Quantification of Dry Matter Content in Hass Avocado by Near-Infrared Spectroscopy (NIRS) Scanning Different Fruit Zones
Abstract
:1. Introduction
2. Results and Discussion
2.1. Number of NIRS Scans Per Fruit Zone for Assessing the Dry Matter Gradient in the Whole Fruit
2.2. Application of Different NIRS Scans by Fruit in Maturity Monitoring of Hass Avocados
2.2.1. Calibration
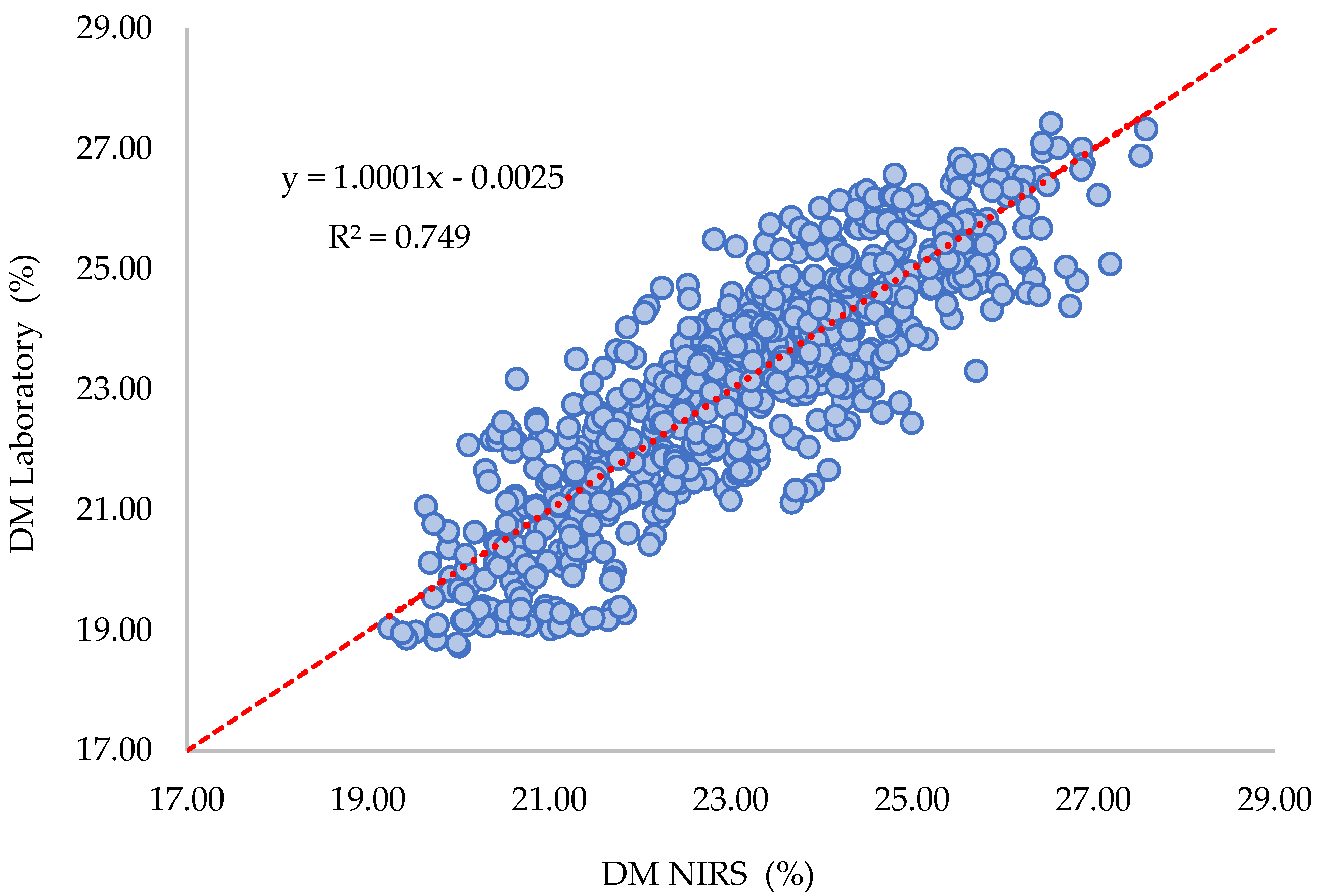
2.2.2. Tests of the Model Using Independent Validation Sets
3. Materials and Methods
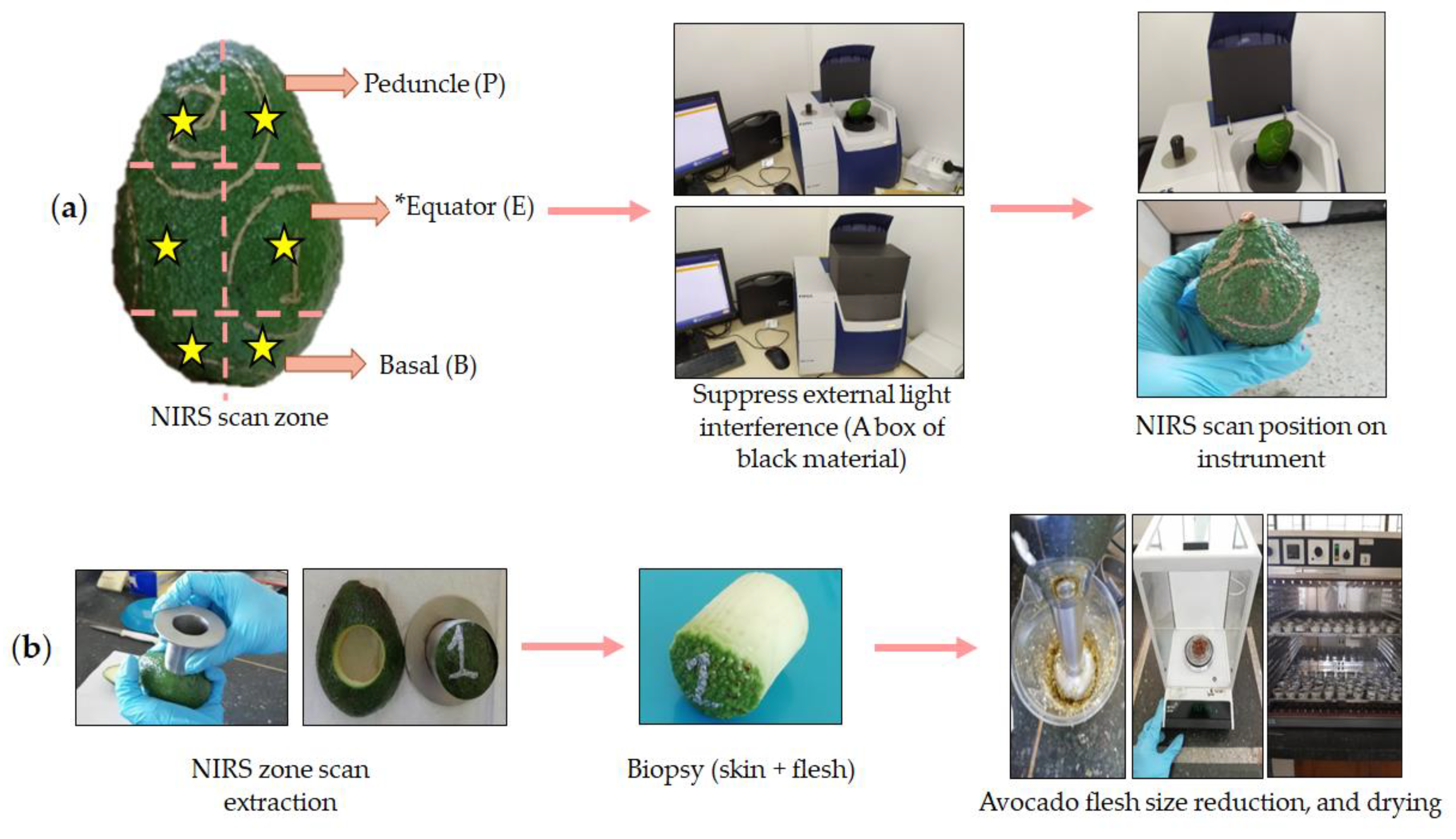
3.1. NIRS Scans Per Fruit Zone for Assessing the Dry Matter Gradient in Whole Fruit
3.1.1. Avocado Sampling
3.1.2. Spectroscopic Measurement and Data Acquisition
3.2. Application of the Method of Different NIRS Scans by Zone in Maturity Monitoring of Hass Avocado
3.2.1. Avocado Fruit Sampling
3.2.2. Spectroscopic Measurement and Data Acquisition
3.3. Dry Matter Analysis with the Reference Method
3.4. Data Analysis
3.4.1. DM Descriptive Statistics and ANOVA
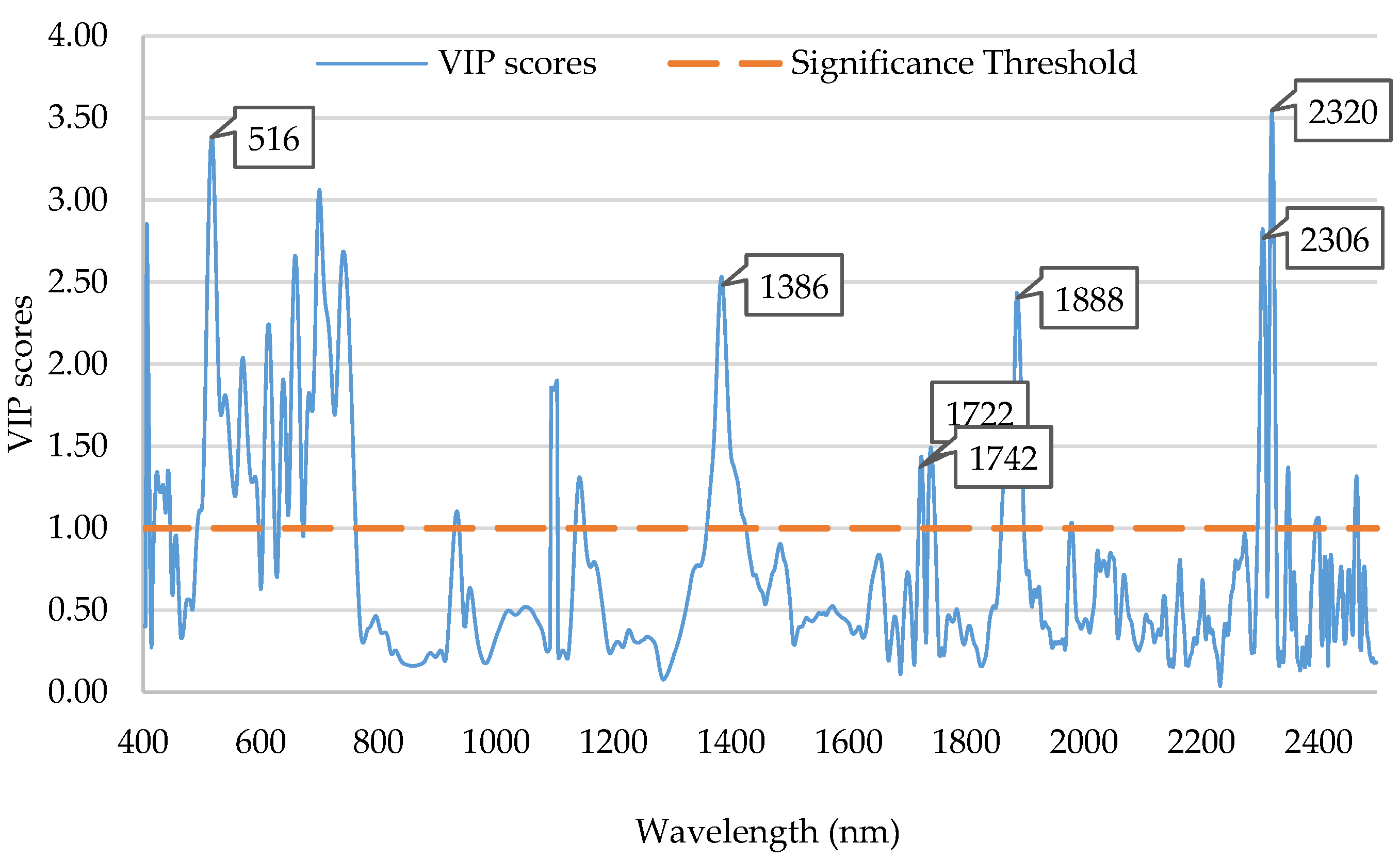
3.4.2. Chemometric Data Analysis
- (a)
- Number of Scans per Fruit zone.
- (b)
- Application of different NIRS scans by fruit in Maturity Monitoring of Hass Avocados.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| Abbreviations | Meaning |
| NIRS | Near-infrared spectroscopy |
| DM | Dry matter |
| OC | Oil content |
| PCA | Principal component analysis |
| PLS | Partial least-squares |
| SNK | Student Newman–Keuls |
| SVD | Singular value decomposition |
| GAP | Gap difference method |
| RMS | Root mean squared |
| RMSi | Root mean squared for each NIRS spectra |
| RMSEC | Root mean squared error of calibration |
| RMSECV | Root mean square error of cross-validation |
| RMSEP | Root mean squared error of prediction |
| SEC | Standard error of calibration |
| SECV | Standard error of cross-validation |
| SEP | Standard error of prediction |
| LV | Latent variables in the PLS model |
References
- Bill, M.; Sivakumar, D.; Thompson, A.K.; Korsten, L. Avocado Fruit Quality Management during the Postharvest Supply Chain. Food Rev. Int. 2014, 30, 169–202. [Google Scholar] [CrossRef]
- Rivera, S.A.; Ferreyra, R.; Robledo, P.; Selles, G.; Arpaia, M.L.; Saavedra, J.; Defilippi, B.G. Identification of Preharvest Factors Determining Postharvest Ripening Behaviors in ‘Hass’ Avocado under Long Term Storage. Sci. Hortic. 2017, 216, 29–37. [Google Scholar] [CrossRef]
- Escobar, J.V.; Rodriguez, P.; Cortes, M.; Correa, G. Influence of Dry Matter as a Harvest Index and Cold Storage Time on Cv. Hass Avocado Quality Produced in High Tropic Region. Inf. Tecnol. 2019, 30, 199–210. [Google Scholar] [CrossRef]
- Lee, S.K.; Young, R.E.; Schiffman, P.M.; Coggins, C.W. Maturity Studies of Avocado Fruit Based on Picking Dates and Dry Weight. J. Am. Soc. Hort. Sci. 1983, 108, 390–394. [Google Scholar] [CrossRef]
- Carvalho, C.P.; Velásquez, M.A.; Van Rooyen, Z. Determination of the minimum dry matter index for the optimun harvest of ‘Hass’ avocado fruits in Colombia. Agron. Colomb. 2014, 32, 399–406. [Google Scholar] [CrossRef]
- Rodriguez, P.; Henao, J.C.; Correa, G.; Aristizabal, A. Identification of Harvest Maturity Indicators for ‘Hass’ Avocado Adaptable to Field Conditions. Horttechnology 2018, 28, 815–821. [Google Scholar] [CrossRef]
- Burdon, J.; Connolly, P.; de Silva, N.; Lallu, N.; Dixon, J.; Pak, H. A Meta-Analysis Using a Logit Non-Linear Mixed Effects Model for “Hass” Avocado Postharvest Performance Data. Postharvest Biol. Technol. 2013, 86, 134–140. [Google Scholar] [CrossRef]
- Olarewaju, O.O.; Bertling, I.; Magwaza, L.S. Non-Destructive Evaluation of Avocado Fruit Maturity Using near Infrared Spectroscopy and PLS Regression Models. Sci. Hortic. 2016, 199, 229–236. [Google Scholar] [CrossRef]
- Donis-González, I.R.; Valero, C.; Momin, M.A.; Kaur, A.; Slaughter, D.C. Performance Evaluation of Two Commercially Available Portable Spectrometers to Non-Invasively Determine Table Grape and Peach Quality Attributes. Agronomy 2020, 10, 148. [Google Scholar] [CrossRef]
- Clark, C.J.; McGlone, V.A.; Requejo, C.; White, A.; Woolf, A.B. Dry Matter Determination in “Hass” Avocado by NIR Spectroscopy. Postharvest Biol. Technol. 2003, 29, 301–308. [Google Scholar] [CrossRef]
- Wedding, B.B.; White, R.D.; Grauf, S.; Wright, C.; Tilse, B.; Hofman, P.; Gadek, P.A. Non-Destructive Prediction of “Hass” Avocado Dry Matter via FT-NIR Spectroscopy. J. Sci. Food Agric. 2011, 91, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.P.; Ying, Y.; Zhou, Y.; Xie, L.J.; Xu, H.R. Application of NIR Spectroscopy for Firmness Evaluation of Peaches. J. Zhejiang Univ. Sci. B 2008, 9, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Blakey, R.J.; Bower, J.P.; Bertling, I. The Potential of Near-Infrared Spectroscopy in the Avocado Industry. Hortic. Sci. 2008, 31, 47–50. [Google Scholar]
- Magwaza, L.S.; Tesfay, S.Z. A Review of Destructive and Non-Destructive Methods for Determining Avocado Fruit Maturity. Food Bioprocess Technol. 2015, 8, 1995–2011. [Google Scholar] [CrossRef]
- Lin, H.; Ying, Y. Theory and Application of near Infrared Spectroscopy in Assessment of Fruit Quality: A Review. Sens. Instrum. Food Qual. Saf. 2009, 3, 130–141. [Google Scholar] [CrossRef]
- Walsh, K.B.; Blasco, J.; Zude-Sasse, M.; Sun, X. Visible-NIR ‘Point’ Spectroscopy in Postharvest Fruit and Vegetable Assessment: The Science behind Three Decades of Commercial Use. Postharvest Biol. Technol. 2020, 168, 111246. [Google Scholar] [CrossRef]
- Schroeder, C.A. Physiological Gradient in Avocado Fruit. Calif. Avocado Soc. Yearb. 1985, 69, 137–143. [Google Scholar]
- Woolf, A.; Clark, C.; Terander, E.; Phetsomphou, V.; Hofshi, R.; Arpaia, M.L.; Boreham, D.; Wong, M.; White, A. Measuring Avocado Maturity; Ongoing Developments. Orchidardist 2003, xx, 40–45. [Google Scholar]
- Wedding, B.B.; Wright, C.; Grauf, S.; White, R.D.; Tilse, B.; Gadek, P. Effects of Seasonal Variability on FT-NIR Prediction of Dry Matter Content for Whole Hass Avocado Fruit. Postharvest Biol. Technol. 2013, 75, 9–16. [Google Scholar] [CrossRef]
- Blakey, R.J. Evaluation of Avocado Fruit Maturity with a Portable Near-Infrared Spectrometer. Postharvest Biol. Technol. 2016, 121, 101–105. [Google Scholar] [CrossRef]
- Ncama, K.; Magwaza, L.S.; Poblete-Echeverría, C.A.; Nieuwoudt, H.H.; Tesfay, S.Z.; Mditshwa, A. On-Tree Indexing of ‘Hass’’ Avocado Fruit by Non-Destructive Assessment of Pulp Dry Matter and Oil Content. Biosyst. Eng. 2018, 174, 41–49. [Google Scholar] [CrossRef]
- Osuna-García, J.A.; Toivonen, P.; Salazar-García, S.; Goenaga, R.; Herrera-González, J.A. A nondestructive model to determine harvest maturity of Hass avocado. In Proceedings of the V Congreso Latinoamericano del Aguacate, Ciudad Guzmán, Mexico, 7 September 2017. [Google Scholar]
- Phetsomphou, V. Evaluation of Various Methods for Dry Matter Content and Firmness of ‘Hass’ Avocados. Bachelor Dissertation, Massey University, Palmerston North, New Zealand, 2000. [Google Scholar]
- Subedi, P.P.; Walsh, K.B. Assessment of Avocado Fruit Dry Matter Content Using Portable near Infrared Spectroscopy: Method and Instrumentation Optimisation. Postharvest Biol. Technol. 2020, 161, 111078. [Google Scholar] [CrossRef]
- Jha, S.N.; Jaiswal, P.; Narsaiah, K.; Gupta, M.; Bhardwaj, R.; Singh, A.K. Non-Destructive Prediction of Sweetness of Intact Mango Using near Infrared Spectroscopy. Sci. Hortic. 2012, 138, 171–175. [Google Scholar] [CrossRef]
- Nordey, T.; Joas, J.; Davrieux, F.; Chillet, M.; Léchaudel, M. Robust NIRS Models for Non-Destructive Prediction of Mango Internal Quality. Sci. Hortic. 2017, 216, 51–57. [Google Scholar] [CrossRef]
- Halliday, K.J.; Martínez-García, J.F.; Josse, E.M. Integration of Light and Auxin Signaling. Cold Spring Harb. Perspect. Biol. 2009, 1, 001586. [Google Scholar] [CrossRef]
- Dos Santos Neto, J.P.; de Assis, M.W.D.; Casagrande, I.P.; Cunha Júnior, L.C.; de Almeida Teixeira, G.H. Determination of ‘Palmer’ Mango Maturity Indices Using Portable near Infrared (VIS-NIR) Spectrometer. Postharvest Biol. Technol. 2017, 130, 75–80. [Google Scholar] [CrossRef]
- Xiaobo, Z.; Jiewen, Z.; Povey, M.J.W.; Holmes, M.; Hanpin, M. Variables Selection Methods in Near-Infrared Spectroscopy. Anal. Chim. Acta 2010, 667, 14–32. [Google Scholar] [CrossRef]
- Guthrie, J.; Greensill, C.; Bowden, R.; Walsh, K. Assessment of Quality Defects in Macadamia Kernels Using NIR Spectroscopy. Aust. J. Agric. Res. 2004, 55, 471–476. [Google Scholar] [CrossRef]
- Wedding, B.B.; Wrightd, C.; Grauf, S.; White, R.D.; Gadek, P.A. Near Infrared Spectroscopy as a Rapid Non-Invasive Tool for Agricultural and Industrial Process Management with Special Reference to Avocado and Sandalwood Industries. Desalination Water Treat. 2011, 32, 365–372. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Prithviraj, V.; Manikantan, M.R.; Kothakota, A.; Rusu, A.; Trif, M.; Mousavi, A. Recent advancements in NIR spectroscopy for assessing the quality and safety of horticultural products: A comprehensive review. Front. Nutr. 2022, 9, 973457. [Google Scholar] [CrossRef]
- Grabska, J.; Beć, K.B.; Ueno, N.; Huck, C.W. Analyzing the Quality Parameters of Apples by Spectroscopy from Vis/NIR to NIR Region: A Comprehensive Review. Foods 2023, 12, 1946. [Google Scholar] [CrossRef]
- Wedding, B.B.; Wright, C.; Grauf, S.; Gadek, P.; White, R.D. The Application of FT-NIRS for the Detection of Bruises and the Prediction of Rot Susceptibility of ‘Hass’ Avocado Fruit. J. Sci. Food Agric. 2019, 99, 1880–1887. [Google Scholar] [CrossRef]
- Mishra, P.; Paillart, M.; Meesters, L.; Woltering, E.; Chauhan, A. Avocado Dehydration Negatively Affects the Performance of Visible and Near-Infrared Spectroscopy Models for Dry Matter Prediction. Postharvest Biol. Technol. 2022, 183, 111739. [Google Scholar] [CrossRef]
- Melado-Herreros, A.; Nieto-Ortega, S.; Olabarrieta, I.; Gutiérrez, M.; Villar, A.; Zufía, J.; Gorretta, N.; Roger, J.M. Postharvest Ripeness Assessment of ‘Hass’ Avocado Based on Development of a New Ripening Index and Vis-NIR Spectroscopy. Postharvest Biol. Technol. 2021, 181, 111683. [Google Scholar] [CrossRef]
- Rodríguez, P.; Soto, I.; Villamizar, J.; Rebolledo, A. Fatty Acids and Minerals as Markers Useful to Classify Hass Avocado Quality: Ripening Patterns, Internal Disorders, and Sensory Quality. Horticulturae 2023, 9, 460. [Google Scholar] [CrossRef]
- Schmilovitch, Z.; Hoffman, A.; Egozi, H.; El-Batzri, R.; Degani, C. Determination of Avocado Maturity by Near-Infrared Spectrometry. Acta Hortic. 2021, 562, 175–179. [Google Scholar] [CrossRef]
- UNECE (United Nations Economic Commission for Europe). Available online: https://unece.org/fileadmin/DAM/trade/agr/meetings/capacity-building/2006_mojmirovce-SK/Dry%20matter%20content%20of%20avocados%20(June%202006).pdf (accessed on 3 May 2023).
- Waissbluth, R.; Valenzuela, J. Determination of the minimum percentage of dry matter to authorize the harvest of Hass avocado pears for export. In Proceedings of the VI World Avocado Congress (Actas VI Congreso Mundial del Aguacate) 2007, Viña Del Mar, Chile, 12–16 November 2007; ISBN 978-956-17-0413-8. [Google Scholar]
- Shenk, J.S.; Westerhaus, M.O. Analysis of Agriculture and Food Products by Near Infrared Reflectance Spectroscopy; Monograph, NIR Systems: Silver Spring, MD, USA, 1995. [Google Scholar]
- Shenk, J.S.; Westerhaus, M.O. Calibration the ISI Way; NIR Publications: Chichester, UK, 1996; pp. 198–202. [Google Scholar]
- Hopkins, D.W. What Is a Norris Derivative? NIR News 2001, 12, 3–5. [Google Scholar] [CrossRef]
- Shenk, J.S.; Westerhaus, M.O. Population Definition, Sample Selection, and Calibration Procedures for Near Infrared Reflectance Spectroscopy. Crop Sci. 1991, 31, 469–474. [Google Scholar] [CrossRef]
- Castrignanò, A.; Buttafuoco, G.; Malegori, C.; Genorini, E.; Iorio, R.; Stipic, M.; Girone, G.; Venezia, A. Assessing the Feasibility of a Miniaturized Near-Infrared Spectrometer in Determining Quality Attributes of San Marzano Tomato. Food Anal. Methods 2019, 12, 1497–1510. [Google Scholar] [CrossRef]
- Burns, D.A.; Ciurczak, E.W. Handbook of Near-Infrared Analysis, 3rd ed.; CRC Press: London, UK, 2007; pp. 585–611. [Google Scholar]
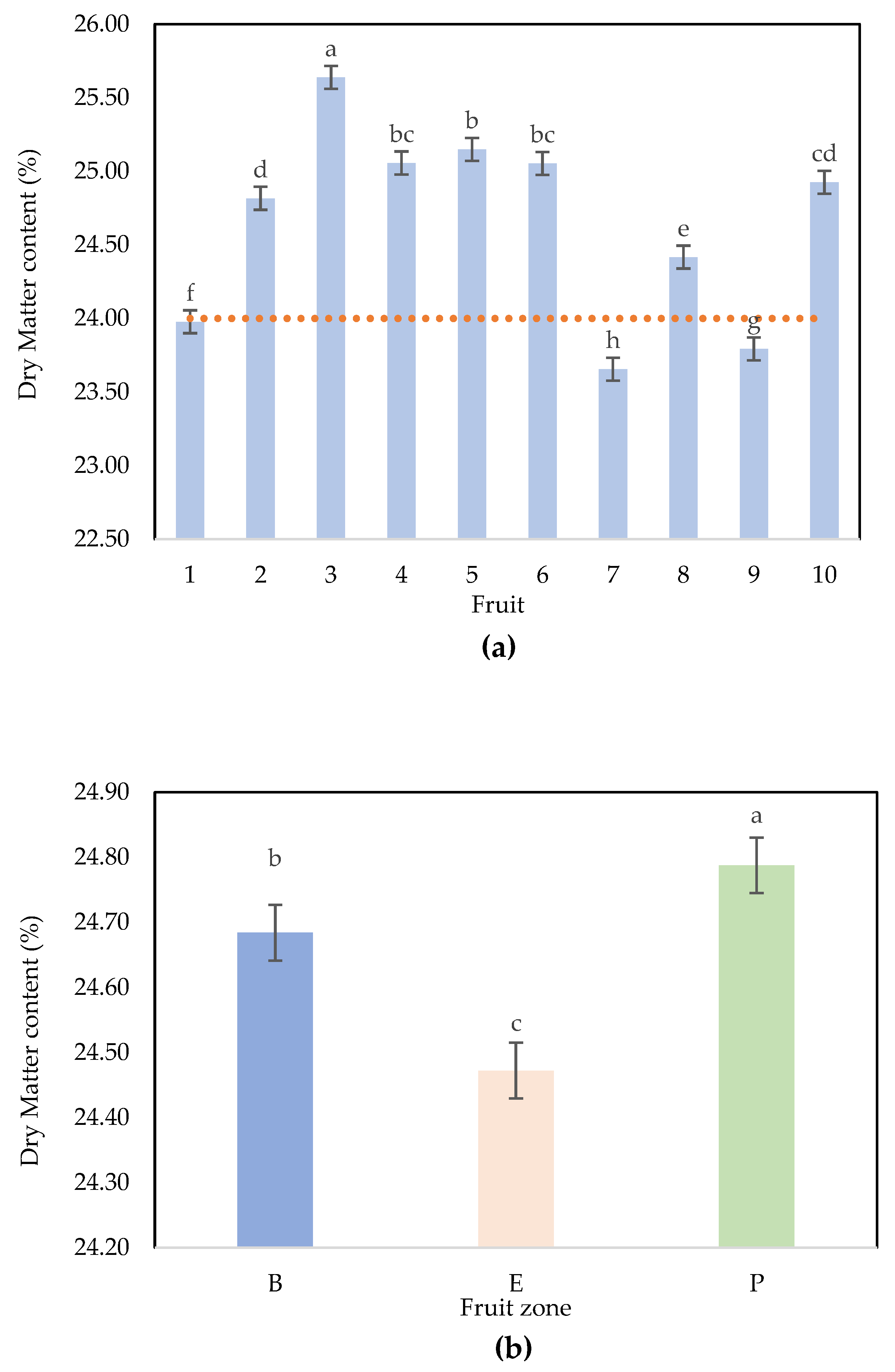
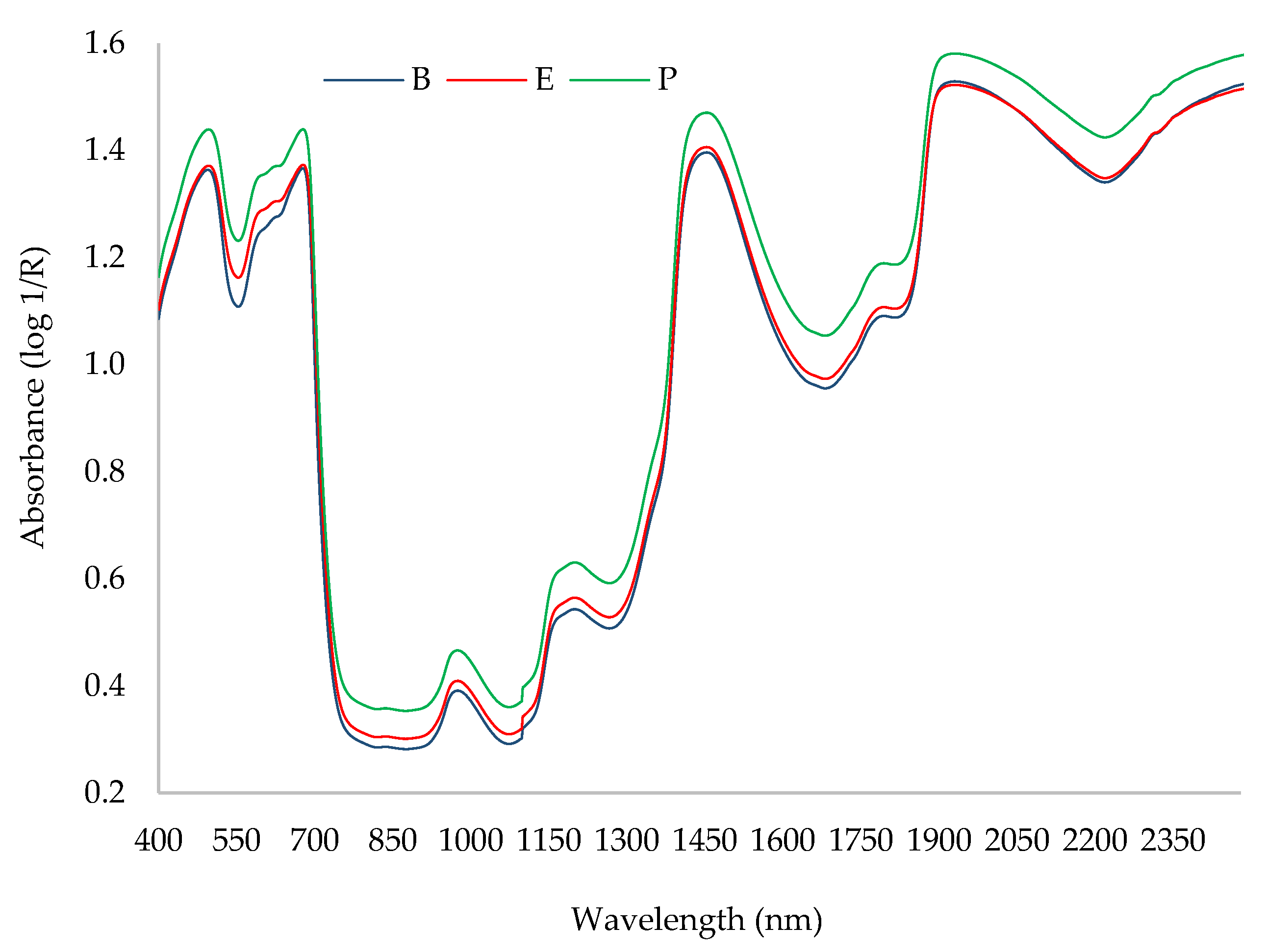
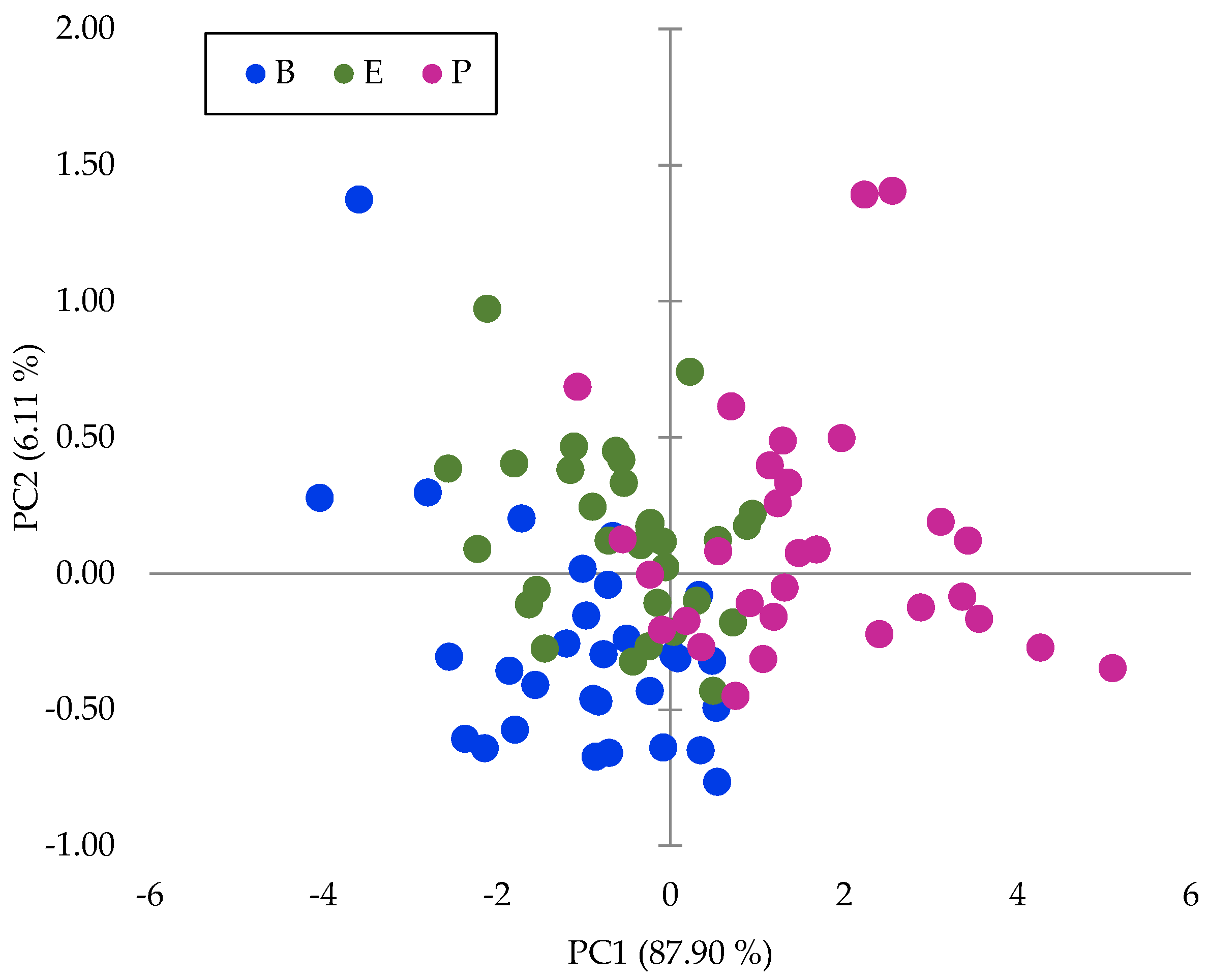
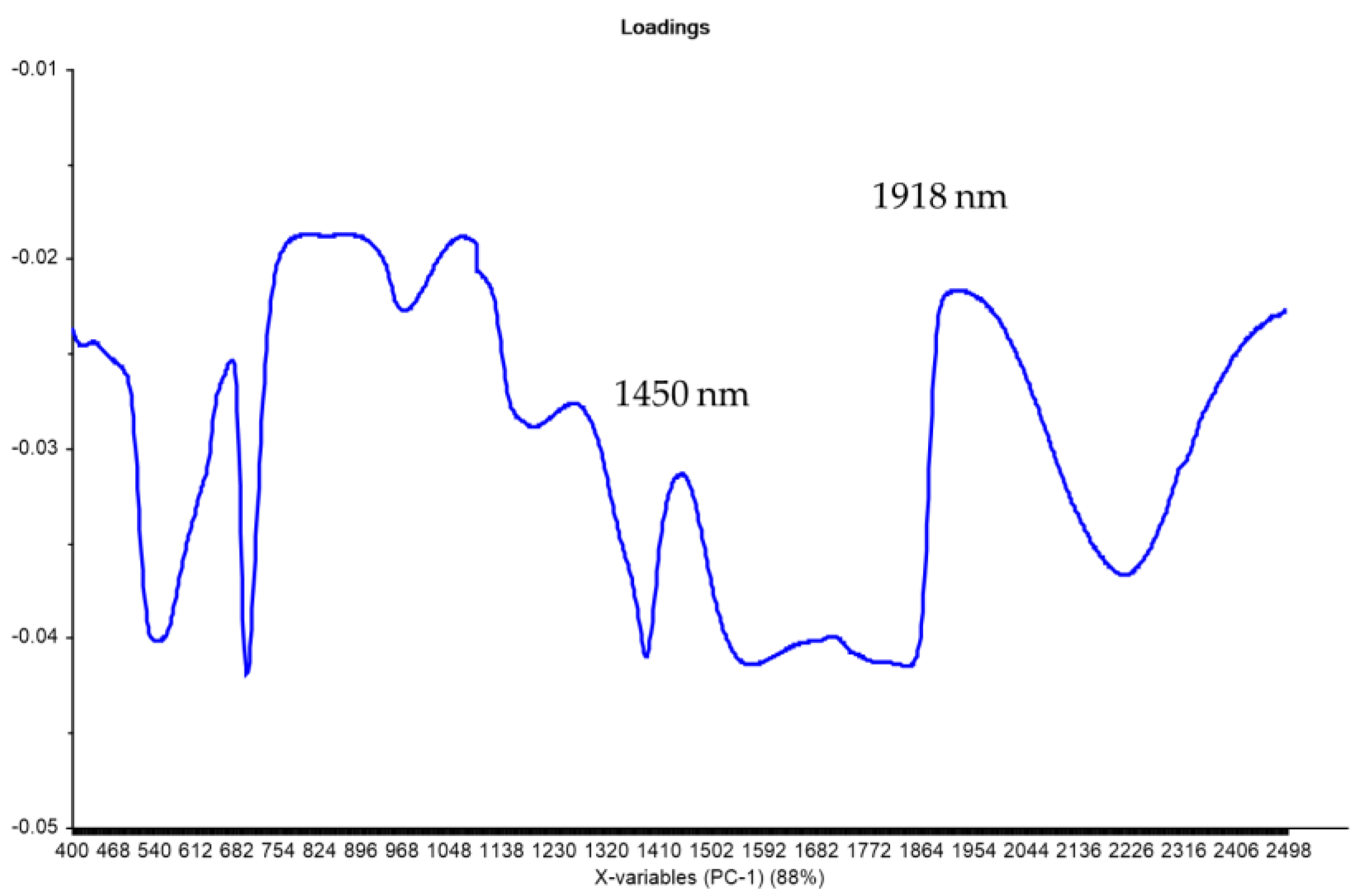

| Factors | Degrees of Freedom | Sum of Squares | Mean Squares | Fisher’s Exact Test | * Pr > F |
|---|---|---|---|---|---|
| Fruit | 9 | 35 | 4 | 282 | 0 |
| Zone | 2 | 2 | 1 | 56 | 0 |
| Harvest | Calibration Nápoles, Sotareño 2022a and Nápoles 2022b_Validation Recuerdo 2022b | |
|---|---|---|
| Statistics | ||
| N calibration | 527 | |
| N prediction | 180 | |
| Range DM Laboratory | 18.73–27.42 | |
| SD | 2.005 | |
| SEC | 0.995 | |
| R2 | 0.754 | |
| Range NIR Prediction | 18.35–27.04 | |
| SECV | 1.151 | |
| #LV | 12 | |
| # Outliers | 13 | |
| SEP | 1.62 | |
| Bias | 1.10 | |
| SEPc | 1.19 | |
| Slope | 0.70 | |
| R2 | 0.70 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, P.; Villamizar, J.; Londoño, L.; Tran, T.; Davrieux, F. Quantification of Dry Matter Content in Hass Avocado by Near-Infrared Spectroscopy (NIRS) Scanning Different Fruit Zones. Plants 2023, 12, 3135. https://doi.org/10.3390/plants12173135
Rodríguez P, Villamizar J, Londoño L, Tran T, Davrieux F. Quantification of Dry Matter Content in Hass Avocado by Near-Infrared Spectroscopy (NIRS) Scanning Different Fruit Zones. Plants. 2023; 12(17):3135. https://doi.org/10.3390/plants12173135
Chicago/Turabian StyleRodríguez, Pablo, Jairo Villamizar, Luis Londoño, Thierry Tran, and Fabrice Davrieux. 2023. "Quantification of Dry Matter Content in Hass Avocado by Near-Infrared Spectroscopy (NIRS) Scanning Different Fruit Zones" Plants 12, no. 17: 3135. https://doi.org/10.3390/plants12173135
APA StyleRodríguez, P., Villamizar, J., Londoño, L., Tran, T., & Davrieux, F. (2023). Quantification of Dry Matter Content in Hass Avocado by Near-Infrared Spectroscopy (NIRS) Scanning Different Fruit Zones. Plants, 12(17), 3135. https://doi.org/10.3390/plants12173135







