Invasive Plants as a Source of Polyphenols with High Radical Scavenging Activity
Abstract
1. Introduction
2. Results
2.1. Polyphenols
2.2. Flavonoids
2.3. Radical Scavenging Activity of Invasive Plant Extracts
2.4. Alkaloid Contents of Different L. polyphyllus Parts
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction of Invasive Plant Biomass
4.3. Extraction of Lupinus Biomass for Determination of Alkaloid Content
4.4. Determination of Total Polyphenolics
4.5. Determination of Total Flavonoids
4.6. Determination of Antioxidative Potential Using DPPH
4.7. Determination of Antioxidative Potential Using ABTS
4.8. Determination of Ferric Reducing Antioxidant Potential Using FRAP
4.9. Determination of CUPARC
4.10. Determination of Alkaloid Content in Lupinus polyphyllus
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coughlan, N.E.; Lyne, L.; Cuthbert, R.N.; Cunningham, E.M.; Lucy, F.E.; Davis, E.; Caffrey, J.M.; Dick, J.T.A. In the black: Information and educational potential amongst international databases for invasive alien species designated as of Union Concern. Glob. Ecol. Conserv. 2020, 24, e01332. [Google Scholar] [CrossRef]
- Pérez, G.; Vilà, M.; Gallardo, B. Potential impact of four invasive alien plants on the provision of ecosystem services in Europe under present and future climatic scenarios. Ecosyst. Serv. 2022, 56, 101459. [Google Scholar] [CrossRef]
- Huebner, C.D. Chapter 18—Effects of global climate change on regeneration of invasive plant species from seeds. In Plant Regeneration from Seeds; Baskin, C.C., Baskin, J.M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 243–257. [Google Scholar]
- Baquero, R.A.; Ayllón, D.; Nicola, G.G. Are the EU biosecurity legislative frameworks sufficiently effective to prevent biological invasions in the Natura 2000 network?—A case study in Mediterranean Europe. Environ. Sci. Policy 2021, 120, 21–28. [Google Scholar]
- Dai, Z.C.; Wan, L.Y.; Qi, S.S.; Rutherford, S.; Ren, G.Q.; Wan, J.S.H.; Du, D.L. Synergy among hypotheses in the invasion process of alien plants: A road map within a timeline. Perspect. Plant Ecol. Evol. Syst. 2020, 47, 125575. [Google Scholar] [CrossRef]
- Matisone, I.; Zumberga, A.; Lībiete, Z.; Gerra-Inohosa, L.; Jansons, J. The impact of forest infrastructure reconstruction on expansion of potentially invasive plant species: First results from a study in Latvia. J. For. Sci. 2018, 64, 353–357. [Google Scholar] [CrossRef]
- Leostrin, A.; Pergl, J. Alien flora in a boreal region of European Russia: An example of Kostroma oblast. Biol. Invasions 2021, 23, 3337–3350. [Google Scholar] [CrossRef]
- Parks, C.G.; Radosevich, S.R.; Endress, B.A.; Naylor, B.J.; Anzinger, D.; Rew, L.J.; Maxwell, B.D.; Dwire, K.A. Natural and land-use history of the Northwest mountain ecoregions (USA) in relation to patterns of plant invasions. Perspect. Plant Ecol. Evol. Syst. 2005, 7, 137–158. [Google Scholar] [CrossRef]
- Vicente, J.R.; Pereira, H.M.; Randin, C.F.; Gonçalves, J.; Lomba, A.; Alves, P.; Metzger, J.; Cezar, M.; Guisan, A.; Honrado, J. Environment and dispersal paths override life strategies and residence time in determining regional patterns of invasion by alien plants. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 1–10. [Google Scholar] [CrossRef]
- Haubrock, P.J.; Turbelin, A.J.; Cuthbert, R.N.; Novoa, A.; Taylor, N.G.; Angulo, E.; Ballesteros-Mejia, L.; Bodey, T.W.; Capinha, C.; Diagne, C.; et al. Economic costs of invasive alien species across Europe. NeoBiota 2021, 67, 153–190. [Google Scholar] [CrossRef]
- López-Núñez, F.A.; Marchante, E.; Heleno, R.; Duarte, L.N.; Palhas, J.; Impson, F.; Freitas, H.; Marchante, H. Establishment, spread and early impacts of the first biocontrol agent against an invasive plant in continental Europe. J. Environ. Manag. 2021, 290, 112545. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Available online: https://ec.europa.eu/environment/nature/invasivealien/list/index_en.htm (accessed on 11 November 2024).
- Evarts-Bunders, P.; Evarte-Bundere, G. Development and approbation of methodology for monitoring invasive plant species: The case of Latvia. Thaiszia J. F Bot. 2020, 30, 059–079. [Google Scholar] [CrossRef]
- Rutkovska, S.; Pučkina, I.; Frolova, O. Inventory of the most invasive alien plant species of Latvia in the “Daugavas loki” Nature Park. Environ. Technol. Resour. Proc. Int. Sci. Pract. Conf. 2017, 1, 246–252. [Google Scholar] [CrossRef]
- Sheng, Q.; Zhao, B.; Huang, M.; Wang, L.; Quan, Z.; Fang, C.; Li, B.; Wu, J. Greenhouse gas emissions following an invasive plant eradication program. Ecol. Eng. 2014, 73, 229–237. [Google Scholar] [CrossRef]
- Yoshida, K.; Hata, K.; Kawakami, K.; Hiradate, S.; Osawa, T.; Kachi, N. Ecosystem changes following the eradication of invasive species: Evaluation of various eradication scenarios by computer simulation. Ecol. Model. 2019, 413, 108831. [Google Scholar] [CrossRef]
- Van Wilgen, B.W.; Wannenburgh, A.; Wilson, J.R.U. A review of two decades of government support for managing alien plant invasions in South Africa. Biol. Conservat. 2022, 274, 109741. [Google Scholar] [CrossRef]
- Sinha, A.; Nath, A.; Lahkar, B.P.; Brahma, N.; Sarma, H.K.; Swargowari, A. Understanding the efficacy of different techniques to manage Chromolaena odorata L., an Invasive Alien Plant in the sub-Himalayan tall grasslands: Toward grassland recovery. Ecol. Eng. 2022, 179, 106618. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Tran, T.V.; Nguyen, T.T.T.; Nguyen, D.H.; Alhassan, M.; Lee, T. New frontiers of invasive plants for biosynthesis of nanoparticles towards biomedical applications: A review. Sci. Total Environ. 2023, 857, 159278. [Google Scholar] [CrossRef]
- Parsova, V.; Jankava, A.; Berzina, M.; Palabinska, A. Planning and use of areas infested with invasive plants: Case of Latvia. Curr. Trends Nat. Sci. 2020, 9, 147–152. [Google Scholar] [CrossRef]
- Darin, G.M.S.; Schoenig, S.; Barney, J.N.F.; Panetta, D.; DiTomaso, J.M. WHIPPET: A novel tool for prioritizing invasive plant populations for regional eradication. J. Environ. Manag. 2011, 92, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Tuck, C.O.; Perez, E.; Horvath, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, B.; Chen, M.; Wu, P.; Lee, X.; Xing, Y. Invasive plants as potential sustainable feedstocks for biochar production and multiple applications: A review. Resour. Conservat. Recycl. 2021, 164, 105204. [Google Scholar] [CrossRef]
- Williams, V.L.; Wojtasik, E.M.; Byrne, M.J. A chronicle of alien medicinal plants used as traditional medicine in South Africa, and their status as invasive species. S. Afr. J. Bot. 2021, 142, 63–72. [Google Scholar] [CrossRef]
- Prabakaran, K.; Li, J.; Anandkumar, A.; Leng, Z.; Zou, C.B.; Du, D. Managing environmental contamination through phytoremediation by invasive plants: A review. Ecol. Eng. 2019, 138, 28–37. [Google Scholar] [CrossRef]
- Pereyra, P.J. Revisiting the use of the invasive species concept: An empirical approach. Austral Ecol. 2016, 41, 519–528. [Google Scholar] [CrossRef]
- Shamprasad, B.R.; Subramani, R.; Subramaniam, S.; Sivasubramanian, A. Environmentally benign, ultrasonication assisted, sustainable valorization for commercially important nutraceutical-Daucosterol from the heartwood of invasive Prosopis juliflora (Sw.) DC. Sustain. Chem. Pharm. 2022, 29, 100810. [Google Scholar] [CrossRef]
- Ahmed, A.; Abu Bakar, M.S.; Hamdani, R.; Park, Y.o.K.; Lam, S.S.; Sukri, R.S.; Hussain, M.; Majeed, K.; Phusunti, N.; Jamil, F.; et al. Valorization of underutilized waste biomass from invasive species to produce biochar for energy and other value-added applications. Environ. Res. 2020, 186, 109596. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.K.; Al-Helal, M.d.A.; Chakraborty, S.K. Management of invasive weed Chromolaena odorata (Siam weed) through vermicomposting: An eco-approach utilizing organic biomass valorization. Environ. Technol. Innov. 2022, 28, 102952. [Google Scholar] [CrossRef]
- Kauser, H.; Pal, S.; Haq, I.; Khwairakpam, M. Evaluation of rotary drum composting for the management of invasive weed Mikania micrantha Kunth and its toxicity assessment. Biores. Technol. 2020, 313, 123678. [Google Scholar] [CrossRef]
- Bandara, W.A.R.T.W.; Ranasinghe, O.; Perera, P.; Vlosky, R.; Kizha, A.R. Potential to use invasive plants in biomass energy production: A case study Prosopis juliflora in coastal wetlands of Sri Lanka. Trees For. People 2022, 10, 100330. [Google Scholar] [CrossRef]
- Niedrite, E.; Klavins, L.; Dobkevica, L.; Purmalis, O.; Ievinsh, G.; Klavins, M. Sustainable control of invasive plants: Compost production, quality, and effects on wheat germination. J. Environ. Manag. 2024, 371, 123149. [Google Scholar] [CrossRef] [PubMed]
- De Lange, W.J.; Stafford, W.H.L.; Forsyth, G.G.; Le Maitre, D.C. Incorporating stakeholder preferences in the selection of technologies for using invasive alien plants as a bio-energy feedstock: Applying the analytical hierarchy process. J. Environ. Manag. 2012, 99, 76–83. [Google Scholar] [CrossRef]
- Wolf, S.; Romeis, J.; Collatz, J. Utilization of plant-derived food sources from annual flower strips by the invasive harlequin ladybird Harmonia axyridis. Biol. Control 2018, 122, 118–126. [Google Scholar] [CrossRef]
- Lin, T.; Vrieling, K.; Laplanche, D.; Klinkhamer, P.G.L.; Lou, Y.; Bekooy, L.; Degen, T.; Bustos-Segura, C.; Turlings, T.C.J.; Desurmont, G.A. Evolutionary changes in an invasive plant support the defensive role of plant volatiles. Curr. Biol. 2021, 31, 3450–3456.e5. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Kulinowski, Ł.; Ciobanu, C.; Zengin, G.; Czerwińska, M.E.; Granica, S.; Xiao, J.; Skalicka-Woźniak, K.; Trifan, A. Phytochemical and multi-biological characterization of two Cynara scolymus L. varieties: A glance into their potential large scale cultivation and valorization as bio-functional ingredients. Ind. Crops Prod. 2022, 178, 114623. [Google Scholar] [CrossRef]
- Peter, A.; Žlabur, J.Š.; Šurić, J.; Voća, S.; Purgar, D.D.; Pezo, L.; Voća, N. Invasive Plant Species Biomass—Evaluation of Functional Value. Molecules 2021, 26, 3814. [Google Scholar] [CrossRef]
- Li, Q.; Mei, H.; Zhang, Z.; Jiang, H.; Zhang, W. Valorization of flavonoid-rich invasive weed—Eupatorium adenophorum in the cleaner production of coloristic and functional wool fabric with a research focus on photofading mechanism. Ind. Crops Prod. 2022, 189, 115835. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Illera, M.; Sánchez, M.; Lodeiro, P.; Torres, M.D.; López-Mosquera, M.E.; Soto, M.; De Vicente, M.S.; Domínguez, H. Integrated valorization of Sargassum muticum in biorefineries. Chem. Eng. J. 2021, 404, 125635. [Google Scholar] [CrossRef]
- Han, C.; Shao, H.; Zhou, S.; Mei, Y.; Cheng, Z.; Huang, L.; Lv, G. Chemical composition and phytotoxicity of essential oil from invasive plant, Ambrosia artemisiifolia L. Ecotox. Environ. Saf. 2021, 211, 111879. [Google Scholar] [CrossRef] [PubMed]
- Adelipour, M.; Cheraghzadeh, M.; Rashidi, M. Polyphenols as epigenetic modulators in treating or preventing of cancers. Gene Rep. 2022, 29, 101710. [Google Scholar] [CrossRef]
- Klavins, M.; Purmalis, O.; Klavina, L.; Niedrite, E.; Ansone-Bertina, L. Biomass of Invasive Plants as a Resource for the Development of the Bioeconomy. BioResources 2024, 19, 9788–9817. [Google Scholar] [CrossRef]
- Duthie, G.; Gardner, P.; Kyle, J. Plant polyphenols: Are they the new magic bullet? Proc. Nutr. Soc. 2003, 62, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- De Elguea-Culebras, G.O.; Bravo, E.M.; Sánchez-Vioque, R. Potential sources and methodologies for the recovery of phenolic compounds from distillation residues of Mediterranean aromatic plants. An approach to the valuation of by-products of the essential oil market—A review. Ind. Crops Prod. 2022, 175, 114261. [Google Scholar] [CrossRef]
- Martins, C.C.; Rodrigues, R.C.; Mercali, G.D.; Rodrigues, E. New insights into non-extractable phenolic compounds analysis. Food Res. Internat. 2022, 157, 111487. [Google Scholar] [CrossRef]
- Rauf, A.; Nengroo, Z. Fatty acid composition, functional group analysis and antioxidant activity of Nymphia alba and Lupinus polyphyllus seed extracts. J. Oleo Sci. 2020, 69, 317–326. [Google Scholar]
- Giacomini, D.; Musumeci, R.; Galletti, P.; Martelli, G.; Assennato, L.; Sacchetti, G.; Guerrini, A.; Calaresu, E.; Martinelli, M.; Cocuzza, C. 4-Alkyliden-azetidinones modified with plant derived polyphenols: Antibacterial and antioxidant properties. Eur. J. Med. Chem. 2017, 140, 604–614. [Google Scholar] [CrossRef]
- Arnoldi, A.; Boschin, G.; Zanoni, C. The health benefits of sweet lupin seed flours and isolated proteins. J. Funct. Foods 2015, 18, 550–563. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Veberic, R.; Hudina, M.; Misic, E. HPLC-DAD-MS Identification and Quantification of Phenolic Components in Japanese Knotweed and American Pokeweed Extracts and Their Phytotoxic Effect on Seed Germination. Plants 2022, 11, 3053. [Google Scholar] [CrossRef]
- Lazzaro, L.; Essl, F.; Lugliè, A.; Padedda, B.M.; Pyšek, P.; Brundu, G. Invasive alien plant impacts on human health and well-being. In Invasive Species and Human Health; Mazza, G., Tricario, E., Eds.; CAB; International Publishing: Wallingford, UK, 2018; pp. 16–33. [Google Scholar]
- Kumar, R.P.; Singh, J.S. Invasive alien plant species: Their impact on environment, ecosystem services and human health. Ecol. Indic. 2020, 111, 106020. [Google Scholar] [CrossRef]
- Ghendov, V.; Izverscaia, T.; Şabanova, G. Heracleum sosnowskyi Manden. (Apiaceae)—An invasive alien plant for the flora of Republic of Moldova. In Horticultură, Viticultură şi Vinificaţie, Silvicultură şi Grădini Publice, Protecţia Plantelor; Universitatea Agrară de Stat din Moldova: Chișinău, Republica Moldova, 2010; Volume 24, pp. 232–237. [Google Scholar]
- Petterson, D.S. Lupin: Overview. In Encyclopedia of Food Grains, 2nd ed.; Colin, W., Harold, C., Koushik, S., Jon, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 280–286. [Google Scholar]
- Erdemoglu, N.; Ozkan, S.; Tosun, F. Alkaloid profile and antimicrobial activity of Lupinus angustifolius L. alkaloid extract. Phytochem. Rev. 2007, 6, 197–201. [Google Scholar] [CrossRef]
- Romeo, F.V.; Fabroni, S.; Ballistreri, G.; Muccilli, S.; Spina, A.; Rapisarda, P. Characterization and antimicrobial activity of alkaloid extracts from seeds of different genotypes of Lupinus spp. Sustainability 2018, 10, 788. [Google Scholar] [CrossRef]
- Osorio, C.E.; Till, B.J. A Bitter-Sweet Story: Unraveling the Genes Involved in Quinolizidine Alkaloid Synthesis in Lupinus albus. Front. Plant Sci. 2022, 12, 795091. [Google Scholar] [CrossRef]
- Leck, M.A.; Parker, V.T.; Simpson, R.L. Ecology of Soil Seed Banks; Academic Press: San Diego, CA, USA, 1989. [Google Scholar]
- Flegel, M.; Schrader, S.; Zhang, H. Influence of food quality on the physical and chemical properties of detritivorous earthworm casts. Appl. Soil Ecol. 1998, 9, 263–269. [Google Scholar] [CrossRef]
- Hosakatte, N.M.; Yaser, H.D.; Dayanand, D.; Nasser, A.S. Comparative physicochemical analysis of seed oils of wild cucumber (Cucumis sativus var. hardwickii (Royle) Alef.), cucumber (Cucumis sativus L. var. sativus), and gherkin (Cucumis anguria L.). S. Afr. J. Bot. 2022, 145, 186–191. [Google Scholar]
- Eleomar, O.P.; Cristina, C.; Carolina, C.G.; Isabel, C.F.R.; Ferreira, L.B. Current status of genus Impatiens: Bioactive compounds and natural pigments with health benefits. Trends Food Sci. Technol. 2021, 117, 106–124. [Google Scholar]
- Borek, S.; Ratajczak, W.; Ratajczak, L. Regulation of storage lipid metabolism in developing and germinating lupin (Lupinus spp.) seeds. Acta Physiol. Plant 2015, 37, 119. [Google Scholar] [CrossRef]
- Kviesis, J.; Kļimenkovs, I.; Arbidans, L.; Podjava, A.; Kļaviņš, M.; Liepiņš, E. Evaluation of furanocoumarins from seeds of the wild parsnip (Pastinaca sativa L. s.l.). J. Chromatogr. B 2019, 1105, 54–66. [Google Scholar] [CrossRef]
- Grzędzicka, E. Invasion of the Giant Hogweed and the Sosnowsky’s Hogweed as a Multidisciplinary Problem with Unknown Future—A Review. Earth 2022, 3, 287–312. [Google Scholar] [CrossRef]
- Rodge, S.; Biradar, S.D. Antioxidant activity of some medicinal plants of family Cucurbitaceae. Worlds J. Pharm. Res. 2017, 6, 946–951. [Google Scholar] [CrossRef][Green Version]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- De Cortes Sánchez, M.; Altares, P.; Pedrosa, M.M.; Burbano, C.; Cuadrado, C.; Goyoaga, C.; Dávila-Ortiz, G. Alkaloid variation during germination in different lupin species. Food Chem. 2005, 90, 347–355. [Google Scholar] [CrossRef]
- Van Meerbeek, K.; Appels, L.; Dewil, R.; Calmeyn, A.; Lemmens, P.; Muys, B.; Hermy, M. Biomass of invasive plant species as a potential feedstock for bioenergy production. Biofuels Bioprod. Biorefining 2015, 9, 273–282. [Google Scholar] [CrossRef]
- Linder, C.R. Adaptive Evolution of Seed Oils in Plants: Accounting for the Biogeographic Distribution of Saturated and Unsaturated Fatty Acids in Seed Oils. Am. Nat. 2000, 156, 442–458. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.L.B.; Capellini, M.C.; Aracava, K.K.; Rodrigues, C.E.C. Corn germ-bran oils extracted with alcoholic solvents: Extraction yield, oil composition and evaluation of protein solubility of defatted meal. Food Bioprod. Proc. 2000, 100, 185–194. [Google Scholar] [CrossRef]
- Szewczyk, K.; Zidorn, C.; Biernasiuk, A.; Komsta, Ł.; Granica, S. Polyphenols from Impatiens (Balsaminaceae) and their antioxidant and antimicrobial activities. Ind. Crops Prod. 2016, 86, 262–272. [Google Scholar] [CrossRef]
- Boinik, V.V.; Akritidu, K.P.; Demeshko, O.V. Phenolic compounds from roots of Lupinus polyphyllus. Chem. Nat. Compd. 2015, 51, 352. [Google Scholar] [CrossRef][Green Version]
- Uysal, A.; Ozer, O.Y.; Zengin, G.; Stefanucci, A.; Mollica, A.; Picot-Allain, C.M.N.; Mahomoodally, M.F. Multifunctional approaches to provide potential pharmacophores for the pharmacy shelf: Heracleum sphondylium L. subsp. Ternatum (Velen.) Brummitt. Comp. Biol. Chem. 2019, 78, 64–73. [Google Scholar] [CrossRef]
- Rysiak, A.; Dresler, S.; Hanaka, A.; Hawrylak-Nowak, B.; Strzemski, M.; Kováčik, J.; Sowa, I.; Latalski, M.; Wójciak, M. High Temperature Alters Secondary Metabolites and Photosynthetic Efficiency in Heracleum sosnowskyi. Int. J. Mol. Sci. 2021, 22, 4756. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Dinparast, L.; Zengin, G. The Genus Heracleum: A Comprehensive Review on Its Phytochemistry, Pharmacology, and Ethnobotanical Values as a Useful Herb. Comprehen. Rev. Food Sci. Food Saf. 2016, 15, 1018–1039. [Google Scholar] [CrossRef] [PubMed]
- Weryszko-Chmielewska, E.; Chwil, M. Localisation of furanocoumarins in the tissues and on the surface of shoots of Heracleum Sosnowskyi. Bot. 2017, 95, 1057–1070. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y.; Padilla-Zakour, O.; Yang, G. Polyphenols, antioxidant and antimicrobial activities of leaf and bark extracts of Solidago canadensis L. Ind. Crops Prod. 2015, 74, 803–809. [Google Scholar] [CrossRef]
- Radusiene, J.; Marska, M.; Ivanauskas, L.; Jakstas, V.; Karpaviciene, B. Assessment of phenolic compound accumulation in two widespread goldenrods. Ind. Crops Prod. 2015, 63, 158–166. [Google Scholar] [CrossRef]
- Dogan, M.; Saygideger, S.D.; Colak, U. Effect of Lead Toxicity on Aquatic Macrophyte Elodea canadensis Michx. Bull Environ. Contam Toxicol 2009, 83, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Mues, R. Species Specific Flavone Glucuronides in Elodea Species. Biochem. Syst. Ecol. 1989, 11, 261–265. [Google Scholar] [CrossRef]
- Erhard, D.; Gross, E.M. Allelopathic activity of Elodea canadensis and Elodea nuttallii against epiphytes and phytoplankton. Aq. Bot. 2006, 85, 203–211. [Google Scholar] [CrossRef]
- Ielciu, I.; Hanganu, D.; Păltinean, R.; Vlase, L.; Frédérich, M.; Gheldiu, A.M.; Benedec, D.; Crişan, G. Antioxidant capacity and polyphenolic content of the Echinocystis lobata (Michx.) Torr. Et A.Gray flowers. Pak. J. Pharm. Sci. 2018, 31, 677–683. [Google Scholar]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and plant allelopathy. Molecules 2010, 7, 8933–8952. [Google Scholar] [CrossRef]
- Vinogradova, Y.; Shelepova, O.; Vergun, O.; Grygorieva, O.; Brindza, J. Phenolic content and antioxidant activity of Echinocystis lobata (Mich.) Torr. ET Gray (Cucurbitaceae). Potravin. Slovak J. Food Sci. 2021, 15, 784–791. [Google Scholar] [CrossRef]
- Cucu, A.A.; Baci, G.M.; Cucu, A.B.; Dezsi, S.; Lujerdean, C.; Hegedus, I.C.; Bobis, O.; Moise, A.R.; Dezmirean, D.S. Calluna vulgaris as a Valuable Source of Bioactive Compounds: Exploring Its Phytochemical Profile, Biological Activities and Apitherapeutic Potential. Plants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Kato-N, H.; Kato, M. Allelopathy and Allelochemicals of Solidago canadensis L. and S. altissima L. for Their Naturalization. Plants 2022, 11, 3235. [Google Scholar] [CrossRef] [PubMed]
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol antioxidant quantity and quality in foods: Fruits. J. Agric. Food Chem. 2001, 49, 5315–5321. [Google Scholar] [CrossRef] [PubMed]
- Bernal, F.A.; Coy-Barrera, E. Composition and Antifungal Activity of the Alkaloidal Fraction of Lupinus mirabilis Leaves: A Biochemometrics-Based Exploration. Molecules 2022, 27, 2832. [Google Scholar] [CrossRef]
- Saito, S.; Okamato, Y.; Kawabata, J. Effects of Alcoholic Solvents on Antiradical Abilities of Protocatechuic Acid and Its Alkyl Esters. Biosci. Biotechnol. Biochem. 2004, 68, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Attard, E. A rapid icrotiter plate Folin-Ciocalteu method for the assessment of polyphenols. Open Life Sci. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, Y.; Chen, J.; Xiao, P.; Bao, J. Nondestructive prediction of total phenolics, flavonoid contents, and antioxidant capacity of rice grain using near-infrared spectroscopy. J. Agric. Food Chem. 2008, 56, 8268–8272. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Granados-Guzmán, G.; Salazar-Aranda, R.; Garza-Tapia, M.; Castro-Ríos, R.; Waksman de Torres, N. Optimization, and validation of two high-throughput methods indicating antiradical activity. Curr. Anal. Chem. 2017, 13, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Klavins, L.; Perkons, I.; Mezulis, M.; Viksna, A.; Klavins, M. Procyanidins from cranberry press residues—Extraction optimization, purification, and characterization. Plants 2022, 11, 3517. [Google Scholar] [CrossRef]
- Khursheed, A.; Ahmad, S.; Saleem, M.; Khan, K.U.R.; Khan, J.; Orhan, I.E.; Khurshid, U. Phytochemical profiling, in vitro biological activity, docking studies, and cytotoxicity assessments of Rondeletia odorata Jacquin: An unexplored plant of the coffee family. Front. Chem. 2022, 10, 1017577. [Google Scholar] [CrossRef]
- Cely-Veloza, W.; Quiroga, D.; Coy-Barrera, E. Quinolizidine-based variations and antifungal activity of eight Lupinus species grown under greenhouse conditions. Molecules 2022, 27, 305. [Google Scholar] [CrossRef]

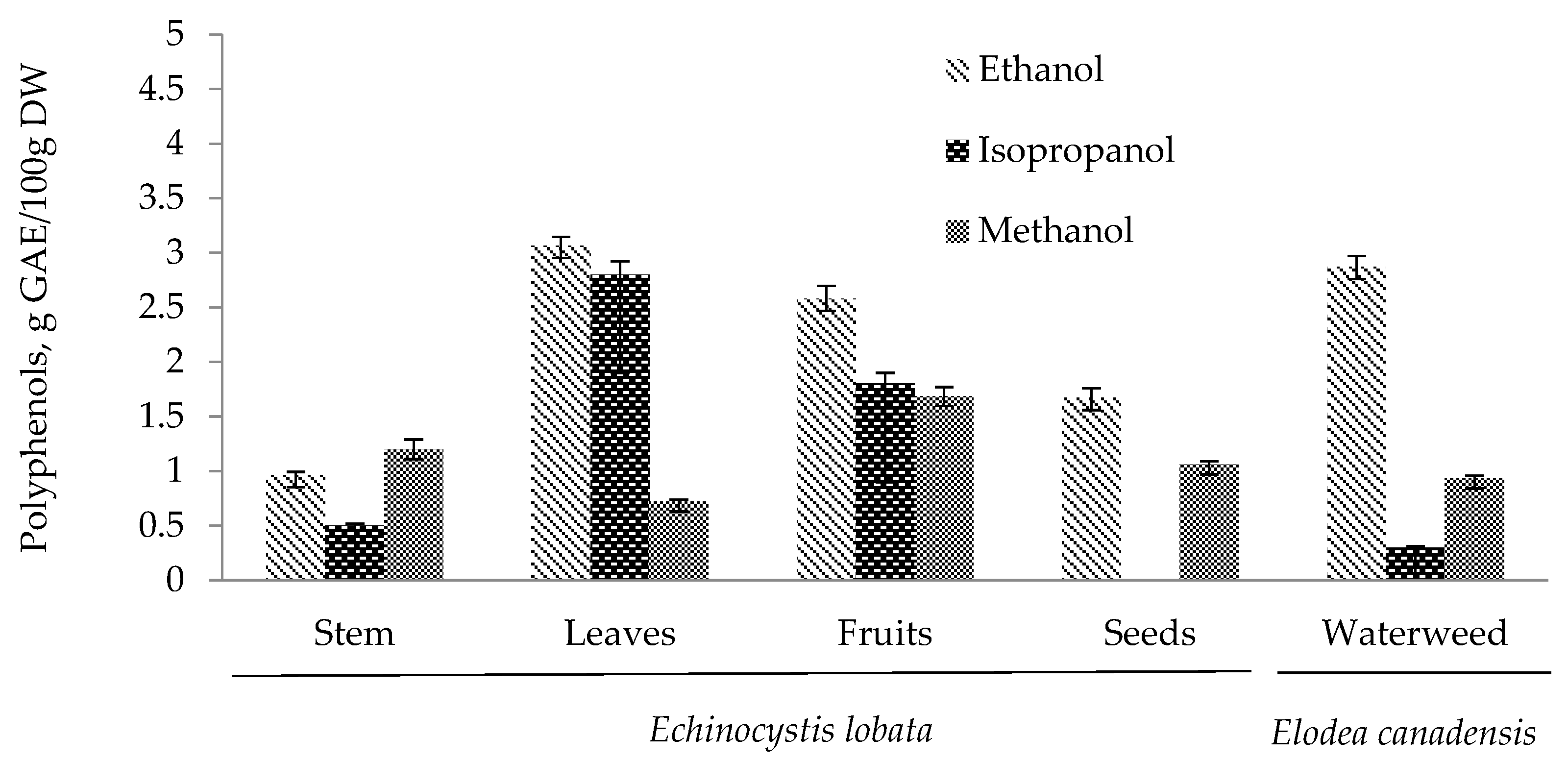
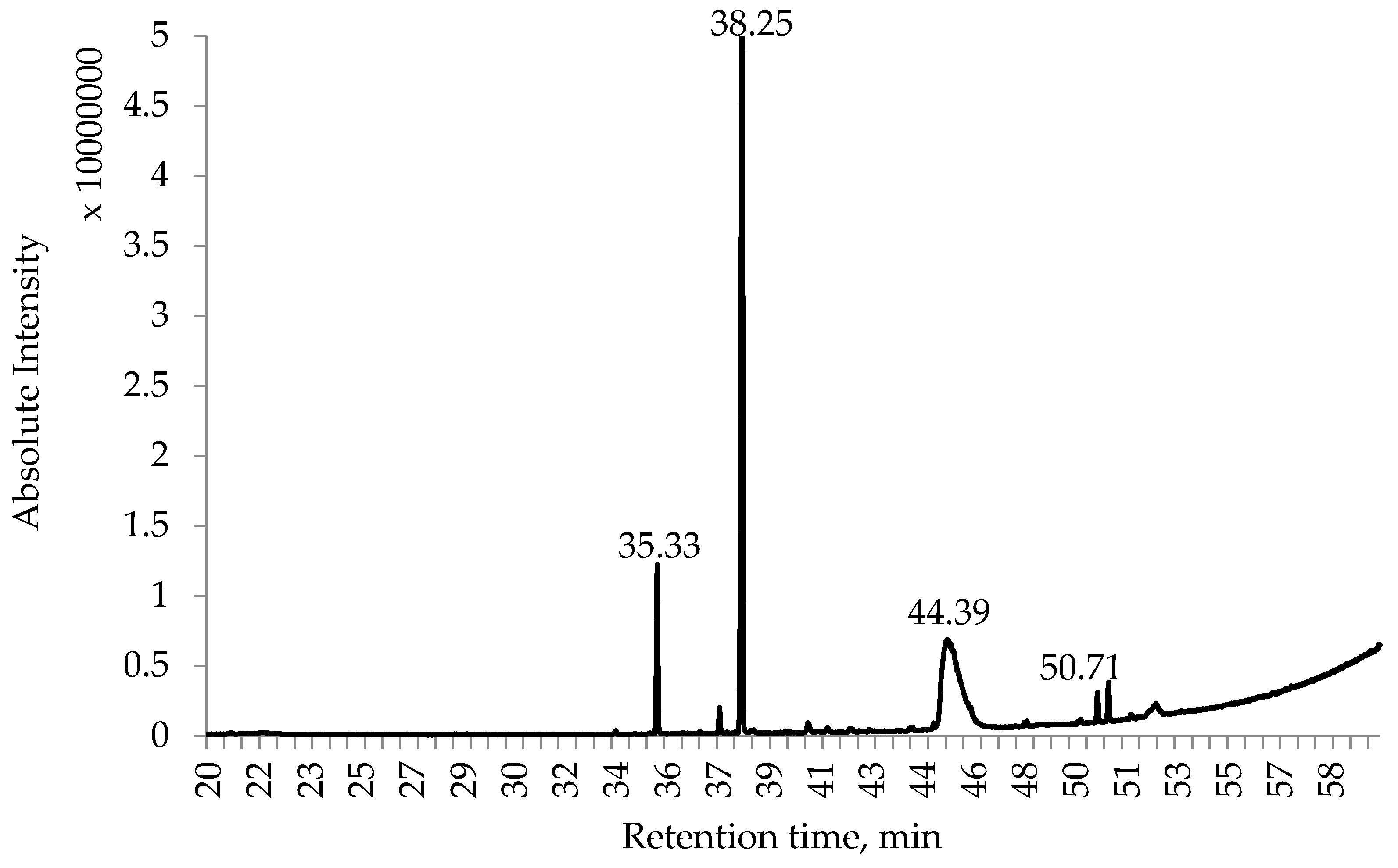
| Plants and Their Parts | MeOH Extract, DW, % | EtOH Extract, DW, % | iPrOH Extract, DW, % | |
|---|---|---|---|---|
| Impatiens glandulifera | Leaves | 13.26 | 8.19 | 8.77 |
| Stem | 14.22 | 18.50 | 1.20 | |
| Root | 10.92 | 6.65 | 0.83 | |
| Seeds | 14.35 | 8.16 | 13.21 | |
| Flowers | 10.78 | 11.57 | 6.87 | |
| Lupinus polyphyllus | Leaves | 14.87 | 17.46 | 6.79 |
| Stem | 15.24 | 9.87 | 12.91 | |
| Root | 9.38 | 14.83 | 1.39 | |
| Seeds | 14.41 | 12.58 | 3.02 | |
| Flowers | 17.71 | 10.90 | 1.26 | |
| Pod | 6.64 | 9.36 | 0.66 | |
| Echinocystis lobata | Leaves | 12.15 | 13.85 | 3.05 |
| Stem | 9.88 | 16.12 | 1.29 | |
| Fruit | 15.53 | 11.11 | 2.29 | |
| Seeds | 12.76 | 8.69 | 16.56 | |
| Heracleum sosnowskyi | Leaves | 15.02 | 14.18 | 2.23 |
| Stem | 14.48 | 14.98 | 1.83 | |
| Root | 15.07 | 14.47 | 3.13 | |
| Flowers | 16.63 | 10.29 | 2.86 | |
| Seeds | 11.69 | 17.39 | 23.41 | |
| Elodea canadensis | Whole plant | 3.95 | 16.26 | 0.69 |
| Solidago canadensis | Leaves | 11.00 | 15.69 | 2.23 |
| Stem | 6.20 | 11.35 | 1.86 | |
| Root | 6.83 | 7.02 | 2.18 | |
| Flowers | 16.52 | 17.54 | 1.93 | |
| Plants and Their Parts | Total Flavonoids, g QEQ/100 g DW | |||
|---|---|---|---|---|
| MeOH | EtOH | iPrOH | ||
| Impatiens glandulifera | Leaves | 1.53 ± 0.06 | 0.48 ± 0.05 | 0.45 ± 0.04 |
| Stem | 0.50 ± 0.05 | 0.44 ± 0.05 | <LOD | |
| Root | 0.53 ± 0.05 | 0.42 ± 0.05 | <LOD | |
| Seeds | <LOD | <LOD | <LOD | |
| Flowers | 2.28 ± 0.06 | 1.57 ± 0.03 | 0.81 ± 0.06 | |
| Lupinus polyphyllus | Leaves | 1.04 ± 0.02 | 2.19 ± 0.11 | 1.78 ± 0.06 |
| Stem | 0.45 ± 0.02 | 0.54 ± 0.02 | <LOD | |
| Root | 0.93 ± 0.03 | 0.63 ± 0.01 | <LOD | |
| Seeds | <LOD | <LOD | <LOD | |
| Flowers | 2.49 ± 0.07 | 2.43 ± 0.01 | 0.46 ± 0.02 | |
| Pod | 1.37 ± 0.04 | 1.54 ± 0.03 | <LOD | |
| Echinocystis lobata | Leaves | 0.66 ± 0.02 | 1.94 ± 0.07 | 0.59 ± 0.03 |
| Stem | 0.62 ± 0.02 | 0.70 ± 0.01 | <LOD | |
| Fruit | 0.37 ± 0.02 | 0.73 ± 0.03 | 0.41 ± 0.02 | |
| Seeds | <LOD | <LOD | <LOD | |
| Heracleum sosnowskyi | Leaves | 1.24 ± 0.02 | 2.28 ± 0.02 | 0.88 ± 0.02 |
| Stem | 0.44 ± 0.03 | 0.53 ± 0.01 | 0.38 ± 0.03 | |
| Root | 0.61 ± 0.03 | 0.47 ± 0.03 | 0.55 ± 0.04 | |
| Flowers | 1.86 ± 0.02 | 2.90 ± 0.05 | 2.41 ± 0.05 | |
| Seeds | <LOD | <LOD | <LOD | |
| Elodea canadensis | Whole plant | 0.39 ± 0.02 | 0.65 ± 0.03 | <LOD |
| Solidago canadensis | Leaves | 0.72 ± 0.05 | 0.53 ± 0.05 | 0.50 ± 0.05 |
| Stem | <LOD | <LOD | <LOD | |
| Root | 0.41 ± 0.04 | 0.38 ± 0.04 | 0.40 ± 0.04 | |
| Flowers | 1.13 ± 0.03 | 1.15 ± 0.03 | 0.52 ± 0.05 | |
| Part of Plant | Solvent | Lupinus polyphyllus | Impatiens glandulifera | Heracleum sosnowskyi | Echinocystis lobata | Solidago canadensis | Elodea canadensis |
|---|---|---|---|---|---|---|---|
| Stem | EtOH | 36.60 ± 0.51 | 257.03 ± 2.41 | 49.8 ± 2.38 | 22.97 ± 0.11 | 37.14 ± 0.39 | |
| iPrOH | 72.39 ± 1.18 | 279.12 ± 2.98 | 15.41 ± 0.66 | 13.52 ± 0.16 | 38.41 ± 0.55 | ||
| MeOH | 45.66 ± 0.67 | 258.04 ± 2.20 | 41.63 ± 1.33 | 36.81 ± 0.15 | 39.36 ± 0.47 | ||
| Root | EtOH | 75.37 ± 1.49 | 180.49 ± 2.55 | 151.78 ± 1.61 | 68.50 ± 0.47 | ||
| iPrOH | 61.63 ± 0.56 | 220.74 ± 3.09 | 183.46 ± 2.60 | 45.68 ± 0.56 | |||
| MeOH | 45.55 ± 0.55 | 172.11 ± 3.30 | 154.46 ± 2.04 | 54.39 ± 0.39 | |||
| Leaves | EtOH | 79.98 ± 0.73 | 128.75 ± 1.52 | 139.19 ± 2.40 | 69.6 ± 0.37 | 178.22 ± 0.92 | 31.90 ± 0.37 |
| iPrOH | 66.85 ± 1.39 | 130.31 ± 0.94 | 85.04 ± 4.46 | 66.19 ± 0.14 | 106.28 ± 0.93 | <LOD | |
| MeOH | 105.3 ± 1.26 | 129.6 ± 0.96 | 114.37 ± 9.46 | 56.38 ± 0.28 | 133.18 ± 0.76 | 11.09 ± 0.15 | |
| Flowers | EtOH | 102.02 ± 0.77 | 335.85 ± 2.11 | 276.33 ± 2.42 | 307.22 ± 1.88 | ||
| iPrOH | 73.56 ± 1.54 | 348.28 ± 2.38 | 245.44 ± 2.13 | 178.71 ± 1.67 | |||
| MeOH | 120.06 ± 1.52 | 343.06 ± 2.24 | 260.6 ± 2.06 | 283.18 ± 1.54 | |||
| Seeds | EtOH | 20.9 ± 0.16 | 34.4 ± 0.63 | 67.8 ± 0.78 | 29.80 ± 0.38 | ||
| iPrOH | 25.63 ± 0.39 | 30.00 ± 0.30 | 45.41 ± 0.56 | <LOD | |||
| MeOH | 31.72 ± 0.48 | 45.21 ± 0.61 | 64.63 ± 0.63 | 16.63 ± 0.33 | |||
| Pod | EtOH | 69.93 ± 0.73 | Fruit | 74.87 ± 0.64 | |||
| iPrOH | 34.93 ± 0.56 | 66.20 ± 0.97 | |||||
| MeOH | 74.93 ± 0.68 | 46.39 ± 0.82 |
| Part of Plant | Solvent | Lupinus polyphyllus | Impatiens glandulifera | Heracleum sosnowskyi | Echinocystis lobata | Solidago canadensis | Elodea canadensis |
|---|---|---|---|---|---|---|---|
| Stem | EtOH | 0.649 ± 0.02 | 0.661 ± 0.03 | 0.408 ± 0.02 | 0.530 ± 0.02 | 0.665 ± 0.03 | |
| iPrOH | 0.546 ± 0.02 | 0.381 ± 0.07 | 0.283 ± 0.02 | 0.324 ± 0.02 | 0.379 ± 0.02 | ||
| MeOH | 0.620 ± 0.01 | 0.608 ± 0.08 | 0.439 ± 0.02 | 0.592 ± 0.03 | 0.422 ± 0.01 | ||
| Root | EtOH | 0.505 ± 0.03 | 0.778 ± 0.03 | 0.936 ± 0.02 | 0.936 ± 0.02 | ||
| iPrOH | 0.665 ± 0.03 | 0.511 ± 0.03 | 0.746 ± 0.03 | 0.746 ± 0.03 | |||
| MeOH | 0.437 ± 0.01 | 0.684 ± 0.03 | 0.902 ± 0.01 | 0.902 ± 0.01 | |||
| Leaves | EtOH | 0.631 ± 0.02 | 0.722 ± 0.02 | 0.851 ± 0.05 | 0.748 ± 0.03 | 0.776 ± 0.02 | 0.332 ± 0.03 |
| iPrOH | 0.572 ± 0.02 | 0.729 ± 0.02 | 0.697 ± 0.03 | 0.849 ± 0.02 | 0.748 ± 0.07 | <LOD | |
| MeOH | 0.668 ± 0.03 | 0.728 ± 0.06 | 0.603 ± 0.05 | 0.777 ± 0.02 | 0.722 ± 0.01 | 0.913 ± 0.07 | |
| Flowers | EtOH | 0.923 ± 0.02 | 0.886 ± 0.05 | 1.521 ± 0.06 | 1.646 ± 0.04 | ||
| iPrOH | 0.498 ± 0.02 | 0.727 ± 0.06 | 1.119 ± 0.04 | 1.234 ± 0.08 | |||
| MeOH | 0.816 ± 0.03 | 1.066 ± 0.12 | 1.289 ± 0.07 | 0.906 ± 0.03 | |||
| Seeds | EtOH | 0.340 ± 0.02 | 0.641 ± 0.02 | 0.689 ± 0.02 | 0.449 ± 0.02 | ||
| iPrOH | 0.281 ± 0.01 | 0.147 ± 0.01 | 0.295 ± 0.01 | <LOD | |||
| MeOH | 0.386 ± 0.02 | 0.724 ± 0.01 | 0.628 ± 0.03 | 0.412 ± 0.03 | |||
| Pod | EtOH | 0.558 ± 0.03 | Fruit | 1.035 ± 0.03 | |||
| iPrOH | 0.373 ± 0.03 | 1.045 ± 0.06 | |||||
| MeOH | 0.522 ± 0.03 | 1.060 ± 0.05 |
| Part of Plant | Solvent | Lupinus polyphyllus | Impatiens glandulifera | Heracleum sosnowskyi | Echinocystis lobata | Solidago canadensis | Elodea canadensis |
|---|---|---|---|---|---|---|---|
| Stem | EtOH | 1.619 ± 0.03 | 1.189 ± 0.08 | 0.860 ± 0.04 | 0.719 ± 0.02 | 0.855 ± 0.04 | |
| iPrOH | 1.622 ± 0.06 | 0.814 ± 0.08 | 0.436 ± 0.04 | 0.407 ± 0.02 | 0.886 ± 0.04 | ||
| MeOH | 1.631 ± 0.03 | 1.414 ± 0.19 | 0.817 ± 0.05 | 0.747 ± 0.03 | 0.865 ± 0.02 | ||
| Root | EtOH | 0.918 ± 0.02 | 0.844 ± 0.03 | 1.881 ± 0.05 | 1.501 ± 0.05 | ||
| iPrOH | 0.844 ± 0.03 | 0.756 ± 0.05 | 1.795 ± 0.05 | 1.199 ± 0.04 | |||
| MeOH | 0.937 ± 0.02 | 0.805 ± 0.05 | 1.864 ± 0.07 | 1.489 ± 0.06 | |||
| Leaves | EtOH | 1.603 ± 0.04 | 1.322 ± 0.07 | 1.093 ± 0.04 | 0.669 ± 0.02 | 0.828 ± 0.04 | 0.671 ± 0.03 |
| iPrOH | 1.147 ± 0.04 | 1.309 ± 0.08 | 0.957 ± 0.04 | 0.728 ± 0.03 | 0.970 ± 0.07 | 0.820 ± 0.03 | |
| MeOH | 1.812 ± 0.05 | 1.403 ± 0.09 | 1.065 ± 0.04 | 0.733 ± 0.01 | 0.880 ± 0.03 | 1.368 ± 0.04 | |
| Flowers | EtOH | 2.455 ± 0.05 | 1.939 ± 0.05 | 2.908 ± 0.07 | 2.919 ± 0.07 | ||
| iPrOH | 2.626 ± 0.06 | 1.657 ± 0.06 | 2.519 ± 0.06 | 2.325 ± 0.09 | |||
| MeOH | 2.424 ± 0.05 | 1.815 ± 0.06 | 2.511 ± 0.06 | 2.880 ± 0.08 | |||
| Seeds | EtOH | 0.896 ± 0.03 | 0.984 ± 0.03 | 0.885 ± 0.04 | 0.784 ± 0.03 | ||
| iPrOH | 0.812 ± 0.03 | 0.780 ± 0.07 | 0.702 ± 0.03 | <LOD | |||
| MeOH | 1.158 ± 0.03 | 0.832 ± 0.05 | 0.895 ± 0.04 | 0.689 ± 0.04 | |||
| Pod | EtOH | 0.856 ± 0.03 | Fruit | 1.541 ± 0.06 | |||
| iPrOH | 0.812 ± 0.03 | 1.704 ± 0.08 | |||||
| MeOH | 0.833 ± 0.03 | 1.233 ± 0.07 |
| Part of Plant | Solvent | Lupinus polyphyllus | Impatiens glandulifera | Heracleum sosnowskyi | Echinocystis lobata | Solidago canadensis | Elodea canadensis |
|---|---|---|---|---|---|---|---|
| Stem | EtOH | 1.898 ± 0.05 | 0.867 ± 0.04 | 1.658 ± 0.05 | 1.042 ± 0.03 | 1.354 ± 0.02 | |
| iPrOH | 1.771 ± 0.03 | 0.533 ± 0.03 | 0.954 ± 0.03 | 1.001 ± 0.07 | 1.420 ± 0.06 | ||
| MeOH | 1.846 ± 0.02 | 0.648 ± 0.02 | 1.303 ± 0.02 | 1.075 ± 0.07 | 1.265 ± 0.03 | ||
| Root | EtOH | 2.223 ± 0.02 | 0.823 ± 0.03 | 2.852 ± 0.06 | 2.802 ± 0.09 | ||
| iPrOH | 3.302 ± 0.07 | 0.629 ± 0.03 | 2.938 ± 0.12 | 3.830 ± 0.07 | |||
| MeOH | 2.209 ± 0.03 | 0.754 ± 0.03 | 2.057 ± 0.04 | 1.990 ± 0.05 | |||
| Leaves | EtOH | 2.278 ± 0.04 | 1.415 ± 0.05 | 2.878 ± 0.07 | 1.560 ± 0.04 | 1.711 ± 0.11 | 0.973 ± 0.02 |
| iPrOH | 1.132 ± 0.05 | 1.275 ± 0.05 | 1.655 ± 0.04 | 1.115 ± 0.05 | 2.855 ± 0.12 | <LOD | |
| MeOH | 2.161 ± 0.04 | 1.440 ± 0.05 | 2.609 ± 0.05 | 1.021 ± 0.06 | 1.650 ± 0.07 | 2.077 ± 0.09 | |
| Flowers | EtOH | 4.165 ± 0.04 | 1.947 ± 0.04 | 5.074 ± 0.09 | 4.361 ± 0.07 | ||
| iPrOH | 2.177 ± 0.06 | 1.776 ± 0.05 | 3.874 ± 0.06 | 3.354 ± 0.07 | |||
| MeOH | 3.909 ± 0.07 | 2.573 ± 0.06 | 4.337 ± 0.06 | 4.186 ± 0.05 | |||
| Seeds | EtOH | 1.265 ± 0.03 | 1.079 ± 0.02 | 1.818 ± 0.02 | 1.354 ± 0.07 | ||
| iPrOH | 0.884 ± 0.03 | 0.637 ± 0.05 | 1.183 ± 0.04 | <LOD | |||
| MeOH | 0.908 ± 0.02 | 0.986 ± 0.04 | 1.475 ± 0.04 | 0.891 ± 0.05 | |||
| Pod | EtOH | 1.872 ± 0.05 | Fruit | 3.368 ± 0.07 | |||
| iPrOH | 1.483 ± 0.04 | 3.031 ± 0.04 | |||||
| MeOH | 1.928 ± 0.04 | 3.999 ± 0.04 |
| Part of Plant | Flowers | Seeds | |||||
|---|---|---|---|---|---|---|---|
| RT, min | Compound/Solvent | EtOH | iPrOH | MeOH | EtOH | iPrOH | MeOH |
| 35.328 | Tetrahydrorhombifoline | 155.18 | 179.33 | 359.47 | 349.74 | 24.66 | 281.78 |
| 35.648 | Phytol | - | - | 94.38 | - | - | - |
| 37.469 | Unknown 1 | - | - | - | 128.87 | 18.01 | 74.26 |
| 38.2 | d-Lupanine | - | - | - | 1338.50 | 36.43 | 975.78 |
| 38.307 | alfa-Lupanine | 132.06 | 67.78 | 232.01 | - | - | - |
| 40.505 | Unknown 2 | - | - | - | 115.93 | - | 62.53 |
| 41.124 | 3-beta-Hydroxylupanine | 69.70 | - | - | 99.01 | - | 57.95 |
| 45.269 | Unknown 3 | - | - | - | 1608.57 | - | 996.47 |
| 47.93 | Heptcosane | 73.30 | 172.78 | 141.06 | - | 17.88 | - |
| 50.338 | Lupanine | 92.50 | 44.14 | 129.86 | 137.47 | 21.56 | 83.86 |
| 50.705 | 13-Tigloyloxylupanine | 96.59 | 43.96 | 130.58 | 152.80 | 19.76 | 88.98 |
| 51.552 | Nonacosane | 76.21 | 568.68 | 158.00 | - | - | - |
| Part of Plant | Stem | Roots | Leaves | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RT, min | Compound/Solvent | EtOH | iPrOH | MeOH | EtOH | iPrOH | MeOH | EtOH | iPrOH | MeOH |
| 26.684 | Isospartaine | - | - | - | - | - | - | - | - | 138.83 |
| 35.328 | Tetrahydrorhombifoline | 113.85 | 37.82 | 204.91 | 87.70 | 16.60 | 93.64 | 380.38 | 57.82 | 168.03 |
| 35.648 | Phytol | - | - | - | - | - | - | 230.94 | 61.11 | 159.03 |
| 38.2 | d-Lupanine | 132.07 | 50.28 | 184.73 | 182.11 | 19.26 | 111.88 | - | - | - |
| 38.307 | alfa-Lupanine | - | - | 121.62 | - | - | - | 835.52 | 77.93 | 587.82 |
| 44.386 | Hydroxylupanine | - | - | 56.29 | - | 49.59 | 185.13 | - | 145.06 | |
| 47.93 | Heptacosane | - | 38.53 | 51.31 | - | 15.28 | 38.04 | 88.67 | 397.24 | - |
| 50.338 | Lupanine | 59.23 | 26.45 | 51.29 | - | 14.64 | - | 135.06 | 37.77 | 92.08 |
| 50.705 | 13-Tigloyloxylupanine | 68.70 | 29.79 | 57.27 | 33.67 | 15.01 | 39.01 | 273.93 | 47.07 | 213.98 |
| 51.552 | Nonacosane | - | 192.32 | 70.27 | - | 15.46 | 39.45 | - | 465.39 | 120.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purmalis, O.; Klavins, L.; Niedrite, E.; Mezulis, M.; Klavins, M. Invasive Plants as a Source of Polyphenols with High Radical Scavenging Activity. Plants 2025, 14, 467. https://doi.org/10.3390/plants14030467
Purmalis O, Klavins L, Niedrite E, Mezulis M, Klavins M. Invasive Plants as a Source of Polyphenols with High Radical Scavenging Activity. Plants. 2025; 14(3):467. https://doi.org/10.3390/plants14030467
Chicago/Turabian StylePurmalis, Oskars, Linards Klavins, Evelina Niedrite, Marcis Mezulis, and Maris Klavins. 2025. "Invasive Plants as a Source of Polyphenols with High Radical Scavenging Activity" Plants 14, no. 3: 467. https://doi.org/10.3390/plants14030467
APA StylePurmalis, O., Klavins, L., Niedrite, E., Mezulis, M., & Klavins, M. (2025). Invasive Plants as a Source of Polyphenols with High Radical Scavenging Activity. Plants, 14(3), 467. https://doi.org/10.3390/plants14030467









