Molecular Links between Flowering and Abiotic Stress Response: A Focus on Poaceae
Abstract
:1. Introduction
2. Molecular Regulators of Flowering Time in Monocotyledons
3. Stress Factors and Flowering Response
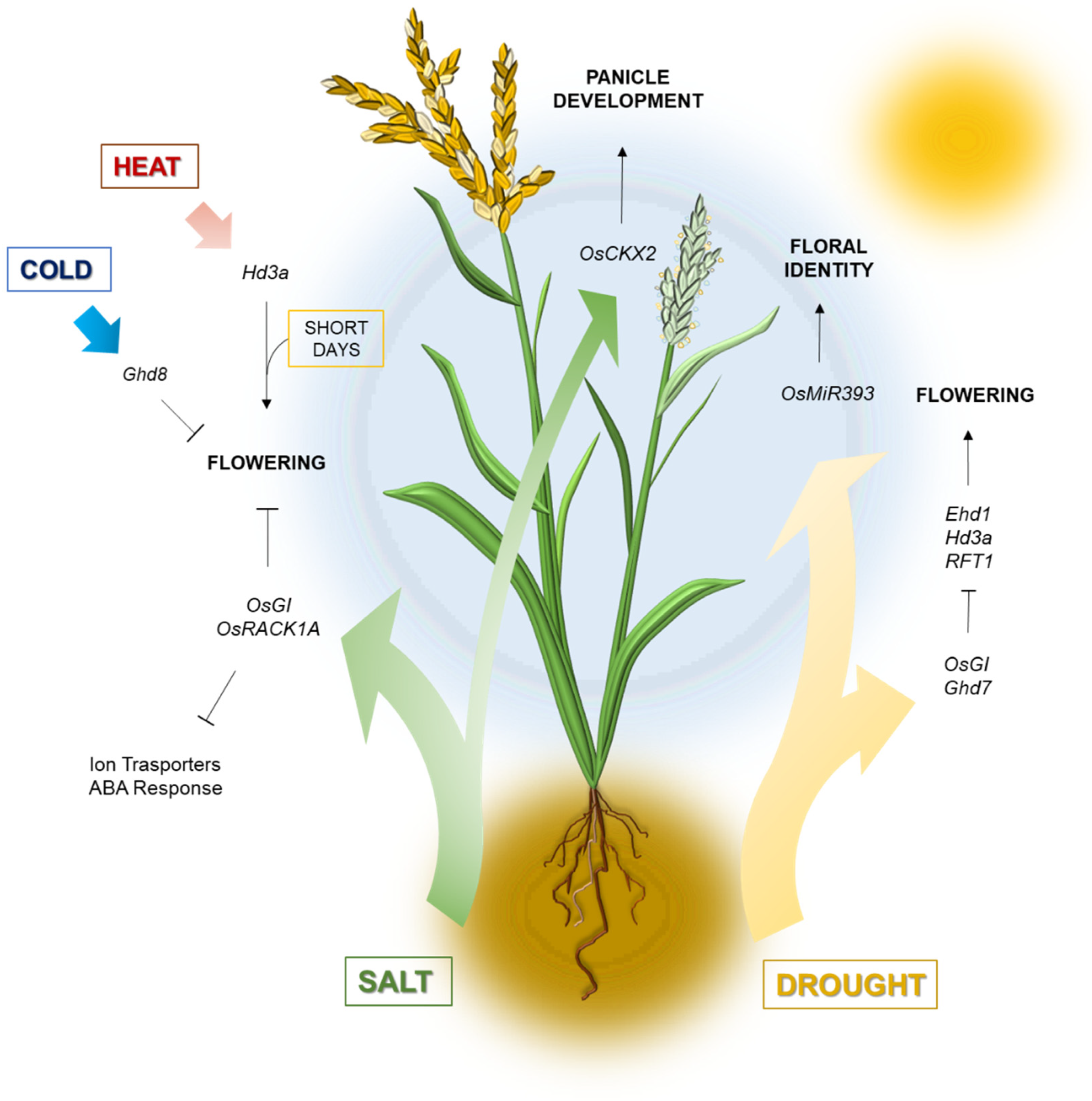
3.1. Water Availability
3.2. Temperature Extremes
3.3. Soil Salinity
4. Conclusions and Perspectives
| Regulator | Species | Stress | Role in Flowering | Reference |
|---|---|---|---|---|
| OsGI | Rice | Drought, salinity | Repressor | [64,68,140] |
| Ehd1 | Rice | Drought | Promoter | [63] |
| OsELF4a | Rice | Salinity | Promoter | [140] |
| OsELF3 | Rice | Salinity | Promoter | [140] |
| OsLUX | Rice | Salinity | Promoter | [140] |
| Ghd7 | Rice | Drought | Repressor | [70] |
| OsRACK1A | Rice | Salinity | Repressor | [142] |
| OsCKX2 | Rice | Salinity | Panicle Development | [147] |
| Ghd8 | Rice | Cold | Repressor | [26] |
| miR393 | Rice | Drought, salinity | Promoter | [102,103] |
| miR172 | Rice, maize, barley | Drought | Panicle Development | [97,99,101,166] |
| ZmCCT | Maize | Drought | Repressor | [71,72] |
| NF-YA3 | Maize | Drought | Promoter | [85] |
| miR164 | Maize, rice | Salinity, drought | Meristem differentiation | [167] |
| Ppd-1 | Barley | Drought, heat | Promoter | [90,127] |
| HvVRN1 | Barley | Heat | Vernalization/Promoter | [125,126,127] |
| HvODDSOC2 | Barley | Heat | Repressor | [125,126] |
| VRN1 | T. monococcum | Cold | Vernalization/Promoter | [113] |
| BdVIL4 | B. dystachion | Heat | Vernalization/Promoter | [168] |
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, J.M.; To, T.K.; Nishioka, T.; Seki, M. Chromatin Regulation Functions in Plant Abiotic Stress Responses. Plant Cell Environ. 2010, 33, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Urano, K.; Kurihara, Y.; Seki, M.; Shinozaki, K. ‘Omics’ Analyses of Regulatory Networks in Plant Abiotic Stress Responses. Curr. Opin. Plant Biol. 2010, 13, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-N.; Zhu, C.; Jiang, J.; Zhang, H.; Zhu, J.-K.; Duan, C.-G. Epigenetic Regulation in Plant Abiotic Stress Responses. J. Integr. Plant Biol. 2020, 62, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic Stress Responses in Plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Danquah, A.; de Zelicourt, A.; Colcombet, J.; Hirt, H. The Role of ABA and MAPK Signaling Pathways in Plant Abiotic Stress Responses. Biotechnol. Adv. 2014, 32, 40–52. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of Abiotic Stress on Plants: A Systems Biology Perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [Green Version]
- Fahad, S.; Nie, L.; Chen, Y.; Wu, C.; Xiong, D.; Saud, S.; Hongyan, L.; Cui, K.; Huang, J. Crop Plant Hormones and Environmental Stress. In Sustainable Agriculture Reviews: Volume 15; Lichtfouse, E., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 371–400. ISBN 978-3-319-09132-7. [Google Scholar]
- Zinn, K.E.; Tunc-Ozdemir, M.; Harper, J.F. Temperature Stress and Plant Sexual Reproduction: Uncovering the Weakest Links. J. Exp. Bot. 2010, 61, 1959–1968. [Google Scholar] [CrossRef] [Green Version]
- De Storme, N.; Geelen, D. The Impact of Environmental Stress on Male Reproductive Development in Plants: Biological Processes and Molecular Mechanisms. Plant Cell Environ. 2014, 37, 1–18. [Google Scholar] [CrossRef]
- Cho, L.-H.; Yoon, J.; An, G. The Control of Flowering Time by Environmental Factors. Plant J. 2017, 90, 708–719. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The Effect of Drought and Heat Stress on Reproductive Processes in Cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, W.-Y.; Pardo, J.M.; Yun, D.-J. Chapter Eight–Molecular Interactions Between Flowering Time and Abiotic Stress Pathways. In International Review of Cell and Molecular Biology; Jeon, K.W., Galluzzi, L., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 327, pp. 371–412. ISBN 1937-6448. [Google Scholar]
- Brambilla, V.; Gómez-Ariza, J.; Cerise, M.; Fornara, F. The Importance of Being on Time: Regulatory Networks Controlling Photoperiodic Flowering in Cereals. Front. Plant Sci. 2017, 8, 665. [Google Scholar] [CrossRef] [Green Version]
- Awika, J.M. Major Cereal Grains Production and Use around the World. In Advances in Cereal Science: Implications to Food Processing and Health Promotion; ACS Symposium Series; American Chemical Society: New York, NY, USA, 2011; Volume 1089, pp. 1–13. ISBN 9780841226364. [Google Scholar]
- Sarwar, H. The Importance of Cereals (Poaceae: Gramineae) Nutrition in Human Health: A Review. J. Cereals Oilseeds 2013, 4, 32–35. [Google Scholar] [CrossRef]
- FAOSTAT. 2022. Available online: https://www.fao.org/faostat/en/#home (accessed on 15 December 2022).
- Hill, C.B.; Li, C. Genetic Architecture of Flowering Phenology in Cereals and Opportunities for Crop Improvement. Front. Plant Sci. 2016, 7, 1906. [Google Scholar] [CrossRef] [Green Version]
- Andrés, F.; Coupland, G. The Genetic Basis of Flowering Responses to Seasonal Cues. Nat. Rev. Genet. 2012, 13, 627–639. [Google Scholar] [CrossRef]
- McCorriston, J.; Hole, F. The Ecology of Seasonal Stress and the Origins of Agriculture in the Near East. Am. Anthropol. 1991, 93, 46–69. [Google Scholar] [CrossRef]
- Greenup, A.; Peacock, W.J.; Dennis, E.S.; Trevaskis, B. The Molecular Biology of Seasonal Flowering-Responses in Arabidopsis and the Cereals. Ann. Bot. 2009, 103, 1165–1172. [Google Scholar] [CrossRef] [Green Version]
- Yano, M.; Kojima, S.; Takahashi, Y.; Lin, H.; Sasaki, T. Genetic Control of Flowering Time in Rice, a Short-Day Plant. Plant Physiol. 2001, 127, 1425–1429. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, X.; Yan, W.; Zhang, Z.; Lu, L.; Han, Z.; Zhao, H.; Liu, H.; Song, P.; Hu, Y.; et al. Combinations of the Ghd7, Ghd8 and Hd1 Genes Largely Define the Ecogeographical Adaptation and Yield Potential of Cultivated Rice. New Phytol. 2015, 208, 1056–1066. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, H.; Qi, F.; Zhang, Z.; Li, Q.; Han, Z.; Xing, Y. Genetic Interactions Among Ghd7, Ghd8, OsPRR37 and Hd1 Contribute to Large Variation in Heading Date in Rice. Rice 2019, 12, 48. [Google Scholar] [CrossRef]
- Klein, R.R.; Miller, F.R.; Dugas, D.V.; Brown, P.J.; Burrell, A.M.; Klein, P.E. Allelic Variants in the PRR37 Gene and the Human-Mediated Dispersal and Diversification of Sorghum. Theor. Appl. Genet. 2015, 128, 1669–1683. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xiong, Y.; Gong, R.; Yang, Y.; Fan, K.; Yu, S. A Key Variant in the Cis-Regulatory Element of Flowering Gene Ghd8 Associated with Cold Tolerance in Rice. Sci. Rep. 2019, 9, 9603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiegmann, M.; Maurer, A.; Pham, A.; March, T.J.; Al-Abdallat, A.; Thomas, W.T.B.; Bull, H.J.; Shahid, M.; Eglinton, J.; Baum, M.; et al. Barley Yield Formation under Abiotic Stress Depends on the Interplay between Flowering Time Genes and Environmental Cues. Sci. Rep. 2019, 9, 6397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatima, Z.; Ahmed, M.; Hussain, M.; Abbas, G.; Ul-Allah, S.; Ahmad, S.; Ahmed, N.; Ali, M.A.; Sarwar, G.; Haque, E.U.; et al. The Fingerprints of Climate Warming on Cereal Crops Phenology and Adaptation Options. Sci. Rep. 2020, 10, 18013. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, T.; Wright, L.; Fujiwara, S.; Cremer, F.; Lee, K.; Onouchi, H.; Mouradov, A.; Fowler, S.; Kamada, H.; Putterill, J.; et al. Distinct Roles of GIGANTEA in Promoting Flowering and Regulating Circadian Rhythms in Arabidopsis. Plant Cell 2005, 17, 2255–2270. [Google Scholar] [CrossRef] [Green Version]
- Murakami, M.; Matsushika, A.; Ashikari, M.; Yamashino, T.; Mizuno, T. Circadian-Associated Rice Pseudo Response Regulators (OsPRRs): Insight into the Control of Flowering Time. Biosci. Biotechnol. Biochem. 2005, 69, 410–414. [Google Scholar] [CrossRef] [Green Version]
- Turner, A.; Beales, J.; Faure, S.; Dunford, R.; Laurie, D. Botany: The Pseudo-Response Regulator Ppd-H1 Provides Adaptation to Photoperiod in Barley. Science 2005, 310, 1031–1034. [Google Scholar] [CrossRef]
- Kitagawa, S.; Shimada, S.; Murai, K. Effect of Ppd-1 on the Expression of Flowering-Time Genes in Vegetative and Reproductive Growth Stages of Wheat. Genes Genet. Syst. 2012, 87, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Liu, T.; Li, B.; Sui, Y.; Chen, J.; Shi, J.; Wing, R.A.; Chen, M. Comparative Sequence Analysis of the Ghd7 Orthologous Regions Revealed Movement of Ghd7 in the Grass Genomes. PLoS ONE 2012, 7, e50236. [Google Scholar] [CrossRef]
- Yan, L.; Loukoianov, A.; Tranquilli, G.; Helguera, M.; Fahima, T.; Dubcovsky, J. Positional Cloning of the Wheat Vernalization Gene VRN1. Proc. Natl. Acad. Sci. USA 2003, 100, 6263–6268. [Google Scholar] [CrossRef]
- Gregis, V.; Sessa, A.; Dorca-Fornell, C.; Kater, M.M. The Arabidopsis Floral Meristem Identity Genes AP1, AGL24 and SVP Directly Repress Class B and C Floral Homeotic Genes. Plant J. 2009, 60, 626–637. [Google Scholar] [CrossRef]
- Distelfeld, A.; Li, C.; Dubcovsky, J. Regulation of Flowering in Temperate Cereals. Curr. Opin. Plant Biol. 2009, 12, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, Q.; Dong, H.; He, Q.; Liang, L.; Tan, C.; Han, Z.; Yao, W.; Li, G.; Zhao, H.; et al. Three CCT Domain-Containing Genes Were Identified to Regulate Heading Date by Candidate Gene-Based Association Mapping and Transformation in Rice. Sci. Rep. 2015, 5, 7663. [Google Scholar] [CrossRef] [Green Version]
- Murphy, R.L.; Morishige, D.T.; Brady, J.A.; Rooney, W.L.; Yang, S.; Klein, P.E.; Mullet, J.E. Ghd7 (Ma6) Represses Sorghum Flowering in Long Days: Ghd7 Alleles Enhance Biomass Accumulation and Grain Production. Plant Genome 2014, 7, 1–10. [Google Scholar] [CrossRef]
- Karlgren, A.; Gyllenstrand, N.; Källman, T.; Sundström, J.F.; Moore, D.; Lascoux, M.; Lagercrantz, U. Evolution of the PEBP Gene Family in Plants: Functional Diversification in Seed Plant Evolution. Plant Physiol. 2011, 156, 1967–1977. [Google Scholar] [CrossRef] [Green Version]
- Taoka, K.; Ohki, I.; Tsuji, H.; Kojima, C.; Shimamoto, K. Structure and Function of Florigen and the Receptor Complex. Trends Plant Sci. 2013, 18, 287–294. [Google Scholar] [CrossRef]
- Li, C.; Lin, H.; Dubcovsky, J. Factorial Combinations of Protein Interactions Generate a Multiplicity of Florigen Activation Complexes in Wheat and Barley. Plant J. 2015, 84, 70–82. [Google Scholar] [CrossRef] [Green Version]
- Lazakis, C.M.; Coneva, V.; Colasanti, J. ZCN8 Encodes a Potential Orthologue of Arabidopsis FT Florigen That Integrates Both Endogenous and Photoperiod Flowering Signals in Maize. J. Exp. Bot. 2011, 62, 4833–4842. [Google Scholar] [CrossRef]
- Wolabu, T.W.; Zhang, F.; Niu, L.; Kalve, S.; Bhatnagar-Mathur, P.; Muszynski, M.G.; Tadege, M. Three FLOWERING LOCUS T-like Genes Function as Potential Florigens and Mediate Photoperiod Response in Sorghum. New Phytol. 2016, 210, 946–959. [Google Scholar] [CrossRef]
- Castelletti, S.; Coupel-Ledru, A.; Granato, I.; Palaffre, C.; Cabrera-Bosquet, L.; Tonelli, C.; Nicolas, S.D.; Tardieu, F.; Welcker, C.; Conti, L. Maize Adaptation across Temperate Climates Was Obtained via Expression of Two Florigen Genes. PLoS Genet. 2020, 16, e1008882. [Google Scholar] [CrossRef]
- Yan, L.; Fu, D.; Li, C.; Blechl, A.; Tranquilli, G.; Bonafede, M.; Sanchez, A.; Valarik, M.; Yasuda, S.; Dubcovsky, J. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc. Natl. Acad. Sci. USA 2006, 103, 19581–19586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Dubcovsky, J. Wheat FT Protein Regulates VRN1 Transcription through Interactions with FDL2. Plant J. 2008, 55, 543–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juraimi, A.; Ahmad Hamdani, M.S.; Begum, M.; Anuar, A.R.; Azmi, M. Influence of Flooding Intensity and Duration on Rice Growth and Yield. Pertanika J. Trop. Agric. Sci. 2009, 32, 195–208. [Google Scholar]
- Haussmann, B.I.G.; Fred Rattunde, H.; Weltzien-Rattunde, E.; Traoré, P.S.C.; vom Brocke, K.; Parzies, H.K. Breeding Strategies for Adaptation of Pearl Millet and Sorghum to Climate Variability and Change in West Africa. J. Agron. Crop. Sci. 2012, 198, 327–339. [Google Scholar] [CrossRef] [Green Version]
- LI, S.; Tompkins, A.M.; Lin, E.; Ju, H. Simulating the Impact of Flooding on Wheat Yield—Case Study in East China. Agric. For. Meteorol. 2016, 216, 221–231. [Google Scholar] [CrossRef]
- Promkhambut, A.; Polthanee, A.; Akkasaeng, C.; Younger, A. Growth, Yield and Aerenchyma Formation of Sweet and Multipurpose Sorghum (Sorghum bicolor L. Moench) as Affected by Flooding at Different Growth Stages. Aust. J. Crop Sci. 2011, 5, 954–965. [Google Scholar]
- Lin, C.; Zhu, T.; Peralta Ogorek, L.; Wang, Y.; Sauter, M.; Pedersen, O. The Pyramiding of Three Key Root Traits Aid Breeding of Flood-Tolerant Rice. Plants 2022, 11, 2033. [Google Scholar] [CrossRef]
- Dat, J.F.; Capelli, N.; Folzer, H.; Bourgeade, P.; Badot, P.-M. Sensing and Signalling during Plant Flooding. Plant Physiol. Biochem. 2004, 42, 273–282. [Google Scholar] [CrossRef]
- Bragina, T.V.; Rodionova, N.A.; Grinieva, G.M. Ethylene Production and Activation of Hydrolytic Enzymes during Acclimation of Maize Seedlings to Partial Flooding. Russ. J. Plant Physiol. 2003, 50, 794–798. [Google Scholar] [CrossRef]
- Larsen, O.; Nilsen, H.-G.; Aarnes, H. Response of Young Barley Plants to Waterlogging, as Related to Concentration of Ethylene and Ethane. J. Plant Physiol. 1986, 122, 365–372. [Google Scholar] [CrossRef]
- Steffens, B. The Role of Ethylene and ROS in Salinity, Heavy Metal, and Flooding Responses in Rice. Front. Plant Sci. 2014, 5, 685. [Google Scholar] [CrossRef]
- Shitsukawa, N.; Ikari, C.; Shimada, S.; Kitagawa, S.; Sakamoto, K.; Saito, H.; Ryuto, H.; Fukunishi, N.; Abe, T.; Takumi, S.; et al. The Einkorn Wheat (Triticum Monococcum) Mutant, Maintained Vegetative Phase, Is Caused by a Deletion in the VRN1 Gene. Genes Genet. Syst. 2007, 82, 167–170. [Google Scholar] [CrossRef]
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; Groot, S.; Soole, K.; Langridge, P. Early Flowering as a Drought Escape Mechanism in Plants: How Can It Aid Wheat Production? Front. Plant Sci. 2017, 8, 1950. [Google Scholar] [CrossRef]
- Abrecht, D.G.; Carberry, P.S. The Influence of Water Deficit Prior to Tassel Initiation on Maize Growth, Development and Yield. Field Crops Res. 1993, 31, 55–69. [Google Scholar] [CrossRef]
- McMaster, G.S.; Wilhelm, W.W. Phenological Responses of Wheat and Barley to Water and Temperature: Improving Simulation Models. J. Agric. Sci. 2003, 141, 129–147. [Google Scholar] [CrossRef] [Green Version]
- Al-Ajlouni, Z.I.; Al-Abdallat, A.M.; Al-Ghzawi, A.L.A.; Ayad, J.Y.; Abu Elenein, J.M.; Al-Quraan, N.A.; Baenziger, P.S. Impact of Pre-Anthesis Water Deficit on Yield and Yield Components in Barley (Hordeum Vulgare L.) Plants Grown under Controlled Conditions. Agronomy 2016, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Jose, J.; Bánfalvi, Z. The Role of GIGANTEA in Flowering and Abiotic Stress Adaptation in Plants. Columella J. Agric. Environ. Sci. 2019, 6, 7–18. [Google Scholar] [CrossRef]
- Mishra, P.; Panigrahi, K.C. GIGANTEA—An Emerging Story. Front. Plant Sci. 2015, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Galbiati, F.; Chiozzotto, R.; Locatelli, F.; Spada, A.; Genga, A.; Fornara, F. Hd3a, RFT1 and Ehd1 Integrate Photoperiodic and Drought Stress Signals to Delay the Floral Transition in Rice. Plant Cell Environ. 2016, 39, 1982–1993. [Google Scholar] [CrossRef]
- Hayama, R.; Yokoi, S.; Tamaki, S.; Yano, M.; Shimamoto, K. Adaptation of Photoperiodic Control Pathways Produces Short-Day Flowering in Rice. Nature 2003, 422, 719–722. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, L.; Fu, G.; Yang, Y.; Zhu, C.; Tao, L. Drought-Induced Proline Accumulation Is Uninvolved with Increased Nitric Oxide, Which Alleviates Drought Stress by Decreasing Transpiration in Rice. J. Plant Res. 2012, 125, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of Plant Heat-Shock Proteins and Molecular Chaperones in the Abiotic Stress Response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Jiang, S.; Zhang, R. The Role of GIGANTEA Gene in Mediating the Oxidative Stress Response and in Arabidopsis. Plant Growth Regul. 2006, 48, 261–270. [Google Scholar] [CrossRef]
- Li, S.; Yue, W.; Wang, M.; Qiu, W.; Zhou, L.; Shou, H. Mutation of OsGIGANTEA Leads to Enhanced Tolerance to Polyethylene Glycol-Generated Osmotic Stress in Rice. Front. Plant Sci. 2016, 7, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, W.; Xing, Y.; Weng, X.; Zhao, Y.; Tang, W.; Wang, L.; Zhou, H.; Yu, S.; Xu, C.; Li, X.; et al. Natural Variation in Ghd7 Is an Important Regulator of Heading Date and Yield Potential in Rice. Nat. Genet. 2008, 40, 761–767. [Google Scholar] [CrossRef]
- Weng, X.; Wang, L.; Wang, J.; Hu, Y.; Du, H.; Xu, C.; Xing, Y.; Li, X.; Xiao, J.; Zhang, Q. Grain Number, Plant Height, and Heading Date7 Is a Central Regulator of Growth, Development, and Stress Response. Plant Physiol. 2014, 164, 735–747. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Liang, J.; Abou-Elwafa, S.F.; Cheng, H.; Dou, D.; Ren, Z.; Xie, J.; Chen, Z.; Gao, F.; Ku, L.; et al. ZmCCT Regulates Photoperiod-Dependent Flowering and Response to Stresses in Maize. BMC Plant Biol. 2021, 21, 453. [Google Scholar] [CrossRef]
- Ku, L.; Tian, L.; Su, H.; Wang, C.; Wang, X.; Wu, L.; Shi, Y.; Li, G.; Wang, Z.; Wang, H.; et al. Dual Functions of the ZmCCT-Associated Quantitative Trait Locus in Flowering and Stress Responses under Long-Day Conditions. BMC Plant Biol. 2016, 16, 239. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Zhao, X.; Guo, S.; Dong, S.; Wen, Y.; Han, Z.; Jin, W.; Chen, Y. ZmCCA1a on Chromosome 10 of Maize Delays Flowering of Arabidopsis Thaliana. Front. Plant Sci. 2020, 11, 78. [Google Scholar] [CrossRef] [Green Version]
- Song, K.; Kim, H.; Shin, S.; Kim, K.-H.; Moon, J.-C.; Kim, J.Y.; Lee, B.-M. Transcriptome Analysis of Flowering Time Genes under Drought Stress in Maize Leaves. Front. Plant Sci. 2017, 8, 267. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Shi, J.; Li, X.; Liu, J.; Geng, Q.; Shi, H.; Ke, Y.; Sun, Q. Transcriptome Analysis Reveals the Molecular Mechanisms of the Defense Response to Gray Leaf Spot Disease in Maize. BMC Genom. 2018, 19, 742. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.; Zhang, Y.; Wang, K.; Meng, Q.; Liu, X.; Ma, L.; Li, Y.; Liu, J.; Ma, L. Expression Profile Analysis of Maize in Response to Setosphaeria Turcica. Gene 2018, 659, 100–108. [Google Scholar] [CrossRef]
- Wei, H.; Xu, H.; Su, C.; Wang, X.; Wang, L. Rice CIRCADIAN CLOCK ASSOCIATED 1 Transcriptionally Regulates ABA Signaling to Confer Multiple Abiotic Stress Tolerance. Plant Physiol. 2022, 190, 1057–1073. [Google Scholar] [CrossRef]
- Li, G.; Zhao, H.; Wang, L.; Wang, Y.; Guo, X.; Baohua, X. The Animal Nuclear Factor Y: An Enigmatic and Important Heterotrimeric Transcription Factor. Am. J. Cancer Res. 2018, 8, 1106–1125. [Google Scholar]
- Swain, S.; Myers, Z.A.; Siriwardana, C.L.; Holt, B.F., 3rd. The Multifaceted Roles of NUCLEAR FACTOR-Y in Arabidopsis Thaliana Development and Stress Responses. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 636–644. [Google Scholar] [CrossRef]
- Laloum, T.; De Mita, S.; Gamas, P.; Baudin, M.; Niebel, A. CCAAT-Box Binding Transcription Factors in Plants: Y so Many? Trends Plant Sci. 2013, 18, 157–166. [Google Scholar] [CrossRef]
- Gnesutta, N.; Kumimoto, R.W.; Swain, S.; Chiara, M.; Siriwardana, C.; Horner, D.S.; Holt, B.F., 3rd; Mantovani, R. CONSTANS Imparts DNA Sequence Specificity to the Histone Fold NF-YB/NF-YC Dimer. Plant Cell 2017, 29, 1516–1532. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Wang, J.; Cai, M.; Zhang, H.; Wu, F.; Xu, Y.; Li, C.; Cheng, Z.; Zhang, X.; Guo, X.; et al. The OsHAPL1-DTH8-Hd1 Complex Functions as the Transcription Regulator to Repress Heading Date in Rice. J. Exp. Bot. 2017, 68, 553–568. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Distelfeld, A.; Comis, A.; Dubcovsky, J. Wheat Flowering Repressor VRN2 and Promoter CO2 Compete for Interactions with NUCLEAR FACTOR-Y Complexes. Plant J. 2011, 67, 763–773. [Google Scholar] [CrossRef] [Green Version]
- Hwang, K.; Susila, H.; Nasim, Z.; Jung, J.-Y.; Ahn, J.H. Arabidopsis ABF3 and ABF4 Transcription Factors Act with the NF-YC Complex to Regulate SOC1 Expression and Mediate Drought-Accelerated Flowering. Mol. Plant 2019, 12, 489–505. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Cao, Y.; Ku, L.; Yao, W.; Cao, Y.; Ren, Z.; Dou, D.; Wang, H.; Ren, Z.; Liu, H.; et al. Dual Functions of ZmNF-YA3 in Photoperiod-Dependent Flowering and Abiotic Stress Responses in Maize. J. Exp. Bot. 2018, 69, 5177–5189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Korff, M.; Grando, S.; Del Greco, A.; This, D.; Baum, M.; Ceccarelli, S. Quantitative Trait Loci Associated with Adaptation to Mediterranean Dryland Conditions in Barley. Theor. Appl. Genet. 2008, 117, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Rollins, J.A.; Drosse, B.; Mulki, M.A.; Grando, S.; Baum, M.; Singh, M.; Ceccarelli, S.; von Korff, M. Variation at the Vernalisation Genes Vrn-H1 and Vrn-H2 Determines Growth and Yield Stability in Barley (Hordeum Vulgare) Grown under Dryland Conditions in Syria. Theor. Appl. Genet. 2013, 126, 2803–2824. [Google Scholar] [CrossRef] [PubMed]
- Habte, E.; Müller, L.M.; Shtaya, M.; Davis, S.J.; Von Korff, M. Osmotic Stress at the Barley Root Affects Expression of Circadian Clock Genes in the Shoot. Plant Cell Environ. 2013, 37, 1321–1337. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, N.; Takao, S.; Kudo, T.; Kiba, T.; Wang, Y.; Kinoshita, T.; Sakakibara, H. Improvement of Arabidopsis Biomass and Cold-, Drought-, and Salinity-Stress Tolerance by Modified Circadian Clock-Associated PSEUDO-RESPONSE REGULATORs. Plant Cell Physiol. 2016, 57, pcw057. [Google Scholar] [CrossRef] [Green Version]
- Gol, L.; Haraldsson, E.B.; von Korff, M. Ppd-H1 Integrates Drought Stress Signals to Control Spike Development and Flowering Time in Barley. J. Exp. Bot. 2021, 72, 122–136. [Google Scholar] [CrossRef]
- Gol, L.; Tomé, F.; von Korff, M. Floral Transitions in Wheat and Barley: Interactions between Photoperiod, Abiotic Stresses, and Nutrient Status. J. Exp. Bot. 2017, 68, 1399–1410. [Google Scholar] [CrossRef] [Green Version]
- Guleria, P.; Goswami, D.; Mahajan, M.; Kumar, V.; Bhardwaj, J.; Yadav, S. MicroRNAs and Their Role in Plants during Abiotic Stresses. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer: New York, NY, USA, 2012; pp. 265–278. [Google Scholar] [CrossRef]
- Chen, Z.; Li, F.; Yang, S.; Dong, Y.; Yuan, Q.; Wang, F.; Li, W.; Jiang, Y.; Jia, S.; Pei, X. Identification and Functional Analysis of Flowering Related MicroRNAs in Common Wild Rice (Oryza Rufipogon Griff.). PLoS ONE 2014, 8, e82844. [Google Scholar] [CrossRef]
- Mathieu, J.; Yant, L.J.; Mürdter, F.; Küttner, F.; Schmid, M. Repression of flowering by the miR172 target SMZ. PLoS Biol. 2009 7, e1000148. [CrossRef] [Green Version]
- Jung, J.-H.; Seo, Y.-H.; Seo, P.J.; Reyes, J.L.; Yun, J.; Chua, N.-H.; Park, C.-M. The GIGANTEA-Regulated MicroRNA172 Mediates Photoperiodic Flowering Independent of CONSTANS in Arabidopsis. Plant Cell 2007, 19, 2736–2748. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Zhang, X.; Wang, W.; Wang, Y.; Ming, F. The Suppression of WRKY44 by GIGANTEA-MiR172 Pathway Is Involved in Drought Response of Arabidopsis Thaliana. PLoS ONE 2013, 8, e73541. [Google Scholar] [CrossRef]
- Zhu, Q.-H.; Upadhyaya, N.M.; Gubler, F.; Helliwell, C.A. Over-Expression of MiR172 Causes Loss of Spikelet Determinacy and Floral Organ Abnormalities in Rice (Oryza Sativa). BMC Plant Biol. 2009, 9, 149. [Google Scholar] [CrossRef]
- Chuck, G.; Meeley, R.; Irish, E.; Sakai, H.; Hake, S. The Maize Tasselseed4 MicroRNA Controls Sex Determination and Meristem Cell Fate by Targeting Tasselseed6/Indeterminate Spikelet1. Nat. Genet. 2007, 39, 1517–1521. [Google Scholar] [CrossRef]
- Brown, R.; Bregitzer, P. A Ds Insertional Mutant of a Barley MiR172 Gene Results in Indeterminate Spikelet Development. Crop. Sci. 2011, 51, 1664. [Google Scholar] [CrossRef]
- Nair, S.K.; Wang, N.; Turuspekov, Y.; Pourkheirandish, M.; Sinsuwongwat, S.; Chen, G.; Sameri, M.; Tagiri, A.; Honda, I.; Watanabe, Y.; et al. Cleistogamous Flowering in Barley Arises from the Suppression of MicroRNA-Guided HvAP2 MRNA Cleavage. Proc. Natl. Acad. Sci. USA 2010, 107, 490–495. [Google Scholar] [CrossRef] [Green Version]
- Kong, Y.M.; Elling, A.A.; Chen, B.; Deng, X.W. Differential Expression of MicroRNAs in Maize Inbred and Hybrid Lines during Salt and Drought Stress. Am. J. Plant Sci. 2010, 1, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Si-Ammour, A.; Windels, D.; Arn-Bouldoires, E.; Kutter, C.; Ailhas, J.; Meins, F.J.; Vazquez, F. MiR393 and Secondary SiRNAs Regulate Expression of the TIR1/AFB2 Auxin Receptor Clade and Auxin-Related Development of Arabidopsis Leaves. Plant Physiol. 2011, 157, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Xia, K.; Wang, R.; Ou, X.; Fang, Z.; Tian, C.; Duan, J.; Wang, Y.; Zhang, M. OsTIR1 and OsAFB2 Downregulation via OsmiR393 Overexpression Leads to More Tillers, Early Flowering and Less Tolerance to Salt and Drought in Rice. PLoS ONE 2012, 7, e30039. [Google Scholar] [CrossRef]
- Gao, P.; Bai, X.; Yang, L.; Lv, D.; Pan, X.; Li, Y.; Cai, H.; Ji, W.; Chen, Q.; Zhu, Y. Osa-MIR393: A Salinity- and Alkaline Stress-Related MicroRNA Gene. Mol. Biol. Rep. 2011, 38, 237–242. [Google Scholar] [CrossRef]
- Feng, X.-M.; You, C.-X.; Qiao, Y.; Mao, K.; Hao, Y.-J. Ectopic Overexpression of Arabidopsis AtmiR393a Gene Changes Auxin Sensitivity and Enhances Salt Resistance in Tobacco. Acta Physiol. Plant. 2010, 32, 997–1003. [Google Scholar] [CrossRef]
- Porter, J.R.; Gawith, M. Temperatures and the Growth and Development of Wheat: A Review. Eur. J. Agron. 1999, 10, 23–36. [Google Scholar] [CrossRef]
- Sánchez, B.; Rasmussen, A.; Porter, J. Temperatures and the Growth and Development of Maize and Rice: A Review. Glob. Chang. Biol. 2013, 20, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Müller, A.E. Flowering Time Control and Applications in Plant Breeding. Trends Plant Sci. 2009, 14, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.; Robertson, M.; Tanner, G.; Peacock, W.; Dennis, E.; Helliwell, C. The Arabidopsis Thaliana Vernalization Response Requires a Polycomb-like Protein Complex That Also Includes VERNALIZATION INSENSITIVE 3. Proc. Natl. Acad. Sci. USA 2006, 103, 14631–14636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, L.; Loukoianov, A.; Blechl, A.; Tranquilli, G.; Ramakrishna, W.; SanMiguel, P.; Bennetzen, J.L.; Echenique, V.; Dubcovsky, J. The Wheat VRN2 Gene Is a Flowering Repressor Down-Regulated by Vernalization. Science 2004, 303, 1640–1644. [Google Scholar] [CrossRef] [Green Version]
- Mckeown, M.; Schubert, M.; Marcussen, T.; Fjellheim, S.; Preston, J. Evidence for an Early Origin of Vernalization Responsiveness in Temperate Pooideae Grasses. Plant Physiol. 2016, 172, 416–426. [Google Scholar] [CrossRef] [Green Version]
- Fornara, F.; de Montaigu, A.; Sánchez-Villarreal, A.; Takahashi, Y.; Ver Loren van Themaat, E.; Huettel, B.; Davis, S.J.; Coupland, G. The GI–CDF Module of Arabidopsis Affects Freezing Tolerance and Growth as Well as Flowering. Plant J. 2015, 81, 695–706. [Google Scholar] [CrossRef] [Green Version]
- Dhillon, T.; Pearce, S.P.; Stockinger, E.J.; Distelfeld, A.; Li, C.; Knox, A.K.; Vashegyi, I.; Vágújfalvi, A.; Galiba, G.; Dubcovsky, J. Regulation of Freezing Tolerance and Flowering in Temperate Cereals: The VRN-1 Connection. Plant Physiol. 2010, 153, 1846–1858. [Google Scholar] [CrossRef] [Green Version]
- Luan, W.; Chen, H.; Fu, Y.; Si, H.; Peng, W.; Song, S.; Liu, W.; Hu, G.; Sun, Z.; Xie, D.; et al. The Effect of the Crosstalk between Photoperiod and Temperature on the Heading-Date in Rice. PLoS ONE 2009, 4, e5891. [Google Scholar] [CrossRef]
- Anwar, M.R.; Liu, D.L.; Macadam, I.; Kelly, G. Adapting Agriculture to Climate Change: A Review. Theor. Appl. Climatol. 2013, 113, 225–245. [Google Scholar] [CrossRef]
- Thines, B.; Harmon, F.G. Ambient Temperature Response Establishes ELF3 as a Required Component of the Core Arabidopsis Circadian Clock. Proc. Natl. Acad. Sci. USA 2010, 107, 3257–3262. [Google Scholar] [CrossRef] [Green Version]
- Laufs, P.; Peaucelle, A.; Morin, H.; Traas, J. MicroRNA Regulation of the CUC Genes Is Required for Boundary Size Control in Arabidopsis Meristems. Development 2004, 131, 4311–4322. [Google Scholar] [CrossRef]
- Hendelman, A.; Stav, R.; Zemach, H.; Arazi, T. The Tomato NAC Transcription Factor SlNAM2 Is Involved in Flower-Boundary Morphogenesis. J. Exp. Bot. 2013, 64, 5497–5507. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Bao, J.; Zhou, B.; Li, M.; Li, X.; Jin, J. The Osa-MiR164 Target OsCUC1 Functions Redundantly with OsCUC3 in Controlling Rice Meristem/Organ Boundary Specification. New Phytol. 2021, 229, 1566–1581. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, S.; Hang, X.; Xiang, Y.; Cheng, Z.; Li, W.; Shi, J.; Huang, L.; Sun, Y. Identification of Heavy-Ion Radiation-Induced MicroRNAs in Rice. Adv. Space Res. 2011, 47, 1054–1061. [Google Scholar] [CrossRef]
- Fang, Y.; Xie, K.; Xiong, L. Conserved MiR164-Targeted NAC Genes Negatively Regulate Drought Resistance in Rice. J. Exp. Bot. 2014, 65, 2119–2135. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Kennedy, A.; Huybrechts, M.; Dochy, N.; Geuten, K. The Effect of Ambient Temperature on Brachypodium Distachyon Development. Front. Plant Sci. 2019, 10, 1011. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, L.; Zhou, J.; Hu, S.; Chen, H.; Xiang, J.; Zhang, Y.; Zeng, Y.; Shi, Q.; Zhu, D.; et al. Research Progress on Heat Stress of Rice at Flowering Stage. Rice Sci. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Sharma, N.; Ruelens, P.; D’hauw, M.; Maggen, T.; Dochy, N.; Torfs, S.; Kaufmann, K.; Rohde, A.; Geuten, K. A Flowering Locus C Homolog Is a Vernalization-Regulated Repressor in Brachypodium and Is Cold Regulated in Wheat. Plant Physiol. 2017, 173, 1301–1315. [Google Scholar] [CrossRef] [Green Version]
- Hemming, M.N.; Walford, S.A.; Fieg, S.; Dennis, E.S.; Trevaskis, B. Identification of High-Temperature-Responsive Genes in Cereals. Plant Physiol. 2012, 158, 1439–1450. [Google Scholar] [CrossRef] [Green Version]
- Greenup, A.G.; Sasani, S.; Oliver, S.N.; Talbot, M.J.; Dennis, E.S.; Hemming, M.N.; Trevaskis, B. ODDSOC2 Is a MADS Box Floral Repressor That Is Down-Regulated by Vernalization in Temperate Cereals. Plant Physiol. 2010, 153, 1062–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ejaz, M.; von Korff, M. The Genetic Control of Reproductive Development under High Ambient Temperature. Plant Physiol. 2017, 173, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.; Xu, Y.; Xu, W.; Wang, X.; Li, N.; Wu, J.; Liang, T.; Chong, K.; Xu, Z.; Tan, K.; et al. Vernalization-Induced Flowering in Wheat Is Mediated by a Lectin-like Gene VER2. Planta 2003, 217, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, S.V.K. Heat Stress during Flowering in Cereals—Effects and Adaptation Strategies. New Phytol. 2020, 226, 1567–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirabayashi, H.; Sasaki, K.; Kambe, T.; Gannaban, R.B.; Miras, M.A.; Mendioro, M.S.; Simon, E.V.; Lumanglas, P.D.; Fujita, D.; Takemoto-Kuno, Y.; et al. QEMF3, a Novel QTL for the Early-Morning Flowering Trait from Wild Rice, Oryza Officinalis, to Mitigate Heat Stress Damage at Flowering in Rice, O. Sativa. J. Exp. Bot. 2015, 66, 1227–1236. [Google Scholar] [CrossRef]
- Corwin, D.L. Climate Change Impacts on Soil Salinity in Agricultural Areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Kholodova, V.; Volkov, K.; Kuznetsov, V. Plants Under Heavy Metal Stress in Saline Environments. In Soil Heavy Metals; Springer: Berlin/Heidelberg, Germany, 2010; pp. 163–183. ISBN 978-3-642-02436-8. [Google Scholar]
- Nikalje, G.C.; Suprasanna, P. Coping with Metal Toxicity—Cues from Halophytes. Front. Plant Sci. 2018, 9, 777. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. Changes in Plant Response to NaCl during Development of Rice (Oryza sativa L.) Varieties Differing in Salinity Resistance. J. Exp. Bot. 1995, 46, 1843–1852. [Google Scholar] [CrossRef]
- Gao, J.-P.; Chao, D.-Y.; Lin, H.-X. Understanding Abiotic Stress Tolerance Mechanisms: Recent Studies on Stress Response in Rice. J. Integr. Plant Biol. 2007, 49, 742–750. [Google Scholar] [CrossRef]
- Zeng, L.; Shannon, M.C.; Grieve, C.M. Evaluation of Salt Tolerance in Rice Genotypes by Multiple Agronomic Parameters. Euphytica 2002, 127, 235–245. [Google Scholar] [CrossRef]
- Hussain, S.; Zhang, J.; Zhong, C.; Zhu, L.; Cao, X.; Yu, S.; Allen Bohr, J.; Hu, J.; Jin, Q. Effects of Salt Stress on Rice Growth, Development Characteristics, and the Regulating Ways: A Review. J. Integr. Agric. 2017, 16, 2357–2374. [Google Scholar] [CrossRef]
- Kim, W.-Y.; Ali, Z.; Park, H.J.; Park, S.J.; Cha, J.-Y.; Perez-Hormaeche, J.; Quintero, F.J.; Shin, G.; Kim, M.R.; Qiang, Z.; et al. Release of SOS2 Kinase from Sequestration with GIGANTEA Determines Salt Tolerance in Arabidopsis. Nat. Commun. 2013, 4, 1352. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; He, Y.; Wei, H.; Wang, L. A Clock Regulatory Module Is Required for Salt Tolerance and Control of Heading Date in Rice. Plant Cell Environ. 2021, 44, 3283–3301. [Google Scholar] [CrossRef]
- Wei, H.; Wang, X.; He, Y.; Xu, H.; Wang, L. Clock Component OsPRR73 Positively Regulates Rice Salt Tolerance by Modulating OsHKT2;1-Mediated Sodium Homeostasis. EMBO J. 2021, 40, e105086. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Shen, J.; Yin, J.; Li, D.; Gao, Y.; Xu, W.; Liang, J. OsRACK1A, Encodes a Circadian Clock-Regulated WD40 Protein, Negatively Affect Salt Tolerance in Rice. Rice 2018, 11, 45. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.; Bai, X.; Yang, L.; Lv, D.; Li, Y.; Cai, H.; Ji, W.; Guo, D.; Zhu, Y. Over-Expression of Osa-MIR396c Decreases Salt and Alkali Stress Tolerance. Planta 2010, 231, 991–1001. [Google Scholar] [CrossRef]
- Zheng, G.; Wei, W.; Li, Y.; Kan, L.; Wang, F.; Zhang, X.; Li, F.; Liu, Z.; Kang, C. Conserved and Novel Roles of MiR164-CUC2 Regulatory Module in Specifying Leaf and Floral Organ Morphology in Strawberry. New Phytol. 2019, 224, 480–492. [Google Scholar] [CrossRef]
- Ma, X.; Qiao, Z.; Chen, D.; Yang, W.; Zhou, R.; Zhang, W.; Wang, M. CYCLIN-DEPENDENT KINASE G2 Regulates Salinity Stress Response and Salt Mediated Flowering in Arabidopsis Thaliana. Plant Mol. Biol. 2015, 88, 287–299. [Google Scholar] [CrossRef]
- Kim, S.-G.; Kim, S.-Y.; Park, C.-M. A Membrane-Associated NAC Transcription Factor Regulates Salt-Responsive Flowering via FLOWERING LOCUS T in Arabidopsis. Planta 2007, 226, 647–654. [Google Scholar] [CrossRef]
- Joshi, R.; Sahoo, K.K.; Tripathi, A.K.; Kumar, R.; Gupta, B.K.; Pareek, A.; Singla-Pareek, S.L. Knockdown of an Inflorescence Meristem-Specific Cytokinin Oxidase—OsCKX2 in Rice Reduces Yield Penalty under Salinity Stress Condition. Plant Cell Environ. 2018, 41, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Rawson, H.M. Effect of Salinity on Salt Accumulation and Reproductive Development in the Apical Meristem of Wheat and Barley. Funct. Plant Biol. 1999, 26, 459–464. [Google Scholar] [CrossRef]
- Ghanem, M.E.; van Elteren, J.; Albacete, A.; Quinet, M.; Martínez-Andújar, C.; Kinet, J.-M.; Pérez-Alfocea, F.; Lutts, S. Impact of Salinity on Early Reproductive Physiology of Tomato (Solanum Lycopersicum) in Relation to a Heterogeneous Distribution of Toxic Ions in Flower Organs. Funct. Plant Biol. 2009, 36, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Taoka, K.I.; Ohki, I.; Tsuji, H.; Furuita, K.; Hayashi, K.; Yanase, T.; Yamaguchi, M.; Nakashima, C.; Purwestri, Y.A.; Tamaki, S.; et al. 14-3-3 Proteins Act as Intracellular Receptors for Rice Hd3a Florigen. Nature 2011, 476, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Roychoudhury, A. Abscisic-Acid-Dependent Basic Leucine Zipper (BZIP) Transcription Factors in Plant Abiotic Stress. Protoplasma 2017, 254, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Gao, C.; Zheng, X.; Han, B. Identification of OsbZIP72 as a Positive Regulator of ABA Response and Drought Tolerance in Rice. Planta 2009, 229, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Tang, N.; Zhang, H.; Li, X.; Xiao, J.; Xiong, L. Constitutive Activation of Transcription Factor OsbZIP46 Improves Drought Tolerance in Rice. Plant Physiol. 2012, 158, 1755–1768. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Wang, G.; Wu, H.; Fan, X.; Liang, L.; Zhao, H.; Li, S.; Hu, Y.; Liu, H.; Ayaad, M.; et al. OsMFT2 Is Involved in the Regulation of ABA Signaling-mediated Seed Germination through Interacting with OsbZIP23/66/72 in Rice. Plant J. 2020, 103, 532–546. [Google Scholar] [CrossRef]
- Shu, K.; Chen, F.; Zhou, W.; Luo, X.; Dai, Y.; Shuai, H.; Yang, W. ABI4 Regulates the Floral Transition Independently of ABI5 and ABI3. Mol. Biol. Rep. 2018, 45, 2727–2731. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Becana, M. Molecular responses of legumes to abiotic stress: Post-translational modifications of proteins and redox signaling. J. Exp. Bot. 2021, 72, 5876–5892. [Google Scholar] [CrossRef]
- Hashiguchi, A.; Komatsu, S. Impact of Post-Translational Modifications of Crop Proteins under Abiotic Stress. Proteomes 2016, 4, 42. [Google Scholar] [CrossRef]
- Friml, J.; Gallei, M.; Gelová, Z.; Johnson, A.; Mazur, E.; Monzer, A.; Rodriguez, L.; Roosjen, M.; Verstraeten, I.; Živanović, B.D.; et al. ABP1–TMK Auxin Perception for Global Phosphorylation and Auxin Canalization. Nature 2022, 609, 575–581. [Google Scholar] [CrossRef]
- Lyzenga, W.J.; Stone, S.L. Abiotic Stress Tolerance Mediated by Protein Ubiquitination. J. Exp. Bot. 2012, 63, 599–616. [Google Scholar] [CrossRef] [Green Version]
- Melo, F.V.; Oliveira, M.M.; Saibo, N.J.M.; Lourenço, T.F. Modulation of Abiotic Stress Responses in Rice by E3-Ubiquitin Ligases: A Promising Way to Develop Stress-Tolerant Crops. Front. Plant Sci. 2021, 12, 640193. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, S.D.; Jang, C.S. Oryza Sativa Drought-, Heat-, and Salt-Induced RING Finger Protein 1 (OsDHSRP1) Negatively Regulates Abiotic Stress-Responsive Gene Expression. Plant Mol. Biol. 2020, 103, 235–252. [Google Scholar] [CrossRef]
- Zörb, C.; Schmitt, S.; Mühling, K.H. Proteomic Changes in Maize Roots after Short-Term Adjustment to Saline Growth Conditions. Proteomics 2010, 10, 4441–4449. [Google Scholar] [CrossRef]
- Wei, K.; Pan, S. Maize Protein Phosphatase Gene Family: Identification and Molecular Characterization. BMC Genom. 2014, 15, 773. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Lv, D.; Ge, P.; Bian, Y.; Chen, G.; Zhu, G.; Li, X.; Yan, Y. Phosphoproteome Analysis Reveals New Drought Response and Defense Mechanisms of Seedling Leaves in Bread Wheat (Triticum aestivum L.). J. Proteom. 2014, 109, 290–308. [Google Scholar] [CrossRef]
- Lv, D.; Zhu, G.; Zhu, D.; Bian, Y.-W.; Liang, X.-N.; Cheng, Z.-W.; Deng, X.; Yan, Y.-M. Proteomic and Phosphoproteomic Analysis Reveals the Response and Defense Mechanism in Leaves of Diploid Wheat T. Monococcum under Salt Stress and Recovery. J. Proteom. 2016, 143, 93–105. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y.; Liu, Z.; Kong, D.; Duan, M.; Luo, L. Genome-Wide Identification and Analysis of Drought-Responsive MicroRNAs in Oryza Sativa. J. Exp. Bot. 2010, 61, 4157–4168. [Google Scholar] [CrossRef]
- Shan, T.; Fu, R.; Xie, Y.; Chen, Q.; Wang, Y.; Li, Z.; Song, X.; Li, P.; Wang, B. Regulatory Mechanism of Maize (Zea mays L.) MiR164 in Salt Stress Response. Russ. J. Genet. 2020, 56, 835–842. [Google Scholar] [CrossRef]
- An, Y.; Guo, Y.; Liu, C.; An, H. BdVIL4 Regulates Flowering Time and Branching through Repressing MiR156 in Ambient Temperature Dependent Way in Brachypodium Distachyon. Plant Physiol. Biochem. 2015, 89, 92–99. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirivì, D.; Betti, C. Molecular Links between Flowering and Abiotic Stress Response: A Focus on Poaceae. Plants 2023, 12, 331. https://doi.org/10.3390/plants12020331
Chirivì D, Betti C. Molecular Links between Flowering and Abiotic Stress Response: A Focus on Poaceae. Plants. 2023; 12(2):331. https://doi.org/10.3390/plants12020331
Chicago/Turabian StyleChirivì, Daniele, and Camilla Betti. 2023. "Molecular Links between Flowering and Abiotic Stress Response: A Focus on Poaceae" Plants 12, no. 2: 331. https://doi.org/10.3390/plants12020331
APA StyleChirivì, D., & Betti, C. (2023). Molecular Links between Flowering and Abiotic Stress Response: A Focus on Poaceae. Plants, 12(2), 331. https://doi.org/10.3390/plants12020331