Effects of Different Heterogeneous Nutrient Environments on the Growth and Activities of Enzymes in the Roots of Fokienia hodginsii Families
Abstract
:1. Introduction
2. Results
2.1. Effects of Different Nutrient Environments on Growth and Root Biomass of F. hodginsii Families
2.2. Effects of Different Nutrient Environments on Root Enzyme Activities of F. hodginsii Families
2.3. Correlation Analysis of Different Nutrient Environments on the Growth of F. hodginsii Families and Root Indexes
2.4. Comprehensive Evaluation of Different Nutrient Environments on the Growth of F. hodginsii Families and Root Indexes
3. Discussion
3.1. Effects of Different Nutrient Environments on Growth and Root Biomass of F. hodginsii
3.2. Effects of Different Nutrient Environments on Root Enzyme Activities of F. hodginsii
3.3. Effects of Different Nutrient Environments on the Growth and Root Enzyme Activities of F. hodginsii Families
4. Materials and Methods
4.1. Overview of the Test Site
4.2. Experimental Materials
4.3. Experimental Design
4.4. Index Measurement
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Hodge, A. Plastic plants and patchy soils. J. Exp. Bot. 2006, 57, 401–411. [Google Scholar] [CrossRef]
- Wang, X.; Tang, H.L.; Shen, J.B. Root responses of maize to spatial heterogenous nitrogen and phosphorus. J. Plant Nutr. Fertil. 2013, 19, 1058–1064. [Google Scholar]
- Yao, J.; Zhou, Z.; Chu, X.; Xu, H.; Tong, J. Effect of neighborhood competition on dry matter accumulation, nitrogen and phosphorus efficiency of three provenances of Schima superba in a heterogeneous nutrient environment. Acta Ecol. Sin. 2018, 38, 1780–1788. [Google Scholar]
- Zhang, Y.; Zhou, Z.; Yang, Q. Genetic variations in root morphology and phosphorus efficiency of Pinus massoniana under heterogeneous and homogeneous low phosphorus conditions. Plant Soil 2013, 364, 93–104. [Google Scholar] [CrossRef]
- Sun, J.L.; Li, H.B.; Zhang, A.P. Effects of nutrient heterogeneity on shoot and root growth of Zea mays and intraspecific competition. J. China Agric. Univ. 2022, 27, 35–45. [Google Scholar]
- Grime, J.P. The Scale-precision trade-off in spacial resource foraging by plants: Restoring perspective. Ann. Bot. 2007, 99, 1017–1021. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Z.C.; Jin, G.Q.; Rao, L.B.; Jiao, Y.L.; Li, Y.G. Differences of foraging behavior between provenances of Pinus massoniana in heterogeneous nutrient environment. Acta Ecol. Sin. 2007, 27, 1350–1358. [Google Scholar]
- Kyle, C.K.; Peairs, S.E.; Ezell, A.W.; Belli, K.L.; Hodges, J.D. Understory Light Conditions Associated with Partial Overstory Removal and Midstory/Understory Control Applications in a Bottomland Hardwood Forest. Forests 2011, 2, 984. [Google Scholar]
- Yan, X.L.; Ma, X. Responses of root morphology and seedling growth in three tree species to heterogeneous supplies of ammonium and nitrate. For. Ecol. Manag. 2021, 479, 118538. [Google Scholar] [CrossRef]
- Drew, M.C. Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot in Barley. New Phytol. 1975, 75, 479–490. [Google Scholar] [CrossRef]
- Brouder, S.M.; Cassman, K.G. Evaluation of a Mechanistic Model of Potassium Uptake by Cotton in Vermiculitic Soil. Soil Sci. Soc. Am. J. 1994, 58, 1174–1183. [Google Scholar] [CrossRef]
- Rose, T.J.; Rengel, Z.; Ma, Q.; Bowden, J.W. Crop species differ in root plasticity response to localised P supply. J. Plant Nutr. Soil Sci. 2009, 172, 360–368. [Google Scholar] [CrossRef]
- Li, B.; Deng, M.; Pan, Y.; Rong, J.; He, T.; Chen, L.; Zheng, Y. Responses of Planting Modes to Photosynthetic Characteristics and Fluorescence Parameters of Fokienia hodginsii Seedlings in a Heterogeneous Nutrient Environment. Forests 2023, 14, 984. [Google Scholar] [CrossRef]
- Wang, P.; Mou, P.; Li, Y. Review of root nutrient foraging plasticity and root competition of plants. Chin. J. Plant Ecol. 2012, 36, 13. [Google Scholar] [CrossRef]
- Freschet, G.T.; Swart, E.M.; Cornelissen, J.H.C. Integrated plant phenotypic responses to contrasting above- and below-ground resources: Key roles of specific leaf area and root mass fraction. New Phytol. 2015, 206, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Glover, G.R.; Zutter, B.R. Loblolly pine and mixed hardwood stand dynamics for 27 years following chemical, mechanical, and manual site preparation. Can. J. For. Res. 1993, 23, 2126–2132. [Google Scholar] [CrossRef]
- Grossman, J.D.; Rice, K.J. Evolution of root plasticity responses to variation in soil nutrient distribution and concentration. Evol. Appl. 2013, 5, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhang, R.; Zhou, Z.; Tong, J.S.; Wang, H. Effects of localized nitrogen supply treatments on growth and root parameters in Pinus massoniana families under phosphorus deficiency. Chin. J. Plant Ecol. 2017, 41, 622–631. [Google Scholar]
- Dao, T.H.H.; Hölscher, D. Fujian cypress and two other threatened tree species in three conservation zones of a nature reserve in north-western Vietnam. For. Ecosyst. 2017, 4, 29. [Google Scholar] [CrossRef]
- Tang, D. Family Variation and Evaluation of Growth Traits On 36-year-old Pinus massoniana Lamb in Fujian Province. For. Res. 2023, 36, 179–184. [Google Scholar]
- Li, B.; Chen, Q.; Wang, X.X.; Rong, J.D.; Chen, L.G.; Zhen, Y.S. Differences in Growth and Nutrients between Pure and Mixed Forest of Fokienia hodginsii with Different Forest Ages. Acta Bot. Boreali Occident. Sin. 2022, 42, 694–704. [Google Scholar]
- Zhen, R.H.; Yang, Z.W.; Shi, J.S.; Huang, D.L.; Huang, X.M. Studies on the Growth rhythm and Genetic variations of traits among plus-tree progeny families of Fokienia Hodginsii at seedling stage. Sci. Silvae Sin. 2003, 39, 179–183. [Google Scholar]
- Wu, P.; Ma, X.; Tigabu, M.; Wang, C.; Odén, P.C. Root morphological plasticity and biomass production of two Chinese fir clones with high phosphorus efficiency under low phosphorus stress. Can. J. For. Res. 2011, 41, 228–234. [Google Scholar] [CrossRef]
- Yao, J.B.; Chu, X.L.; Zhou, Z.C.; Xu, H.B.; Zhen, X.J. Response of Seedlings of Three Schima superba Provenances to Different Light Environments When Mixed Planting with Cunninghamia lanceolata. For. Res. 2018, 31, 144–153. [Google Scholar]
- Yan, X.L.; Hu, W.J.; Ma, Y.F.; Huo, Y.F.; Wang, T.; Ma, X.Q. Nitrogen Uptake Preference of Cunninghamia lanceolata, Pinus massoniana, and Schima superba under Heterogeneous Nitrogen Supply Environment and their Root Foraging Strategies. Sci. Silvae Sin. 2020, 56, 1–11. [Google Scholar]
- Mommer, L.; Visser, E.J.W.; Ruijven, J.V.; Caluwe, H.D.; Pierik, R.; Kroon, H.D. Contrasting root behaviour in two grass species: A test of functionality in dynamic heterogeneous conditions. Plant Soil 2011, 344, 347–360. [Google Scholar] [CrossRef]
- Ma, X.H.; Zhou, Z.C.; Zhang, Y.; Jin, G.Q. Foraging Behaviors and Growth Responses of Pinus massoniana Seeding in the Heterogeneous Nutrient Environment with Different Nutrient Patches. For. Res. 2010, 23, 697–702. [Google Scholar]
- Jin, S.H.; Huang, J.Q.; Li, X.Q.; Zheng, B.S.; Wu, J.S.; Wang, Z.J.; Liu, G.H.; Chen, M. Effects of potassium supply on limitations of photosynthesis by mesophyll diffusion conductance in Carya cathayensis. Tree Physiol. 2011, 31, 1142–1151. [Google Scholar] [CrossRef]
- Wu, P.; Wang, G.; Farooq, T.H.; Li, Q.; Zou, X.; Ma, X. Low phosphorus and competition affect Chinese fir cutting growth and root organic acid content: Does neighboring root activity aggravate P nutrient deficiency? J. Soils Sediments 2017, 17, 2775–2785. [Google Scholar] [CrossRef]
- Sun, B.; Liao, H.; Su, Y.H.; Xu, W.F.; Jiang, Y.J. Progress in the study of some key synergistic mechanisms affecting nitrogen and phosphorus utilization in soil-root-microbial systems. Soils 2015, 47, 10. [Google Scholar]
- Li, X.; Zhang, L.; Li, Y.; Ma, L.; Bu, N.; Ma, C. Changes in photosynthesis, antioxidant enzymes and lipid peroxidation in soybean seedlings exposed to UV-B radiation and/or Cd. Plant Soil 2012, 352, 377–387. [Google Scholar] [CrossRef]
- Zou, X.H.; Wu, P.F.; Jia, Y.Y.; Ma, X.Q. Periodical response of Chinese fir root to the phosphorus concentrations in patches and heterogeneous distribution in different growing stages. J. Plant Nutr. Fertil. 2016, 22, 1056–1063. [Google Scholar]
- Yan, M.; Wu, Y.M.; Huang, S.X.; Huang, X.L. Resistance Physiological Response of Different Fast-Growing Eucalyptus Clones to Acid-Aluminum Stresses. Sci. Silvae Sin. 2011, 47, 181–187. [Google Scholar]
- Chen, B.J.W.; During, H.J.; Vermeulen, P.J.; De Kroon, H.; Poorter, H.; Anten, N.P.R. Corrections for rooting volume and plant size reveal negative effects of neighbour presence on root allocation in pea. Funct. Ecol. 2015, 29, 1383–1391. [Google Scholar] [CrossRef]
- Wu, R.J.; Zhuang, J.; Huang, J.; Chen, W.P. Responses and Resistance Mechanismof Pinus massoniana under the Stresses of Simulated Acid Rainand Aluminum. Sci. Silvae Sin. 2009, 12, 8. [Google Scholar]
- Yu, D.J.; Xia, L.D.; Yin, D.Y.; Zhou, C.F. Effects of Phosphorus on Aluminum Tolerance of Chinese Fir Seedlings. Sci. Silvae Sin. 2018, 54, 12. [Google Scholar]
- Song, P.; Zhang, R.; Zhang, Y.; Zhou, Z.C.; Feng, Z.P. Effects of simulated nitrogen deposition on fine root morphology, nitrogen and phosphorus efficiency of Pinus massoniana clone under phosphorus deficiency. Chin. J. Plant Ecol. 2016, 40, 1136–1144. [Google Scholar]
- Xiao, Y.; Chu, X.L.; Yin, Z.F.; Jiang, J.M.; Wang, H.; Zhou, Z.C. Analyses on differences in seedling growth, photosynthetic physiology and height growth rhythm of each family of Taxus wallichiana var. mairei from different locations. J. Plant Resour. Environ. 2016, 25, 34–42. [Google Scholar]
- Yao, J.B.; Chu, X.L.; Zhou, Z.C.; Tong, J.S.; Wang, H.; Yu, J.Z. Different responses of growth and root development of Schima superba provenance to the adjacent plant competition in different nutrient conditions. Chin. J. Appl. Ecol. 2017, 28, 1087–1093. [Google Scholar]
- Zhang, J.J.; Xu, S.S.; Cao, G.Q.; Lin, S.Z.; Pan, Y.M.; Ye, Y.Q. Effects of Nitrogen Forms on the Chlorophyll Fluorescence Parameters and Chloroplast Ultra-structure of Cunninghamia lanceolata. J. Northwest For. Univ. 2020, 35, 24–31. [Google Scholar]
- Savicka, M.; Škute, N. Effects of high temperature on malondialdehyde content, superoxide production and growth changes in wheat seedlings (Triticum aestivum L.). Ekologija 2010, 56, 26–33. [Google Scholar] [CrossRef]
- Ye, L.S.; Chen, S.L. Antioxidant system response to different forms and ratios of nitrogen in leaves and roots of Phyllostachys violascens. J. Zhejiang A F Univ. 2017, 34, 14–19. [Google Scholar]
- Li, B.; Deng, M.; Pan, Y.; Chen, W.; Rong, J.; He, T.; Chen, L.; Zheng, Y. Responses of Growth andRoot Vitality of Fokienia hodginsii Seedling to the Neighbor Competition in Different Heterogeneous Nutrient Environments. Forests 2023, 14, 2278. [Google Scholar] [CrossRef]
- Mou, P.; Jones, R.H.; Tan, Z.; Bao, Z.; Chen, H. Morphological and physiological plasticity of plant roots when nutrients are both spatially and temporally heterogeneous. Plant Soil 2013, 364, 373–384. [Google Scholar] [CrossRef]
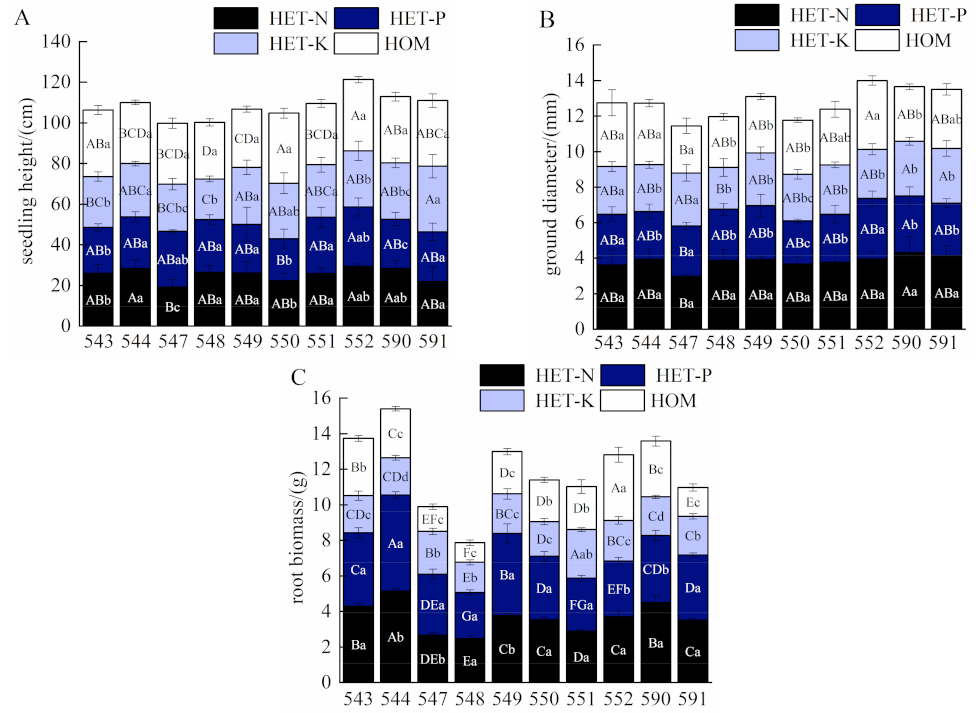
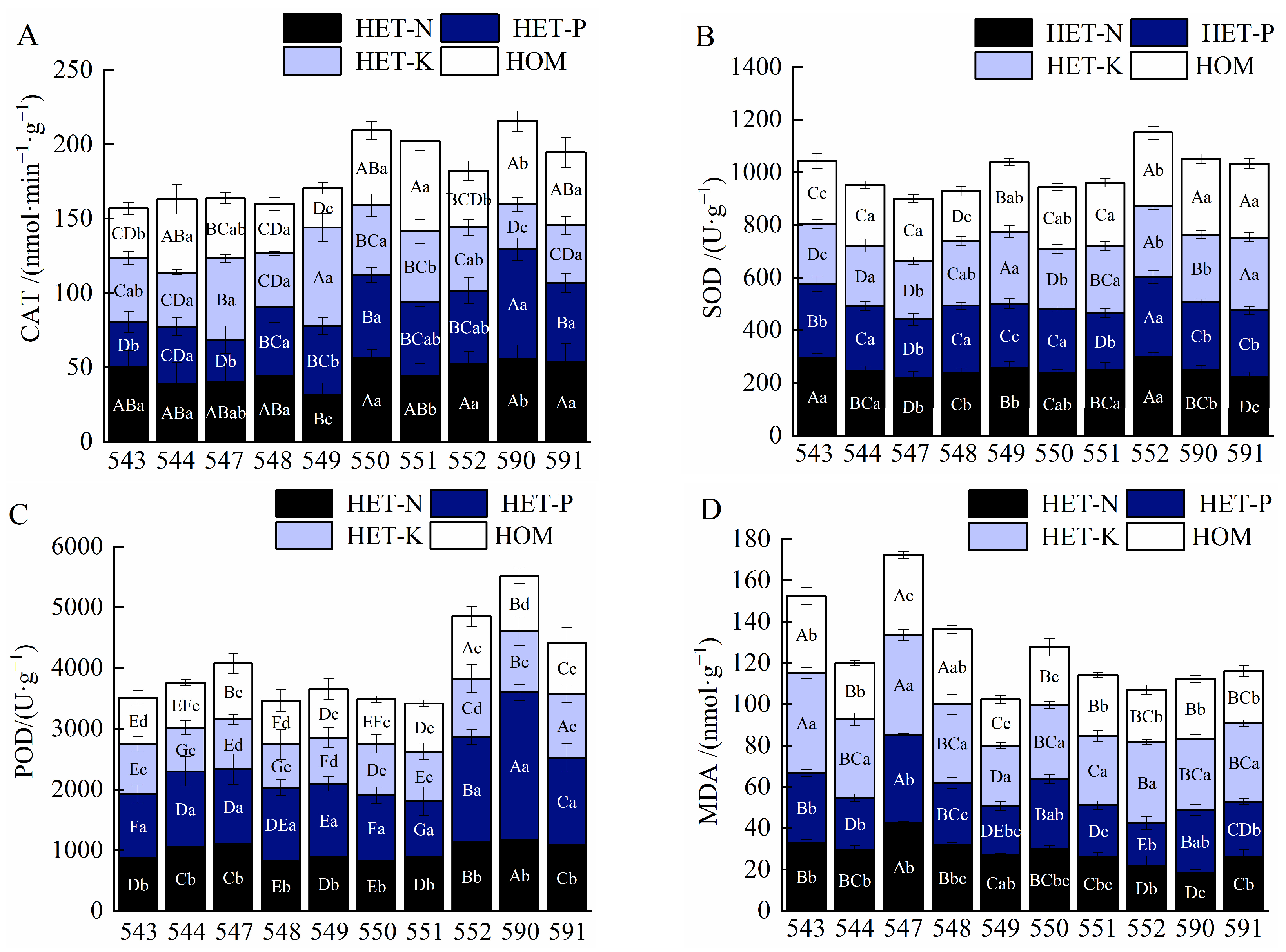
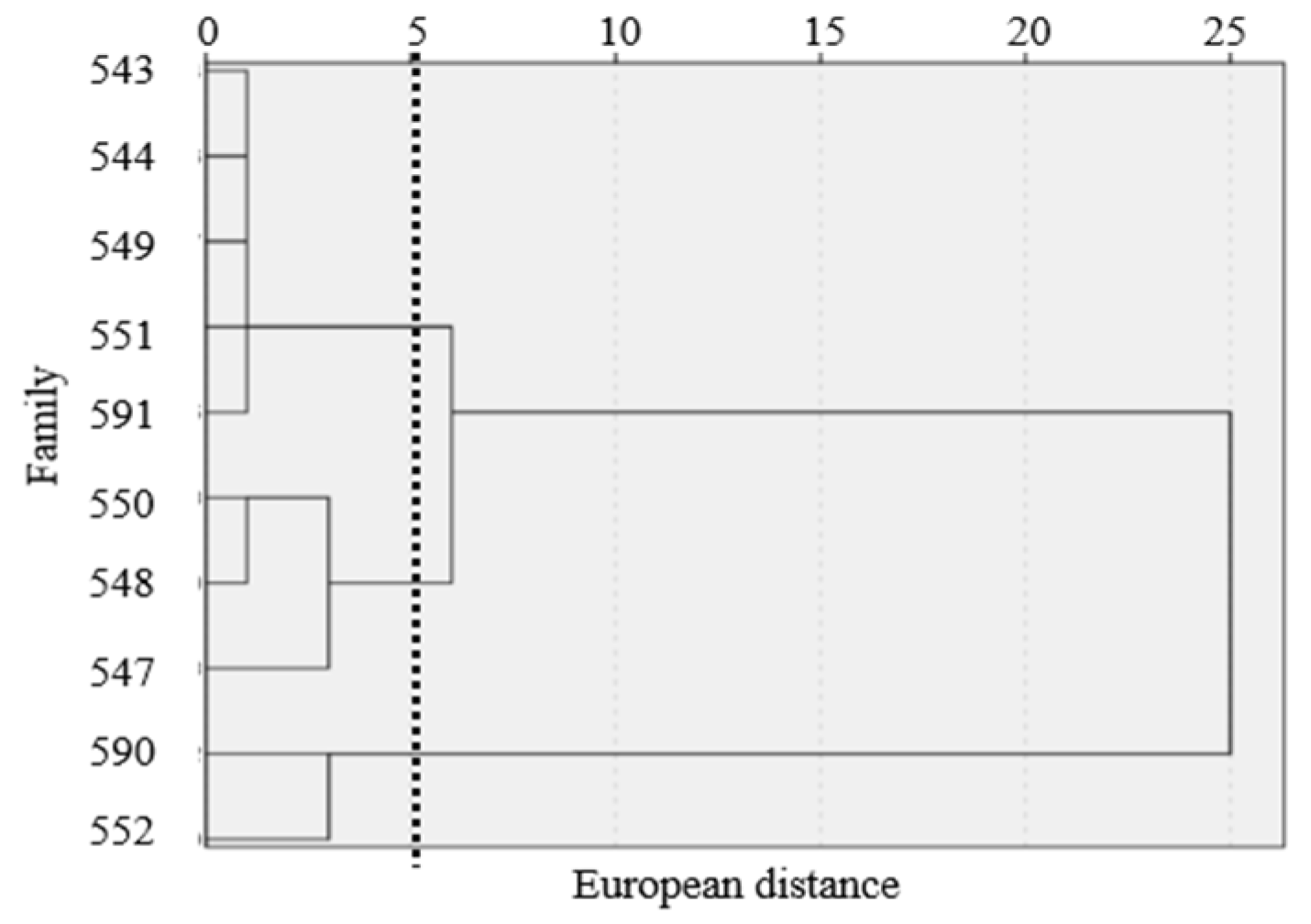
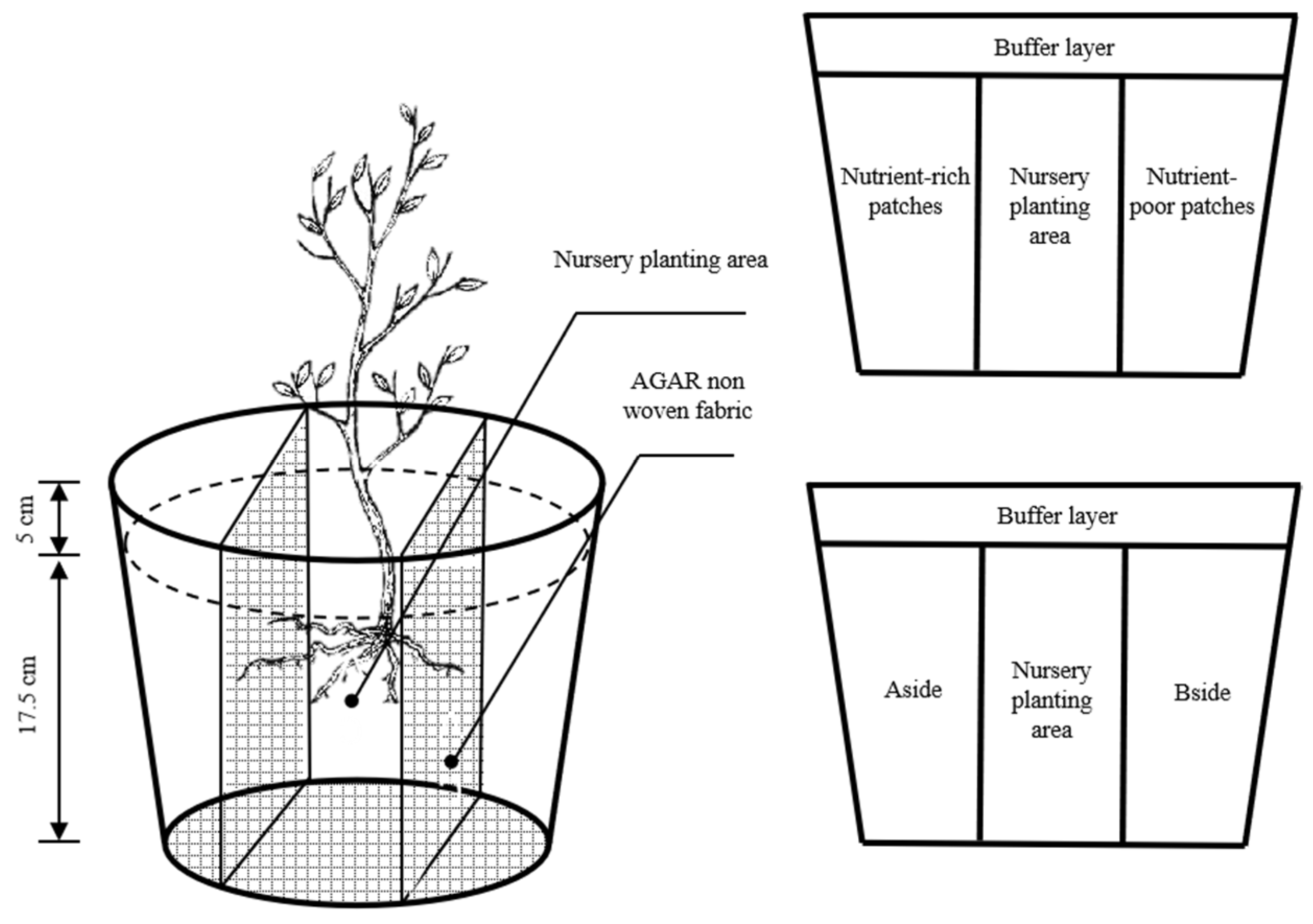
| Factor | Family | Nutrient Environment | Family × Nutrient Environment |
|---|---|---|---|
| seedling height | 2.474 * | 19.042 ** | 1.675 ** |
| ground diameter | 2.281 * | 26.27 ** | 0.877 ns |
| root biomass | 104.721 ** | 551.43 ** | 25.391 ** |
| Factor | Family | Nutrient Environment | Family × Nutrient Environment |
|---|---|---|---|
| CAT | 6.246 ** | 1.57 ns | 5.92 ** |
| SOD | 112.57 ** | 1.926 ns | 11.16 ** |
| POD | 1232.28 ** | 6156.08 ** | 394.48 ** |
| MDA | 61.141 ** | 99.091 ** | 6.096 ** |
| Parameter | Seedling Height | Ground Diameter | Root Biomass | CAT | SOD | POD | MDA |
|---|---|---|---|---|---|---|---|
| seedling height | 1 | ||||||
| ground diameter | 0.881 ** | 1 | |||||
| root biomass | 0.548 * | 0.534 * | 1 | ||||
| CAT | 0.392 | 0.257 | 0.032 | 1 | |||
| SOD | 0.841 ** | 0.917 ** | 0.467 | 0.173 | 1 | ||
| POD | 0.533 * | 0.493 | 0.622 * | 0.089 | 0.249 | 1 | |
| MDA | −0.691 * | −0.700 * | −0.361 | −0.490 | −0.559 * | −0.288 | 1 |
| Index | Principal Component | |
|---|---|---|
| 1 | 2 | |
| seedling height | 0.948 | −0.060 |
| ground diameter | 0.944 | −0.004 |
| root biomass | 0.676 | 0.528 |
| POD | 0.602 | 0.525 |
| SOD | 0.851 | −0.047 |
| CAT | 0.394 | −0.716 |
| MDA | −0.777 | 0.374 |
| Eigenvalue | 4.091 | 1.212 |
| Contribution rate/% | 58.45 | 27.32 |
| Cumulative contribution rate/% | 58.45 | 85.77 |
| Family | 543 | 544 | 547 | 548 | 549 | 550 | 551 | 552 | 590 | 591 |
|---|---|---|---|---|---|---|---|---|---|---|
| Comprehensive scores | −0.11 | 0.91 | −1.75 | −1.62 | 0.32 | −0.92 | −0.43 | 1.84 | 1.30 | 0.45 |
| Comprehensive rank | 6 | 3 | 10 | 9 | 5 | 8 | 7 | 1 | 2 | 4 |
| Nutrient Patch | Heterogeneous Nutrient Patch | Homogeneous Nutrient Patches | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutrient-Rich Patches | Nutrient-Poor Patches | Side A | Side B | |||||||||
| N | P | K | N | P | K | N | P | K | N | P | K | |
| HET-N | 100 | 125 | 75 | 0 | 125 | 75 | 50 | 125 | 75 | 50 | 125 | 75 |
| HET-P | 50 | 250 | 75 | 50 | 0 | 75 | ||||||
| HET-K | 50 | 125 | 150 | 50 | 125 | 0 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, M.; Li, B.; Pan, Y.; Chen, W.; He, T.; Rong, J.; Chen, L.; Zheng, Y. Effects of Different Heterogeneous Nutrient Environments on the Growth and Activities of Enzymes in the Roots of Fokienia hodginsii Families. Plants 2023, 12, 4152. https://doi.org/10.3390/plants12244152
Deng M, Li B, Pan Y, Chen W, He T, Rong J, Chen L, Zheng Y. Effects of Different Heterogeneous Nutrient Environments on the Growth and Activities of Enzymes in the Roots of Fokienia hodginsii Families. Plants. 2023; 12(24):4152. https://doi.org/10.3390/plants12244152
Chicago/Turabian StyleDeng, Mi, Bingjun Li, Yanmei Pan, Wenchen Chen, Tianyou He, Jundong Rong, Liguang Chen, and Yushan Zheng. 2023. "Effects of Different Heterogeneous Nutrient Environments on the Growth and Activities of Enzymes in the Roots of Fokienia hodginsii Families" Plants 12, no. 24: 4152. https://doi.org/10.3390/plants12244152






