Exploring the Effects of Selenium and Brassinosteroids on Photosynthesis and Protein Expression Patterns in Tomato Plants under Low Temperatures
Abstract
:1. Introduction
2. Results
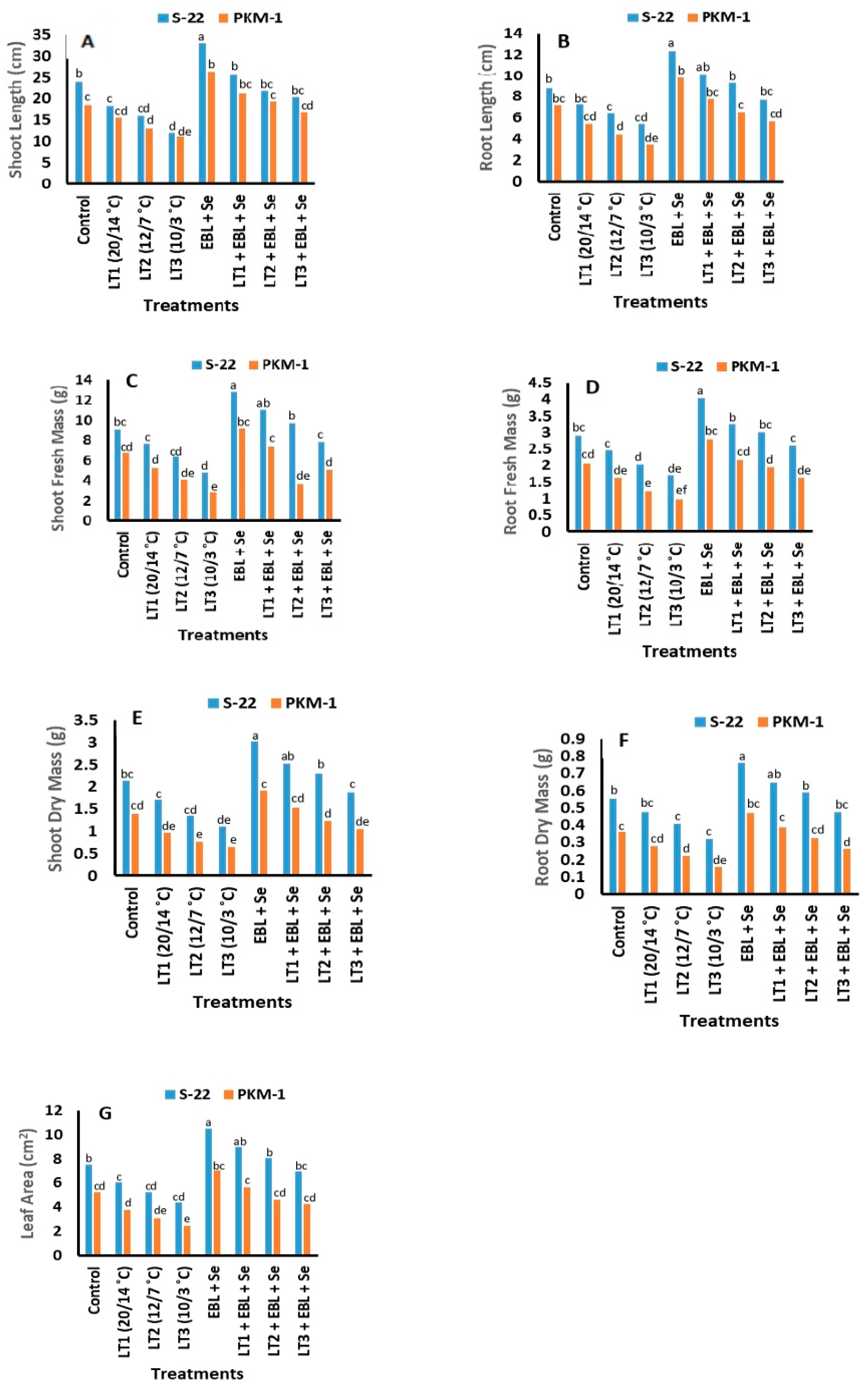
2.1. Growth Traits
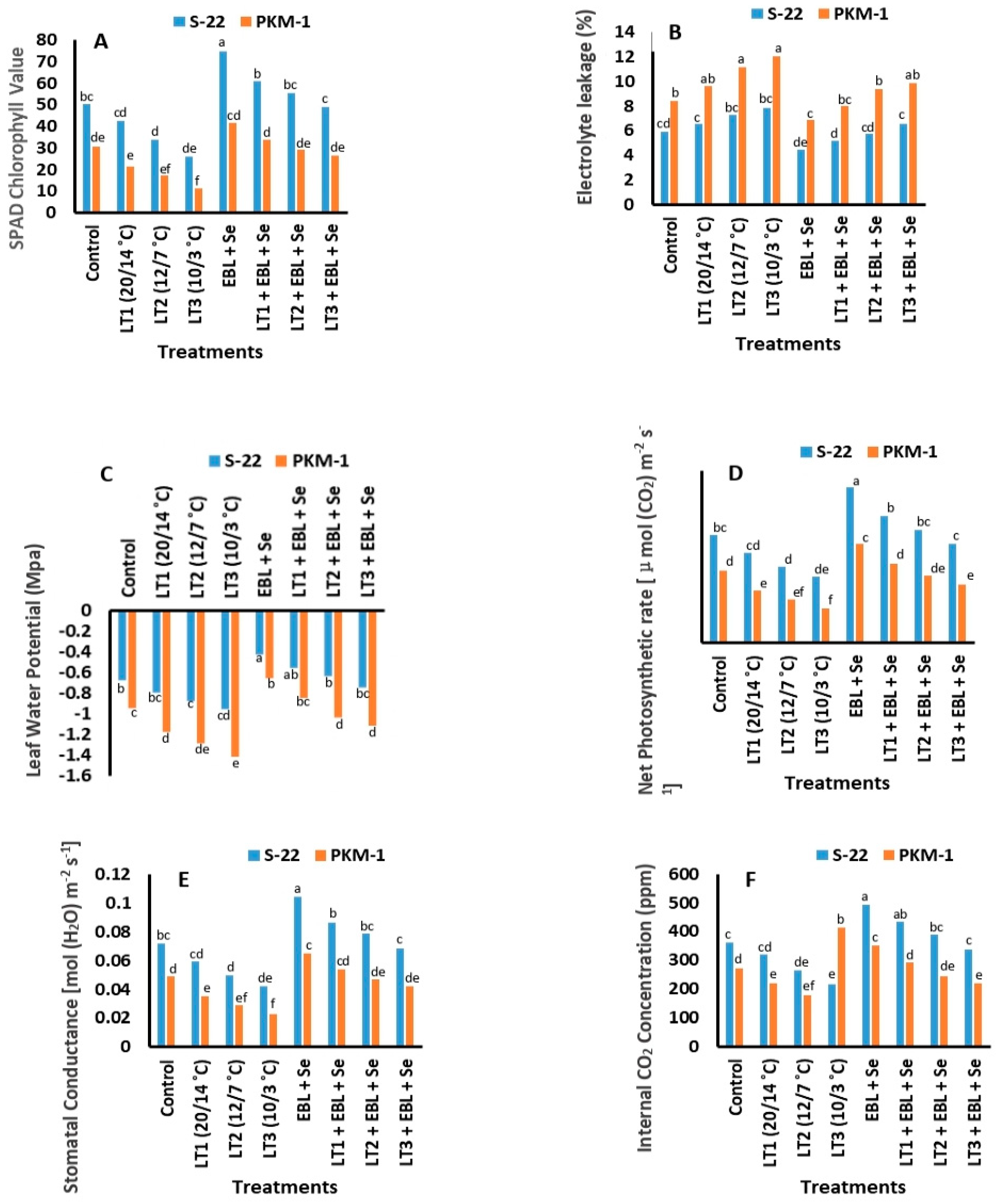
2.2. SPAD Value of Chlorophyll
2.3. Electrolyte Leakage
2.4. Leaf Water Potential (LWP)
2.5. Photosynthesis and Associated Traits
2.6. Photosystem II (PS II; Fv/Fm)
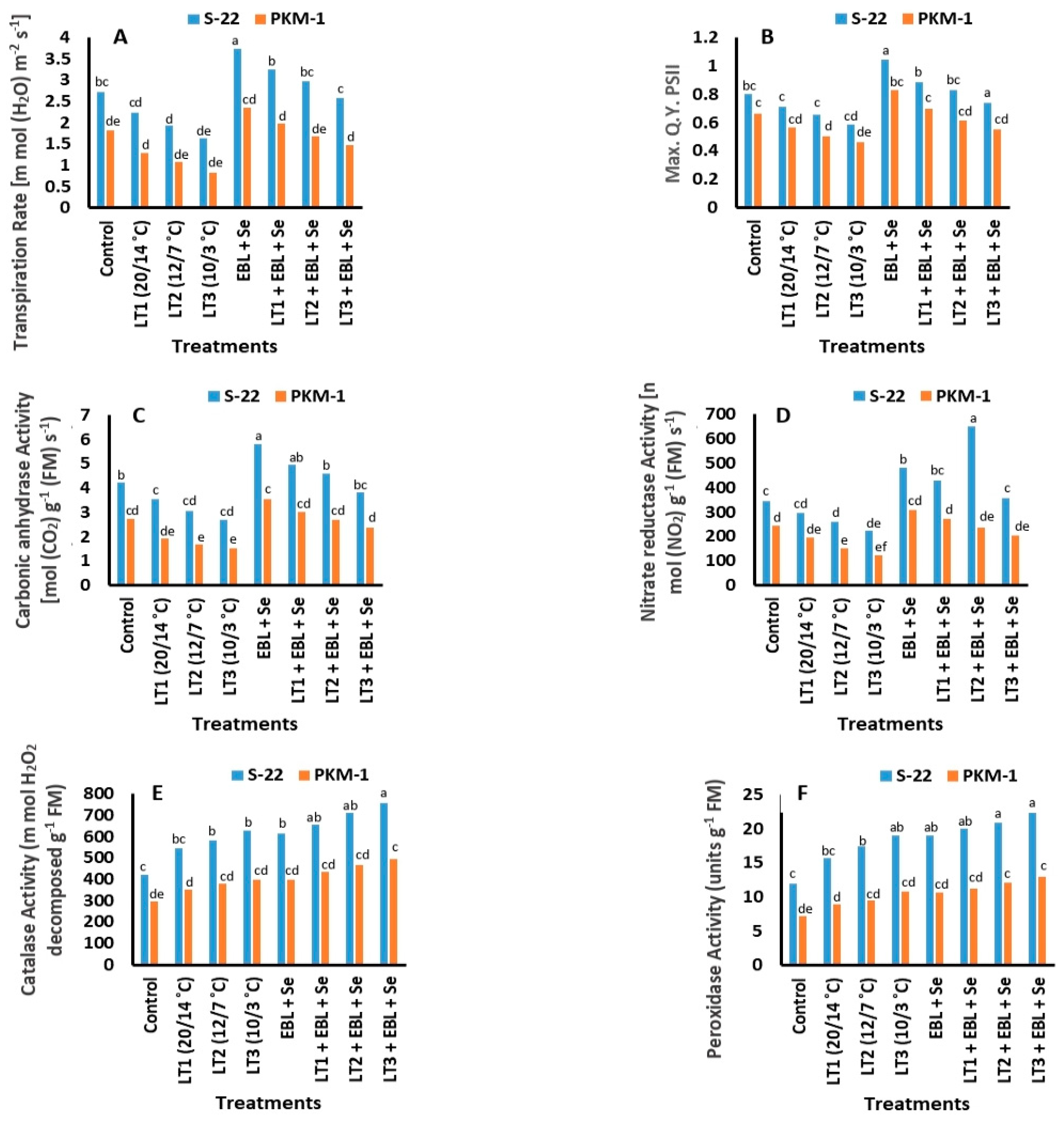
2.7. Activities of Nitrate Reductase (NR) and Carbonic Anhydrase (CA)
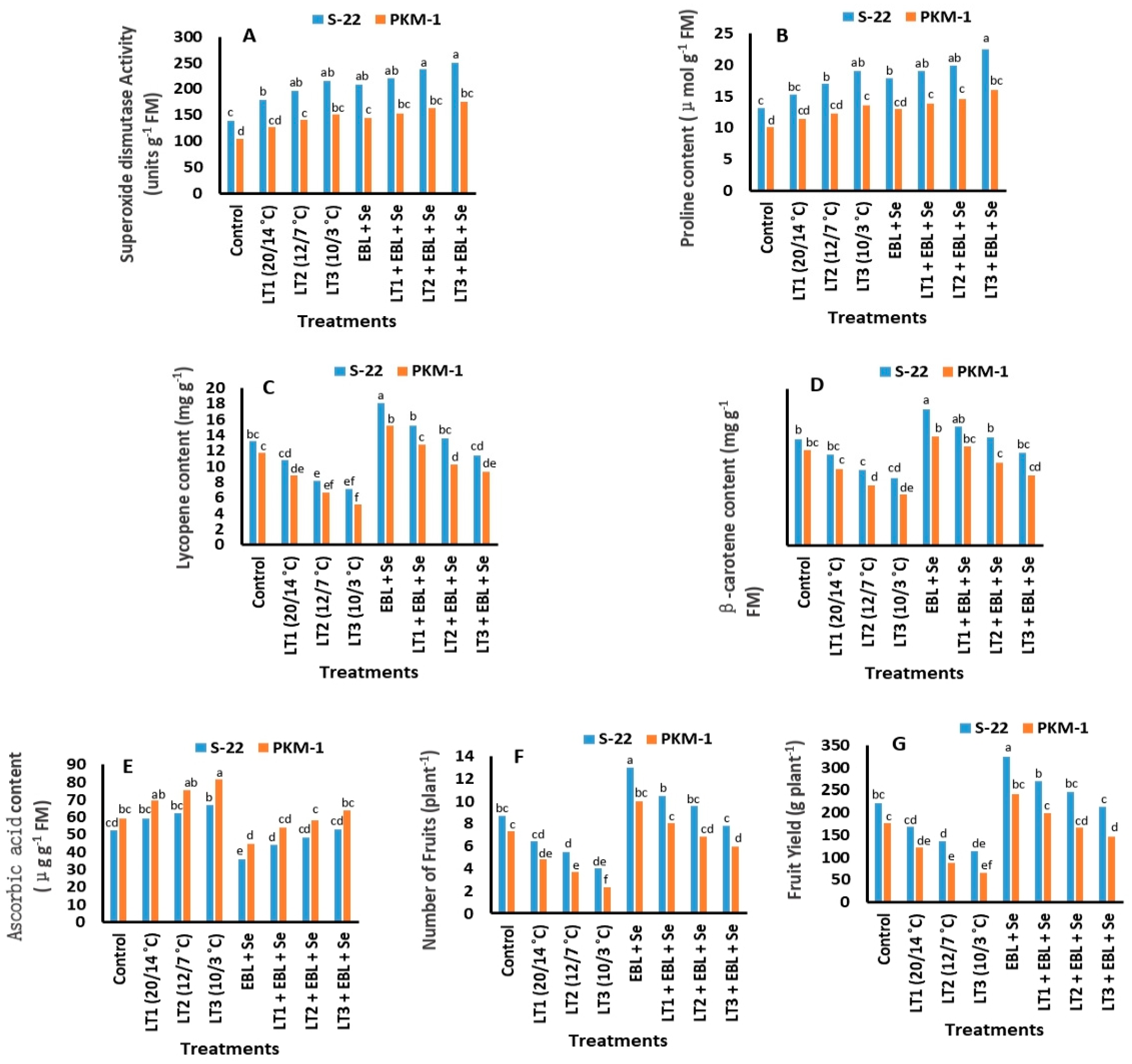
2.8. Activities of Antioxidant Enzymes
2.9. Proline Content
2.10. Contents of Lycopene, Beta-Carotene, and Ascorbic Acid
2.11. Yield and Number of Fruits
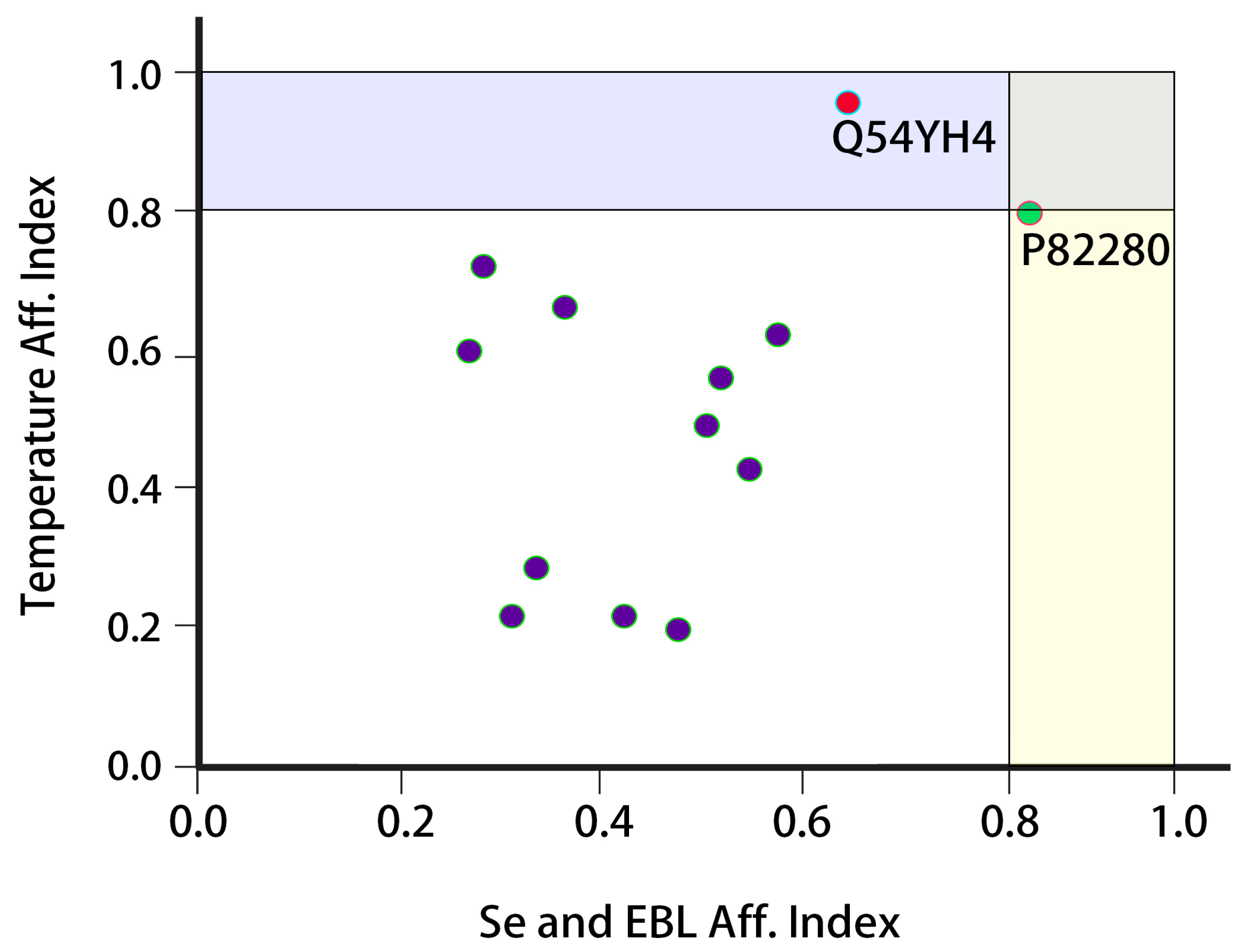
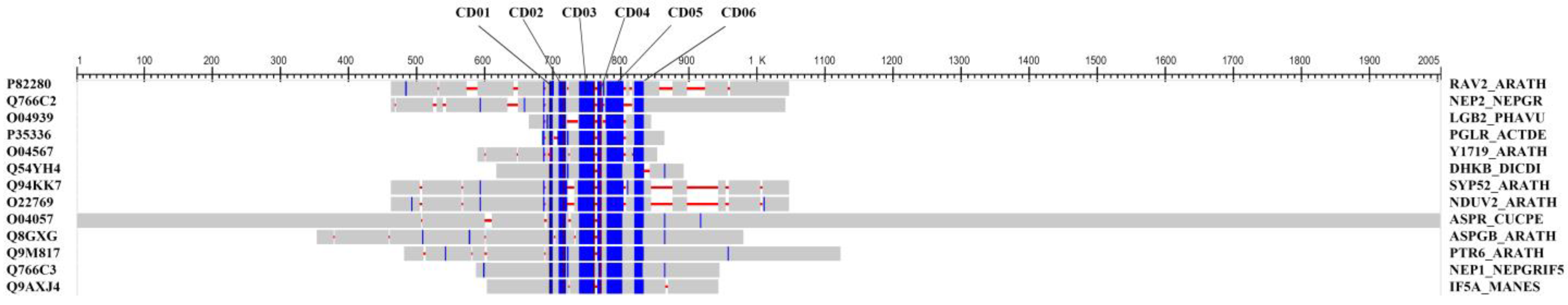
2.12. Analysis of Protein Expression
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Preparation of EBL and Se
4.3. Experimental Setup
4.4. Analysis of Plant Growth
4.5. Physiological Analysis
4.6. Biochemical Analysis
4.7. Analyses of Protein Expression
4.8. Identification and Image Analysis
4.9. Identification of Protein Function
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaynab, M.; Kanwal, S.; Furqan, M.; Islam, W.; Noman, A.; Ali, G.M. Proteomic approach to address low seed germination in Cyclobalnopsis gilva. Biotechnol. Lett. 2017, 39, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Fariduddin, Q.; Yusuf, M. Lycopersicon esculentum under low temperature stress: An approach toward enhanced antioxidants and yield. Environ. Sci. Pollut. Res. 2015, 22, 14178–14188. [Google Scholar] [CrossRef]
- Nazir, F.; Fariduddin, Q.; Hussain, A.; Khan, T.A. Brassinosteroid and hydrogen peroxide improve photosynthetic efficiency and maintain chloroplast ultrastructure, stomatal movement, root morphology, cell viability and reduce Cu- triggered oxidative burst in tomato. Ecotoxicol. Environ. Saf. 2021, 207, 111081. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Aslam, M.; Fakher, B.; Ashraf, M.A.; Cheng, Y.; Wang, B.; Qin, Y. Plant Low-Temperature Stress: Signaling and Response. Agronomy 2022, 12, 702. [Google Scholar] [CrossRef]
- Farooq, M.; Aziz, T.; Wahid, A.; Lee, D.J.; Siddique, K.H.M. Chilling tolerance in maize: Agronomics and physiological approaches. Crop Pasture Sci. 2009, 60, 501–516. [Google Scholar] [CrossRef]
- Kazemi-Shahandashti, S.S.; Maali-Amiri, R.; Zeinali, H.; Khazaei, M.; Talei, A.; Ramezanpour, S.S. Effect of short-term cold stress on oxidative damage and transcript accumulation of defense-related genes in chickpea seedlings. J. Plant Physiol. 2014, 171, 1106–1116. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Pilon-Smits, E.A.H.; Zhao, F.J.; Williams, P.N.; Meharg, A.A. Selenium in higher plants: Understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci. 2009, 14, 437–442. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Hossain, M.A.; Siddiqui, M.N.; Fujita, M.; Tran, L.P. Phenotypical, physiological and biochemical analyses provide insight into selenium-induced phytotoxicity in rice plants. Chemosphere 2017, 178, 212–223. [Google Scholar] [CrossRef]
- Stroud, J.L.; Zhao, F.J.; Buchner, P.; Shinmachi, F.; McGrath, S.P.; Abecassis, J.; Shewry, P.R. Impacts of sulphur nutrition on selenium and molybdenum concentrations in wheat grain. J. Cereal Sci. 2010, 52, 111–113. [Google Scholar] [CrossRef]
- Smith, F.W.; Hawkesford, M.J.; Ealing, P.M.; Clarkson, D.T.; van den Berg, P.J.; Belcher, A.R.; Warrilow, A.G.S. Regulation of expression of a cDNA from barley roots encoding a high affinity sulphate transporter. Plant J. 1997, 12, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Turakainen, M.; Hartikainen, H.; Seppänen, M.M. Effects of selenium treatments on potato (Solanum tuberosum L.) growth and concentrations of soluble sugars and starch. J. Agric. Food Chem. 2004, 52, 5378–5382. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhong, Y.; Huang, Z.; Yang, Y. Selenium accumulation in unicellular green Alga Chlorella vulgaris and its effects on antioxidant enzymes and content of photosynthetic pigments. PLoS ONE 2014, 9, e112270. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Blinov, A.V.; Serov, A.V.; Gvozdenko, A.A.; Kravtsov, A.A.; Nagdalian, A.A.; Raffa, V.V.; Maglakelidze, D.G.; Blinova, A.A.; Kobina, A.V.; et al. Effect of Selenium Nanoparticles on Germination of Hordéum Vulgáre Barley Seeds. Coatings 2021, 11, 862. [Google Scholar] [CrossRef]
- Naz, F.S.; Yusuf, M.; Khan, T.A.; Fariduddin, Q.; Ahmad, A. Low level of selenium increases the efficacy of 24-epibrassinolide through altered physiological and biochemical traits of Brassica juncea plants. Food Chem. 2015, 185, 441–448. [Google Scholar] [CrossRef]
- Yusuf, M.; Khan, T.A.; Fariduddin, Q. Interaction of Epibrassinolide and selenium ameliorates the excess copper in Brassica juncea through altered proline metabolism and antioxidant. Ecotoxicol. Environ. Saf. 2016, 129, 25–34. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, X.; Pang, Y.; Huang, Q.; Yi, Q. Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Environ. Exp. Bot. 2014, 107, 39–45. [Google Scholar] [CrossRef]
- Hartikainen, H.; Xue, T.L.; Piironen, V. Selenium as an antioxidant and pro-oxidant in ryegrass. Plant Soil 2000, 225, 193–200. [Google Scholar] [CrossRef]
- Lyons, G.H.; Genc, Y.; Soole, K.; Stangoulis, J.C.R.; Liu, F.; Graham, R.D. Selenium increases seed production in Brassica. Plant Soil 2009, 318, 73–80. [Google Scholar] [CrossRef]
- Ekanayake, L.J.; Thavarajah, D.; Vial, E.; Schatz, B.; McGee, R.; Thavarajah, P. Selenium fertilization on lentil (Lens culinaris Medikus) grain yield, seed selenium concentration, and antioxidant activity. Field Crops Res. 2015, 177, 9–14. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biol. Trace Elem. Res. 2011, 143, 1704–1721. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zheng, Q.; Liu, Z.; Xu, W.; Liu, L.; Zhao, G.; Long, X. Overexpression of Arabidopsis thaliana Na+/H+ antiporter gene enhanced salt resistance in transgenic poplar (Populus× euramericana‘Neva’). Trees 2012, 26, 685–694. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.V.; Seppänen, M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol. Biochem. 2010, 48, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Fariduddin, Q.; Yusuf, M.; Ahmad, I.; Ahmad, A. Brassinosteroids and their role in response of plants to abiotic stresses. Biol. Plant. 2014, 58, 9–17. [Google Scholar] [CrossRef]
- Vardhini, B.V. Modifications of morphological and anatomical characteristics of plants by application of brassinosteroids under various abiotic stress conditions—A review. Plant Gene 2017, 11, 70–89. [Google Scholar] [CrossRef]
- Kim, T.W.; Wang, Z.Y. Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2011, 61, 681–704. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Yusuf, M.; Chalkoo, S.; Hayat, S.; Ahmad, A. 28-homobrassinolide improves growth and photosynthesis in Cucumis sativus L. through an enhanced antioxidant system in the presence of chilling stress. Photosynthetica 2011, 49, 55–64. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Wang, Y.T.; Pan, X.B.; Xi, Z.M. Amelioration of cold-induced oxidative stress by exogenous 24-epibrassinolide treatment in grapevine seedlings: Toward regulating the ascorbate-glutathione cycle. Sci. Hortic. 2019, 244, 379–387. [Google Scholar] [CrossRef]
- Yusuf, M.; Saeed, T.; Almenhali, H.A.; Azzam, F.; Hamzah, A.I.A.H.; Khan, T.A. Melatonin improved efficiency of 24-epibrassinolide to counter the collective stress of drought and salt through osmoprotectant and antioxidant system in pea plants. Sci. Hortic. 2023, 323, 112453. [Google Scholar] [CrossRef]
- Chandrakar, V.; Yadu, B.; Meena, R.K.; Dubey, A.; Keshavkant, S. Arsenic-induced genotoxic responses and their amelioration by diphenyleneiodonium, 24-epibrassinolide and proline in Glycine max L. Plant Physiol. Biochem. 2017, 112, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Dalyan, E.; Yuzbasioglu, E.; Akpinar, I. Effect of 24-epibrassinolide on antioxidativedefence system against lead-induced oxidative stress in the roots of Brassica juncea L. seedlings. Russ. J. Plant Physiol. 2018, 65, 570–578. [Google Scholar] [CrossRef]
- Khan, T.A.; Fariduddin, Q.; Yusuf, M. Low temperature stress: Is phytohormones application a remedy? Environ. Sci. Pollut. Res. 2017, 24, 21574–21590. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Yusuf, M.; Ahmad, A.; Bashir, Z.; Saeed, T.; Fariduddin, Q.; Hayat, S.; Mock, H.P.; Wu, T. Proteomic and physiological assessment of stress sensitive and tolerant variety of tomato treated with brassinosteroids and hydrogen peroxide under low-temperature stress. Food Chem. 2019, 289, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Khan, T.A.; Yusuf, M.; Fariduddin, Q. Silicon mediated role of 24-epibrassinolide in wheat under high temperature stress. Environ. Sci. Pollut. Res. 2019, 26, 17163–17172. [Google Scholar] [CrossRef]
- Gill, M.B.; Cai, K.; Zhang, G.; Zeng, F. Brassinolide alleviates the drought induced adverse effects in barley by modulation of enzymatic antioxidants and ultrastructure. Plant Growth Regul. 2017, 82, 447–455. [Google Scholar] [CrossRef]
- Li, H.; Mo, Y.; Cui, Q.; Yang, X.Z.; Huao, Y.L.; Wei, C.H.; Yang, J.; Zhang, Y.; Ma, J.X.; Zhang, X. Transcriptomic and physiological analyses reveal drought adaptation strategies in drought-tolerant and -susceptible watermelon genotypes. Plant Sci. 2019, 278, 32–43. [Google Scholar] [CrossRef]
- Cao, S.; Xu, Q.; Cao, Y.; Qian, K.; An, K.; Zhu, Y.; Binzeng, H.; Zhao, H.; Kuai, B. Loss of function mutation in DET2 gene lead to an enhanced resistance to oxidative stress in Arabidopsis. Physiol. Plant 2005, 123, 57–66. [Google Scholar] [CrossRef]
- Dong, Y.J.; Wang, W.W.; Hu, G.Q.; Chen, W.F.; Zhuge, Y.P.; Wang, Z.L.; He, M.R. Role of exogenous 24-epibrassinolide in enhancing the salt tolerance of wheat seedlings. J. Soil Sci. Plant Nutr. 2017, 17, 554–569. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Khan, T.A.; Yusuf, M.; Aafaqee, S.T.; Khalil, R.R.A.E. Ameliorative role of salicylic acid and spermidine in the presence of excess salt in Lycopersicon esculentum. Photosynthetica 2018, 56, 750–762. [Google Scholar] [CrossRef]
- Ali, B.; Hayat, S.; Ahmad, A. 28-Homobrassinolide ameliorates the saline stress in Cicer arietinum L. Environ. Exp. Bot. 2007, 59, 217–223. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, T.A.; Mubeen, S.; Shahzadi, I.; Akram, W.; Saeed, T.; Bashir, Z.; Wang, R.; Alam, M.; Ahmed, S.; et al. Metabolic and Proteomic Perspectives of Augmentation of Nutritional Contents and Plant Defense in Vigna unguiculata. Biomolecules 2020, 10, 224. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Ahmed, M.; Mir, B.A.; Yusuf, M.; Khan, T.A. 24-Epibrassinolide mitigates the adverse effects of manganese induced toxicity through improved antioxidant system and photosynthetic attributes in Brassica juncea. Environ. Sci. Pollut. Res. 2015, 22, 11349–11359. [Google Scholar] [CrossRef] [PubMed]
- Lukatkin, A.S.; Brazaitytė, A.; Bobinas, C.; Duchovskis, P. Chilling injury in chilling-sensitive plants: A review. Agriculture 2012, 99, 111–124. [Google Scholar]
- Naseem, M.; Anwar-ul-Haq, M.; Wang, X.; Farooq, N.; Awais, M.; Sattar, H.; Malik, H.A.; Mustafa, A.; Ahmad, J.; El-Esawi, M.A. Influence of Selenium on Growth, Physiology, and Antioxidant Responses in Maize Varies in a Dose-Dependent Manner. J. Food Qual. 2021, 2021, 6642018. [Google Scholar] [CrossRef]
- Ramos, S.J.; Faquin, V.; Guilherme, L.R.G.; Castro, E.M.; Avila, F.W.; Carvalho, G.S.; Bastos, C.E.A.; Oliveira, C. Selenium biofortification and antioxidant activity in lettuce plants feed with selenate and selenite. Plant Soil Environ. 2010, 12, 583–587. [Google Scholar]
- Boldrin, P.F.; de Figueiredo, M.A.; Yang, Y.; Luo, H.; Giri, S.; Hart, J.J.; Li, L. Selenium promotes sulfur accumulation and plant growth in wheat (Triticum aestivum). Physiol. Plant. 2016, 158, 80–91. [Google Scholar] [CrossRef]
- Catterou, M.; Dubois, F.; Schaller, H.; Aubanelle, L.; Vilcot, B.; Sangwan-Norreel, B.S.; Sangwan, R.S. Brassinosteroids, microtubules and cell elongation in Arabidopsis thaliana. II. Effects of brassinosteroids on microtubules and cell elongation in the bul1 mutant. Planta 2001, 212, 673–683. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.A.; Arteca, R.N.; Foolad, M.R. The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Crit. Rev. Plant Sci. 2010, 29, 162–190. [Google Scholar] [CrossRef]
- Bajguz, A.; Tretyn, A. The chemical characteristic and distribution of brassinosteroids in plants. Phytochemical 2003, 62, 1027–1046. [Google Scholar] [CrossRef]
- Cakmark, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Lu, K.X.; Cao, B.H.; Feng, X.P.; He, Y.; Jiang, D.A. Photosynthetic response of salt tolerant and sensitive soybean varieties. Photosynthetica 2009, 47, 381–387. [Google Scholar] [CrossRef]
- Wu, X.X.; He, J.; Zhu, Z.W.; Yang, S.J.; Zha, D.S. Protection of photosynthesis and antioxidative system by 24-epibrassinolide in Solanum melongena under cold stress. Biol. Plant. 2014, 58, 185–188. [Google Scholar] [CrossRef]
- Liu, H.; Xiao, C.; Qiu, T.; Deng, J.; Cheng, H.; Cong, X.; Cheng, S.; Rao, S.; Zhang, Y. Selenium Regulates Antioxidant, Photosynthesis, and Cell Permeability in Plants under Various Abiotic Stresses: A Review. Plants 2023, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Hola, A. Brassinosteroids and photosynthesis. In Brassinosteroids: A Class of Plant Hormone; Hayat, S., Ahmad, A., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 143–192. [Google Scholar]
- Yusuf, M.; Fariduddin, Q.; Khan, T.A.; Hayat, S. Epibrassinolide reverses the stress generated by combination of excess aluminum and salt in two wheat cultivars through altered proline metabolism and antioxidants. S. Afr. J. Bot. 2017, 112, 391–398. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Beneficial effects of exogenous selenium in cucumber seedlings subjected to salt stress. Biol. Trace Elem. Res. 2009, 132, 259–269. [Google Scholar] [CrossRef]
- Abbas, S.M. Effects of low temperature and selenium application on growth and the physiological changes in sorghum seedlings. J. Stress Physiol. Biochem. 2012, 8, 268–286. [Google Scholar]
- Abbas, S.M. Low levels of selenium application attenuate low temperature stress in sorghum (Sorghum bicolor L.) seedlings. Pak. J. Bot. 2013, 45, 1597–1604. [Google Scholar]
- Xia, X.J.; Huang, L.F.; Zhou, Y.H.; Mao, W.H.; Shi, K.; Wu, J.X.; Asami, T.; Chen, Z.; Yu, J.Q. Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 2009, 230, 1185–1196. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhao, Z.G.; Si, J.; Di, C.X.; Han, J.; An, L.Z. Brassinosteroids alleviate chilling-induced oxidative damage by enhancing antioxidant defense system in suspension cultured cells of Chorispora bungeana. Plant Growth Regul. 2009, 59, 207–214. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, T.; Khan, T.A.; Ahmad, A.; Yusuf, M.; Kappachery, S.; Fariduddin, Q.; Mudgal, G.; Gururani, M.A. Exploring the Effects of Selenium and Brassinosteroids on Photosynthesis and Protein Expression Patterns in Tomato Plants under Low Temperatures. Plants 2023, 12, 3351. https://doi.org/10.3390/plants12193351
Saeed T, Khan TA, Ahmad A, Yusuf M, Kappachery S, Fariduddin Q, Mudgal G, Gururani MA. Exploring the Effects of Selenium and Brassinosteroids on Photosynthesis and Protein Expression Patterns in Tomato Plants under Low Temperatures. Plants. 2023; 12(19):3351. https://doi.org/10.3390/plants12193351
Chicago/Turabian StyleSaeed, Taiba, Tanveer Alam Khan, Aqeel Ahmad, Mohammad Yusuf, Sajeesh Kappachery, Qazi Fariduddin, Gaurav Mudgal, and Mayank Anand Gururani. 2023. "Exploring the Effects of Selenium and Brassinosteroids on Photosynthesis and Protein Expression Patterns in Tomato Plants under Low Temperatures" Plants 12, no. 19: 3351. https://doi.org/10.3390/plants12193351
APA StyleSaeed, T., Khan, T. A., Ahmad, A., Yusuf, M., Kappachery, S., Fariduddin, Q., Mudgal, G., & Gururani, M. A. (2023). Exploring the Effects of Selenium and Brassinosteroids on Photosynthesis and Protein Expression Patterns in Tomato Plants under Low Temperatures. Plants, 12(19), 3351. https://doi.org/10.3390/plants12193351







