Biodiversity, Ecology and Distribution of Mediterranean Charophytes in Southern Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Algae Identification and Analysis
2.3. Water Analysis
2.4. Sediment Analysis
2.5. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, R. The Characeae (Charales, Charophyceae) of Sardinia (Italy): Habitats, distribution and conservation. Webbia 2019, 74, 83–101. [Google Scholar] [CrossRef]
- Crum, G.H. Distribution, Taxonomy, and Ecology of Charophytes in Iowa. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1975. [Google Scholar] [CrossRef]
- Lambert, S. Stoneworts: Their Habitats, Ecological Requirements and Conservation; Science Report: SC030202; Technical Report; Environment Agency: Bristol, UK, 2009.
- Caisová, L.; Gąbka, M. Charophytes (Characeae, Charophyta) in the Czech Republic: Taxonomy, autecology and distribution. Fottea 2009, 9, 1–43. [Google Scholar] [CrossRef]
- Mjelde, M.; Swe, T.; Langangen, A.; Ballot, A. A contribution to the knowledge of charophytes in Myanmar; morphological and genetic identification and ecology notes. Bot. Lett. 2021, 168, 102–109. [Google Scholar] [CrossRef]
- Nurashov, S.; Jumakhanova, G.; Barinova, S.; Romanov, R.; Sametova, E.; Jiyenbekov, A.; Shalgimbayeva, S.; Smith, T.E. Charophytes (Charophyceae, Charales) of South Kazakhstan: Diversity, distribution, and tentative Red List. Plants 2023, 12, 368. [Google Scholar] [CrossRef]
- Azzella, M. Italian volcanic lakes: A diversity hotspot and refuge for European charophytes. J. Limnol. 2014, 73, 502–510. [Google Scholar] [CrossRef]
- Becker, R.; Schubert, H.; Nowak, P. Chara zeylanica J.G. Klein Ex Willd. (Charophyceae, Charales, Characeae): First European Record from the Island of Sardinia, Italy. Plants 2021, 10, 2069. [Google Scholar] [CrossRef]
- Auderset Joye, D.; Castella, E.; Lachavanne, J.B. Occurrence of Characeae in Switzerland over the last two centuries (1800–2000). Aquat. Bot. 2002, 72, 369–385. [Google Scholar] [CrossRef]
- Rodrigo, M.A.; Rojo, C.; Segura, M.; Alonso-Guillén, J.L.; Martín, M.; Vera, P. The role of charophytes in a Mediterranean pond created for restoration purposes. Aquat. Bot. 2015, 120, 101–111. [Google Scholar] [CrossRef]
- Council of European Union. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora; Council of European Union: Brussels, Belgium, 1992.
- Council of European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy; Council of European Union: Brussels, Belgium, 2000.
- Blindow, I.; Carlsson, M.; van de Weyer, K. Re-Establishment Techniques and Transplantations of Charophytes to Support Threatened Species. Plants 2021, 10, 1830. [Google Scholar] [CrossRef]
- Schneider, S.C.; Nowak, P.; Ammon, U.V.; Ballot, A. Species differentiation in the genus Chara (Charophyceae): Considerable Phenotypic Plasticity Occurs within Homogeneous Genetic Groups. Eur. J. Phycol. 2016, 51, 282–293. [Google Scholar] [CrossRef]
- Domozych, D.S.; Bagdan, K. The cell biology of charophytes: Exploring the past and models for the future. Plant Physiol. 2022, 190, 1588–1608. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; Technical Report; National University of Ireland: Galway, Ireland, 2021. [Google Scholar]
- Hindáková, A.; Gąbka, M.; Hrivnák, R. Checklist, red list, and distribution pattern of Charophytes (Charophyceae, Charales) in Slovakia based on critical revision of herbarium specimens. Diversity 2022, 14, 897. [Google Scholar] [CrossRef]
- Coops, H. Ecology of charophytes: An introduction. Aquat. Bot. 2002, 72, 205–208. [Google Scholar] [CrossRef]
- Rybak, A.S.; Woyda-Ploszczyca, A.M. Ecology and distribution patterns of Chara connivens (Charophyta, Characeae) on the Canary Islands—The First Record from Fuerteventura. Oceanol. Hydrobiol. Stud. 2019, 48, 368–380. [Google Scholar] [CrossRef]
- Tindall, D. The Systematics and Ecology of the Characeae (Nitella and Chara) of Southwestern United States and Northern Mexico. Ph.D. Thesis, University of Louisville, Louisville, KY, USA, 1966. [Google Scholar] [CrossRef]
- Kufel, L.; Kufel, I. Chara beds acting as nutrient sinks in shallow lakes—A review. Aquat. Bot. 2002, 72, 249–260. [Google Scholar] [CrossRef]
- Guarino, R.; Marcenò, C.; Ilardi, V.; Mannino, A.M.; Troia, A. One Chara does not make Charetea Mediterranean aquatic vegetation. Webbia 2019, 74, 139–147. [Google Scholar] [CrossRef]
- Panzeca, P.; Troia, A.; Madonia, P. Aquatic Macrophytes Occurrence in Mediterranean Farm Ponds: Preliminary Investigations in North-Western Sicily (Italy). Plants 2021, 10, 1292. [Google Scholar] [CrossRef]
- Saber, A.A.; Gontcharov, A.A.; Nikulin, A.Y.; Nikulin, V.Y.; Rayan, W.A.; Cantonati, M. Integrative taxonomic, ecological and genotyping study of charophyte populations from the Egyptian western-desert oases and Sinai Peninsula. Plants 2021, 10, 1157. [Google Scholar] [CrossRef]
- Rojo, C.; Puche, E.; Rodrigo, M.A. The antagonistic effect of UV radiation on warming or nitrate enrichment depends on ecotypes of freshwater macroalgae (Charophytes). J. Phycol. 2019, 55, 714–729. [Google Scholar] [CrossRef]
- Kolada, A. Charophyte variation in sensitivity to eutrophication affects their potential for the trophic and ecological status indication. Knowl. Manag. Aquat. Ecosyst. 2021, 422, 30. [Google Scholar] [CrossRef]
- Flor-Arnau, N.; Sánchez, J.C. Biodiversity Changes of Charophytes in Lakes and Ponds of the Duero Basin (NW-Spain) over a Twenty-year Period. Wetlands 2014, 35, 159–169. [Google Scholar] [CrossRef]
- Khuram, I.; Ahmad, N.; Barinova, S. Effect of water quality on the spatial distribution of charophytes in the Peshawar Valley, Khyber Pakhtunkhwa, Pakistan. Oceanol. Hydrobiol. Stud. 2021, 50, 359–372. [Google Scholar] [CrossRef]
- Steinman, A.D.; Havens, K.E.; Rodusky, A.J.; Sharfstein, B.; James, R.; Harwell, M.C. The influence of environmental variables and a managed water recession on the growth of charophytes in a large, subtropical lake. Aquat. Bot. 2002, 72, 297–313. [Google Scholar] [CrossRef]
- Blaženčić, J.; Kashta, L.; Vesić, A.; Biberdžić, V.; Stevanović, B. Charophytes (Charales) of Lake Skadar/Shkodra: Ecology and distribution. In The Skadar/Shkodra Lake Environment; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 169–202. [Google Scholar] [CrossRef]
- Bučas, M.; Sinkevičienė, Z.; Kataržytė, M.; Vaičiūtė, D.; Petkuvienė, J.; Stragauskaitė, V.; Ilginė, R. How much can the occurrence and coverage of charophytes in an estuarine lagoon (Curonian Lagoon) be explained by environmental factors? Estuar. Coast. Shelf Sci. 2019, 216, 128–138. [Google Scholar] [CrossRef]
- Schwarz, A.M.; de Winton, M.; Hawes, I. Species-specific depth zonation in New Zealand charophytes as a function of light availability. Aquat. Bot. 2002, 72, 209–217. [Google Scholar] [CrossRef]
- Nuñez, M.M.A.; Hussner, A.; Mauersberger, R.; Brämick, U.; Hühn, D.; He, L.; Hilt, S. Periphyton and benthivorous fish affect charophyte abundance and indicate hidden nutrient loading in oligo- and mesotrophic temperate hardwater lakes. Freshw. Biol. 2022, 68, 312–324. [Google Scholar] [CrossRef]
- Meurer, T.; Bueno, N.C. The genera Chara and Nitella (Chlorophyta, Characeae) in the Subtropical Itaipu Reservoir, Brazil. Braz. J. Bot. 2012, 35, 219–232. [Google Scholar] [CrossRef]
- Troia, A. Macrophytes in Inland Waters: From Knowledge to Management. Plants 2023, 12, 582. [Google Scholar] [CrossRef]
- Azzella, M.; Rosati, L.; Iberite, M.; Bolpagni, R.; Blasi, C. Changes in aquatic plants in the Italian volcanic-lake system detected using current data and historical records. Aquat. Bot. 2014, 112, 41–47. [Google Scholar] [CrossRef]
- Rodrigo, M.A.; Alonso-Guillén, J.L. The charophyte flora in a Ramsar Mediterranean wetland (Albufera de València Natural Park, Spain) during the period 2007–2010. Bot. Serbica 2016, 40, 205–215. [Google Scholar]
- Milovanović, V.; Popović, S.; Predojević, D.; Simić, G.S.; Ržaničanin, A.; Sekulić, J.Š.; Trbojević, I. Oospore Features among Morphologically Similar and Closely Related Charophyte Species: Consistency and Variability. Cryptogam. Algol. 2022, 43, 189–200. [Google Scholar] [CrossRef]
- Bellino, A.; Alfani, A.; De Riso, L.; Gregorio, R.; Pellegrino, T.; Baldantoni, D. Long-established and new active biomonitors jointly reveal potentially toxic element gradients across spatial scales in freshwater ecosystems. Ecol. Indic. 2020, 118, 106742. [Google Scholar] [CrossRef]
- Bellino, A.; Alfani, A.; De Riso, L.; Gregorio, R.; Pellegrino, T.; Baldantoni, D. A promising cosmopolitan biomonitor of potentially toxic elements in freshwater ecosystems: Concentration gradients in sensitive areas. Ecol. Indic. 2020, 109, 105801. [Google Scholar] [CrossRef]
- Soulié-Märsche, I.; García, A. Gyrogonites and oospores, complementary viewpoints to improve the study of the charophytes (Charales). Aquat. Bot. 2015, 120, 7–17. [Google Scholar] [CrossRef]
- Calero, S.; Rodrigo, M.A. A quantitative method to analyse the sexual development of charophytes (Charophyceae): A baseline for phenological studies. Phycologia 2022, 61, 354–362. [Google Scholar] [CrossRef]
- Andrews, M. Phosphate uptake by the component parts of Chara hispida. Br. Phycol. J. 1987, 22, 49–53. [Google Scholar] [CrossRef]
- Vermeer, C.P.; Escher, M.; Portielje, R.; de Klein, J.J. Nitrogen uptake and translocation by Chara. Aquat. Bot. 2003, 76, 245–258. [Google Scholar] [CrossRef]
- Bazzichelli, G.; Abdelahad, N. Flora Analitica delle Caroficee; Ministero dell’Ambiente, Sapienza University of Rome: Rome, Italy, 2009. [Google Scholar]
- Urbaniak, J.; Gąbka, M. Polish Charophytes—An Illustrated Guide to Identification; Uniwersytet Przyrodniczy we Wrocławiu: Wrocław, Poland, 2014. [Google Scholar]
- WCSP. World Checklist of Selected Plant Families. Facilitated by the Royal Botanic Gardens, Kew; Technical Report; Royal Botanic Gardens, Kew: Richmond, UK, 2021. [Google Scholar]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Sviben, S.; Kepčija, R.M.; Vidaković-Cifrek, Ž.; Perić, M.S.; Kružić, P.; Popijač, A.; Primc, B. Chara spp. exhibit highly heterogeneous light adaptation, calcite encrustation and epiphyton patterns in a marl lake. Aquat. Bot. 2018, 147, 1–10. [Google Scholar] [CrossRef]
- Bellino, A.; Alfani, A.; Selosse, M.A.; Guerrieri, R.; Borghetti, M.; Baldantoni, D. Nutritional regulation in mixotrophic plants: New insights from Limodorum abortivum. Oecologia 2014, 175, 875–885. [Google Scholar] [CrossRef]
- Küpper, H.; Seibert, S.; Parameswaran, A. Fast, sensitive, and inexpensive alternative to analytical pigment HPLC: Quantification of chlorophylls and carotenoids in crude extracts by fitting with Gauss Peak Spectra. Anal. Chem. 2007, 79, 7611–7627. [Google Scholar] [CrossRef]
- Delbecque, E.J.P. Periphyton on nymphaeids: An evaluation of methods and separation techniques. Hydrobiologia 1985, 124, 85–93. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A Graph. User Interface Rietveld Refinement Program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. Cluster: Cluster Analysis Basics and Extensions; CRAN: Chicago, IL, USA, 2022. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Hamilton, N.E.; Ferry, M. ggtern: Ternary Diagrams Using ggplot2. J. Stat. Softw. Code Snippets 2018, 87, 1–17. [Google Scholar] [CrossRef]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased Recursive Partitioning: A Conditional Inference Framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef]
- Hothorn, T.; Zeileis, A. partykit: A Modular Toolkit for Recursive Partytioning in R. J. Mach. Learn. Res. 2015, 16, 3905–3909. [Google Scholar]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package; R Package Version 2.6-4; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Bellino, A.; Alfani, A.; De Riso, L.; Baldantoni, D. Multivariate spatial analysis for the identification of criticalities and of the subtended causes in river ecosystems. Environ. Sci. Pollut. Res. 2019, 27, 30969–30976. [Google Scholar] [CrossRef]
- Castaldi, V.; Bellino, A.; Baldantoni, D. The ecology of bladderworts: The unique hunting-gathering-farming strategy in plants. Food Webs 2023, 35, e00273. [Google Scholar] [CrossRef]
- Romanov, R.; Napolitano, T.; Weyer, K.V.D.; Troia, A. New records and observations to the Characean flora (Charales, Charophyceae) of Sicily (Italy). Webbia 2019, 74, 111–119. [Google Scholar] [CrossRef]
- Romanov, R.E.; Barinova, S.S. The Charophytes of Israel: Historical and contemporary species richness, distribution, and ecology. Biodivers. Res. Conserv. 2012, 25, 67–74. [Google Scholar] [CrossRef]
- Wade, P.M. The colonisation of disturbed freshwater habitats by Characeae. Folia Geobot. Phytotaxon. 1990, 25, 275–278. [Google Scholar] [CrossRef]
- Sametova, E.; Jumakhanova, G.; Nurashov, S.; Barinova, S.; Jiyenbekov, A.; Smith, T. Microalgae indicators of Charophyte habitats of South and Southeast Kazakhstan. Diversity 2022, 14, 530. [Google Scholar] [CrossRef]
- Alderton, E.; Sayer, C.D.; Davies, R.; Lambert, S.J.; Axmacher, J.C. Buried alive: Aquatic plants survive in ‘ghost ponds’ under agricultural fields. Biol. Conserv. 2017, 212, 105–110. [Google Scholar] [CrossRef]
- Rodrigo, M.A.; Alonso-Guillén, J.L.; Soulié-Märsche, I. Reconstruction of the former charophyte community out of the fructifications identified in Albufera de València lagoon sediments. Aquat. Bot. 2010, 92, 14–22. [Google Scholar] [CrossRef]
- Lemonnier, H.; Royer, F.; Caradec, F.; Lopez, E.; Hubert, C.; Rabiller, É.; Desclaux, T.; Fernandez, J.M.; Andrieux-Loyer, F. Diagenetic processes in aquaculture ponds showing metal accumulation on shrimp gills. Front. Mar. Sci. 2021, 8, 625789. [Google Scholar] [CrossRef]

 ), C. gymnophylla (
), C. gymnophylla ( ), C. vulgaris (
), C. vulgaris ( ) and C. vulgaris var papillata (
) and C. vulgaris var papillata ( ), and the type of environment (A: artificial ponds, K: karst resurgences, N: natural ponds, R: rivers). Numbered populations, labeled according to previous studies conducted in the area [39,40,60], are the ones on which the ecological, morphological, and biochemical traits were investigated. Cyan and yellow lines indicate the course of the main rivers in the area and the boundaries of the “Cilento Vallo di Diano and Alburni” National Park, respectively.
), and the type of environment (A: artificial ponds, K: karst resurgences, N: natural ponds, R: rivers). Numbered populations, labeled according to previous studies conducted in the area [39,40,60], are the ones on which the ecological, morphological, and biochemical traits were investigated. Cyan and yellow lines indicate the course of the main rivers in the area and the boundaries of the “Cilento Vallo di Diano and Alburni” National Park, respectively.
 ), C. gymnophylla (
), C. gymnophylla ( ), C. vulgaris (
), C. vulgaris ( ) and C. vulgaris var papillata (
) and C. vulgaris var papillata ( ), and the type of environment (A: artificial ponds, K: karst resurgences, N: natural ponds, R: rivers). Numbered populations, labeled according to previous studies conducted in the area [39,40,60], are the ones on which the ecological, morphological, and biochemical traits were investigated. Cyan and yellow lines indicate the course of the main rivers in the area and the boundaries of the “Cilento Vallo di Diano and Alburni” National Park, respectively.
), and the type of environment (A: artificial ponds, K: karst resurgences, N: natural ponds, R: rivers). Numbered populations, labeled according to previous studies conducted in the area [39,40,60], are the ones on which the ecological, morphological, and biochemical traits were investigated. Cyan and yellow lines indicate the course of the main rivers in the area and the boundaries of the “Cilento Vallo di Diano and Alburni” National Park, respectively.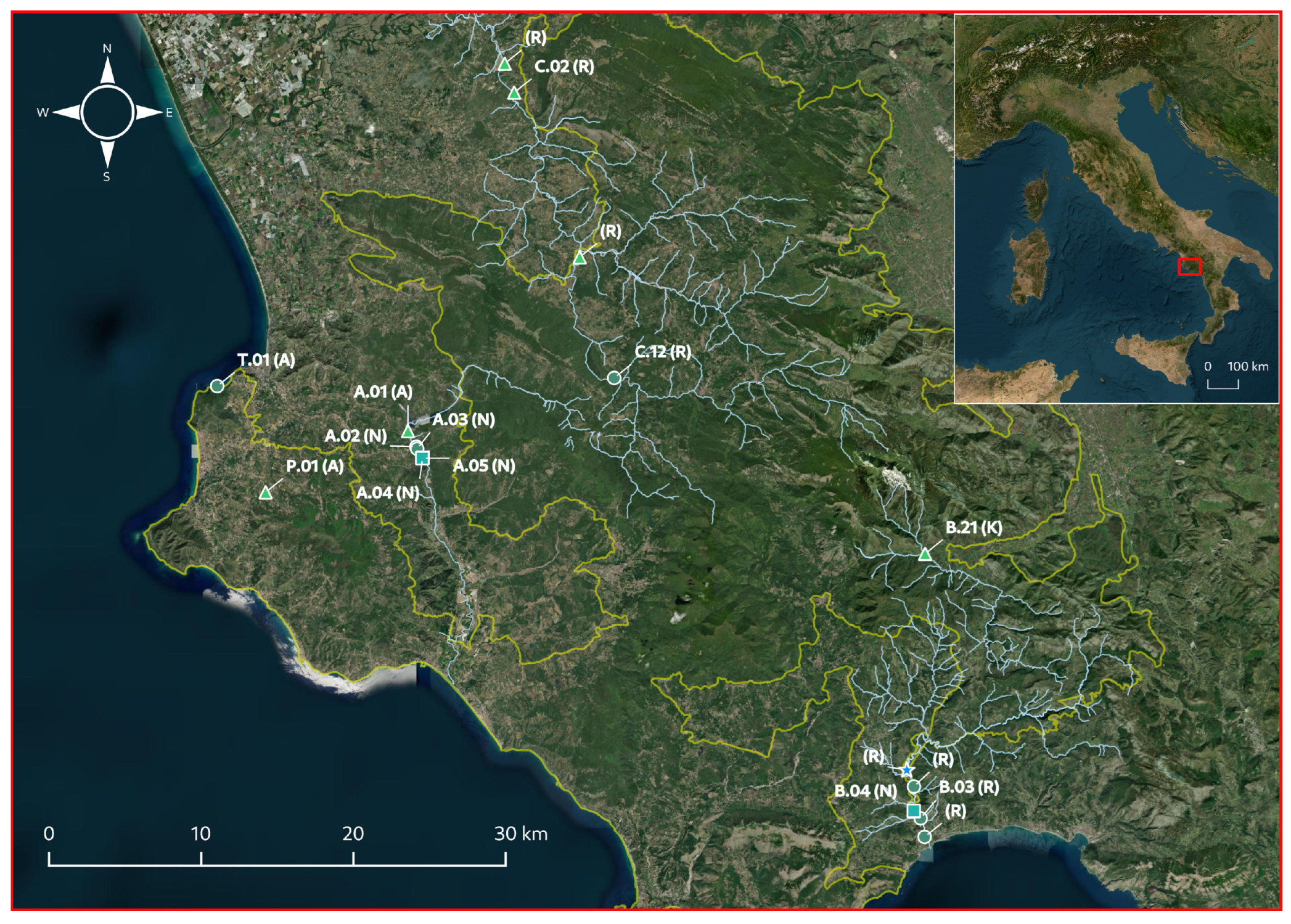
 : C. globularis,
: C. globularis,  : C. gymnophylla,
: C. gymnophylla,  : C. vulgaris). Populations belonging to each species, indicated by different colors (
: C. vulgaris). Populations belonging to each species, indicated by different colors ( : C. globularis,
: C. globularis,  : C. gymnophylla,
: C. gymnophylla,  : C. vulgaris) are further differentiated in the conditional inference tree (right) based on their morphological traits (values in m), labeled according to Table 1 and Figure 1 and indicated on the nodes with their respective p-values and the splitting rules.
: C. vulgaris) are further differentiated in the conditional inference tree (right) based on their morphological traits (values in m), labeled according to Table 1 and Figure 1 and indicated on the nodes with their respective p-values and the splitting rules.
 : C. globularis,
: C. globularis,  : C. gymnophylla,
: C. gymnophylla,  : C. vulgaris). Populations belonging to each species, indicated by different colors (
: C. vulgaris). Populations belonging to each species, indicated by different colors ( : C. globularis,
: C. globularis,  : C. gymnophylla,
: C. gymnophylla,  : C. vulgaris) are further differentiated in the conditional inference tree (right) based on their morphological traits (values in m), labeled according to Table 1 and Figure 1 and indicated on the nodes with their respective p-values and the splitting rules.
: C. vulgaris) are further differentiated in the conditional inference tree (right) based on their morphological traits (values in m), labeled according to Table 1 and Figure 1 and indicated on the nodes with their respective p-values and the splitting rules.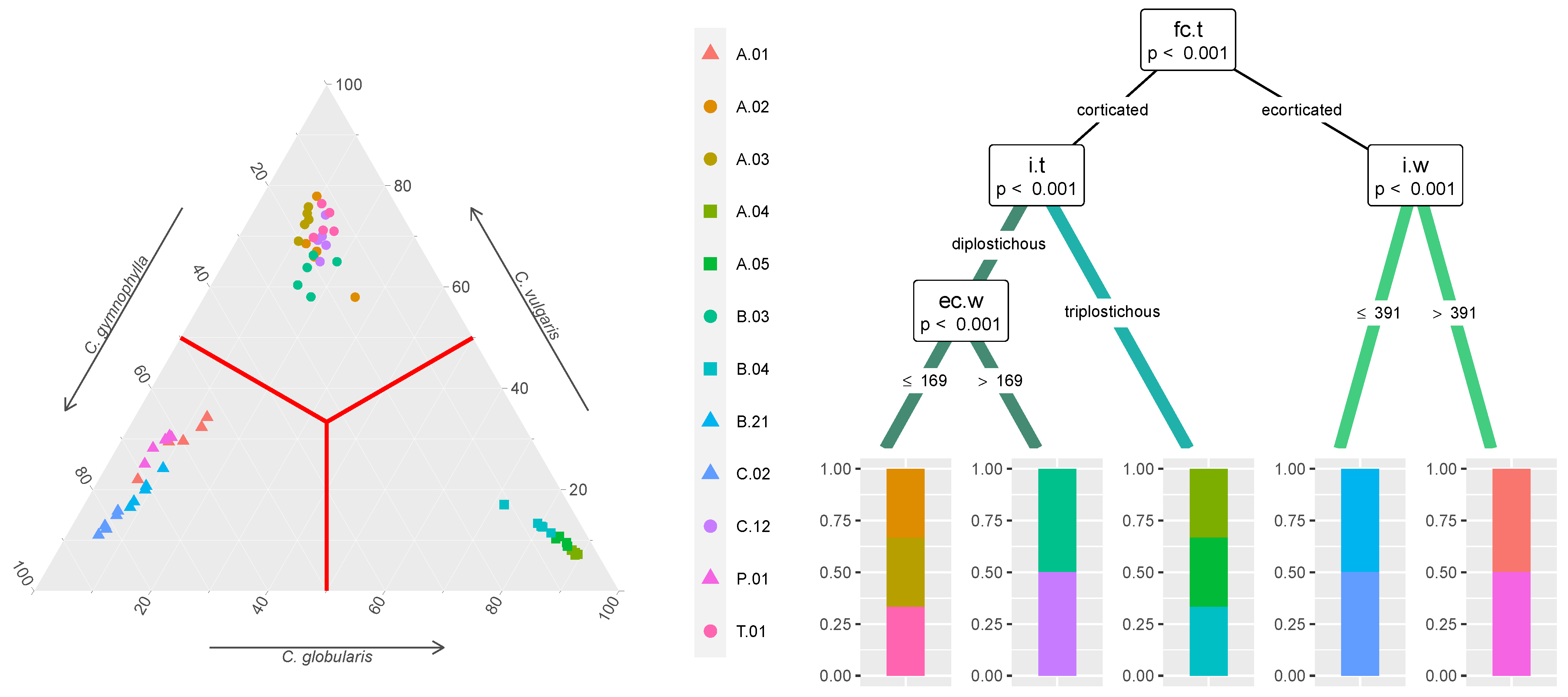
 : C. globularis,
: C. globularis,  : C. gymnophylla,
: C. gymnophylla,  : C. vulgaris). Abbreviations for populations are reported in Figure 2, with those indicating morphological traits in Table 1 and Figure 1, whereas those indicating environmental characteristics in Section 2.3 and Section 2.4.
: C. vulgaris). Abbreviations for populations are reported in Figure 2, with those indicating morphological traits in Table 1 and Figure 1, whereas those indicating environmental characteristics in Section 2.3 and Section 2.4.
 : C. globularis,
: C. globularis,  : C. gymnophylla,
: C. gymnophylla,  : C. vulgaris). Abbreviations for populations are reported in Figure 2, with those indicating morphological traits in Table 1 and Figure 1, whereas those indicating environmental characteristics in Section 2.3 and Section 2.4.
: C. vulgaris). Abbreviations for populations are reported in Figure 2, with those indicating morphological traits in Table 1 and Figure 1, whereas those indicating environmental characteristics in Section 2.3 and Section 2.4.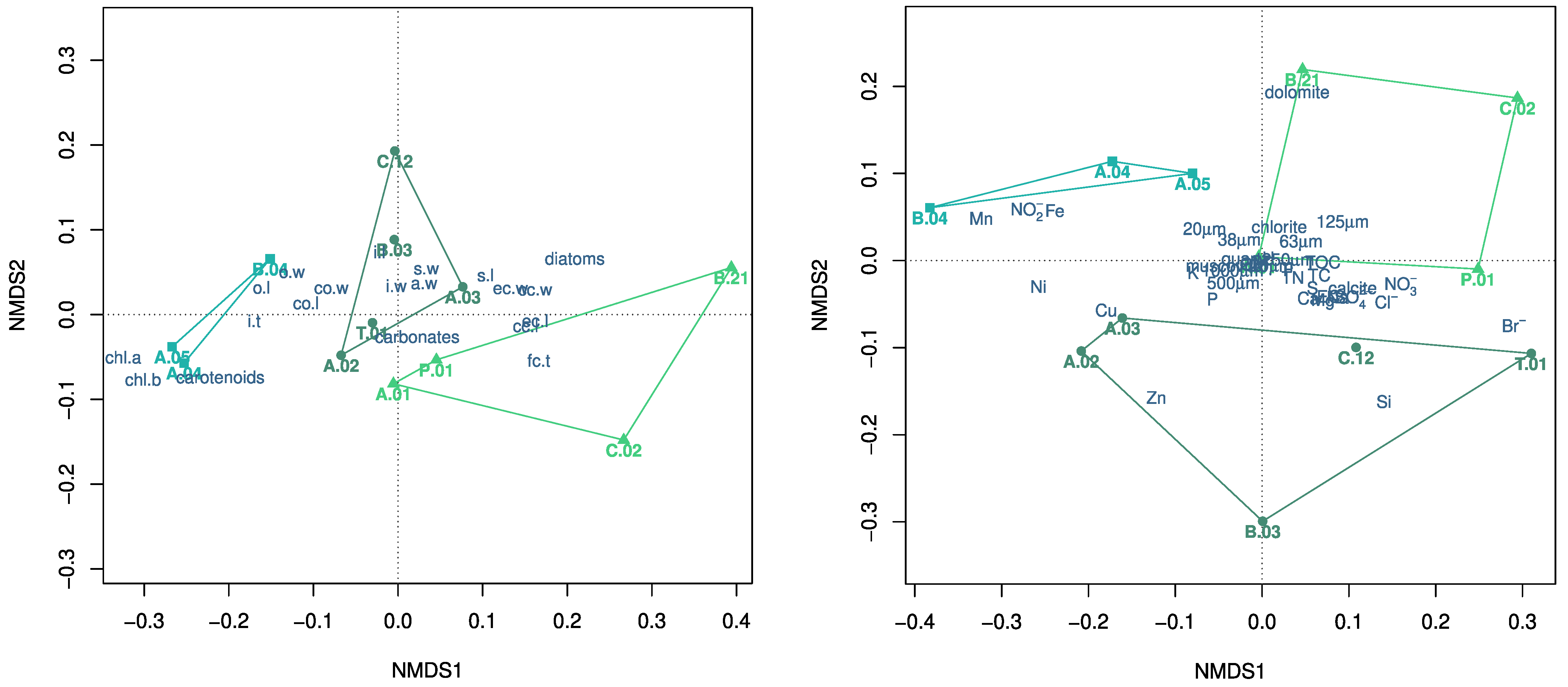
| Length | Diameter | Cortification | |
|---|---|---|---|
| Internodes | i.l | i.w | i.t a |
| Arrangement of cortical cells | c.t b | ||
| Spine cells | s.l | s.w | |
| Ecorticated branchlet segments | ec.l | ec.w | |
| Corticated branchlet segments | cc.l | cc.w | |
| Oosporangia | o.l | o.w | |
| Antheridia | a.w c | ||
| Coronula | c.l | c.w | |
| Branchlet cells below nodes with gametangia | fc.t d | ||
| Stipulodes | s.t |
| i.l (mm) | i.w (m) | s.l (m) | s.w (m) | cc.l (mm) | cc.w (m) | ec.l (mm) | ec.w (m) | |
|---|---|---|---|---|---|---|---|---|
| A.01 | 25.3 ± 1.8 | 500 ± 72 | 55 ± 14 | 23.2 ± 7.8 | 1.23 ± 0.46 | 176 ± 41 | 0.99 ± 0.40 | 151 ± 33 |
| A.02 | 18.7 ± 1.2 | 490 ± 100 | 63 ± 17 | 37 ± 16 | 1.26 ± 0.51 | 166 ± 56 | 1.13 ± 0.55 | 154 ± 47 |
| A.03 | 46.9 ± 2.8 | 640 ± 160 | 100 ± 18 | 44.9 ± 5.7 | 1.42 ± 0.46 | 185 ± 42 | 1.02 ± 0.44 | 155 ± 48 |
| A.04 | 12.35 ± 0.66 | 388 ± 57 | 49 ± 10 | 49 ± 11 | 1.15 ± 0.42 | 158 ± 27 | 0.99 ± 0.64 | 141 ± 47 |
| A.05 | 24.8 ± 1.7 | 386 ± 65 | 73 ± 15 | 67 ± 43 | 1.07 ± 0.57 | 146 ± 28 | 1.02 ± 0.42 | 142 ± 32 |
| B.03 | 23.7 ± 1.7 | 430 ± 140 | 87 ± 30 | 33.3 ± 4.7 | 1.17 ± 0.66 | 237 ± 68 | 1.31 ± 0.72 | 217 ± 69 |
| B.04 | 14.3 ± 1.2 | 292 ± 66 | 63 ± 23 | 53 ± 22 | 1.40 ± 0.51 | 189 ± 44 | 1.33 ± 0.61 | 177 ± 48 |
| B.21 | 9.67 ± 0.46 | 315 ± 37 | 101 ± 40 | 67 ± 17 | 2.07 ± 0.73 | 367 ± 91 | 2.07 ± 0.74 | 305 ± 72 |
| C.02 | 14.3 ± 1.2 | 360 ± 110 | 79 ± 36 | 65 ± 25 | 2.22 ± 0.69 | 236 ± 48 | 2.00 ± 0.94 | 192 ± 57 |
| C.12 | 47.4 ± 3.5 | 750 ± 260 | 103 ± 23 | 91 ± 33 | 1.25 ± 0.51 | 213 ± 60 | 1.07 ± 0.59 | 182 ± 64 |
| P.01 | 29.8 ± 3.4 | 560 ± 140 | 86 ± 31 | 30 ± 12 | 1.22 ± 0.49 | 171 ± 45 | 1.14 ± 0.37 | 162 ± 66 |
| T.01 | 28.4 ± 2.2 | 466 ± 79 | 50 ± 12 | 38 ± 19 | 1.22 ± 0.43 | 169 ± 50 | 1.03 ± 0.64 | 157 ± 36 |
| o.l (m) | o.w (m) | co.l (m) | co.w (m) | a.w (m) | i.t | c.t | fc.t | |
| A.01 | 401 ± 53 | 297 ± 26 | 127 ± 19 | 136 ± 22 | 250 ± 72 | dip. | aul. | eco. |
| A.02 | 428 ± 96 | 227 ± 70 | 106 ± 37 | 119 ± 25 | 184 ± 36 | dip. | aul. | cor. |
| A.03 | 381 ± 55 | 210 ± 35 | 71 ± 17 | 103 ± 21 | 207 ± 48 | dip. | aul. | cor. |
| A.04 | 534 ± 47 | 314 ± 35 | 135 ± 30 | 128.0 ± 8.7 | 204 ± 21 | tri. | iso. | cor. |
| A.05 | 580 ± 57 | 333 ± 43 | 113 ± 13 | 122 ± 25 | 144 ± 64 | tri. | iso. | cor. |
| B.03 | 514 ± 48 | 343 ± 25 | 126 ± 47 | 165 ± 34 | 196 ± 13 | dip. | aul. | cor. |
| B.04 | 566 ± 72 | 305 ± 49 | 148 ± 46 | 130 ± 25 | 219 ± 34 | tri. | iso. | cor. |
| B.21 | 276 ± 82 | 203 ± 70 | 90 ± 40 | 98 ± 42 | 234 ± 67 | dip. | iso. | eco. |
| C.02 | 166 ± 45 | 110 ± 38 | 56.8 ± 8.9 | 68 ± 13 | 187 ± 42 | dip. | aul. | eco. |
| C.12 | 464 ± 37 | 355 ± 37 | 97.9 ± 9.6 | 131 ± 24 | 306 ± 39 | dip. | aul. | cor. |
| P.01 | 430 ± 47 | 226 ± 48 | 116 ± 20 | 118 ± 14 | 194 ± 20 | dip. | aul. | eco. |
| T.01 | 418 ± 76 | 248 ± 65 | 112 ± 19 | 125 ± 15 | 228 ± 30 | dip. | iso. | cor. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellino, A.; Baldantoni, D. Biodiversity, Ecology and Distribution of Mediterranean Charophytes in Southern Italy. Plants 2023, 12, 3434. https://doi.org/10.3390/plants12193434
Bellino A, Baldantoni D. Biodiversity, Ecology and Distribution of Mediterranean Charophytes in Southern Italy. Plants. 2023; 12(19):3434. https://doi.org/10.3390/plants12193434
Chicago/Turabian StyleBellino, Alessandro, and Daniela Baldantoni. 2023. "Biodiversity, Ecology and Distribution of Mediterranean Charophytes in Southern Italy" Plants 12, no. 19: 3434. https://doi.org/10.3390/plants12193434
APA StyleBellino, A., & Baldantoni, D. (2023). Biodiversity, Ecology and Distribution of Mediterranean Charophytes in Southern Italy. Plants, 12(19), 3434. https://doi.org/10.3390/plants12193434







