, 273–278. [Google Scholar] [CrossRef]
- Sun, X.-H.; Zhu, K.-X.; Zhou, H.-M. Optimization of a novel backward extraction of defatted wheat germ protein from reverse micelles. Innov. Food Sci. Emerg. Technol. 2009, 10, 328–333. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Mlyuka, E.; Mbifile, M.; Zhang, S.; Zheng, Z.; Chen, J. Strategic applications and the challenges of subcritical water extraction technology in food industries. Chiang Mai J. Sci. 2018, 45, 1015–1029. [Google Scholar]
- Álvarez-Viñas, M.; Rodríguez-Seoane, P.; Flórez-Fernández, N.; Torres, M.D.; Díaz-Reinoso, B.; Moure, A.; Domínguez, H. Subcritical water for the extraction and hydrolysis of protein and other fractions in biorefineries from agro-food wastes and algae: A review. Food Bioprocess Technol. 2021, 14, 373–387. [Google Scholar]
- Zhi, W.-J.; Wang, L.-F.; Hu, X.-J. Recent advances in the effects of microwave radiation on brains. Mil. Med. Res. 2017, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Brnčić, S.R. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibanez, E. Pressurized liquid extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–398. [Google Scholar]
- Kitrytė, V.; Kavaliauskaitė, A.; Tamkutė, L.; Pukalskienė, M.; Syrpas, M.; Venskutonis, P.R. Zero waste biorefining of lingonberry (Vaccinium vitis-idaea L.) pomace into functional ingredients by consecutive high pressure and enzyme assisted extractions with green solvents. Food Chem. 2020, 322, 126767. [Google Scholar] [CrossRef]
- Teles, A.S.C.; Chávez, D.W.H.; Coelho, M.A.Z.; Rosenthal, A.; Gottschalk, L.M.F.; Tonon, R.V. Combination of enzyme-assisted extraction and high hydrostatic pressure for phenolic compounds recovery from grape pomace. J. Food Eng. 2021, 288, 110128. [Google Scholar] [CrossRef]
- Tang, S.; Hettiarachchy, N.S.; Shellhammer, T.H. Protein extraction from heat-stabilized defatted rice bran. 1. Physical processing and enzyme treatments. J. Agric. Food Chem. 2002, 50, 7444–7448. [Google Scholar] [CrossRef]
- Ntourtoglou, G.; Tsapou, E.A.; Drosou, F.; Bozinou, E.; Lalas, S.; Tataridis, P.; Dourtoglou, V. Pulsed electric field extraction of α and β-acids from pellets of Humulus lupulus (Hop). Front. Bioeng. Biotechnol. 2020, 8, 297. [Google Scholar]
- Käferböck, A.; Smetana, S.; de Vos, R.; Schwarz, C.; Toepfl, S.; Parniakov, O. Sustainable extraction of valuable components from Spirulina assisted by pulsed electric fields technology. Algal Res. 2020, 48, 101914. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed electric field-assisted extraction of valuable compounds from microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 530–552. [Google Scholar] [CrossRef]
- Yu, X.; Gouyo, T.; Grimi, N.; Bals, O.; Vorobiev, E. Pulsed electric field pretreatment of rapeseed green biomass (stems) to enhance pressing and extractives recovery. Bioresour. Technol. 2016, 199, 194–201. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef]
- Xing, Q.; Utami, D.P.; Demattey, M.B.; Kyriakopoulou, K.; de Wit, M.; Boom, R.M.; Schutyser, M.A. A two-step air classification and electrostatic separation process for protein enrichment of starch-containing legumes. Innov. Food Sci. Emerg. Technol. 2020, 66, 102480. [Google Scholar] [CrossRef]
- Sumner, A.K.; Gebre-Egziabher, A.; Tyler, R.T.; Rossnagel, B.G. Composition and properties of pearled and fines fractions from hulled and hull-less barley. Cereal Chem. 1985, 62, 112–116. [Google Scholar]
- Du, Z.; Yu, Y.-L.; Wang, J.-H. Extraction of proteins from biological fluids by use of an ionic liquid/aqueous two-phase system. Chem. Eur. J. 2007, 13, 2130–2137. [Google Scholar] [CrossRef]
- Varghese, T.; Pare, A. Effect of microwave assisted extraction on yield and protein characteristics of soymilk. J. Food Eng. 2019, 262, 92–99. [Google Scholar] [CrossRef]
- Ayim, I.; Ma, H.; Alenyorege, E.A. Optimizing and predicting degree of hydrolysis of ultrasound assisted sodium hydroxide extraction of protein from tea (Camellia sinensis L.) residue using response surface methodology. J. Food Sci. Technol. 2018, 55, 5166–5174. [Google Scholar] [CrossRef]
- Carullo, D.; Pataro, G.; Donsì, F.; Ferrari, G. Pulsed electric fields-assisted extraction of valuable compounds from arthrospira platensis: Effect of pulse polarity and mild heating. Front. Bioeng. Biotechnol. 2020, 8, 551272. Available online: https://www.frontiersin.org/articles/10.3389/fbioe.2020.551272 (accessed on 18 July 2023). [CrossRef] [PubMed]
- Pai, S.; Hebbar, A.; Selvaraj, S. A critical look at challenges and future scopes of bioactive compounds and their incorporations in the food, energy, and pharmaceutical sector. Environ. Sci. Pollut. Res. Int. 2022, 29, 35518–35541. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Niu, D.; Zhang, Y.; Zeng, X.A. Physicochemical properties, antioxidant and antiproliferative activities of polysaccharides from Morinda citrifolia L. (Noni) based on different extraction methods. Int. J. Biol. Macromol. 2020, 150, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research. Bioactive Ingredients Market Growth & Trends. 2023. Available online: https://www.grandviewresearch.com/press-release/global-bioactive-ingredients-market (accessed on 20 July 2023).
- Fu, Y.; Luo, J.; Qin, J.; Yang, M. Screening techniques for the identification of bioactive compounds in natural products. J. Pharm. Biomed. Anal. 2019, 168, 189–200. [Google Scholar] [CrossRef]
- Muscat, A.; de Olde, E.M.; Ripoll-Bosch, R.; Van Zanten, H.H.; Metze, T.A.; Termeer, C.J.; van Ittersum, M.K.; de Boer, I.J. Principles, drivers and opportunities of a circular bioeconomy. Nat. Food 2021, 2, 561–566. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of fruit waste for bioactive compounds and their applications in the food industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef]
| Food By-Product | Bioactive Compounds | References | ||
|---|---|---|---|---|
| Class | Concentration (mg/kg) | Major Compounds | ||
| Vegetable By-Products | ||||
| Beetroot Pomace | Phenolic acids | 1513 | Ferulic acid, Caffeic acid, p-Hydroxybenzoic acid, Vanillic acid, Protocatechuic acid | [21] |
| Flavonoids | 386 | Catechin, Epicatechin | ||
| Betalains | 558.8 | Betaxanthins, Betacyanins (isobetanin and betanin) | ||
| Potato Pulp and Peel | Carbohydrates | / | Pectin | |
| Glycoalkaloid | 639–3580 | α-Solanine, α-Chaconine | [22,23,24] | |
| Phenolic acids | 1830–9130 | Caffeic acid, Chlorogenic acid | ||
| Broccoli leaves | Glucosinolates | 1332–1594 | Glucoiberin, Gluconasturtiin, Glucoraphanin, Glucobrassicin, Neoglucobrassin, 4-Methoxy-glucobrassicin | |
| Broccoli stalks and florets | Flavonoids | 56.6 | Quercetin, Kaempferol | [25,26] |
| Phenolic acid | 74.6–193.8 | Neochlorogenic acid, Chlorogenic acid, Sinapic acid | ||
| Glucosinolates | 1836.6–5775.6 | Glucoiberiin, Glucoraphanin, Glucoalyssin, Neoglucobrassin, Glucobrassicin, Glucoerucin, Gluconapin | ||
| Carrot Peel | Carotenoids | 205.6 | Lutein, α-Carotene, β-Carotene, Lycopene | |
| Cauliflower Stem and leaves | Phenolic acids | / | Sinapic acid, Ferulic acid | [27,28] |
| Isothiocyanate | / | / | [29] | |
| Flavonoids | Quercetin, Kaempferol, Glycosides | [30] | ||
| Fruit By-Products | ||||
| Apple Pomace | Phenolic acids | 523–1542 | Caffeic acid, p-coumaric acid, Sinapic acid, Chlorogenic acid, Ferulic acid, p-coumaroylquinic acid | [31,32,33,34,35,36] |
| Anthocyanins | 50–130 | Cyanidin-3-O-galactoside | ||
| Triterpenoids | / | Oleanolic acid, Ursolic acid | ||
| Carbohydrates | / | Pectin, Pectin oligosaccharides | ||
| Flavonoids | 2153–3734 | Isorhamentin, Quercetin, Glycoconjugates, Kaemferol, Rhamnetin, Epicatechin | ||
| Dihydrochalcones | 688–2535 | Phloretein, Phlorizin | ||
| Grape pomace | Phenolic acids | Hydroxybenzoic acids (Gallic acid, Syringic acid); Hydroxycinnamic acids (Caffeic acid, p-Coumaric acid, Ferulic acid); | [37,38,39,40,41,42,43] | |
| Anthocyanins | 13,169–78,537 | |||
| Flavanols | 1000–12,886 | Procyanidin B1, (+)-Catechin, Procyanidin B2, (-)-Epicatechin, (-)-Gallocatechin, (-)-Gallocatechin gallate, (-)-Epicatechin gallate | ||
| Flavonols | Kaempferol-3-glucoside, Quercetin | |||
| Plum Pomace | Flavonols | 40.3 | Quercetin, Kaempferol, Glycosides, Rutinoside | [44] |
| Phenolic acid | 95.7 | Chlorogenic acid, Neochlorogenic acid | ||
| Anthocyanins | 6.5 | Cyanidin, Peonidin | ||
| Mango Peel | Carotenoids | 1900 | β-cryptoxanthin, β-carotene, Lutein | |
| Mango Kernel Seed | Flavonoids | 7200–13,000 | Fisetin, Isoquercetin, Quercetin | [45,46,47] |
| Xanthanoids | 13,600 | Mangiferin | ||
| Phenoic acids | / | Gallic acid | ||
| Catechins | / | Epicatecin, Epigallocatechin | ||
| Berries Press Residue | Anthhocyanins | 84,120 (blueberries) 27,890 (lingonberries) 284,950 (bilberries) 43,530 (cranberries) | Malvidin, Cyanidin, Petunidin, Delphinidin | |
| Banana Peel | Flavonols | 1019.6 | Rutin, Kaempferol, Laricitrin, Quercetin, Myricitin | [48,49] |
| Catecholamines | 4720 | Dopamine | ||
| Phenolic acids | 99.5 | Ferulic acid, Sinapic acids, p-Coumaric acid, Caffeic acid | ||
| Catechins | / | Epicatechin, Catechin, Gallocatechin | ||
| Citrus Peel and Pulp | Phenolic acids | 560 (orange) 276 (lemon) | Caffeic acid, Hydroxybenzoic acid | [50,51] |
| Flavanones | 22298 (orange) 10646 (lemon) | Hesperidin, Eriocitrin, Narirutin | ||
| Flavones | 55 (orange) 1659 (lemon) | Diosmetin glucoside, Apigenin glucoside | ||
| Source | Bioactive Compounds | Concentration | Reference |
|---|---|---|---|
| Wheat Bran | Thiamin | 0.65 mg/100 g | [58] |
| Riboflavin | 0.51 mg/100 g | ||
| Niacin | 28 mg/100 g | ||
| Pantothenic acid | 3.15 mg/100 g | ||
| Pyridoxine | 1 mg/100 g | ||
| Folate | 0.23 mg/100 g | ||
| Total Carotenoids | 4.2 ug/g | [59] | |
| Lignan | 4.75 mg/100 g | [58] | |
| Phytosterol | 158 mg/100 g | ||
| Betaine | 868 mg/100 g | ||
| Choline | 172 mg/100 g | ||
| Total Flavonoids | 3000–4300 ug/g | [60] | |
| Alkyresorcinol | 489–1429 ug/g | [61] | |
| Phytosterols | 4.73–2020 ug/g | [58] | |
| Ferulic acid | 1376–1918 ug/g | [62] | |
| Total Phenolic content | 4206.16 ug/g | [63] | |
| Rice Husk | p-coumaric acid | 265.4 mg/100 g | [64] |
| Ferulic acid | 33.64 mg/100 g | ||
| Total Flavonoids | 3.08 mg CE/g | ||
| Total Phenolics | 14.90 mg GAE/g | ||
| Caffeic acid | 3.68 mg/100 g | ||
| p-hydroxybenzoic acid | 12.55 mg/100 g | ||
| Corn Bran | Total Phenolic content | 1925 mg GAE/100 g | [59] |
| Total Carotenoids | 32.0 μg/g | ||
| Corn Germ meal | Total Carotenoids | 57.9 μg/g |
| Plant | Ref | Protein % | Glutelin % | Albumin % | Prolamin % | Globulin % |
|---|---|---|---|---|---|---|
| Cereals | ||||||
| Maize | [87] | 10.3 | 40 | 5 | 50 | 5 |
| Rice | [88] | 6–8 | 75–81 | 5–10 | 3–6 | 7–17 |
| Wheat | [89] | 8–13 | 42–62.5 | 15–20 | 28–42 | 15–20 |
| Barley | [90] | 10–12 | 25 | 2–3 | 30 | 2–3 |
| Oats | [91] | 16.9 | 35–40 | 20–25 | 10–15 | 20–25 |
| Oil seeds | ||||||
| Rapeseed | [92] | 38 | 10 | <1 | 20 | 70 |
| Sunflower | [93] | 20–40 | 17 | 38 | 5.5 | 39 |
| Microalgae | ||||||
| Cyanophyceae | [94] | 43–77 * | / | / | / | / |
| Chlorophyceae | [94] | 11–55 * | / | / | / | / |
| Millet, legumes and nuts proteins | ||||||
| Millet | [95] | 9–13 | 30 | 15 | 30 | 8 |
| Peas | [96] | 25.6 | 19.1 | 13.8 | 3.08 | 57.2 |
| Lupins | [97] | 44 | 5.7–11.9 | 8.9–23.9 | 0.6–1.8 | 46.5 |
| Soy | [98] | 40 | 30.4 | 22.9 | 0.3 | 46.5 |
| Lentils | [99] | 26 | 2.1–3.5 | 56.3–64.0 | 1.4–2.0 | 26.5–29.5 |
| Cashew | [100] | 20–25 | 11.7 | 45.6 | 0.4 | 42.4 |
| Pistachios | [101] | 20 | 7.3 | 25 | 2 | 66 |
| Almonds | [102] | 22.7–29.9 | <5 | 21 | <5 | 74 |
| Walnuts | [103] | 18–24 | 72 | 7.5 | 4.7 | 15.7 |
| Extraction Method | Technique | Protein Yield/% | Ref |
|---|---|---|---|
| Dry Extraction | Air classification techniques | 18 | [165] |
| Sieving | 17 | [166] | |
| Wet Extraction | Aqueous two-phase system extraction | 90 | [167] |
| Subcritical water extraction | 80 | [140] | |
| Reverse micelles extraction | 37 | [143] | |
| Enzyme-assisted extraction | 74 | [137] | |
| Novel Green methods of Extraction | High pressure-assisted extraction | 66.3 | [134] |
| Microwave-assisted extraction | 24 | [168] | |
| Ultrasound-assisted extraction | 63 | [169] | |
| Pulsed electric field-assisted extraction | 25.4 | [170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, Z.S.; Amir, S.; Sokač Cvetnić, T.; Jurinjak Tušek, A.; Benković, M.; Jurina, T.; Valinger, D.; Gajdoš Kljusurić, J. Sustainable Isolation of Bioactive Compounds and Proteins from Plant-Based Food (and Byproducts). Plants 2023, 12, 2904. https://doi.org/10.3390/plants12162904
Khan ZS, Amir S, Sokač Cvetnić T, Jurinjak Tušek A, Benković M, Jurina T, Valinger D, Gajdoš Kljusurić J. Sustainable Isolation of Bioactive Compounds and Proteins from Plant-Based Food (and Byproducts). Plants. 2023; 12(16):2904. https://doi.org/10.3390/plants12162904
Chicago/Turabian StyleKhan, Zakir Showkat, Saira Amir, Tea Sokač Cvetnić, Ana Jurinjak Tušek, Maja Benković, Tamara Jurina, Davor Valinger, and Jasenka Gajdoš Kljusurić. 2023. "Sustainable Isolation of Bioactive Compounds and Proteins from Plant-Based Food (and Byproducts)" Plants 12, no. 16: 2904. https://doi.org/10.3390/plants12162904





