Genetic Basis of Resistance to Warrior (-) Yellow Rust Race at the Seedling Stage in Current Central and Northern European Winter Wheat Germplasm
Abstract
1. Introduction
2. Results
2.1. Phenotypic Evaluation for Warrior (-) Yellow Rust
2.2. GWAS for Resistance to Warrior (-) Yellow Rust at Seedling Stage
2.3. Correspondence to Published Yr Loci
3. Discussion
4. Materials and Methods
4.1. Seedling Stage Yellow Rust Assessment
4.2. Statistical Analysis
4.3. SNP Genotyping, Population Structure, and LD Analyses
4.4. Association Mapping
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Line, R.F. Stripe rust of wheat and barley in North America, a retrospective historical review. Annu. Rev. Phytopathol. 2002, 40, 75–118. [Google Scholar] [CrossRef] [PubMed]
- Waqar, A.; Khattak, S.H.; Begum, S.; Rehman, T.; Rabia, R.; Shehzad, A.; Ajmal, W.; Zia, S.S.; Siddiqi, I.; Ali, G.M. Stripe Rust: A Review of the Disease, Yr Genes and its Molecular Markers. Sarhad J. Agric. 2018, 34, 188–201. [Google Scholar] [CrossRef]
- Laidig, F.; Feike, T.; Hadasch, S.; Rentel, D.; Klocke, B.; Miedaner, T.; Piepho, H.P. Breeding progress of disease resistance and impact of disease severity under natural infections in winter wheat variety trials. Theor. Appl. Genet. 2021, 134, 1281–1302. [Google Scholar] [CrossRef]
- Chen, X. Epidemiology and control of stripe rust [Puccinia striiformis f. sp. tritici] on wheat. Can. J. Plant Pathol. 2005, 27, 314–337. [Google Scholar] [CrossRef]
- Yao, F.; Guan, F.; Duan, L.; Long, L.; Tang, H.; Jiang, Y.; Li, H.; Jiang, Q.; Wang, J.; Qi, P.; et al. Genome-Wide Association Analysis of Stable Stripe Rust Resistance Loci in a Chinese Wheat Landrace Panel Using the 660K SNP Array. Front. Plant Sci. 2021, 12, 783830. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wellings, C.; Chen, X.; Kang, Z.; Liu, T. Wheat stripe (yellow) rust caused by Puccinia striiformis f. sp. tritici. Mol. Plant Pathol. 2014, 15, 433–446. [Google Scholar] [CrossRef]
- Chen, X. Review Article: High-Temperature Adult-Plant Resistance, Key for Sustainable Control of Stripe Rust. Am. J. Plant Sci. 2013, 4, 608–627. [Google Scholar] [CrossRef]
- Maccaferri, M.; Zhang, J.; Bulli, P.; Abate, Z.; Chao, S.; Cantu, D.; Bossolini, E.; Chen, X.; Pumphrey, M.; Dubcovsky, J. A Genome-Wide Association Study of Resistance to Stripe Rust (Puccinia striiformis f. sp. tritici) in a Worldwide Collection of Hexaploid Spring Wheat (Triticum aestivum L.). G3 Genes Genomes Genet. 2015, 5, 449–465. [Google Scholar] [CrossRef]
- Hovmøller, M.S.; Walter, S.; Bayles, R.A.; Hubbard, A.; Flath, K.; Sommerfeldt, N.; Leconte, M.; Czembor, P.; Rodriguez-Algaba, J.; Thach, T.; et al. Replacement of the European wheat yellow rust population by new races from the centre of diversity in the near-Himalayan region. Plant Pathol. 2015, 65, 402–411. [Google Scholar] [CrossRef]
- Flath, K.; Schulz, P.; Klocke, B. RustWatch—Das erste Frühwarnsystem für Getreideroste in Europa. In Julius Kühn–Institut (Hrsg) 62. Deutsche Pflanzenschutztagung, Gesunde Pflanzen in Verantwortung für unsere Welt. 21–23 September 2021; Julius–Kühn–Archiv 467: Quedlinburg, German; pp. 211–212.
- Draz, I.S.; Serfling, A.; Muqaddasi, Q.H.; Röder, M.S. Quantitative trait loci for yellow rust resistance in spring wheat doubled haploid populations developed from the German Federal ex situ genebank genetic resources. Plant Genome 2021, 14, e20142. [Google Scholar] [CrossRef]
- Rosewarne, G.M.; Herrera-Foessel, S.A.; Singh, R.P.; Huerta-Espino, J.; Lan, C.X.; He, Z.H. Quantitative trait loci of stripe rust resistance in wheat. Theor. Appl. Genet. 2013, 126, 2427–2449. [Google Scholar] [CrossRef]
- Bulli, P.; Zhang, J.; Chao, S.; Chen, X.; Pumphrey, M. Genetic Architecture of Resistance to Stripe Rust in a Global Winter Wheat Germplasm Collection. G3 Genes Genomes Genet. 2016, 6, 2237–2253. [Google Scholar] [CrossRef] [PubMed]
- Tehseen, M.M.; Tonk, F.A.; Tosun, M.; Amri, A.; Sansaloni, C.P.; Kurtulus, E.; Yazbek, M.; Al-Sham’Aa, K.; Ozseven, I.; Bin Safdar, L.; et al. Genome-wide association study of resistance to PstS2 and Warrior races of Puccinia striiformis f. sp. tritici (stripe rust) in bread wheat landraces. Plant Genome 2020, 14, e20066. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Z.; Ali, M.; Mirza, J.I.; Fayyaz, M.; Majeed, K.; Naeem, M.K.; Aziz, A.; Trethowan, R.; Ogbonnaya, F.C.; Poland, J.; et al. Genome-Wide Association and Genomic Prediction for Stripe Rust Resistance in Synthetic-Derived Wheats. Front. Plant Sci. 2022, 13, 788593. [Google Scholar] [CrossRef] [PubMed]
- Shahinnia, F.; Geyer, M.; Schürmann, F.; Rudolphi, S.; Holzapfel, J.; Kempf, H.; Stadlmeier, M.; Löschenberger, F.; Morales, L.; Buerstmayr, H.; et al. Genome-wide association study and genomic prediction of resistance to stripe rust in current Central and Northern European winter wheat germplasm. Theor. Appl. Genet. 2022, 135, 3583–3595. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Fan, J.-B.; Campbell, D.; Chang, W.; Chen, J.; Doucet, D.; Yeakley, J.; Bibikova, M.; Garcia, E.W.; McBride, C.; et al. High-throughput SNP genotyping on universal bead arrays. Mutat. Res. Mol. Mech. Mutagen. 2005, 573, 70–82. [Google Scholar] [CrossRef] [PubMed]
- The International Wheat Genome Sequencing Consortium (IWGSC); Appels, R.; Eversole, K.; Feuillet, C.; Keller, B.; Rogers, J.; Stein, N.; Pozniak, C.J.; Stein, N.; Choulet, F.; et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef]
- Gao, L.; Turner, M.K.; Chao, S.; Kolmer, J.; Anderson, J.A. Genome Wide Association Study of Seedling and Adult Plant Leaf Rust Resistance in Elite Spring Wheat Breeding Lines. PLoS ONE 2016, 11, e0148671. [Google Scholar] [CrossRef] [PubMed]
- Rollar, S.; Geyer, M.; Hartl, L.; Mohler, V.; Ordon, F.; Serfling, A. Quantitative Trait Loci Mapping of Adult Plant and Seedling Resistance to Stripe Rust (Puccinia striiformis Westend.) in a Multiparent Adv. Gener. Intercross Wheat Population. Front. Plant Sci. 2021, 12, 684671. [Google Scholar] [CrossRef] [PubMed]
- Zetzsche, H.; Serfling, A.; Ordon, F. Breeding Progress in Seedling Resistance against Various Races of Stripe and Leaf Rust in European Bread Wheat. Crop. Breed. Genet. Genom. 2019, 1, e190021. [Google Scholar] [CrossRef]
- Lin, F.; Chen, X.M. Quantitative trait loci for non-race-specific, high-temperature adult-plant resistance to stripe rust in wheat cultivar Express. Theor. Appl. Genet. 2008, 118, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Bariana, H.S.; Bansal, U.; Schmidt, A.; Lehmensiek, A.; Kaur, J.; Miah, H.; Howes, N.; McIntyre, C. Molecular mapping of adult plant stripe rust resistance in wheat and identification of pyramided QTL genotypes. Euphytica 2010, 176, 251–260. [Google Scholar] [CrossRef]
- Zwart, R.S.; Thompson, J.P.; Milgate, A.W.; Bansal, U.K.; Williamson, P.M.; Raman, H.; Bariana, H.S. QTL mapping of multiple foliar disease and root-lesion nematode resistances in wheat. Mol. Breed. 2010, 26, 107–124. [Google Scholar] [CrossRef]
- Bansal, U.K.; Kazi, A.G.; Singh, B.; Hare, R.A.; Bariana, H.S. Mapping of durable stripe rust resistance in a durum wheat cultivar Wollaroi. Mol. Breed. 2013, 33, 51–59. [Google Scholar] [CrossRef]
- Melichar, J.P.E.; Berry, S.; Newell, C.; MacCormack, R.; Boyd, L.A. QTL identification and microphenotype characterisation of the developmentally regulated yellow rust resistance in the UK wheat cultivar Guardian. Theor. Appl. Genet. 2008, 117, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Basnet, B.R.; Singh, R.P.; Ibrahim, A.M.; Herrera-Foessel, S.A.; Huerta-Espino, J.; Lan, C.; Rudd, J.C. Characterization of Yr54 and other genes associated with adult plant resistance to yellow rust and leaf rust in common wheat Quaiu 3. Mol. Breed. 2014, 33, 385–399. [Google Scholar] [CrossRef]
- Lan, C.; Rosewarne, G.M.; Singh, R.P.; Herrera-Foessel, S.A.; Huerta-Espino, J.; Basnet, B.R.; Zhang, Y.; Yang, E. QTL characterization of resistance to leaf rust and stripe rust in the spring wheat line Francolin#1. Mol. Breed. 2014, 34, 789–803. [Google Scholar]
- William, M.; Singh, R.P.; Huerta-Espino, J.; Islas, S.O.; Hoisington, D. Molecular marker mapping of leaf rust resistance gene Lr46 and its association with stripe rust resistance gene Yr29 in wheat. Phytopathology 2003, 93, 153–159. [Google Scholar] [CrossRef]
- Pradhan, A.K.; Kumar, S.; Singh, A.K.; Budhlakoti, N.; Mishra, D.C.; Chauhan, D.; Mittal, S.; Grover, M.; Kumar, S.; Gangwar, O.P.; et al. Identification of QTLs/Defense Genes Effective at Seedling Stage Against Prevailing Races of Wheat Stripe Rust in India. Front. Genet. 2020, 11, 572975. [Google Scholar] [CrossRef]
- Zhang, P.; Yan, X.; Gebrewahid, T.-W.; Zhou, Y.; Yang, E.; Xia, X.; He, Z.; Li, Z.; Liu, D. Genome-wide association mapping of leaf rust and stripe rust resistance in wheat accessions using the 90K SNP array. Theor. Appl. Genet. 2021, 134, 1233–1251. [Google Scholar] [CrossRef]
- Boukhatem, N.; Baret, P.V.; Mingeot, D.; Jacquemin, J.M. Quantitative trait loci for resistance against Yellow rust in two wheat-derived recombinant inbred line populations. Theor. Appl. Genet. 2002, 104, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; He, Z.; Li, J.; Lillemo, M.; Wu, L.; Bai, B.; Lu, Q.; Zhu, H.; Zhou, G.; Du, J.; et al. QTL mapping of adult-plant resistance to stripe rust in a population derived from common wheat cultivars Naxos and Shanghai 3/Catbird. Theor. Appl. Genet. 2012, 125, 1211–1221. [Google Scholar] [CrossRef]
- Rosewarne, G.M.; Singh, R.P.; Huerta-Espino, J.; Herrera-Foessel, S.A.; Forrest, K.L.; Hayden, M.J.; Rebetzke, G.J. Analysis of leaf and stripe rust severities reveals pathotype changes and multiple minor QTLs associated with resistance in an Avocet × Pastor wheat population. Theor. Appl. Genet. 2012, 124, 1283–1294. [Google Scholar] [CrossRef]
- Chen, J.; Chu, C.; Souza, E.J.; Guttieri, M.J.; Chen, X.; Xu, S.; Hole, D.; Zemetra, R. Genome-wide identification of QTL conferring high-temperature adult-plant (HTAP) resistance to stripe rust (Puccinia striiformis f. sp. tritici) in wheat. Mol. Breed. 2011, 29, 791–800. [Google Scholar] [CrossRef]
- Mu, J.; Liu, L.; Liu, Y.; Wang, M.; See, D.R.; Han, D.; Chen, X. Genome-Wide Association Study and Gene Specific Markers Identified 51 Genes or QTL for Resistance to Stripe Rust in U.S. Winter Wheat Cultivars and Breeding Lines. Front. Plant Sci. 2020, 11, 998. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, M.; Zhang, Z.; See, D.R.; Chen, X. Identification of stripe rust resistance loci in U.S. spring wheat cultivars and breeding lines using genome-wide association mapping and YrGene markers. Plant Dis. 2020, 104, 2181–2192. [Google Scholar] [CrossRef]
- Akdemir, D.; Rio, S.; Sánchez, J.I.Y. TrainSel: An R Package for Selection of Training Populations. Front. Genet. 2021, 12, 655287. [Google Scholar] [CrossRef]
- McNeal, F.H.; Konzak, C.F.; Smith, E.P.; Tate, W.S.; Russell, T.S. A Uniform System for Recording and Processing Cereal Research Data. Agric. Res. Serv. Bull. 1971, 34, 121–143. [Google Scholar]
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Earl, D.A.; vonHoldt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, R.A.; Yamazaki, Y.; Dubcovsky, J.; Rogers, J.; Morris, C.; Appels, R. Catalogue of gene symbols for wheat. In Proceedings of the 12th International Wheat Genetics Symposium, Yokohama Japan, 8 September 2013. [Google Scholar]
- Agenbag, G.M.; Pretorius, Z.A.; Boyd, L.A.; Bender, C.M.; Prins, R. Identification of adult plant resistance to stripe rust in the wheat cultivar Cappelle-Desprez. Theor. Appl. Genet. 2012, 125, 109–120. [Google Scholar]
- Bariana, H.; Kant, L.; Qureshi, N.; Forrest, K.; Miah, H.; Bansal, U. Identification and Characterisation of Stripe Rust Resistance Genes Yr66 and Yr67 in Wheat Cultivar VL Gehun 892. Agronomy 2022, 12, 318. [Google Scholar]
- Basnet, B.R.; Ibrahim, A.; Chen, X.; Singh, R.; Mason, E.; Bowden, R.L.; Liu, S.; Hays, D.B.; Devkota, R.N.; Subramanian, N.K.; et al. Molecular mapping of stripe rust resistance in hard red winter wheat TAM 111 adapted to the U.S. high plains. Crop Sci. 2014, 54, 1361–1373. [Google Scholar]
- Liu, J.; Chang, Z.; Zhang, X.; Yang, Z.; Li, X.; Jia, J.; Zhan, H.; Guo, H.; Wang, J. Putative Thinopyrum intermedium‐derived stripe rust resistance gene Yr50 maps on wheat chromosome arm 4BL. Theor. Appl. Genet. 2013, 126, 265–274. [Google Scholar]
- Lu, Y.; Lan, C.; Liang, S.; Zhou, X.; Liu, D.; Zhou, G.; Lu, Q.; Jing, J.; Wang, M.; Xia, X.; et al. QTL mapping for adult‐plant resistance to stripe rust in Italian common wheat cultivars Libellula and Strampelli. Theor. Appl. Genet. 2009, 119, 1349–1359. [Google Scholar]
- Prins, R.; Pretorius, Z.; Bender, C.; Lehmensiek, A. QTL mapping of stripe, leaf and stem rust resistance genes in a Kariega × Avocet S doubled haploid wheat population. Mol. Breed. 2011, 27, 259–270. [Google Scholar]
- William, H.M.; Singh, R.; Huerta-Espino, J.; Palacios, G.; Suenaga, K. Characterization of genetic loci conferring adult plant resistance to leaf rust and stripe rust in spring wheat. Genome 2006, 49, 977–990. [Google Scholar]
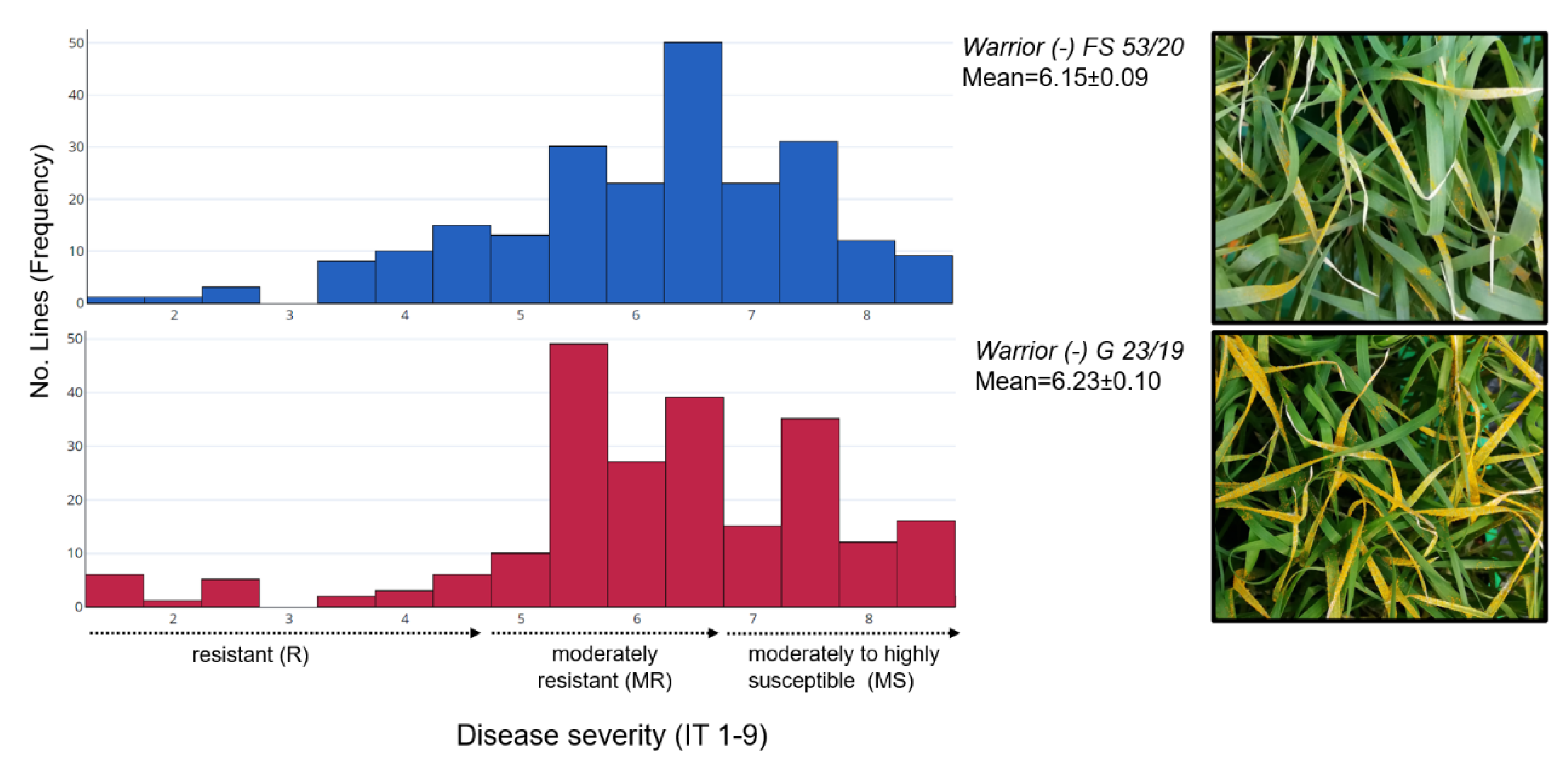
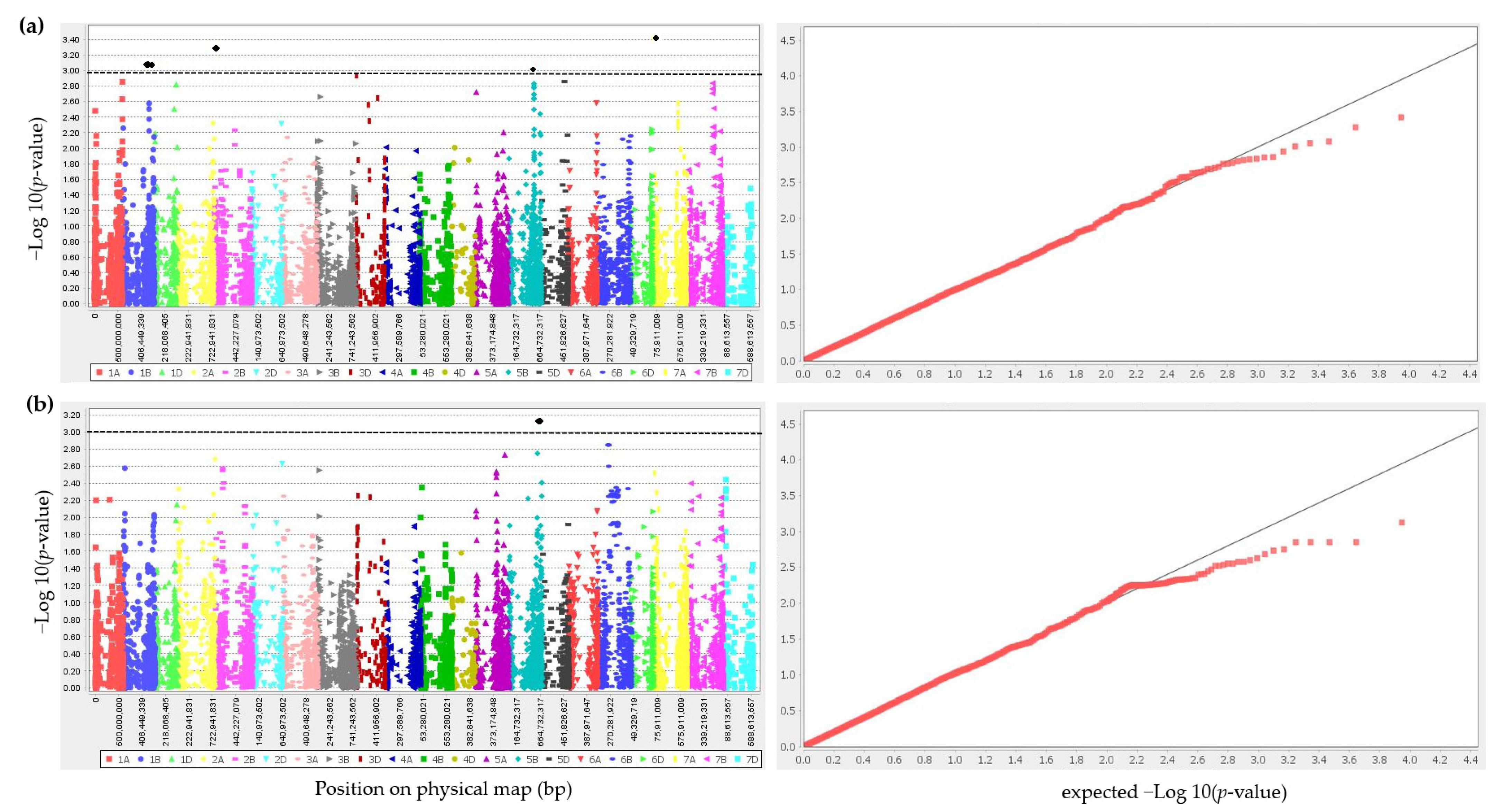
| Pathotype | Marker | Chromosome | Position (bp) | R2 | SNPs | Number of Accessions with Resistant Allele | Number of Accessions with Susceptible Allele | Number of Accessions with Missing Alleles | IT | Effect | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Warrior (-) FS 53/20 | |||||||||||
| Kukri_c5357_323 | 1B | 637,621,766 | 0.051 | G/T | 18 | 210 | 1 | 5.00/6.24 | 1.20 | 0.0008 | |
| Tdurum_contig13879_352 | 1B | 680,162,719 | 0.051 | A/G | 193 | 34 | 2 | 6.00/6.91 | 0.91 | 0.0009 | |
| BS00098033_51 | 2A | 778,724,092 | 0.055 | C/T | 19 | 205 | 5 | 5.02/6.26 | 1.16 | 0.0005 | |
| CAP12_c703_150 | 5B | 550,377,847 | 0.053 | C/T | 95 | 126 | 8 | 5.89/6.38 | 0.76 | 0.0010 | |
| BS00062869_51 | 7A | 17,454,693 | 0.061 | A/G | 21 | 205 | 3 | 5.06/6.27 | 1.14 | 0.0004 | |
| Warrior (-) G 23/19 | |||||||||||
| Excalibur_c17489_804 | 5B | 670,829,789 | 0.052 | C/T | 104 | 120 | 5 | 6.48/6.01 | 0.81 | 0.0008 | |
| SNP/IT | Kukri_c5357_323 | Tdurum_contig13879_352 | BS00098033_51 | CAP12_c703_150 | BS00062869_51 | Excalibur_c17489_804 | IT Against Warrior (-)FS 53/20 | IT Against Warrior (-) G23/19 |
|---|---|---|---|---|---|---|---|---|
| Line/Allele | G | A | C | C | A | T | ||
| TS049 | X | X | X | 3.67 | 2.33 | |||
| TS111 | X | X | X | X | X | 1.67 | 4.33 | |
| TS185 | X | X | X | X | X | 2.33 | 2.67 | |
| TS229 | X | X | X | X | 2.01 | 2.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahinnia, F.; Mohler, V.; Hartl, L. Genetic Basis of Resistance to Warrior (-) Yellow Rust Race at the Seedling Stage in Current Central and Northern European Winter Wheat Germplasm. Plants 2023, 12, 420. https://doi.org/10.3390/plants12030420
Shahinnia F, Mohler V, Hartl L. Genetic Basis of Resistance to Warrior (-) Yellow Rust Race at the Seedling Stage in Current Central and Northern European Winter Wheat Germplasm. Plants. 2023; 12(3):420. https://doi.org/10.3390/plants12030420
Chicago/Turabian StyleShahinnia, Fahimeh, Volker Mohler, and Lorenz Hartl. 2023. "Genetic Basis of Resistance to Warrior (-) Yellow Rust Race at the Seedling Stage in Current Central and Northern European Winter Wheat Germplasm" Plants 12, no. 3: 420. https://doi.org/10.3390/plants12030420
APA StyleShahinnia, F., Mohler, V., & Hartl, L. (2023). Genetic Basis of Resistance to Warrior (-) Yellow Rust Race at the Seedling Stage in Current Central and Northern European Winter Wheat Germplasm. Plants, 12(3), 420. https://doi.org/10.3390/plants12030420







