Eugenol: In Vitro and In Ovo Assessment to Explore Cytotoxic Effects on Osteosarcoma and Oropharyngeal Cancer Cells
Abstract
:1. Introduction
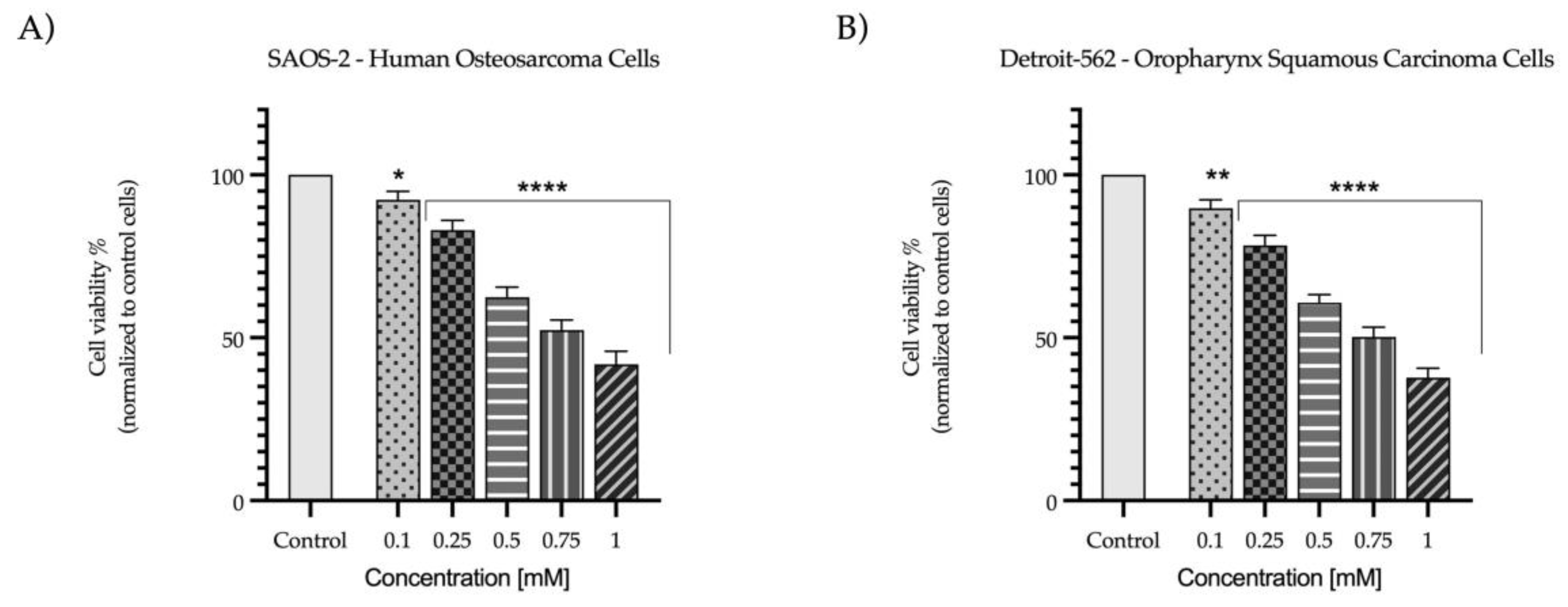
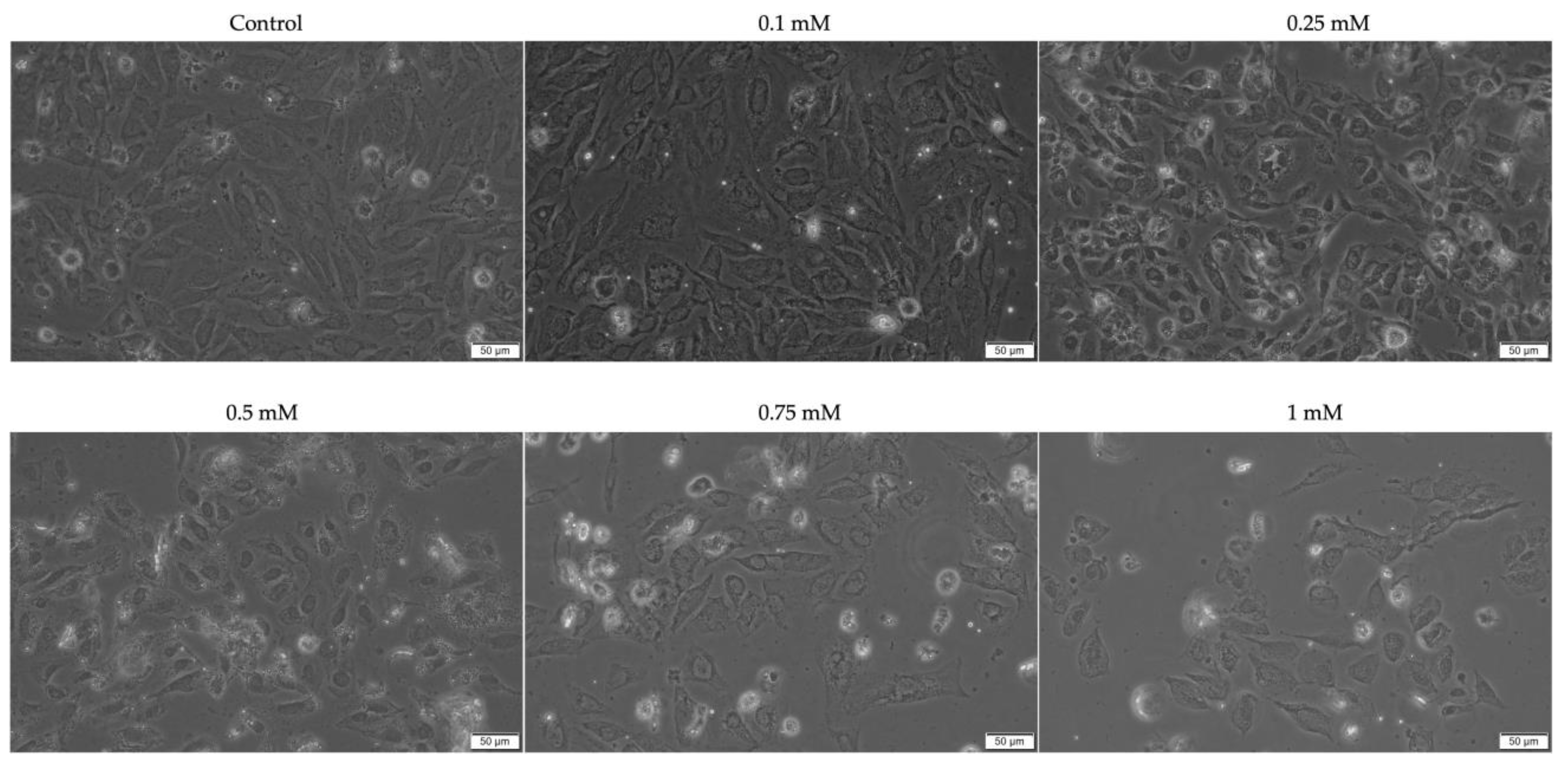
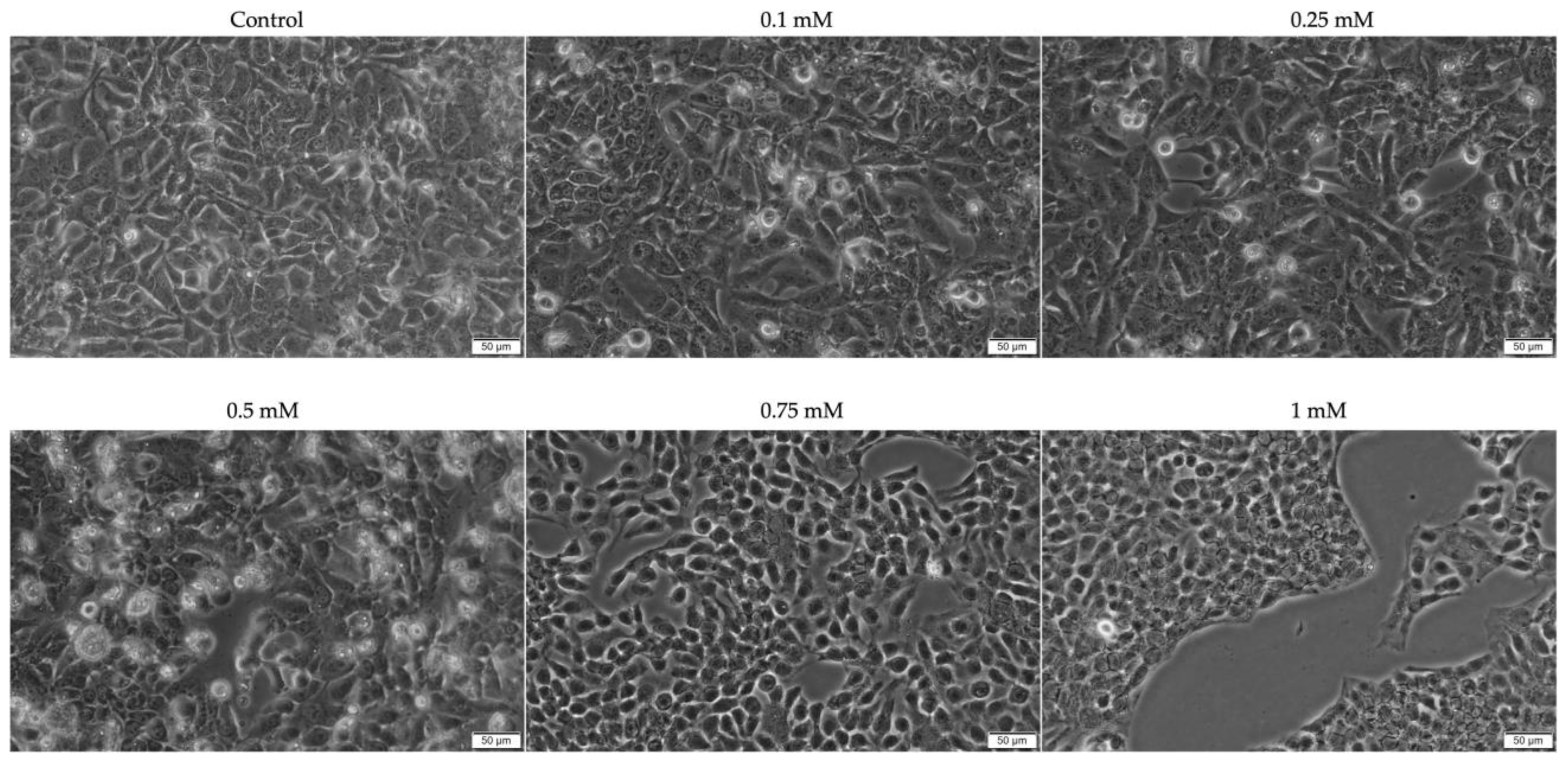
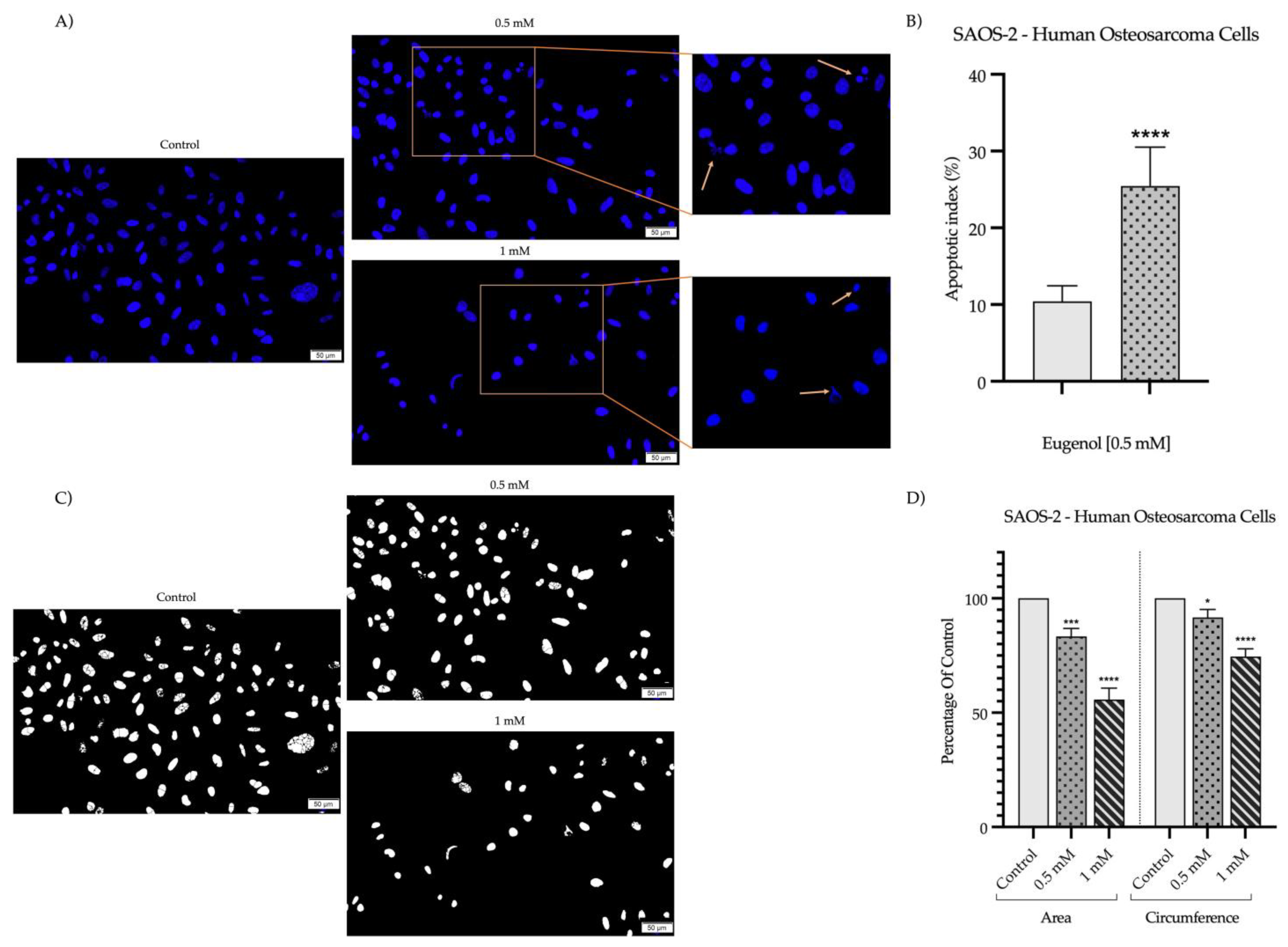
2. Results
2.1. Evaluation of the Cytotoxic Profile
2.2. Detection and Quantification of Nuclear Morphology
2.3. Real Time PCR Study and Caspases Activity
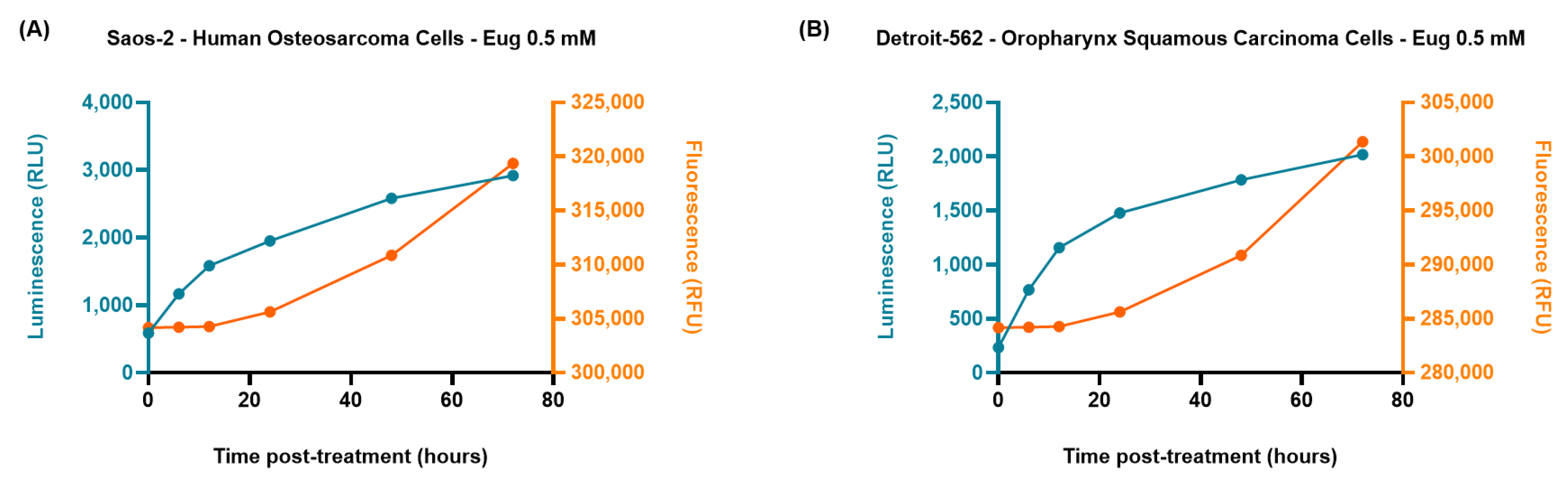
2.4. The RealTime-Glo™ Annexin V Apoptosis and Necrosis Assay
2.5. Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM)
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Cellular Viability Evaluation
4.4. Cellular Morphology Evaluation
4.5. Nuclear Morphology Evaluation
4.6. Gene Expression Ratio
4.7. Caspase-3/7, -8, and -9 Activity
4.8. The RealTime-Glo™ Annexin V Apoptosis and Necrosis Assay
4.9. Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM)
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef]
- Tobeiha, M.; Rajabi, A.; Raisi, A.; Mohajeri, M.; Yazdi, S.M.; Davoodvandi, A.; Aslanbeigi, F.; Vaziri, M.S.; Hamblin, M.R.; Mirzaei, H. Potential of Natural Products in Osteosarcoma Treatment: Focus on Molecular Mechanisms. Biomed. Pharmacother. 2021, 144, 112257. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, Y.; Gupta, V.K.; Jaitak, V. Anticancer Activity of Essential Oils: A Review. J. Sci. Food Agric. 2013, 93, 3643–3653. [Google Scholar] [CrossRef] [PubMed]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. Evid.-Based Complement. Altern. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef] [PubMed]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An Overview on the Anti-Inflammatory Potential and Antioxidant Profile of Eugenol. Oxid. Med. Cell Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef]
- Park, S.H.; Sim, Y.B.; Lee, J.K.; Kim, S.M.; Kang, Y.J.; Jung, J.S.; Suh, H.W. The Analgesic Effects and Mechanisms of Orally Administered Eugenol. Arch. Pharm. Res. 2011, 34, 501–507. [Google Scholar] [CrossRef]
- Benencia, F.; Courrèges, M.C. In Vitro and in Vivo Activity of Eugenol on Human Herpesvirus. Phytother. Res. 2000, 14, 495–500. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant Activity of Eugenol: A Structure–Activity Relationship Study. J. Med. Food. 2011, 14, 975–985. [Google Scholar] [CrossRef]
- Zari, A.T.; Zari, T.A.; Hakeem, K.R. Anticancer Properties of Eugenol: A Review. Molecules 2021, 26, 7407. [Google Scholar] [CrossRef]
- Khalil, A.A.; Rahman, U.U.; Khan, M.R.; Sahar, A.; Mehmood, T.; Khan, M. Essential Oil Eugenol: Sources, Extraction Techniques and Nutraceutical Perspectives. RSC Adv. 2017, 7, 32669–32681. [Google Scholar] [CrossRef]
- Fadilah, F.; Yanuar, A.; Arsianti, A.; Andrajati, R. Phenylpropanoids, Eugenol Scaffold, and Its Derivatives as Anticancer. Asian J. Pharm. Clin. Res. 2017, 10, 41–46. [Google Scholar] [CrossRef]
- Begum, S.N.; Ray, A.S.; Rahaman, C.H. A Comprehensive and Systematic Review on Potential Anticancer Activities of Eugenol: From Pre-Clinical Evidence to Molecular Mechanisms of Action. Phytomedicine 2022, 107, 154456. [Google Scholar] [CrossRef] [PubMed]
- Beird, H.C.; Bielack, S.S.; Flanagan, A.M.; Gill, J.; Heymann, D.; Janeway, K.A.; Livingston, J.A.; Roberts, R.D.; Strauss, S.J.; Gorlick, R. Osteosarcoma. Nat. Rev. Dis. Primers 2022, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. International Osteosarcoma Incidence Patterns in Children and Adolescents, Middle Ages, and Elderly Persons. Int. J. Cancer 2009, 125, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Walsh, M.F.; Wu, G.; Edmonson, M.N.; Gruber, T.A.; Easton, J.; Hedges, D.; Ma, X.; Zhou, X.; Yergeau, D.A.; et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N. Engl. J. Med. 2015, 373, 2336–2346. [Google Scholar] [CrossRef]
- Cundy, T. Paget’s Disease of Bone. Metabolism 2018, 80, 5–14. [Google Scholar] [CrossRef]
- Tucker, M.A.; D’Angio, G.J.; Boice, J.D.; Strong, L.C.; Li, F.P.; Stovall, M.; Stone, B.J.; Green, D.M.; Lombardi, F.; Newton, W.; et al. Bone Sarcomas Linked to Radiotherapy and Chemotherapy in Children. N. Engl. J. Med. 1987, 317, 588–593. [Google Scholar] [CrossRef]
- Martins-Neves, S.R.; Sampaio-Ribeiro, G.; Gomes, C.M.F. Self-Renewal and Pluripotency in Osteosarcoma Stem Cells’ Chemoresistance: Notch, Hedgehog, and Wnt/β-Catenin Interplay with Embryonic Markers. Int. J. Mol. Sci. 2023, 24, 8401. [Google Scholar] [CrossRef]
- Ferrari, D.; Moneghini, L.; Allevi, F.; Bulfamante, G.; Biglioli, F. Osteosarcoma of the Jaw: Classification, Diagnosis and Treatment. In Osteosarcoma-Biology, Behavior and Mechanisms; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Q.; Gong, X.; Liu, J.; Ma, Y. Osteosarcoma: A Review of Current and Future Therapeutic Approaches. Biomed. Eng. Online 2021, 20, 24. [Google Scholar] [CrossRef]
- Smeland, S.; Bielack, S.S.; Whelan, J.; Bernstein, M.; Hogendoorn, P.; Krailo, M.D.; Gorlick, R.; Janeway, K.A.; Ingleby, F.C.; Anninga, J.; et al. Survival and Prognosis with Osteosarcoma: Outcomes in More than 2000 Patients in the EURAMOS-1 (European and American Osteosarcoma Study) Cohort. Eur. J. Cancer 2019, 109, 36–50. [Google Scholar] [CrossRef]
- Jamal, Z.; Anjum, F. Oropharyngeal Squamous Cell Carcinoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563268/ (accessed on 27 April 2023).
- Panarese, I.; Aquino, G.; Ronchi, A.; Longo, F.; Montella, M.; Cozzolino, I.; Roccuzzo, G.; Colella, G.; Caraglia, M.; Franco, R. Oral and Oropharyngeal Squamous Cell Carcinoma: Prognostic and Predictive Parameters in the Etiopathogenetic Route. Expert Rev. Anticancer Ther. 2019, 19, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Fauzi, F.H.; Hamzan, N.I.; Rahman, N.A.; Suraiya, S.; Mohamad, S. Detection of Human Papillomavirus in Oropharyngeal Squamous Cell Carcinoma. J. Zhejiang Univ. Sci. B 2020, 21, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Stern, P.L.; Dalianis, T. Oropharyngeal Squamous Cell Carcinoma Treatment in the Era of Immune Checkpoint Inhibitors. Viruses 2021, 13, 1234. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Tseng, Y.H. The Potential of Phytochemicals in Oral Cancer Prevention and Therapy: A Review of the Evidence. Biomolecules 2020, 10, 1150. [Google Scholar] [CrossRef]
- Padhy, I.; Paul, P.; Sharma, T.; Banerjee, S.; Mondal, A. Molecular Mechanisms of Action of Eugenol in Cancer: Recent Trends and Advancement. Life 2022, 12, 1795. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Costello, L.; Toner, M.; Pierse, D.; Stassen, L.F.A. Osteosarcoma (Osteogenic Sarcoma) of the Jaws Presenting in General Dental Practice—A Series of Four Cases. Br. Dent. J. 2021, 230, 583–586. [Google Scholar] [CrossRef]
- Golusiński, W.; Golusińska-Kardach, E. Current Role of Surgery in the Management of Oropharyngeal Cancer. Front. Oncol. 2019, 9, 388. [Google Scholar] [CrossRef]
- Ali Abdalla, Y.O.; Subramaniam, B.; Nyamathulla, S.; Shamsuddin, N.; Arshad, N.M.; Mun, K.S.; Awang, K.; Nagoor, N.H. Natural Products for Cancer Therapy: A Review of Their Mechanism of Actions and Toxicity in the Past Decade. J. Trop. Med. 2022, 2022, 5794350. [Google Scholar] [CrossRef]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological Properties and Health Benefits of Eugenol: A Comprehensive Review. Oxid. Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- Luiz De Sá Júnior, P.; Aparecida, D.; Câmara, D.; Santos Costa, A.; Luis, J.; Ruiz, M.; Levy, D.; Azevedo, R.A.; Fernanda, K.; Pasqualoto, M.; et al. Apoptotic Effect of Eugenol Envolves G2/M Phase Abrogation Accompanied by Mitochondrial Damage and Clastogenic Effect on Cancer Cell in Vitro. Phytomedicine 2016, 23, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Al Wafai, R.; El-Rabih, W.; Katerji, M.; Safi, R.; El Sabban, M.; El-Rifai, O.; Usta, J. Chemosensitivity of MCF-7 Cells to Eugenol: Release of Cytochrome-c and Lactate Dehydrogenase. Sci. Rep. 2017, 7, 43730. [Google Scholar] [CrossRef] [PubMed]
- Ramazani, E.; YazdFazeli, M.; Ahmad Emami, S.; Mohtashami, L.; Javadi, B.; Asili, J.; Tayarani-Najaran, Z. Protective Effects of Cinnamomum Verum, Cinnamomum Cassia and Cinnamaldehyde against 6-OHDA-Induced Apoptosis in PC12 Cells. Mol. Biol. Rep. 2020, 47, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Park, J.H.; Kim, G.C.; Park, B.S.; Gil, Y.G.; Kim, C.H. The Mechanism of Apoptosis Induced by Eugenol in Human Osteosarcoma Cells. J. Korean Assoc. Oral Maxillofac. Surg. 2007, 33, 20–27. [Google Scholar]
- Surducan, D.A.; Racea, R.C.; Cabuta, M.; Olariu, I.; Macasoi, I.; Rusu, L.C.; Chiriac, S.D.; Chioran, D.; Dinu, S.; Pricop, M.O. Eugenol Induces Apoptosis in Tongue Squamous Carcinoma Cells by Mediating the Expression of Bcl-2 Family. Life 2023, 13, 22. [Google Scholar] [CrossRef]
- Racea, R.C.; Merghes, P.E.; Gurgus, D.; Macasoi, I.; Rusu, L.C.; Chioran, D.; Dinu, S.; Breban-Schwarzkopf, D.; Szuhanek, C.; Rivis, M. Eugenol: In Vitro Characterization Of The Cytotoxic Profile At The Level Of Colorectal Carcinoma Cells. Farmacia 2023, 71, 288–295. [Google Scholar] [CrossRef]
- ISO 10993-5:2009(En); Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009. Available online: https://www.iso.org/obp/ui/#iso:std:iso:10993:-5:ed-3:v1:en (accessed on 25 July 2023).
- Razak, M.A.I.A.; Hamid, H.A.; Othman, R.N.I.R.; Moktar, S.A.M.; Miskon, A. Improved Drug Delivery System for Cancer Treatment by D-Glucose Conjugation with Eugenol From Natural Product. Curr. Drug Deliv. 2020, 18, 312–322. [Google Scholar] [CrossRef]
- Rothzerg, E.; Pfaff, A.L.; Koks, S. Innovative Approaches for Treatment of Osteosarcoma. Exp. Biol. Med. 2022, 247, 310–316. [Google Scholar] [CrossRef]
- Wen, Y.; Grandis, J.R. Emerging Drugs for Head and Neck Cancer. Expert Opin. Emerg. Drugs 2015, 20, 313–329. [Google Scholar] [CrossRef]
- Kis, A.M.; Macasoi, I.; Paul, C.; Radulescu, M.; Buzatu, R.; Watz, C.G.; Cheveresan, A.; Berceanu, D.; Pinzaru, I.; Dinu, S.; et al. Methotrexate and Cetuximab—Biological Impact on Non-Tumorigenic Models: In Vitro and In Ovo Assessments. Medicina 2022, 58, 167. [Google Scholar] [CrossRef] [PubMed]
- Sramek, M.; Neradil, J.; Sterba, J.; Veselska, R. Non-DHFR-Mediated Effects of Methotrexate in Osteosarcoma Cell Lines: Epigenetic Alterations and Enhanced Cell Differentiation. Cancer Cell Int. 2016, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, A.; Shanehbandi, D.; Yousefi, B.; Soleimanpour, J. Thymoquinone Potentiates Methotrexate Mediated-Apoptosis in Saos-2 Osteosarcoma Cell Line. Drug Res. 2022, 72, 390–395. [Google Scholar]
- Young, N.R.; Soneru, C.; Liu, J.; Grushko, T.A.; Hardeman, A.; Olopade, O.I.; Baum, A.; Solca, F.; Cohen, E.E.W. Afatinib Efficacy against Squamous Cell Carcinoma of the Head and Neck Cell Lines in Vitro and in Vivo. Target Oncol. 2015, 10, 501–508. [Google Scholar] [CrossRef]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.-F. Natural Compounds as Potential Adjuvants to Cancer Therapy: Preclinical Evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef]
- Duan, Y.; Huang, X.; Qiao, B.; Ma, R.; Li, J. Eugenol Inhibits the Biological Activities of an Oral Squamous Cell Carcinoma Cell Line SCC9 via Targeting MIF. Anticancer Agents Med. Chem. 2022, 22, 2799–2806. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, B.S. The Effect of Eugenol on the Induction of Apoptosis in HSC-2 Human Oral Squamous Cell Carcinoma. Orig. Artic. J. Korean Soc. Dent. Hyg. 2015, 15, 523–532. [Google Scholar] [CrossRef]
- Yoo, C.-B.; Han, K.-T.; Cho, K.-S.; Ha, J.; Park, H.-J.; Nam, J.-H.; Kil, U.-H.; Lee, K.-T. Eugenol Isolated from the Essential Oil of Eugenia Caryophyllata Induces a Reactive Oxygen Species-Mediated Apoptosis in HL-60 Human Promyelocytic Leukemia Cells. Cancer Lett. 2005, 225, 41–52. [Google Scholar] [CrossRef]
- das Chagas Pereira de Andrade, F.; Mendes, A.N. Computational Analysis of Eugenol Inhibitory Activity in Lipoxygenase and Cyclooxygenase Pathways. Sci. Rep. 2020, 10, 16204. [Google Scholar] [CrossRef]
- Fangjun, L.; Zhijia, Y. Tumor Suppressive Roles of Eugenol in Human Lung Cancer Cells. Thorac. Cancer 2018, 9, 25–29. [Google Scholar] [CrossRef]
- Fathy, M.; Fawzy, M.A.; Hintzsche, H.; Nikaido, T.; Dandekar, T.; Othman, E.M. Eugenol Exerts Apoptotic Effect and Modulates the Sensitivity of HeLa Cells to Cisplatin and Radiation. Molecules 2019, 24, 3979. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, U.; Groscurth, P. Morphological Features of Cell Death. Physiology 2004, 19, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Menyhárt, O.; Harami-Papp, H.; Sukumar, S.; Schäfer, R.; Magnani, L.; de Barrios, O.; Győrffy, B. Guidelines for the Selection of Functional Assays to Evaluate the Hallmarks of Cancer. Biochim. Biophys. Acta Rev. Cancer. 2016, 1866, 300–319. [Google Scholar] [CrossRef] [PubMed]
- Doonan, F.; Cotter, T.G. Morphological Assessment of Apoptosis. Methods 2008, 44, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Vidhya, N.; Devaraj, N. Induction of Apoptosis by Eugenol in Human Breast Cancer Cell. Indian J. Exp. Biol. 2011, 49, 871–878. [Google Scholar]
- Das, A.; Harshadha, K.; Dhinesh Kannan, S.K.; Hari Raj, K.; Jayaprakash, B. Evaluation of Therapeutic Potential of Eugenol-A Natural Derivative of Syzygium aromaticum on Cervical Cancer. Asian Pac. J. Cancer Prev. 2018, 19, 1977–1985. [Google Scholar]
- Mandelkow, R.; GüMBEL, D.; Ahrend, H.; Kaul, A.; Zimmermann, U.; Burchardt, M.; Stope, M.B. Detection and Quantification of Nuclear Morphology Changes in Apoptotic Cells by Fluorescence Microscopy and Subsequent Analysis of Visualized Fluorescent Signals. Anticancer Res. 2017, 37, 2239–2244. [Google Scholar] [CrossRef]
- Permatasari, H.K.; Effendi, A.B.; Qhabibi, F.R.; Fawwaz, F.; Dominique, A. Eugenol Isolated from Syzygium aromaticum Inhibits HeLa Cancer Cell Migration by Altering Epithelial-Mesenchymal Transition Protein Regulators. J. Appl. Pharm. Sci. 2021, 11, 49–53. [Google Scholar]
- Kim, G.C.; Choi, D.S.; Lim, J.S.; Jeong, H.C.; Kim, I.R.; Lee, M.H.; Park, B.S. Caspases-Dependent Apoptosis in Human Melanoma Cell by Eugenol. Korean J. Anat. 2016, 39, 245–253. [Google Scholar] [CrossRef]
- Lindenboim, L.; Zohar, H.; Worman, H.J.; Stein, R. The Nuclear Envelope: Target and Mediator of the Apoptotic Process. Cell Death Discov. 2020, 6, 29. [Google Scholar] [CrossRef]
- Islam, S.S.; Al-Sharif, I.; Sultan, A.; Al-Mazrou, A.; Remmal, A.; Aboussekhra, A. Eugenol Potentiates Cisplatin Anti-Cancer Activity through Inhibition of ALDH-Positive Breast Cancer Stem Cells and the NF-ΚB Signaling Pathway. Mol. Carcinog. 2018, 57, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Hemaiswarya, S.; Doble, M. Combination of Phenylpropanoids with 5-Fluorouracil as Anti-Cancer Agents against Human Cervical Cancer (HeLa) Cell Line. Phytomedicine 2013, 20, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Panda, C.K.; Das, S. Clove (Syzygium aromaticum L.), a Potential Chemopreventive Agent for Lung Cancer. Carcinogenesis 2006, 27, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Bhattacharjee, S.; Mandal, D.P. Induction of Apoptosis by Eugenol and Capsaicin in Human Gastric Cancer AGS Cells-Elucidating the Role of P53. Asian Pac. J. Cancer Prev. 2015, 16, 6753–6759. [Google Scholar] [CrossRef]
- Dhani, S.; Zhao, Y.; Zhivotovsky, B. A Long Way to Go: Caspase Inhibitors in Clinical Use. Cell Death Dis. 2021, 12, 949. [Google Scholar] [CrossRef] [PubMed]
- Kupcho, K.; Shultz, J.; Hurst, R.; Hartnett, J.; Zhou, W.; Machleidt, T.; Grailer, J.; Worzella, T.; Riss, T.; Lazar, D.; et al. A Real-Time, Bioluminescent Annexin V Assay for the Assessment of Apoptosis. Apoptosis 2019, 24, 184–197. [Google Scholar] [CrossRef]
- RealTime-GloTM Annexin V Apoptosis Assay|Annexin V Staining|Apoptosis Assay. Available online: https://www.promega.ro/products/cell-health-assays/apoptosis-assays/realtime-glo-annexin-v-apoptosis-assay/?catNum=JA1011 (accessed on 5 October 2023).
- Winter, G.; Koch, A.B.F.; Löffler, J.; Jelezko, F.; Lindén, M.; Li, H.; Abaei, A.; Zuo, Z.; Beer, A.J.; Rasche, V. In Vivo PET/MRI Imaging of the Chorioallantoic Membrane. Front. Phys. 2020, 8, 151. [Google Scholar] [CrossRef]
- Budai, P.; Kormos, É.; Buda, I.; Somody, G.; Lehel, J. Comparative Evaluation of HET-CAM and ICE Methods for Objective Assessment of Ocular Irritation Caused by Selected Pesticide Products. Toxicol. Vitr. 2021, 74, 105150. [Google Scholar] [CrossRef]
- Ahmad, N.; Jalees Ahmad, F.; Bedi, S.; Sharma, S.; Umar, S.; Azam Ansari, M. A Novel Nanoformulation Development of Eugenol and Their Treatment in Inflammation and Periodontitis. Saudi Pharm. J. 2019, 27, 778–790. [Google Scholar] [CrossRef]
- Thanekar, D.; Dhodi, J.; Gawali, N.; Raju, A.; Deshpande, P.; Degani, M.; Juvekar, A. Evaluation of Antitumor and Anti-Angiogenic Activity of Bioactive Compounds from Cinnamomum Tamala: In Vitro, in Vivo and in Silico Approach. S. Afr. J. Bot. 2016, 104, 6–14. [Google Scholar] [CrossRef]
- Gad El-Hak, H.; Gerges, M. Evaluating the teratogenic effect of Eugenol in the development of the chick embryos. J. Biol. Stud. 2018, 1, 59–75. [Google Scholar]
- Gag, O.; Macasoi, I.; Pinzaru, I.; Dinu, S.; Popovici, R.; Cosoroaba, M.R.; Buzatu, R.; Cabuta, M.; Chiriac, S.D. In Vitro Assessment of the Impact of Ultraviolet B Radiation on Oral Healthy and Tumor Cells. Photonics 2023, 10, 464. [Google Scholar] [CrossRef]
- Eidet, J.R.; Pasovic, L.; Maria, R.; Jackson, C.J.; Utheim, T.P. Objective Assessment of Changes in Nuclear Morphology and Cell Distribution Following Induction of Apoptosis. Diagn. Pathol. 2014, 9, 92. [Google Scholar] [CrossRef]
- Rednic, R.; Macasoi, I.; Pinzaru, I.; Dehelean, C.A.; Tomescu, M.C.; Susan, M.; Feier, H. Pharmaco-Toxicological Assessment of the Combined Cytotoxic Effects of Digoxin and Betulinic Acid in Melanoma Cells. Life 2022, 12, 1855. [Google Scholar] [CrossRef] [PubMed]
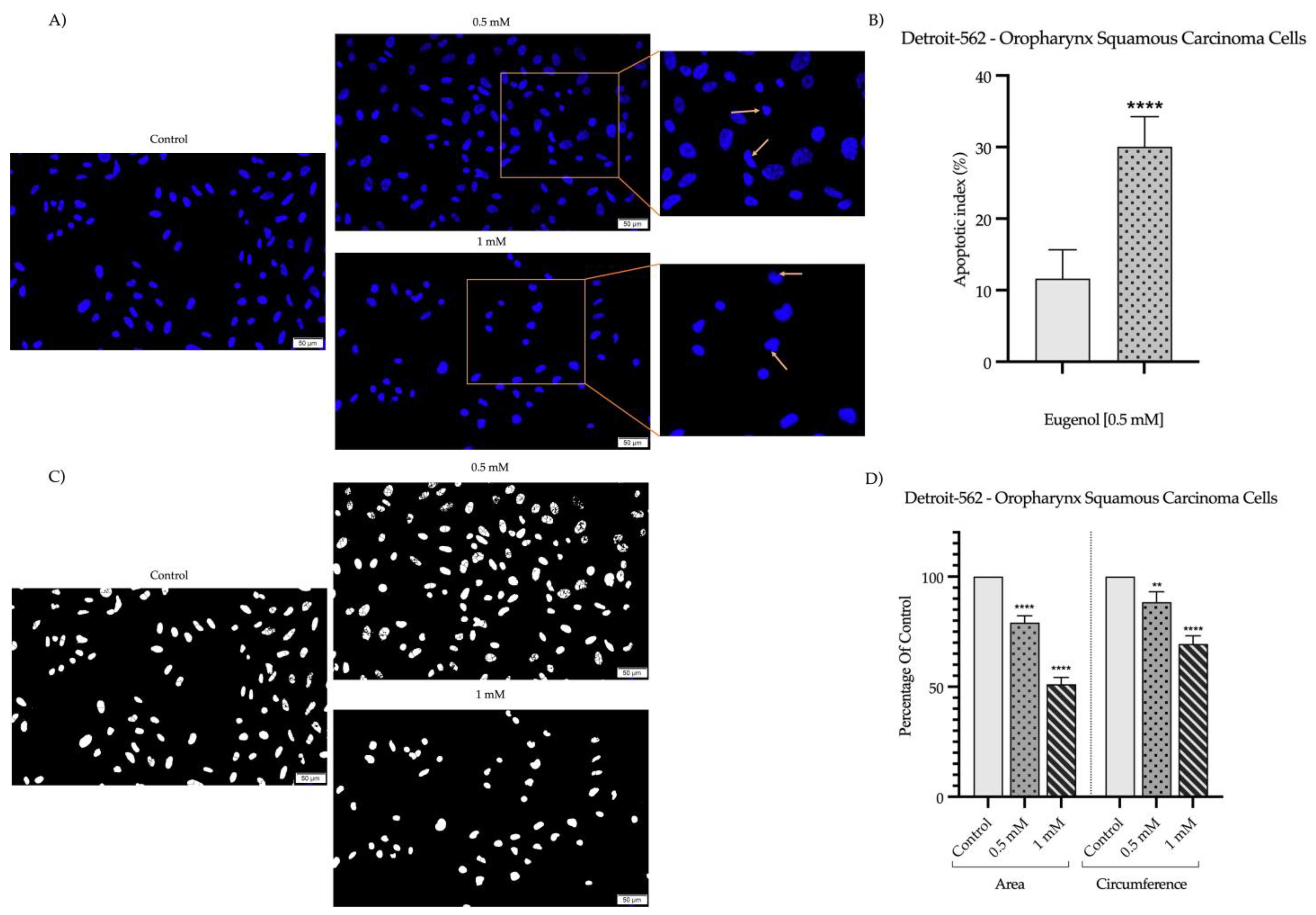

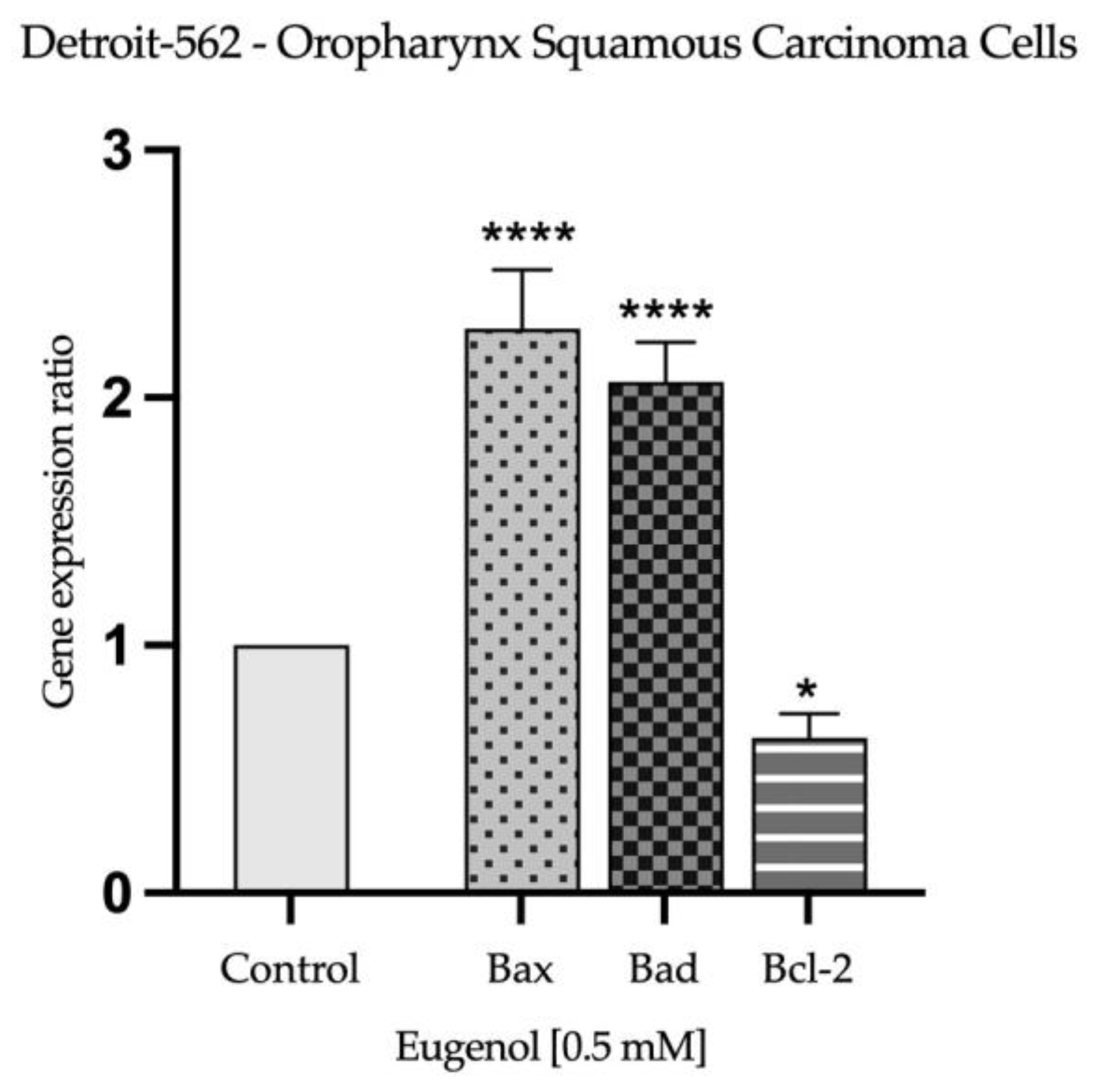
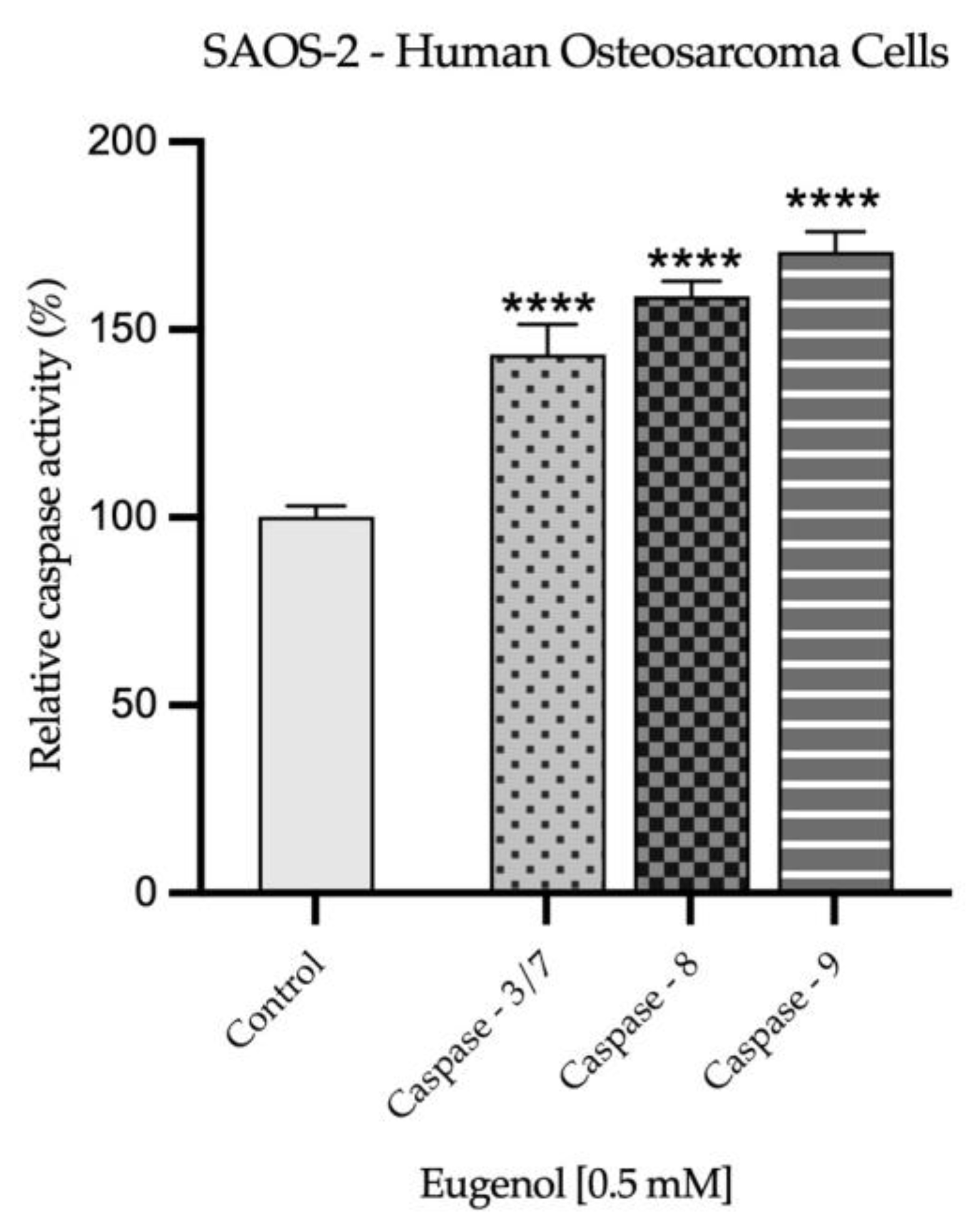
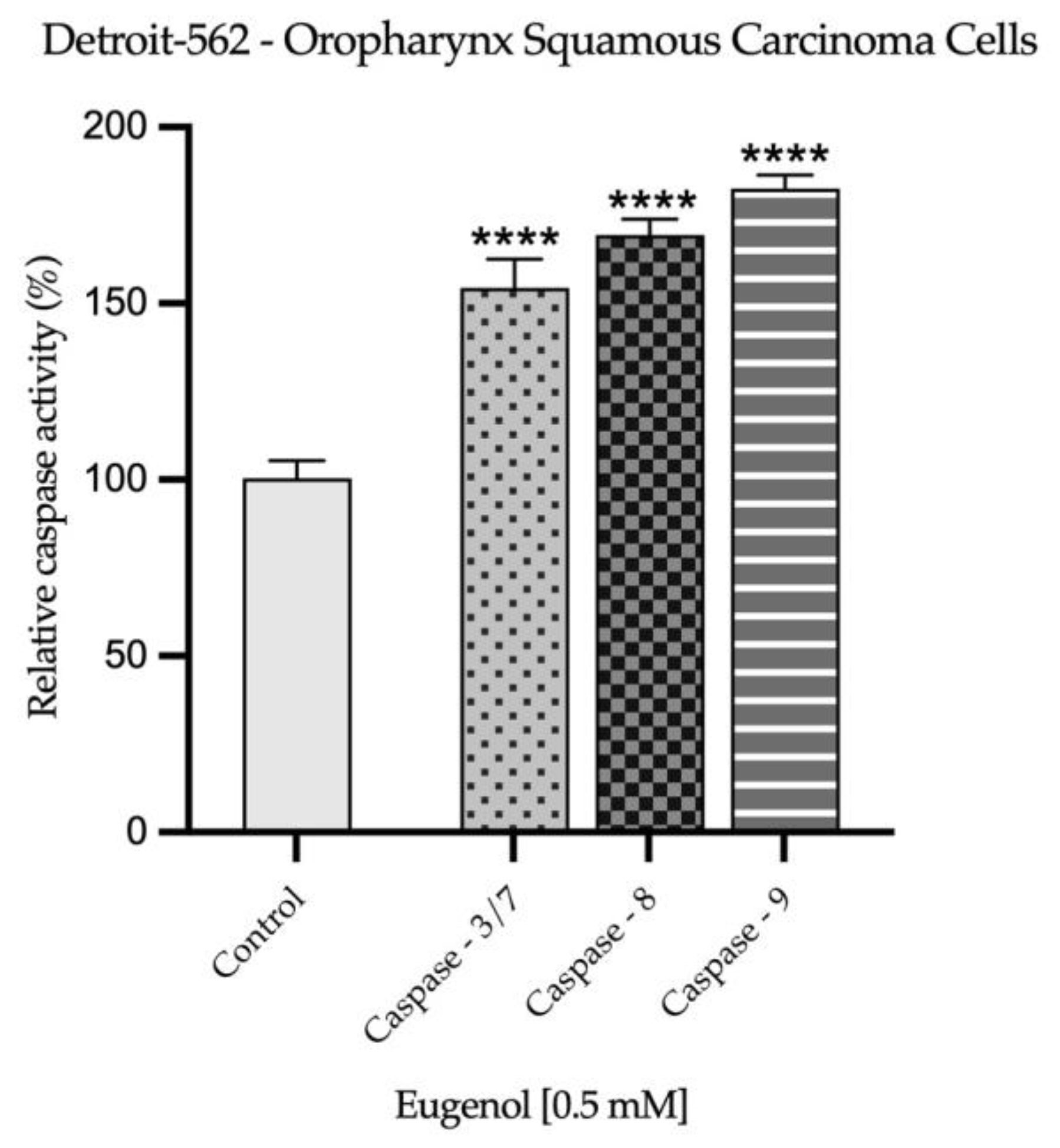
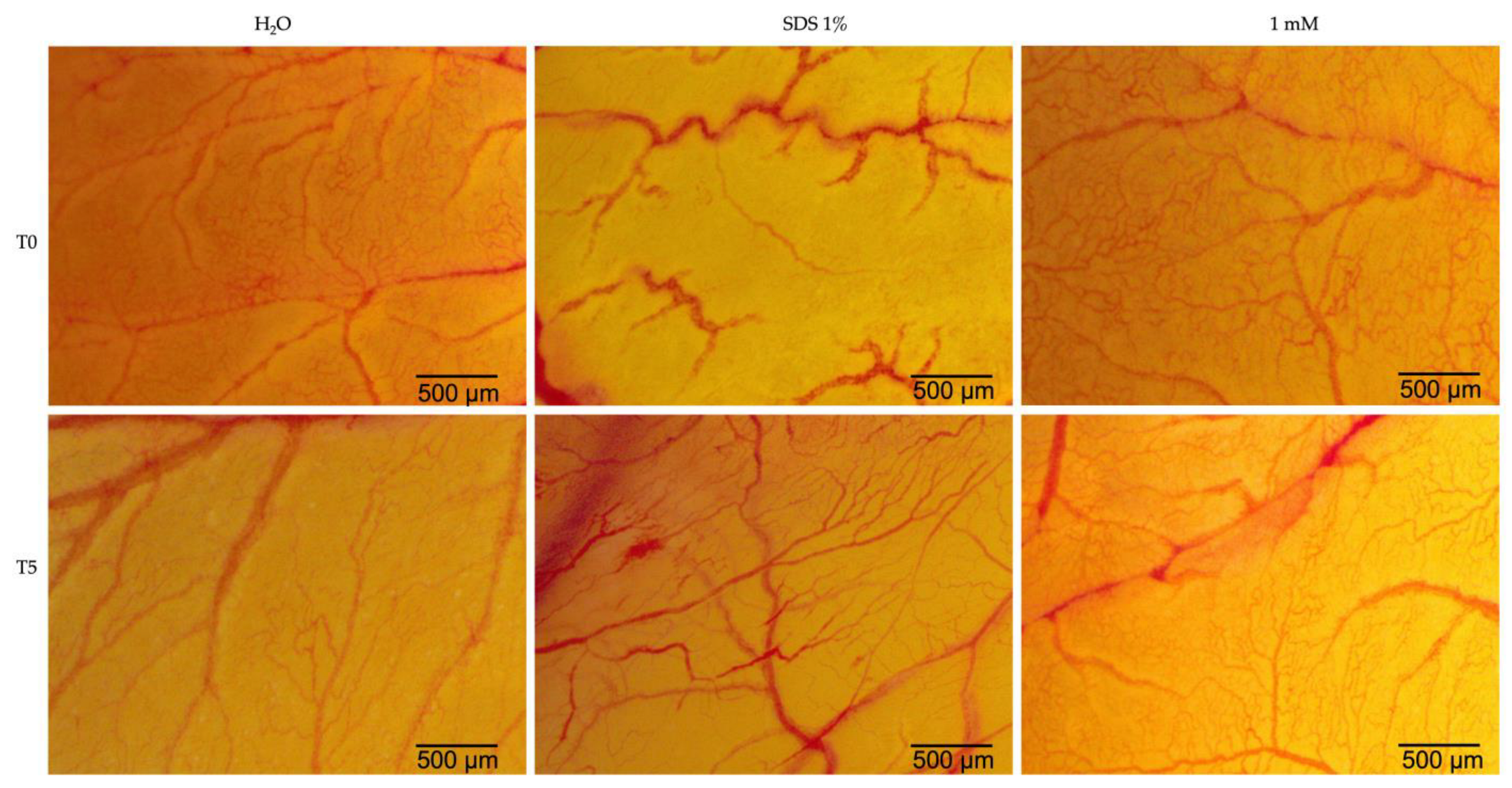
| H2O | SDS 1% | Eug 1 mM | |
|---|---|---|---|
| IS | 0.15 | 19.87 | 1.69 |
| tH | 300 | 21 | 300 |
| tL | 299 | 17 | 278 |
| tC | 298 | 15 | 263 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Racea, R.-C.; Macasoi, I.-G.; Dinu, S.; Pinzaru, I.; Marcovici, I.; Dehelean, C.; Rusu, L.-C.; Chioran, D.; Rivis, M.; Buzatu, R. Eugenol: In Vitro and In Ovo Assessment to Explore Cytotoxic Effects on Osteosarcoma and Oropharyngeal Cancer Cells. Plants 2023, 12, 3549. https://doi.org/10.3390/plants12203549
Racea R-C, Macasoi I-G, Dinu S, Pinzaru I, Marcovici I, Dehelean C, Rusu L-C, Chioran D, Rivis M, Buzatu R. Eugenol: In Vitro and In Ovo Assessment to Explore Cytotoxic Effects on Osteosarcoma and Oropharyngeal Cancer Cells. Plants. 2023; 12(20):3549. https://doi.org/10.3390/plants12203549
Chicago/Turabian StyleRacea, Robert-Cosmin, Ioana-Gabriela Macasoi, Stefania Dinu, Iulia Pinzaru, Iasmina Marcovici, Cristina Dehelean, Laura-Cristina Rusu, Doina Chioran, Mircea Rivis, and Roxana Buzatu. 2023. "Eugenol: In Vitro and In Ovo Assessment to Explore Cytotoxic Effects on Osteosarcoma and Oropharyngeal Cancer Cells" Plants 12, no. 20: 3549. https://doi.org/10.3390/plants12203549







