Soil Water Deficit Reduced Root Hydraulic Conductivity of Common Reed (Phragmites australis)
Abstract
:1. Introduction
2. Results
2.1. Root Morphology
2.2. Root Anatomy
2.3. Hydraulic Conductivity
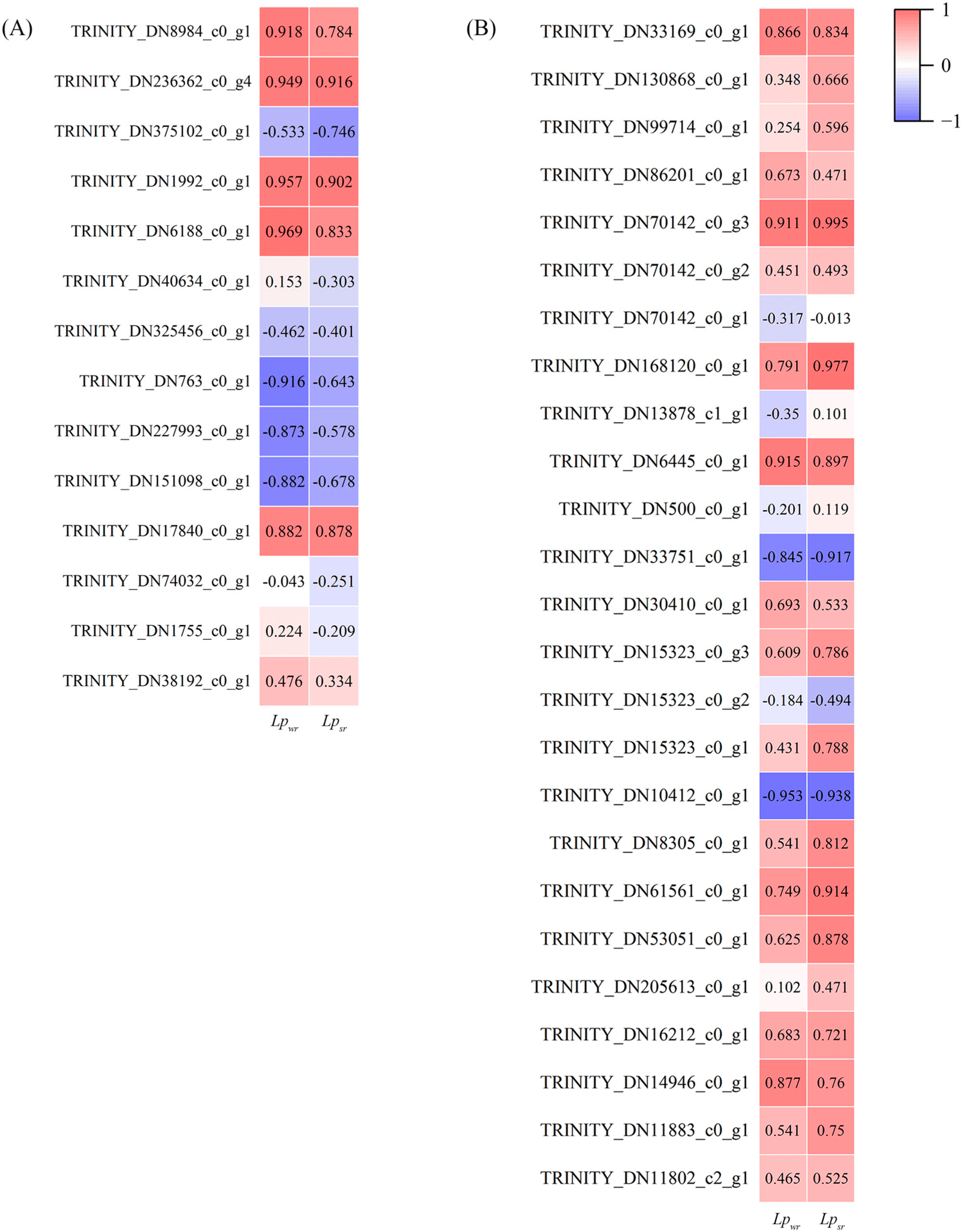
2.4. The Relationship of Lpwr with Root Morphological Features and Anatomical Characteristics
2.5. RNA-Seq Assembly and Annotation
2.6. Analysis of DEGs
2.7. The Expression Levels of ABA and AQP Genes and Their Relationship with Lpr
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Measurement of Root Morphological Traits
4.3. Measurement of Root Anatomy
4.4. Hydraulic Conductivity of Roots
4.5. Transcriptome Sequencing, De Novo Assembly, and Functional Annotation
4.6. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| NCED | 9-cis-epoxycarotenoid dioxygenase |
| AAO3 | abscisic-aldehyde oxidase 3 |
| AQPs | aquaporins |
| PIPs | plasma membrane intrinsic proteins |
| TIPs | tonoplast intrinsic proteins |
| SIPs | small basic intrinsic proteins |
| NIPs | nodulin 26-like intrinsic proteins |
| FC | field capacity |
| FAA | formalin aceto-alcohol |
| Lpr | root hydraulic conductivity |
| Lpwr | whole-root hydraulic conductivity |
| Lpsr | single-root hydraulic conductivity |
| RD | root diameter |
| CT | cortex thickness |
| VD | vessel diameter |
| RCA | root cross-sectional area |
| DEGs | differentially expressed genes |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
References
- Sun, X.-S.; Chen, Y.-H.; Zhuo, N.; Cui, Y.; Luo, F.-L.; Zhang, M.-X. Effects of salinity and concomitant species on growth of Phragmites australis populations at different levels of genetic diversity. Sci. Total Environ. 2021, 780, 146516. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Yu, J.; Hou, A.; Han, G.; Wang, G.; Qu, F.; Xia, J.; Wang, X. The ecological adaptability of Phragmites australis to interactive effects of water level and salt stress in the Yellow River Delta. Aquat. Ecol. 2016, 51, 107–116. [Google Scholar] [CrossRef]
- Ren, J.; Chen, J.; Xu, C.; van de Koppel, J.; Thomsen, M.S.; Qiu, S.; Cheng, F.; Song, W.; Liu, Q.-X.; Xu, C.; et al. An invasive species erodes the performance of coastal wetland protected areas. Sci. Adv. 2021, 7, eabi8943. [Google Scholar] [CrossRef]
- Ummenhofer, C.C.; Meehl, G.A. Extreme weather and climate events with ecological relevance: A review. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160135. [Google Scholar] [CrossRef]
- Sarika, M.; Zikos, A. Coastal Salt Marshes: Structure and Function of Plant Communities. In Handbook of Halophytes; Grigore, M.-N., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–39. ISBN 978-3-030-17854-3. [Google Scholar]
- Han, G.; Sun, B.; Chu, X.; Xing, Q.; Song, W.; Xia, J. Precipitation events reduce soil respiration in a coastal wetland based on four-year continuous field measurements. Agric. For. Meteorol. 2018, 256–257, 292–303. [Google Scholar] [CrossRef]
- Sousa, C.A.; Cunha, M.E.; Ribeiro, L. Tracking 130 years of coastal wetland reclamation in Ria Formosa, Portugal: Opportunities for conservation and aquaculture. Land Use Policy 2020, 94, 104544. [Google Scholar] [CrossRef]
- Zhang, L.; Lan, S.; Angelini, C.; Yi, H.; Zhao, L.; Chen, L.; Han, G. Interactive effects of crab herbivory and spring drought on a Phragmites australis-dominated salt marsh in the Yellow River Delta. Sci. Total Environ. 2020, 766, 144254. [Google Scholar] [CrossRef]
- Opitz, N.; Marcon, C.; Paschold, A.; Malik, W.A.; Lithio, A.; Brandt, R.; Piepho, H.-P.; Nettleton, D.; Hochholdinger, F. Extensive tissue-specific transcriptomic plasticity in maize primary roots upon water deficit. J. Exp. Bot. 2015, 67, 1095–1107. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Liu, S.; Ferjani, A.; Li, J.; Yan, J.; Yang, X.; Qin, F. Genetic variation in ZmVPP1 contributes to drought tolerance in maize seedlings. Nat. Genet. 2016, 48, 1233–1241. [Google Scholar] [CrossRef]
- Hu, L.; Xie, Y.; Fan, S.; Wang, Z.; Wang, F.; Zhang, B.; Li, H.; Song, J.; Kong, L. Comparative analysis of root transcriptome profiles between drought-tolerant and susceptible wheat genotypes in response to water stress. Plant Sci. 2018, 272, 276–293. [Google Scholar] [CrossRef]
- Assefa, Y.; Staggenborg, S.A.; Prasad, V.P.V. Grain Sorghum Water Requirement and Responses to Drought Stress: A Review. Crop Manag. 2010, 9, 1–11. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, W.; Wang, Q.; Cao, Y.; Xu, F.; Dodd, I.C.; Xu, W. ABA regulation of root growth during soil drying and recovery can involve auxin response. Plant Cell Environ. 2021, 45, 871–883. [Google Scholar] [CrossRef]
- Maurel, C.; Nacry, P. Root architecture and hydraulics converge for acclimation to changing water availability. Nat. Plants 2020, 6, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Ahmed, M.A.; Abdalla, M.; Carminati, A. Root hydraulic phenotypes impacting water uptake in drying soils. Plant Cell Environ. 2022, 45, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Steudle, E. Water uptake by roots: Effects of water deficit. J. Exp. Bot. 2000, 51, 1531–1542. [Google Scholar] [CrossRef]
- Mu, Z.; Zhang, S.; Zhang, L.; Liang, A.; Liang, Z. Hydraulic Conductivity of Whole Root System Is Better than Hydraulic Conductivity of Single Root in Correlation with the Leaf Water Status of Maize. Bot. Stud. 2006, 47, 145–151. [Google Scholar]
- Cai, G.; Ahmed, M.A. The role of root hairs in water uptake: Recent advances and future perspectives. J. Exp. Bot. 2022, 73, 3330–3338. [Google Scholar] [CrossRef]
- Kato, Y.; Okami, M. Root morphology, hydraulic conductivity and plant water relations of high-yielding rice grown under aerobic conditions. Ann. Bot. 2011, 108, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, S.; Hazak, O. Understanding the root xylem plasticity for designing resilient crops. Plant Cell Environ. 2021, 45, 664–676. [Google Scholar] [CrossRef]
- Domec, J.-C.; King, J.S.; Carmichael, M.J.; Overby, A.T.; Wortemann, R.R.; Smith, W.K.; Miao, G.; Noormets, A.; Johnson, D.M. Aquaporins, and not changes in root structure, provide new insights into physiological responses to drought, flooding, and salinity. J. Exp. Bot. 2021, 72, 4489–4501. [Google Scholar] [CrossRef]
- Lynch, J.P. Harnessing root architecture to address global challenges. Plant J. 2021, 109, 415–431. [Google Scholar] [CrossRef]
- Lynch, J.P.; Mooney, S.J.; Strock, C.F.; Schneider, H.M. Future roots for future soils. Plant Cell Environ. 2022, 45, 620–636. [Google Scholar] [CrossRef]
- Knipfer, T.; Fricke, W. Water uptake by seminal and adventitious roots in relation to whole-plant water flow in barley (Hordeum vulgare L.). J. Exp. Bot. 2010, 62, 717–733. [Google Scholar] [CrossRef]
- Wasson, A.P.; Richards, R.A.; Chatrath, R.; Misra, S.C.; Prasad, S.V.S.; Rebetzke, G.J.; Kirkegaard, J.A.; Christopher, J.; Watt, M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 2012, 63, 3485–3498. [Google Scholar] [CrossRef] [PubMed]
- Eshel, A.; Beeckman, T. Plant Roots: The Hidden Half, 4th ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-1-4398-4649-0. [Google Scholar]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Zwieniecki, M.A.; Thompson, M.V.; Holbrook, N.M. Understanding the Hydraulics of Porous Pipes: Tradeoffs Between Water Uptake and Root Length Utilization. J. Plant Growth Regul. 2002, 21, 315–323. [Google Scholar] [CrossRef]
- Hernandez-Espinoza, L.H.; Barrios-Masias, F.H. Physiological and anatomical changes in tomato roots in response to low water stress. Sci. Hortic. 2020, 265, 109208. [Google Scholar] [CrossRef]
- Parent, B.; Hachez, C.; Redondo, E.; Simonneau, T.; Chaumont, F.; Tardieu, F. Drought and Abscisic Acid Effects on Aquaporin Content Translate into Changes in Hydraulic Conductivity and Leaf Growth Rate: A Trans-Scale Approach. Plant Physiol. 2009, 149, 2000–2012. [Google Scholar] [CrossRef]
- Kumar, S.; Shah, S.H.; Vimala, Y.; Jatav, H.S.; Ahmad, P.; Chen, Y.; Siddique, K.H.M. Abscisic acid: Metabolism, transport, crosstalk with other plant growth regulators, and its role in heavy metal stress mitigation. Front. Plant Sci. 2022, 13, 972856. [Google Scholar] [CrossRef]
- Rosales, M.A.; Maurel, C.; Nacry, P. Abscisic Acid Coordinates Dose-Dependent Developmental and Hydraulic Responses of Roots to Water Deficit. Plant Physiol. 2019, 180, 2198–2211. [Google Scholar] [CrossRef]
- Jackson, M.B.; Davies, W.J.; Else, M.A. Pressure–Flow Relationships, Xylem Solutes and Root Hydraulic Conductance in Flooded Tomato Plants. Ann. Bot. 1996, 77, 17–24. [Google Scholar] [CrossRef]
- Wilkinson, S.; Davies, W.J. ABA-based chemical signalling: The co-ordination of responses to stress in plants. Plant Cell Environ. 2002, 25, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Törnroth-Horsefield, S.; Wang, Y.; Hedfalk, K.; Johanson, U.; Karlsson, M.; Tajkhorshid, E.; Neutze, R.; Kjellbom, P. Structural mechanism of plant aquaporin gating. Nature 2005, 439, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Pagter, M.; Bragato, C.; Brix, H. Tolerance and physiological responses of Phragmites australis to water deficit. Aquat. Bot. 2005, 81, 285–299. [Google Scholar] [CrossRef]
- Fang, Y.; Du, Y.; Wang, J.; Wu, A.; Qiao, S.; Xu, B.; Zhang, S.; Siddique, K.H.M.; Chen, Y. Moderate Drought Stress Affected Root Growth and Grain Yield in Old, Modern and Newly Released Cultivars of Winter Wheat. Front. Plant Sci. 2017, 8, 672. [Google Scholar] [CrossRef]
- Khan, F.; Feng, Y.; Palta, J.A.; Chen, Y.; Sadras, V.O.; Siddique, K.H.M. Selection for yield over five decades favored anisohydric and phenological adaptations to early-season drought in Australian wheat. Plant Soil 2022, 476, 511–526. [Google Scholar] [CrossRef]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef]
- Lynch, J.P. Root phenotypes for improved nutrient capture: An underexploited opportunity for global agriculture. New Phytol. 2019, 223, 548–564. [Google Scholar] [CrossRef]
- Enstone, D.E.; Peterson, C.A.; Ma, F. Root Endodermis and Exodermis: Structure, Function, and Responses to the Environment. J. Plant Growth Regul. 2002, 21, 335–351. [Google Scholar] [CrossRef]
- Clark, L.J.; Whalley, W.R.; Barraclough, P.B. How Do Roots Penetrate Strong Soil? In Roots: The Dynamic Interface between Plants and the Earth: The 6th Symposium of the International Society of Root Research, 11–15 November 2001, Nagoya, Japan; Abe, J., Ed.; Developments in Plant and Soil Sciences; Springer: Dordrecht, The Netherlands, 2003; pp. 93–104. ISBN 978-94-017-2923-9. [Google Scholar]
- Strock, C.F.; Lynch, J.P. Root secondary growth: An unexplored component of soil resource acquisition. Ann. Bot. 2020, 126, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, M.; Eastham, J.; Gregory, P.J.; Turner, N.C. A comparison of plant hydraulic conductances in wheat and lupins. J. Exp. Bot. 1996, 47, 233–239. [Google Scholar] [CrossRef]
- Rehschuh, R.; Cecilia, A.; Zuber, M.; Faragó, T.; Baumbach, T.; Hartmann, H.; Jansen, S.; Mayr, S.; Ruehr, N.K. Drought-Induced Xylem Embolism Limits the Recovery of Leaf Gas Exchange in Scots Pine. Plant Physiol. 2020, 184, 852–864. [Google Scholar] [CrossRef]
- Evans, D.E. Aerenchyma formation. New Phytol. 2003, 161, 35–49. [Google Scholar] [CrossRef]
- Zhu, J.; Brown, K.M.; Lynch, J.P. Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant Cell Environ. 2010, 33, 740–749. [Google Scholar] [CrossRef]
- Henry, A.; Cal, A.J.; Batoto, T.C.; Torres, R.O.; Serraj, R. Root attributes affecting water uptake of rice (Oryza sativa) under drought. J. Exp. Bot. 2012, 63, 4751–4763. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.; Zhong, J.; Zhang, T.; Li, D.; Ba, T.; Xu, T.; Chang, L.; Zhang, Q.; Sun, M. Root Physiological Traits and Transcriptome Analyses Reveal that Root Zone Water Retention Confers Drought Tolerance to Opisthopappus taihangensis. Sci. Rep. 2020, 10, 2627. [Google Scholar] [CrossRef]
- Doussan, C.; Pierret, A.; Garrigues, E.; Pagès, L. Water Uptake by Plant Roots: II—Modelling of Water Transfer in the Soil Root-system with Explicit Account of Flow within the Root System—Comparison with Experiments. Plant Soil 2006, 283, 99–117. [Google Scholar] [CrossRef]
- Vandeleur, R.K.; Mayo, G.; Shelden, M.C.; Gilliham, M.; Kaiser, B.N.; Tyerman, S.D. The Role of Plasma Membrane Intrinsic Protein Aquaporins in Water Transport through Roots: Diurnal and Drought Stress Responses Reveal Different Strategies between Isohydric and Anisohydric Cultivars of Grapevine. Plant Physiol. 2009, 149, 445–460. [Google Scholar] [CrossRef]
- Vadez, V. Root hydraulics: The forgotten side of roots in drought adaptation. Field Crop. Res. 2014, 165, 15–24. [Google Scholar] [CrossRef]
- Rowse, H.R.; Goodman, D. Axial Resistance to Water Movement in Broad Bean (Vicia faba) Roots. J. Exp. Bot. 1981, 32, 591–598. [Google Scholar] [CrossRef]
- Schneider, H.M.; Wojciechowski, T.; Postma, J.A.; Brown, K.M.; Lücke, A.; Zeisler, V.; Schreiber, L.; Lynch, J.P. Root cortical senescence decreases root respiration, nutrient content and radial water and nutrient transport in barley. Plant Cell Environ. 2017, 40, 1392–1408. [Google Scholar] [CrossRef]
- Rieger, M.; Litvin, P. Root system hydraulic conductivity in species with contrasting root anatomy. J. Exp. Bot. 1999, 50, 201–209. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, L.; Ren, Y.; Zhang, T.; Zhang, S.; Li, H.; Chen, Y.; Zhang, S. The Higher Water Absorption Capacity of Small Root System Improved the Yield and Water Use Efficiency of Maize. Plants 2022, 11, 2300. [Google Scholar] [CrossRef]
- Hachez, C.; Moshelion, M.; Zelazny, E.; Cavez, D.; Chaumont, F. Localization and Quantification of Plasma Membrane Aquaporin Expression in Maize Primary Root: A Clue to Understanding their Role as Cellular Plumbers. Plant Mol. Biol. 2006, 62, 305–323. [Google Scholar] [CrossRef]
- Kim, Y.X.; Ranathunge, K.; Lee, S.; Lee, Y.; Lee, D.; Sung, J. Composite Transport Model and Water and Solute Transport across Plant Roots: An Update. Front. Plant Sci. 2018, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Kreszies, T.; Schreiber, L.; Ranathunge, K. Suberized transport barriers in Arabidopsis, barley and rice roots: From the model plant to crop species. J. Plant Physiol. 2018, 227, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Pantin, F.; Monnet, F.; Jannaud, D.; Costa, J.M.; Renaud, J.; Muller, B.; Simonneau, T.; Genty, B. The dual effect of abscisic acid on stomata. New Phytol. 2012, 197, 65–72. [Google Scholar] [CrossRef]
- Yao, Y.; Dong, C.-H.; Yi, Y.; Li, X.; Zhang, X.; Liu, J. Regulatory function of AMP1 in ABA biosynthesis and drought resistance in arabidopsis. J. Plant Biol. 2014, 57, 117–126. [Google Scholar] [CrossRef]
- Aroca, R.; Ferrante, A.; Vernieri, P.; Chrispeels, M.J. Drought, Abscisic Acid and Transpiration Rate Effects on the Regulation of PIP Aquaporin Gene Expression and Abundance in Phaseolus vulgaris Plants. Ann. Bot. 2006, 98, 1301–1310. [Google Scholar] [CrossRef]
- Zupin, M.; Sedlar, A.; Kidrič, M.; Meglič, V. Drought-induced expression of aquaporin genes in leaves of two common bean cultivars differing in tolerance to drought stress. J. Plant Res. 2017, 130, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.-L.; Yu, X.; Lane, D.; Sun, W.-N.; Tang, Z.-C.; Su, W.-A. Upland rice and lowland rice exhibited different PIP expression under water deficit and ABA treatment. Cell Res. 2006, 16, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Fricke, W. Changes in root hydraulic conductivity facilitate the overall hydraulic response of rice (Oryza sativa L.) cultivars to salt and osmotic stress. Plant Physiol. Biochem. 2017, 113, 64–77. [Google Scholar] [CrossRef]
- Siefritz, F.; Tyree, M.T.; Lovisolo, C.; Schubert, A.; Kaldenhoff, R. PIP1 Plasma Membrane Aquaporins in Tobacco: From Cellular Effects to Function in Plants. Plant Cell 2002, 14, 869–876. [Google Scholar] [CrossRef]
- Martre, P.; Porter, J.R.; Jamieson, P.D.; Triboï, E. Modeling Grain Nitrogen Accumulation and Protein Composition to Understand the Sink/Source Regulations of Nitrogen Remobilization for Wheat. Plant Physiol. 2003, 133, 1959–1967. [Google Scholar] [CrossRef]
- Ranathunge, K.; Schreiber, L. Water and solute permeabilities of Arabidopsis roots in relation to the amount and composition of aliphatic suberin. J. Exp. Bot. 2011, 62, 1961–1974. [Google Scholar] [CrossRef]
- López-Bucio, J.; Cruz-Ramírez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Miller, D.M. Studies of root function in Zea mays I. Apparatus and methods. Can. J. Bot. 1980, 58, 351–360. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Hattori, M.; Aoki-Kinoshita, K.F.; Itoh, M.; Kawashima, S.; Katayama, T.; Araki, M.; Hirakawa, M. From genomics to chemical genomics: New developments in KEGG. Nucleic Acids Res. 2006, 34, D354–D357. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the Unification of Biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
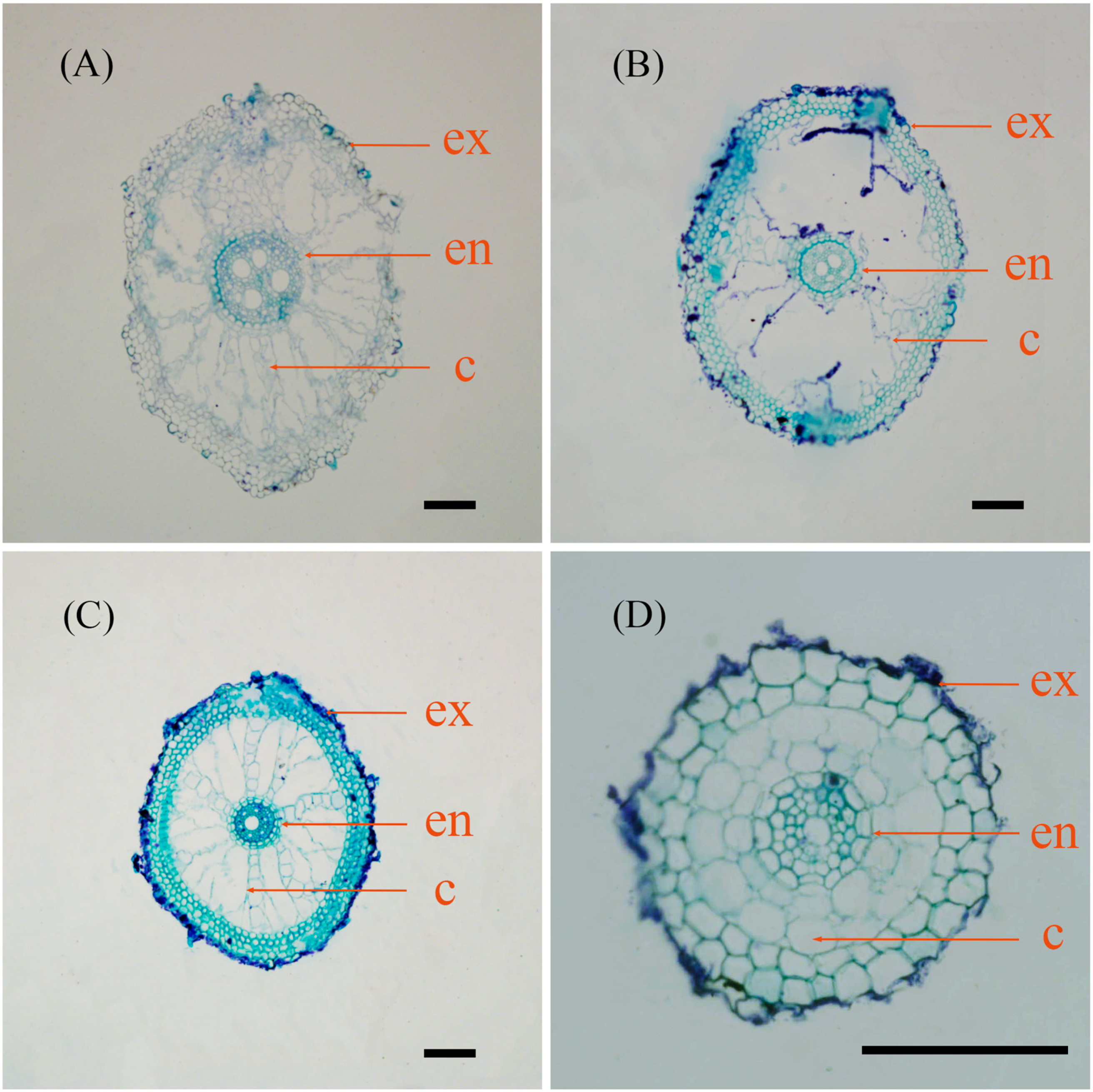


| Treatment | Total Root Length (cm) | Surface Area (cm2) | Volume (cm3) | Average Diameter (μm) |
|---|---|---|---|---|
| FC | 415.13 ± 59.56 a | 125.24 ± 33.58 a | 2.65 ± 0.91 a | 1042.57 ± 219.89 a |
| 80% | 285.83 ± 83.04 ab | 65.15 ± 21.12 b | 1.67 ± 0.71 ab | 970.37 ± 212.8 a |
| 60% | 212.77 ± 49.58 b | 53.48 ± 13.68 b | 0.85 ± 0.29 b | 837.3 ± 166.38 a |
| 40% | 203.89 ± 56.57 b | 38.14 ± 8.79 b | 0.57 ± 0.12 b | 627.45 ± 88.39 a |
| Treatment | RD (μm) | CT (μm) | VD (μm) | RCA (mm2) |
|---|---|---|---|---|
| FC | 808.49 ± 20.61 a | 298.15 ± 8.03 a | 54.82 ± 2.24 a | 0.42 ± 0.03 a |
| 80% | 601.51 ± 23.62 b | 235.94 ± 14.22 b | 28.64 ± 0.94 c | 0.24 ± 0.02 b |
| 60% | 580.91 ± 35.66 bc | 238.28 ± 16.8 b | 29.66 ± 2.22 c | 0.19 ± 0.02 bc |
| 40% | 516.1 ± 13.67 c | 178.84 ± 6.21 c | 44 ± 2.59 b | 0.14 ± 0.01 c |
| Root Hydraulics | Soil Moisture Contents | Lpr (10−7 m∙s−1∙MPa−1) |
|---|---|---|
| Lpwr | FC | 1.64 ± 0.26 a |
| 80% | 0.83 ± 0.71 ab | |
| 60% | 0.23 ± 0.08 b | |
| 40% | 0.19 ± 0.09 b | |
| Lpsr | FC | 1.8 ± 0.51 a |
| 80% | 0.92 ± 0.38 a | |
| 60% | 0.89 ± 0.62 a | |
| 40% | 0.96 ± 0.54 a |
| Characteristics | Lpwr | Lpsr |
|---|---|---|
| Total root length | 0.949 ** | |
| Surface area | 0.971 ** | |
| Volume | 0.952 ** | |
| Average diameter | 0.866 ** | |
| Root diameter | 0.800 ** | |
| Cortex thickness | 0.755 ** | |
| Vessel diameter | 0.462 | |
| Root cross-sectional area | 0.707 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Zhang, Z.; Wang, H.; Chen, Y.; Zhang, M. Soil Water Deficit Reduced Root Hydraulic Conductivity of Common Reed (Phragmites australis). Plants 2023, 12, 3543. https://doi.org/10.3390/plants12203543
Wang R, Zhang Z, Wang H, Chen Y, Zhang M. Soil Water Deficit Reduced Root Hydraulic Conductivity of Common Reed (Phragmites australis). Plants. 2023; 12(20):3543. https://doi.org/10.3390/plants12203543
Chicago/Turabian StyleWang, Ruiqing, Zhenming Zhang, Haoyue Wang, Yinglong Chen, and Mingxiang Zhang. 2023. "Soil Water Deficit Reduced Root Hydraulic Conductivity of Common Reed (Phragmites australis)" Plants 12, no. 20: 3543. https://doi.org/10.3390/plants12203543
APA StyleWang, R., Zhang, Z., Wang, H., Chen, Y., & Zhang, M. (2023). Soil Water Deficit Reduced Root Hydraulic Conductivity of Common Reed (Phragmites australis). Plants, 12(20), 3543. https://doi.org/10.3390/plants12203543









