Phytochemical Analysis and Antioxidant Activity of Centella Asiatica Extracts: An Experimental and Theoretical Investigation of Flavonoids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Drying of C. asiatica
2.2. Preparation of Various C. asiatica Extracts
2.3. Phytochemical Studies
2.3.1. Determination of TPC and TFC
2.3.2. TLC Analysis
2.3.3. DPPH Assay
2.3.4. Ferric Reducing Antioxidant Power (FRAP) Assay
2.3.5. ABTS Assay
2.3.6. GC-MS Analysis
2.3.7. LC-MS Analysis
2.4. Computational Detail
3. Results and Discussion
3.1. Phytochemical Test Results
3.2. TLC Analysis of C. asiatica Extract
3.3. Total Polyphenol and Flavonoid Contents
3.4. Antioxidant Activity Analysis via Radical Scavenging Assay
3.5. GC-MS Analysis
3.6. LC-MS Analysis
3.7. Theoretical Analysis
3.7.1. Molecular Electrostatic Potential (MEP) Analysis
3.7.2. Frontier Molecular Orbital Analysis
3.7.3. Global Reactivity Parameters
3.7.4. BDE through H-atom Abstraction
3.7.5. ADME Analysis
3.7.6. Molecular Target Prediction Analysis
3.7.7. Molecular Docking Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.-Q.; Zhang, D.; Deng, J.; Liu, Y.; Li, W.; Nie, X. Preparation and Safety Evaluation of Centella asiatica Total Glycosides Nitric Oxide Gel and Its Therapeutic Effect on Diabetic Cutaneous Ulcers. Evid.-Based Complement. Altern. Med. 2022, 2022, 1419146. [Google Scholar] [CrossRef] [PubMed]
- Kunjumon, R.; Johnson, A.J.; Sukumaryamma Remadevi, R.K.; Baby, S. Assessment of major centelloside ratios in Centella asiatica accessions grown under identical ecological conditions, bioconversion clues and identification of elite lines. Sci. Rep. 2022, 12, 8177. [Google Scholar] [CrossRef] [PubMed]
- Delbo, M.; Calapai, G. Assessment Report on Centella asiatica (L.) Urban, Herba; European Medicines Agency Science Medicines Health; European Medicines Agency: London, UK, 2010. [Google Scholar]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic Potential of Centella asiatica and Its Triterpenes: A Review. Front. Pharmacol. 2020, 11, 568032. [Google Scholar] [CrossRef] [PubMed]
- Kunjumon, R.; Johnson, A.J.; Baby, S. Centella asiatica: Secondary metabolites, biological activities and biomass sources. Phytomed. Plus 2022, 2, 100176. [Google Scholar] [CrossRef]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J. The Role of Plants in Traditional Medicine and Current Therapy. J. Altern. Complement. Med. 1995, 1, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, P.P.; Paul, S.B. History of Indian Traditional Medicine: A Medical Inheritance. Asian J. Pharm. Clin. Res. 2018, 11, 421–426. [Google Scholar] [CrossRef]
- Agarwa, P.; Sharma, B.; Fatima, A.; Jain, S.K. An update on Ayurvedic herb Convolvulus pluricaulis Choisy. Asian Pac. J. Trop. Biomed. 2014, 4, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Diwanay, S.; Patki, P.; Patwardhan, B. Studies on immunomodulatory activity of Withania somnifera (Ashwagandha) extracts in experimental immune inflammation. J. Ethnopharmacol. 1999, 67, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.; Verma, D.K.; Thakur, M.; Chakravorty, N.; Singh, S.; Srivastav, P.P. Recent trends in extraction, identification and quantification methods of Centella asiatica phytochemicals with potential applications in food industry and therapeutic relevance: A review. Food Biosci. 2022, 49, 101864. [Google Scholar] [CrossRef]
- Buraphaka, H.; Putalun, W. Stimulation of health-promoting triterpenoids accumulation in Centella asiatica (L.) Urban leaves triggered by postharvest application of methyl jasmonate and salicylic acid elicitors. Ind. Crops Prod. 2020, 146, 112171. [Google Scholar] [CrossRef]
- Bari, M.S.; Khandokar, L.; Haque, E.; Romano, B.; Capasso, R.; Seidel, V.; Haque, M.A.; Rashid, M.A. Ethnomedicinal uses, phytochemistry, and biological activities of plants of the genus Gynura. J. Ethnopharmacol. 2021, 271, 113834. [Google Scholar] [CrossRef] [PubMed]
- Niamnuy, C.; Charoenchaitrakool, M.; Mayachiew, P.; Devahastin, S. Bioactive compounds and bioactivities of Centella asiatica (L.) Urban prepared by different drying methods and conditions. Dry. Technol. 2013, 31, 2007–2015. [Google Scholar] [CrossRef]
- Ariffin, F.; Heong Chew, S.; Bhupinder, K.; Karim, A.A.; Huda, N. Huda Antioxidant capacity and phenolic composition of fermented Centella asiatica herbal teas. J. Sci. Food Agric. 2011, 91, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Anbazhakan, K.; Sadasivam, K.; Praveena, R.; Salgado, G.; Cardona, W.; Mitnik, D.G.; Gerli, L. Theoretical assessment of antioxidant property of polyproponoid and its derivatives. Struct. Chem. 2020, 31, 1089–1094. [Google Scholar] [CrossRef]
- Dahanukar, S.; Kulkarni, R.; Rege, N. Pharmacology of medicinal plants and natural products. Indian J. Pharmacol. 2000, 32, S81–S118. [Google Scholar]
- Shen, X.; Guo, M.; Yu, H.; Liu, D.; Lu, Z.; Lu, Y. Propionibacterium acnes related anti-inflammation and skin hydration activities of madecassoside, a pentacyclic triterpene saponin from Centella asiatica. Biosci. Biotechnol. Biochem. 2019, 83, 561–568. [Google Scholar] [CrossRef]
- Anbazhakan, K.; Praveena, R.; Sadasivam, K. Prediction of biological activities of phytoestrogen and its derivative–A Insilico study. AIP Conf. Proc. 2019, 2142, 190003. [Google Scholar]
- Praveena, R.; Anbazhakan, K.; Sadasivam, K.; Nivetha, K. Density functional theory calculations for interactions of 2-bromo 9H carbazole and 2, 7-dibromo 9H carbazole with human serum albumin. Mater. Today Proc. 2021, 45, 2540–2543. [Google Scholar] [CrossRef]
- Shukla, A.; Rasik, A.M.; Jain, G.K.; Shankar, R.; Kulshrestha, D.K.; Dhawan, B.N. In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica. J. Ethnopharmacol. 1999, 65, 1–11. [Google Scholar] [CrossRef]
- Gray, N.E.; Magana, A.A.; Lak, P.; Wright, K.M.; Quinn, J.; Stevens, J.F.; Maier, C.S.; Soumyanath, A. Centella asiatica: Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem. Rev. 2018, 17, 161–194. [Google Scholar] [CrossRef] [PubMed]
- Ondeko, D.; Juma, B.; Baraza, D.; Nyongesa, P. LC-ESI/MS and GC-MS Methanol Extract Analysis, Phytochemical and Antimicrobial Activity Studies of Centella asiatica. Asian J. Chem. Sci. 2020, 8, 32–51. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Tongkao-On, W.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K.; Li, G.Q. Seasonal variation of triterpenes and phenolic compounds in Australian Centella asiatica (L.) Urb. Phytochem. Anal. 2015, 26, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Antognoni, F.; Perellino, N.C.; Crippa, S.; Toso, R.D.; Danieli, B.; Minghetti, A.; Poli, F.; Pressi, G. Irbic acid, a dicaffeoylquinic acid derivative from Centella asiatica cell cultures. Fitoterapia 2011, 82, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Praveena, R.; Balasankar, A.; Aruchamy, K.; Oh, T.; Polisetti, V.; Ramasundaram, S.; Anbazhakan, K. Structural Activity and HAD Inhibition Efficiency of Pelargonidin and Its Glucoside—A Theoretical Approach. Molecules 2022, 27, 8016. [Google Scholar] [CrossRef] [PubMed]

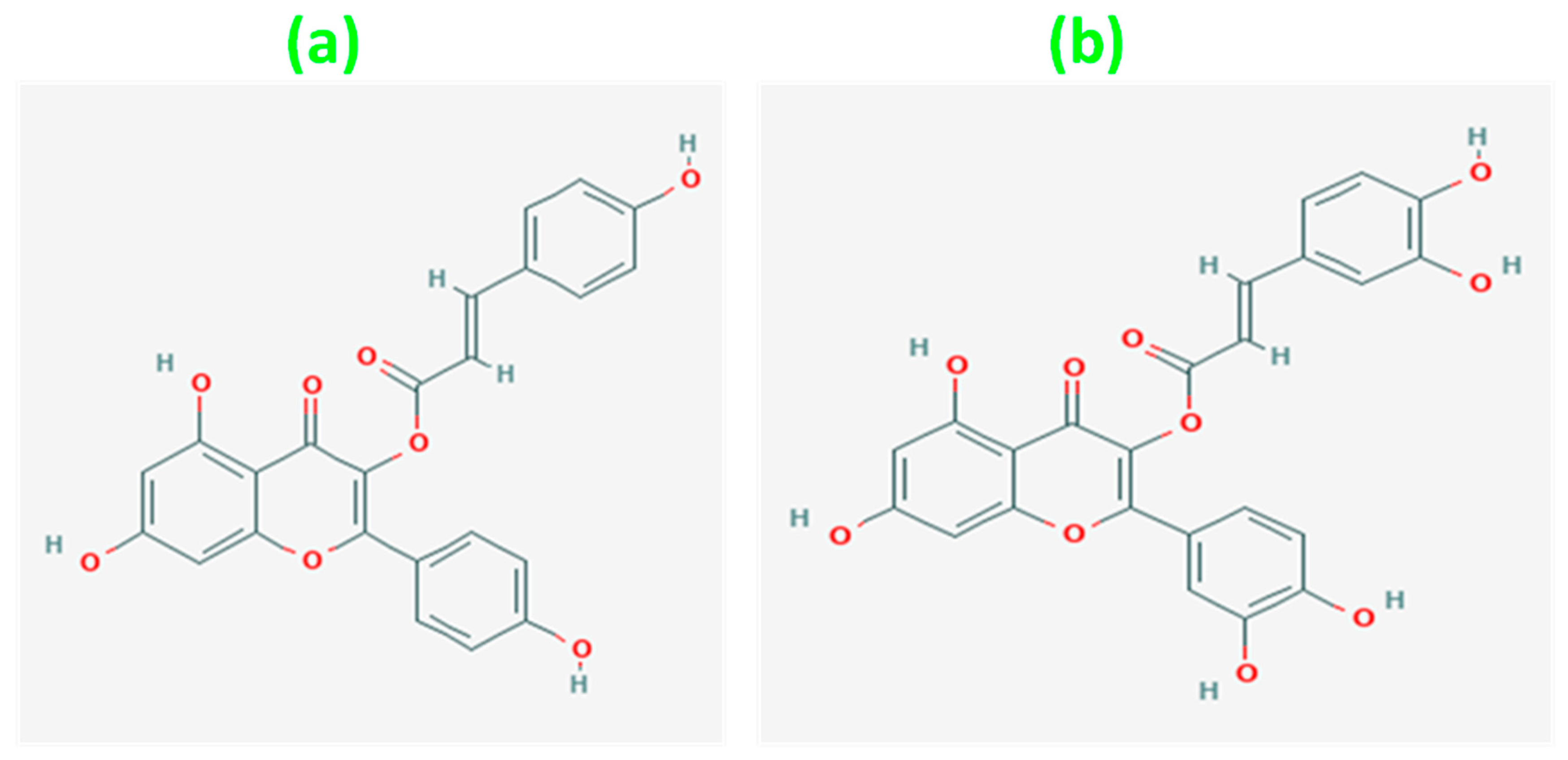


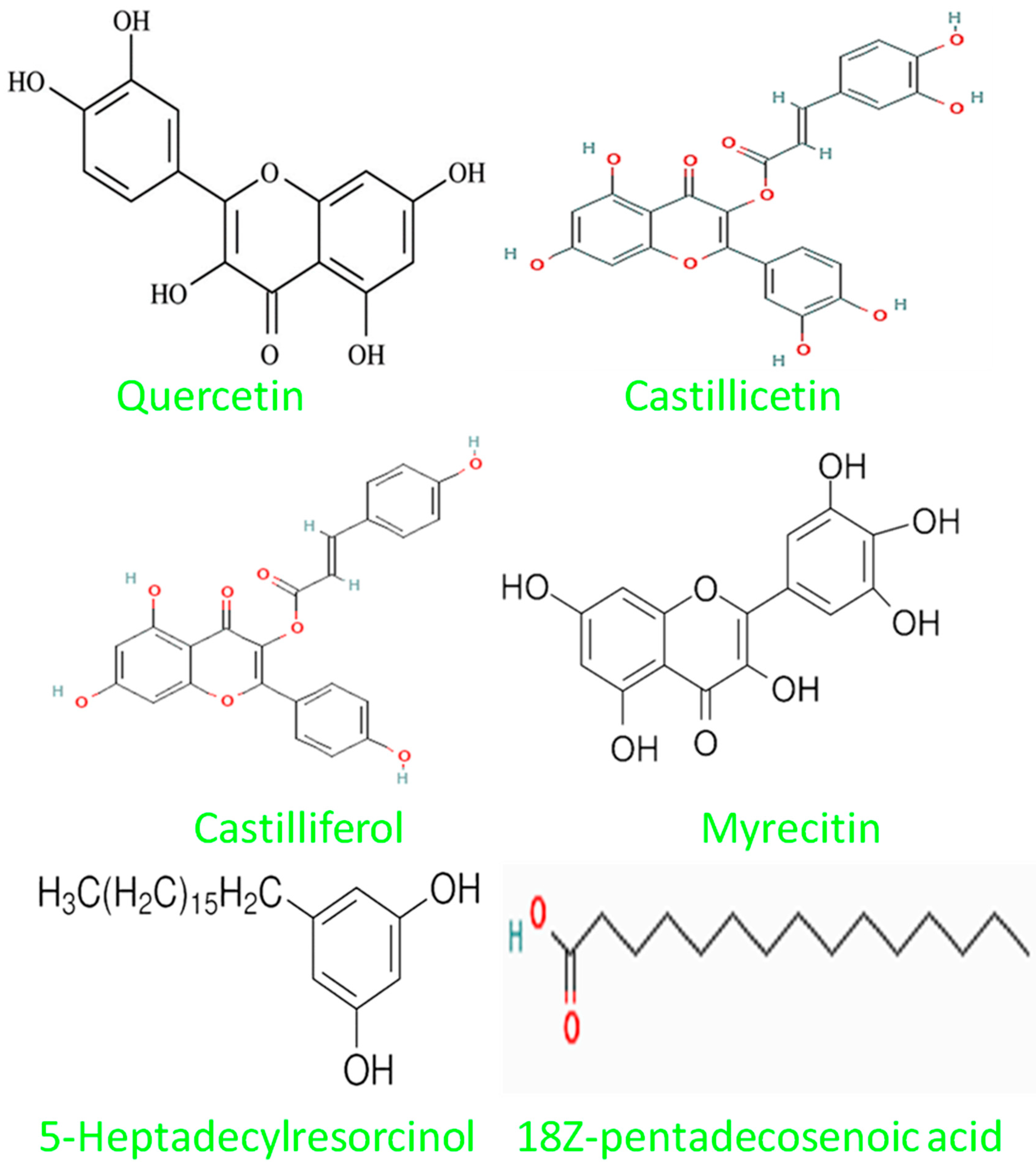
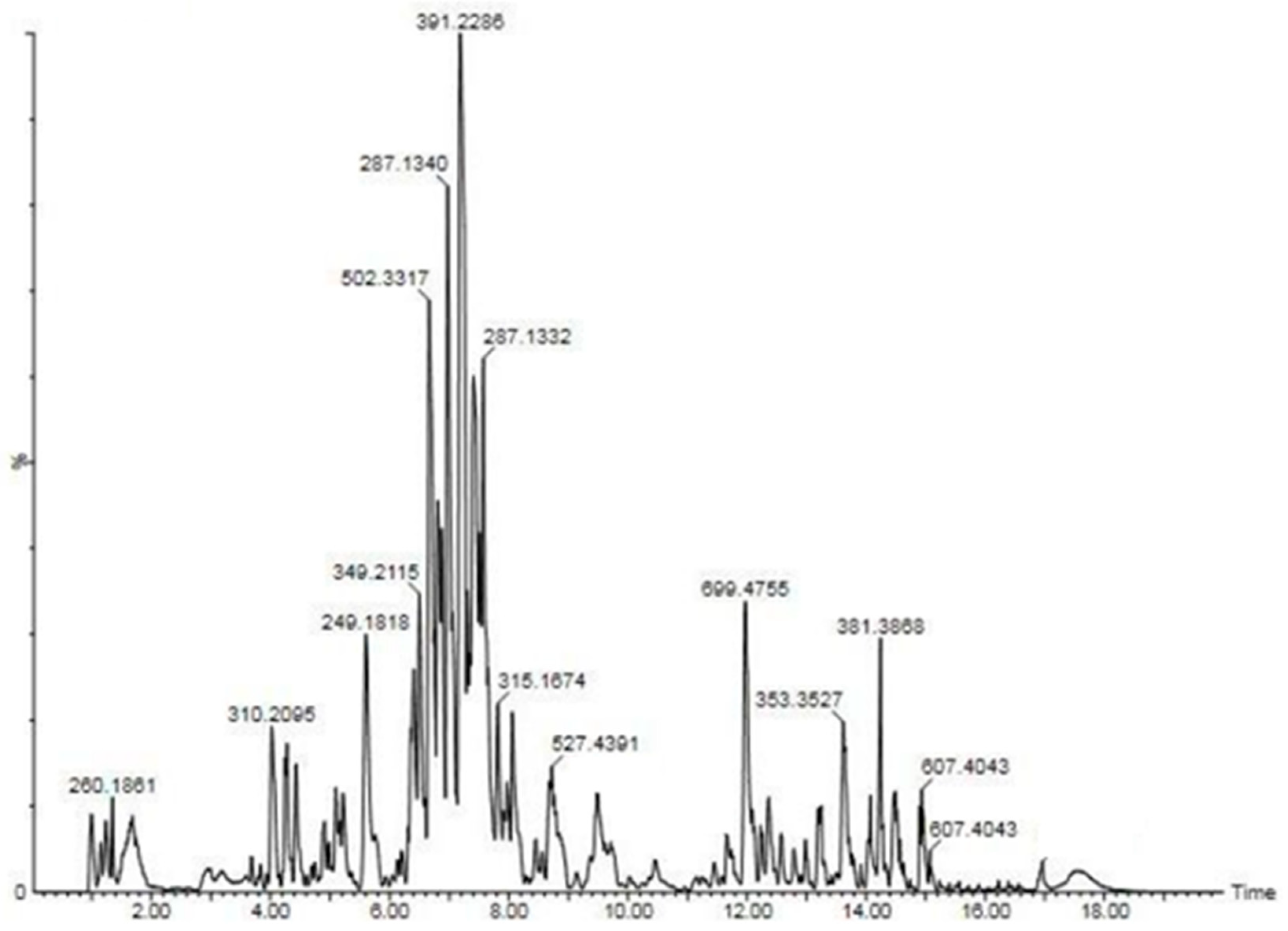




| Solvent | C. asiatica Extract |
|---|---|
| CW | 60.1 ± 1.12 |
| MeOH | 19.5 ± 1.0 |
| EtOH | 28.4 ± 0.11 |
| Extract | Test | |||
|---|---|---|---|---|
| Flavonoids | Phenolics | Tannins | Alkaloids | |
| H2O | + | + | ++ | ++ |
| MeOH | +++ | +++ | ++ | ++ |
| EtOH | +++ | +++ | + | + |
| Extract | Polyphenol (GAE mg/g) | Flavonoid (QE mg/g) |
|---|---|---|
| CW | 34.4 ± 1.1 | 7.5 ± 0.1 |
| MeOH | 132.6 ± 8.4 | 15.1 ± 1.4 |
| EtOH | 106.2 ± 7.5 | 14.8 ± 0.3 |
| Extract (AEAC mg/g) | Radical Scavenging Activity (IC50) | FRAP (AE mg/g) | |
|---|---|---|---|
| ABTS | DPPH | ||
| CW | 46.5 ± 0.8 | 21.9 ± 1.1 | 34.1 ± 0.6 |
| MeOH | 65.4 ± 1.2 | 40.7 ± 1.2 | 55.7 ± 0.9 |
| EtOH | 66.6 ± 0.3 | 37.2 ± 1.2 | 56.3 ± 1.2 |
| S. No | Compound | TIC tR(min) | M + H(M/Z) | CID Product ions (M/Z) |
|---|---|---|---|---|
| Alkaloid | ||||
| 1 | Dioncopeltine A | 1.15 | 395 | 274,122 |
| 2 | Dipyridamole | 4.258 | 514 | 265,186,142 |
| Anthocyanins | ||||
| 3 | Delphinidin 3,5-O-diglucoside | 5.223 | 623 | 409,307 |
| 4 | Delphinidin 3-O-glucosyl-glucoside | 5.242 | 620 | 413,307 |
| 5 | Pelargonidin-3-O-(6”-malonyl-glucoside) | 12.412 | 531 | 375,353,243 |
| Flavonoid Glycosides | ||||
| 6 | Isorhamnetin 3-O-rutinoside | 7.122 | 471 | 287,145 |
| 7 | Kaempferol 7-O-glucoside | 5.162 | 448 | 402,307 |
| 8 | Dihydroquercetin 3-O-rhamnoside | 7.427 | 448 | 333,145 |
| 9 | Isorhamnetin 3-O-glucuronide | 14.64 | 505 | 441,163 |
| Coumarin | ||||
| 10 | Coumestrol | 4.615 | 269 | 163 |
| Polyaromatic | ||||
| 11 | 6,13-Dihexyl-2,3,9,10-termethylpentacene- 1,4,8,11-tetrone | 4.921 | 563 | 411,307,209 |
| Lignans | ||||
| 12 | Todolactol A | 4.422 | 377 | 163,145 |
| Phenolic Acid | ||||
| 13 | Dihydrocaffeic acid | 1.002 | 186 | 139 |
| 14 | Cinnamoyl glucose | 4.025 | 311 | 292,166 |
| Phytosterols | ||||
| 15 | Stigmasterol | 14.18 | 425 | 413, |
| Lipids | ||||
| 16 | Delta-carotene-1,2-epoxide | 21.68 | 563 | 453,317,203 |
| Stilbenes | ||||
| 17 | Resveratol 3-O-glucoside | 7.28 | 396 | 309,189,171 |
| Triterpene Glycoside | ||||
| 18 | Ziziphin | 8.115 | 985 | 453,291,154 |
| Xanthones | ||||
| 19 | 8-Desoxygartatin | 1.54 | 385 | 248,203,182 |
| Molecular Descriptors | Eₒ (eV) of Castilliferol | Eₒ(eV) of Castillicetin |
|---|---|---|
| IP (eV) | 6.013 | 5.947 |
| EA(eV) | 1.953 | 1.996 |
| ω(eV) | 2.030 | 1.975 |
| S(eV) | 0.246 | 0.253 |
| X(eV) | 3.983 | 3.972 |
| ɳ(eV) | 3.908 | 3.993 |
| -OH Sites | Castilliferol (k cal/mol) | Castillicetin (k cal/mol) |
|---|---|---|
| 3’OH | - | 41.85 |
| 4’OH | 37.23 | 40.34 |
| 5OH | 58.54 | 74.21 |
| 7OH | 44.28 | 53.26 |
| 7OH(cin) | - | 46.24 |
| 8OH(cin) | 41.24 | 47.38 |
| (a) | |||||
| Molecule | TPSA | iLogP | XLogP3 | WLogP | MLogP |
| Castilliferol | 137.43 | 2.67 | 4.36 | 3.79 | 1.17 |
| Castillicetin | 177.89 | 1.83 | 3.65 | 3.2 | 0.16 |
| (b) | |||||
| Molecule | CYP1A2 Inhibitor | CYP2C19 Inhibitor | CYP2C9 Inhibitor | CYP2D6 Inhibitor | CYP3A4 Inhibitor |
| Castilliferol | No | No | Yes | No | No |
| Castillicetin | Yes | No | Yes | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandasamy, A.; Aruchamy, K.; Rangasamy, P.; Varadhaiyan, D.; Gowri, C.; Oh, T.H.; Ramasundaram, S.; Athinarayanan, B. Phytochemical Analysis and Antioxidant Activity of Centella Asiatica Extracts: An Experimental and Theoretical Investigation of Flavonoids. Plants 2023, 12, 3547. https://doi.org/10.3390/plants12203547
Kandasamy A, Aruchamy K, Rangasamy P, Varadhaiyan D, Gowri C, Oh TH, Ramasundaram S, Athinarayanan B. Phytochemical Analysis and Antioxidant Activity of Centella Asiatica Extracts: An Experimental and Theoretical Investigation of Flavonoids. Plants. 2023; 12(20):3547. https://doi.org/10.3390/plants12203547
Chicago/Turabian StyleKandasamy, Anbazhakan, Kanakaraj Aruchamy, Praveena Rangasamy, Deepha Varadhaiyan, Chandrasekar Gowri, Tae Hwan Oh, Subramaniyan Ramasundaram, and Balasankar Athinarayanan. 2023. "Phytochemical Analysis and Antioxidant Activity of Centella Asiatica Extracts: An Experimental and Theoretical Investigation of Flavonoids" Plants 12, no. 20: 3547. https://doi.org/10.3390/plants12203547
APA StyleKandasamy, A., Aruchamy, K., Rangasamy, P., Varadhaiyan, D., Gowri, C., Oh, T. H., Ramasundaram, S., & Athinarayanan, B. (2023). Phytochemical Analysis and Antioxidant Activity of Centella Asiatica Extracts: An Experimental and Theoretical Investigation of Flavonoids. Plants, 12(20), 3547. https://doi.org/10.3390/plants12203547








