Effect of Natural Liquid Hydroabsorbents on Ammonia Emission from Liquid Nitrogen Fertilizers and Plant Growth of Maize (Zea Mays L.) under Drought Conditions
Abstract
1. Introduction
2. Results and Discussion
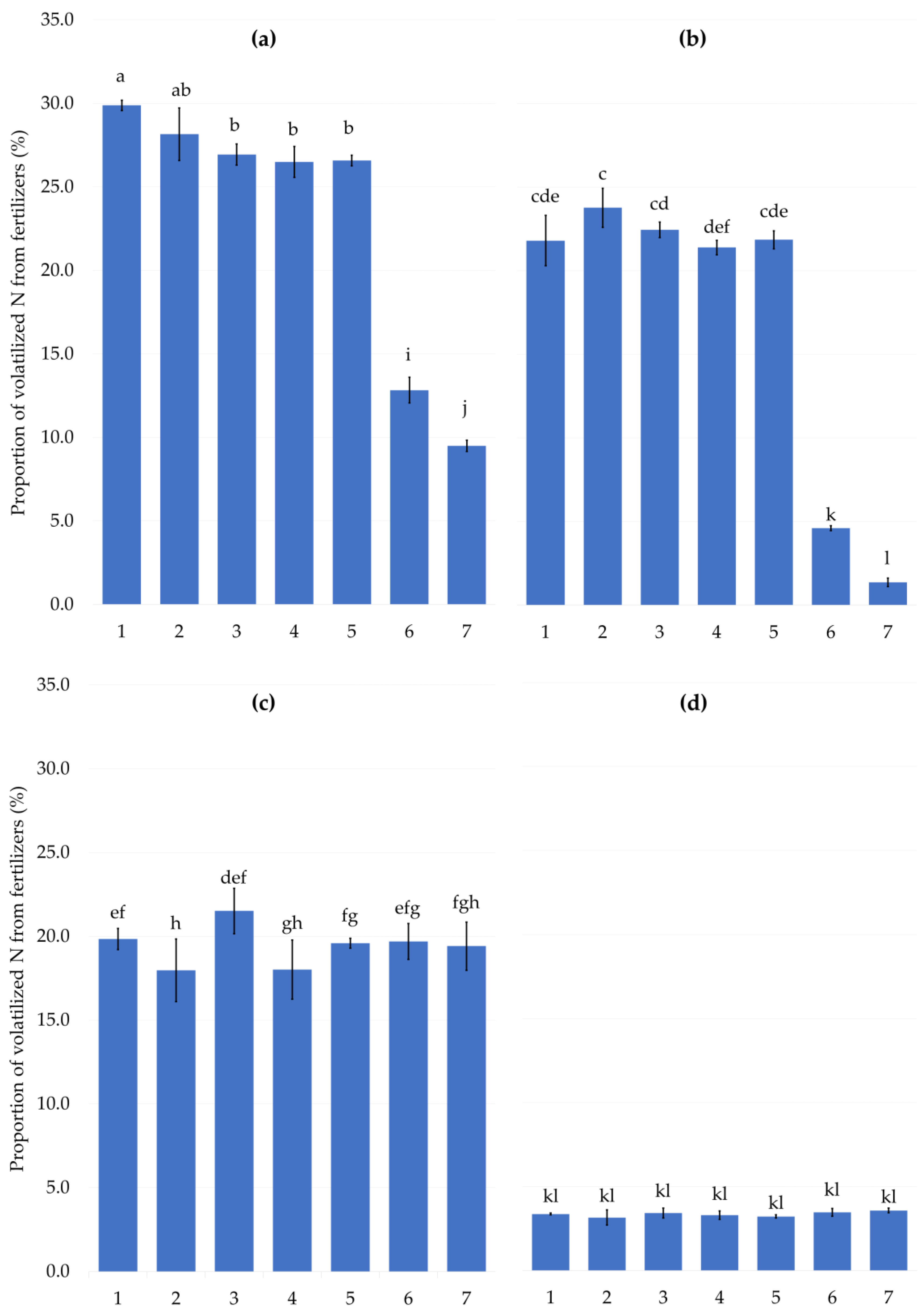
2.1. The Laboratory Experiment
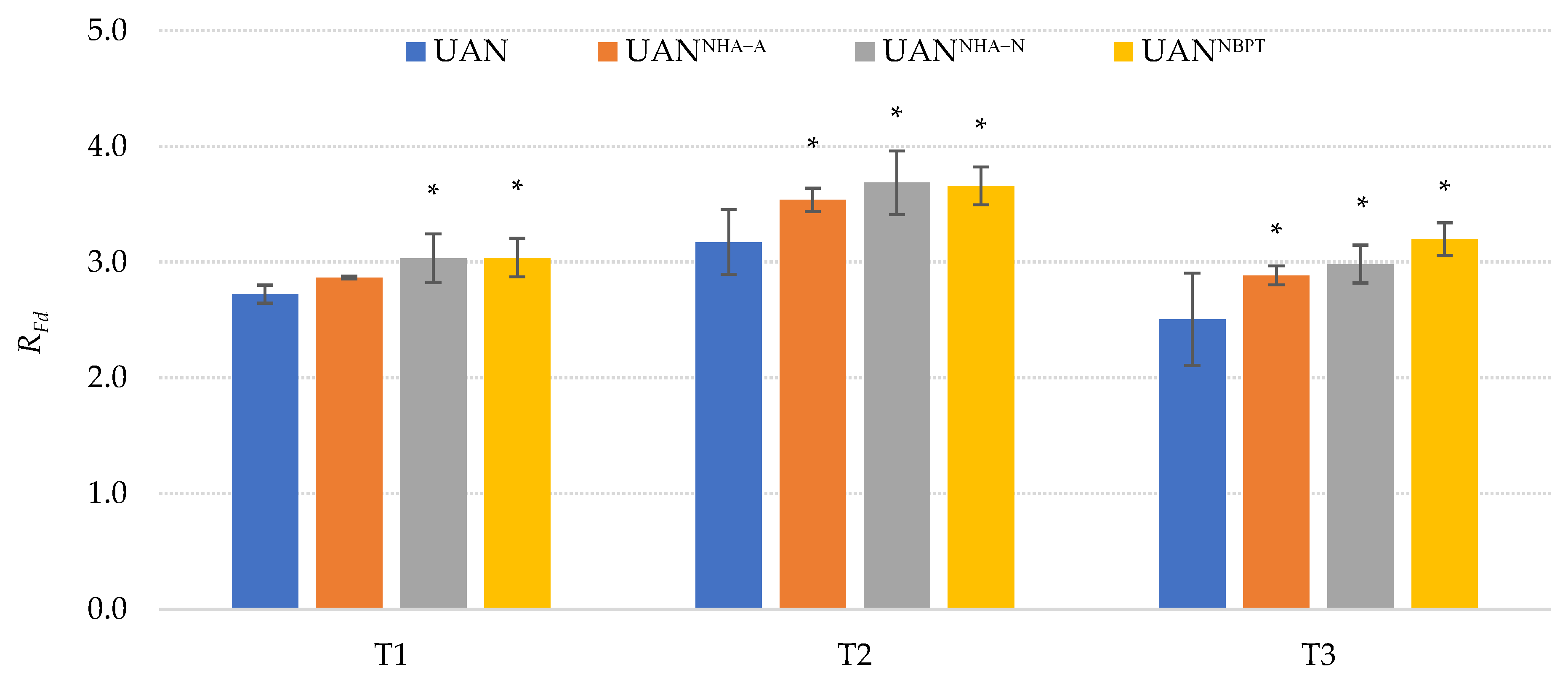
2.2. The Greenhouse Experiment
3. Materials and Methods
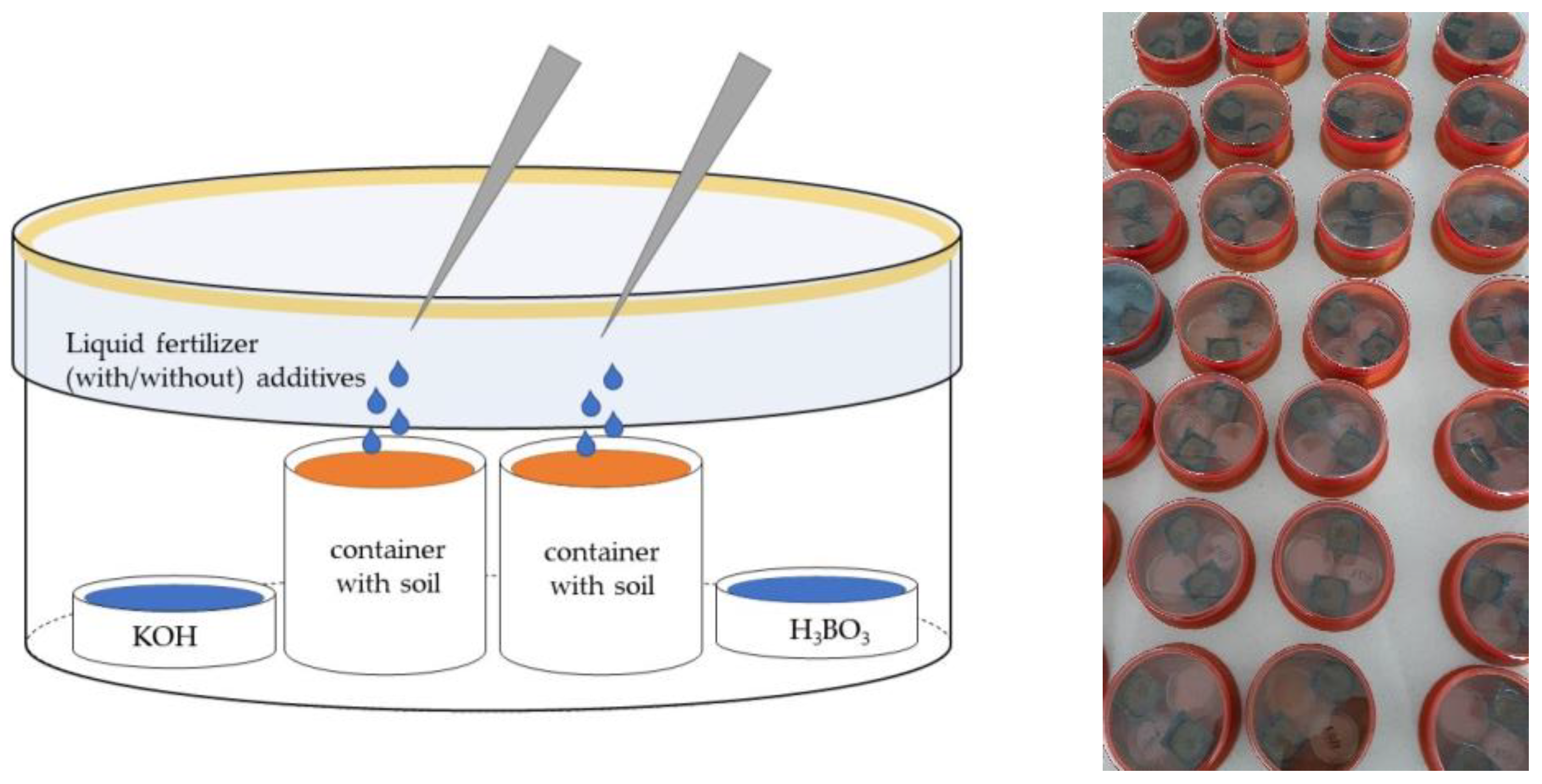
3.1. The Laboratory Experiment
3.1.1. Materials
3.1.2. Experimental Design and Measurements
3.2. The Greenhouse Experiment
3.2.1. Materials and Experimental Design
3.2.2. Measurement of Maize Growth Parameters
3.3. Statistical Data Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IFA. Consumption Nitrogen World. Available online: https://www.ifastat.org/market-outlooks (accessed on 10 October 2022).
- Tian, Z.; Liu, X.; Yu, J.; Gu, S.; Zhang, J.; Jiang, D.; Cao, W.; Dai, T. Early nitrogen deficiency favors high nitrogen recovery efficiency by improving deeper soil root growth and reducing nitrogen loss in wheat. Arch. Agron. Soil Sci. 2020, 66, 1384–1398. [Google Scholar] [CrossRef]
- Santillano-Cázares, J.; Núñez-Ramírez, F.; Ruíz-Alvarado, C.; Cárdenas-Castañeda, M.E.; Ortiz-Monasterio, I. Assessment of Fertilizer Management Strategies Aiming to Increase Nitrogen Use Efficiency of Wheat Grown under Conservation Agriculture. Agronomy 2018, 8, 304. [Google Scholar] [CrossRef]
- Millar, N.; Robertson, G.P.; Grace, P.R.; Gehl, R.J.; Hoben, J.P. Nitrogen fertilizer management for nitrous oxide (N2O) mitigation in intensive corn (Maize) production: An emissions reduction protocol for US Midwest agriculture. Mitig. Adapt. Strateg. Glob. Chang. 2010, 15, 185–204. [Google Scholar] [CrossRef]
- Sundaram, P.K.; Mani, I.; Lande, S.D.; Parray, R.A. Evaluation of urea ammonium nitrate application on the performance of wheat. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1956–1963. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Yao, J.; Bao, D.J.; He, A.L.; Luo, X.S.; Du, J. Effects of ammonium nitrate solution on yield, quality and nutrient uptake in maize. J. Agric. Sci. Technol. 2018, 20, 113–121. [Google Scholar]
- Wang, Y.; Xu, Z.; Li, B.N.; Gao, Q.; Feng, G.Z.; Li, C.L.; Yan, L.; Wang, S. Effects of urea ammonium nitrate solution on grain yield and nitrogen uptake of spring maize in black soil region. Sci. Agric. Sin. 2018, 51, 718–727. [Google Scholar]
- Soares, J.R.; Cantarella, H.; Menegale, M.L. Ammonia volatilization losses from surface-applied urea with urease and nitrification inhibitors. Soil Biol. Biochem. 2012, 52, 82–89. [Google Scholar] [CrossRef]
- Hu, Y.; Gaßner, M.P.; Weber, A.; Schraml, M.; Schmidhalter, U. Direct and Indirect Effects of Urease and Nitrification Inhibitors on N2O-N Losses from Urea Fertilization to Winter Wheat in Southern Germany. Atmosphere 2020, 11, 782. [Google Scholar] [CrossRef]
- Turner, D.A.; Edis, R.B.; Chen, D.; Freney, J.R.; Denmead, O.T.; Christie, R. Determination and mitigation of ammonia loss from urea applied to winter wheat with N-(n-butyl) thiophosphorictriamide. Agric. Ecosyst. Environ. 2010, 137, 261–266. [Google Scholar] [CrossRef]
- Wang, Z.H.; Liu, X.J.; Ju, X.T.; Zhang, F.S.; Malhi, S.S. Ammonia Volatilization Loss from Surface-Broadcast Urea: Comparison of Vented and Closed-Chamber Methods and Loss in Winter Wheat–Summer Maize Rotation in North China Plain. Commun. Soil Sci. Plant Anal. 2004, 35, 2917–2939. [Google Scholar] [CrossRef]
- Rochette, P.; Angers, D.A.; Chantigny, M.H.; Gasser, M.O.; Macdonald, J.D.; Pelster, D.E.; Bertrand, N. Ammonia Volatilization and Nitrogen Retention: How Deep to Incorporate Urea? J. Environ. Qual. 2013, 42, 1635–1642. [Google Scholar] [CrossRef]
- Black, A.S.; Sherlock, R.R.; Smith, N.P. Effect of timing of simulated rainfall on ammonia volatilization from urea, applied to soil of varyingmoisture content. J. Soil Sci. 1987, 38, 679–687. [Google Scholar] [CrossRef]
- Khan, I.; Zaman, M.; Khan, M.J.; Iqbal, M.; Babar, M.N. How to improve yield and quality of potatoes: Effects of two rates of urea N, urease inhibitor and Cytozyme nutritional program. J. Soil Sci. Plant Nutr. 2014, 14, 268–276. [Google Scholar] [CrossRef]
- Cantarella, H.; Otto, R.; Soares, J.R.; Silva, A.G. Agronomic efficiency of NBPT as a urease inhibitor: A review. J. Adv. Res. 2018, 13, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Suter, H.; Sultana, H.; Turner, D.; Davies, R.; Walker, C.; Chen, D. Influence of urea fertiliser formulation, urease inhibitor and season on ammonia loss from ryegrass. Nutr. Cycl. Agroecosyst. 2013, 95, 175–185. [Google Scholar] [CrossRef]
- Rawluk, C.D.L.; Grant, C.A.; Racz, G.J. Ammonia volatilization from soils fertilized with urea and varying rates of urease inhibitor NBPT. Can. J. Soil Sci. 2001, 81, 239–246. [Google Scholar] [CrossRef]
- Sanz-Cobena, A.; Misselbrook, T.; Camp, V.; Vallejo, A. Effect of water addition and the urease inhibitor NBPT on the abatement of ammonia emission from surface applied urea. Atmos. Environ. 2011, 45, 1517–1524. [Google Scholar] [CrossRef]
- Gill, J.S.; Bijay-Singh; Khind, C.S.; Yadvinder-Singh. Efficiency of N-(n-butyl) thiophosphoric triamide in retarding hydrolysis of urea and ammonia volatilization losses in a flooded sandy loam soil amended with organic materials. Nutr. Cycl. Agroecosyst. 1999, 53, 203–207. [Google Scholar] [CrossRef]
- Horie, K.; Barón, M.; Fox, R.B.; He, J.; Hess, M.; Kahovec, J.; Kitayama, T.; Kubisa, P.; Maréchal, E.; Mormann, W.; et al. Definitions of terms relating to reactions of polymers and to functional polymeric materials (IUPAC Recommendations 2003). Pure Appl. Chem. 2004, 76, 889–906. [Google Scholar] [CrossRef]
- Khadem, S.A.; Galavi, M.; Ramrodi, M.; Mousavi, S.R.; Rousta, M.J.; Rezvani-Moghadam, P. Effect of Animal Manure and Superabsorbent Polymer on Corn Leaf Relative Water Content, Cell Membrane Stability and Leaf Chlorophyll Content under Dry Condition. Aust. J. Crop Sci. 2010, 4, 642–647. [Google Scholar]
- Islam, M.R.; Zeng, Z.; Mao, J.; Eneji, A.E.; Xue, X.; Hu, Y. Feasibility of summer corn (Zea mays L.) production in drought affected areas of northern China using watersaving superabsorbent polymer. Plant Soil Environ. 2011, 57, 279–285. [Google Scholar] [CrossRef]
- Pedroza-Sandoval, A.; Yáñez-Chávez, L.G.; Sánchez-Cohen, I.; Samaniego-Gaxiola, J.A.; Trejo-Calzada, R. Hydrogel, biocompost and its effect on photosynthetic activity and production of forage maize plants (Zea mays L.). Acta Agron. 2016, 66, 63–68. [Google Scholar] [CrossRef]
- Bečka, D.; Borokov, P.; Mikšík, V.; Vašák, J. Performance Comparison of Winter Oilseed Rape Varieties in the Slovakia in 2017/18. In Prosperous Oil Crops 2018, Proceedings of the Conference Prosperous Oil Crops 2018, Nové Strašecí, Czech Republic, 4 December 2018; Czech University Life Sciences Prague: Prague, Czech Republic, 2018; pp. 32–35. [Google Scholar]
- Hou, X.; Li, R.; He, W.; Dai, X.; Ma, K.; Liang, Y. Superabsorbent polymers influence soil physical properties and increase potato tuber yield in a dry-farming region. J. Soils Sediments 2018, 18, 816–826. [Google Scholar] [CrossRef]
- Zhang, L.; Loh, K.C.; Sarvanantharajah, S.; Shen, Y.; Tong, Y.W.; Wang, C.V.; Dai, Y. Recovery of Nitrogen and Phosphorus Nutrition from Anaerobic Digestate by Natural Superabsorbent Fiber-Based Adsorbent and Reusing as an Environmentally Friendly Slow-Release Fertilizer for Horticultural Plants. Waste Biomass Valorization 2020, 11, 5223–5237. [Google Scholar] [CrossRef]
- Verma, A.K.; Sindhu, S.S.; Anand, P.; Singh, A.; Chauhan, V.B.S.; Verma, S.K. Vermi products and biodegradable superabsorbent polymer improve physiological activities and leaf nutrient contents of gerbera. Res. J. Biotechnol. 2018, 13, 8–18. [Google Scholar]
- Kwon, Y.R.; Hong, S.J.; Lim, S.H.; Kim, J.S.; Chang, Y.W.; Choi, J.; Kim, D.H. Ionic-bonded superabsorbent polymers and their surface-crosslinking using modified silica. Polym.-Plast. Technol. Mater. 2021, 60, 724–733. [Google Scholar] [CrossRef]
- Ni, B.; Liu, M.; Lü, S. Multifunctional slow-release urea fertilizer from ethylcellulose and superabsorbent coated formulations. J. Chem. Eng. 2009, 155, 892–898. [Google Scholar] [CrossRef]
- Teodorescu, M.; Lungu, A.; Stanescu, P.O. Preparation and Properties of Novel Slow-Release NPK Agrochemical Formulations Based on Poly(acrylic acid) Hydrogels and Liquid Fertilizers. Ind. Eng. Chem. Res. 2009, 48, 6527–6534. [Google Scholar] [CrossRef]
- Mikkelsen, R.L.; Behel, A.D.; Williams, H.M. Addition of gel-forming hydrophilic polymers to nitrogen fertilizer solutions. Fertil. Res. 1993, 36, 55–61. [Google Scholar] [CrossRef]
- Alam, M.N.; Christopher, L.P. Natural Cellulose-Chitosan Cross-Linked Superabsorbent Hydrogels with Superior Swelling Properties. ACS Sustain. Chem. Eng. 2018, 6, 8736–8742. [Google Scholar] [CrossRef]
- Skarpa, P.; Jancar, J.; Lepcio, P.; Antosovsky, J.; Klofac, D.; Kriska, T.; Abdel-Mohsen, A.M.; Brtnicky, M. Effect of fertilizers enriched with bio-based carriers on selected growth parameters, grain yield and grain quality of maize (Zea mays L.). Eur. J. Agron. 2023, 143, 126714. [Google Scholar] [CrossRef]
- Demitri, C.; Scalera, F.; Madaghiele, M.; Sannino, A.; Maffezzoli, A. Potential of Cellulose-Based Superabsorbent Hydrogels as Water Reservoir in Agriculture. Int. J. Polym. Sci. 2013, 2013, 435073. [Google Scholar] [CrossRef]
- Kong, W.; Li, Q.; Li, X.; Su, Y.; Yue, Q.; Gao, B. A biodegradable biomass-based polymeric composite for slow release and water retention. J. Environ. Manag. 2019, 230, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Senna, A.M.; Braga do Carmo, J.; Santana da Silva, J.M.; Botaro, V.R. Synthesis, characterization and application of hydrogel derived from cellulose acetate as a substrate for slow-release NPK fertilizer and water retention in soil. J. Environ. Chem. Eng. 2015, 3, 996–1002. [Google Scholar] [CrossRef]
- Teli, M.D.; Mallick, A. Application of Sorghum Starch for Preparing Superabsorbent. J. Polym. Environ. 2018, 26, 1581–1591. [Google Scholar] [CrossRef]
- Milani, P.; França, D.; Balieiro, A.G.; Faez, R. Polymers and its applications in agriculture. Polimeros 2017, 27, 256–266. [Google Scholar] [CrossRef]
- Kolackova, I.; Gruberova, H.A.; Kratochvil, O.; Baholet, D.; Skladanka, J.; Jancar, J.; Skarpa, P. Effect of foliar copper-containing superabsorbent polymers on nutritional characteristics and mycotoxin contamination of wheat kernel. Acta Univ. Agric. Silvic. Mendel. Brun. 2021, 69, 71–78. [Google Scholar] [CrossRef]
- Triplett, G.B.; Dick, W.A. No-tillage crop production: A revolution in agriculture. Agron. J. 2008, 100, 153–156. [Google Scholar] [CrossRef]
- Holcomb, J.C.; Sullivan, D.M.; Horneck, D.A.; Clough, G.H. Effect of irrigation rate on ammonia volatilization. Soil Sci. Soc. Am. J. 2011, 75, 2341–2347. [Google Scholar] [CrossRef]
- Harty, M.; McGeough, K.L.; Ramsey, R.; Muller, C. Gross nitrogen transformations in grassland soil react differently to urea stabilisers under laboratory and field conditions. Soil Biol. Biochem. 2017, 109, 23–34. [Google Scholar] [CrossRef]
- Li, D.; Watson, C.J.; Yan, M.J.; Lalor, S.; Rafique, R.; Hyde, B.; Lanigan, G.; Richards, K.G.; Holden, N.M.; Humphreys, J.A. review of nitrous oxide mitigation by farm nitrogen management in temperate grassland-based agriculture. J. Environ. Manag. 2013, 128, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Al-Kanani, T.; MacKenzie, A.F.; Blenkhorn, H. Volatilization of ammonia from urea-ammonium nitrate solutions as influenced by organic and inorganic additives. Fertil. Res. 1990, 23, 113–119. [Google Scholar] [CrossRef]
- Nikolajsen, M.T.; Pacholski, A.S.; Sommer, S.G. Urea Ammonium Nitrate Solution Treated with Inhibitor Technology: Effects on Ammonia Emission Reduction, Wheat Yield, and Inorganic N in Soil. Agronomy 2020, 10, 161. [Google Scholar] [CrossRef]
- Turner, D.A.; Edis, R.E.; Chen, D.; Freney, J.R.; Denmead, O.T. Ammonia volatilization from nitrogen fertilizers applied to cereals in two cropping areas of southern Australia. Nutr. Cycl. Agroecosyst. 2012, 93, 113–126. [Google Scholar] [CrossRef]
- Gezgin, S.; Bayrakll, F. Ammonia volatilization from ammonium sulphate, ammonium nitrate, and urea surface applied to winter wheat on a calcareous soil. J. Plant Nutr. 1995, 18, 2483–2494. [Google Scholar] [CrossRef]
- Fenn, L.B.; Miyamoto, S. Ammonia loss and associated reactions of urea in calcareous soils. J. Soil Sci. Soc. Am. 1981, 45, 537–540. [Google Scholar] [CrossRef]
- Byrnes, B.H.; Freney, J.R. Recent developments on the use of urease inhibitors in the tropics. Fertil. Res. 1995, 42, 251–259. [Google Scholar] [CrossRef]
- Bremner, J.M. Recent research on problems in the use of urea as a nitrogen fertilizer. Fertil. Res. 1995, 42, 321–329. [Google Scholar] [CrossRef]
- Phongpan, S.; Freney, J.R.; Keerthisinghe, D.G.; Chaiwanakupt, P. Use of phenylphosphorodiamidate and N-(n-butyl)thiophosphorictriamide to reduce ammonia loss and increase grain-yield following application of urea to flooded rice. Fertil. Res. 1995, 41, 59–66. [Google Scholar] [CrossRef]
- Abalos, D.; Sanz-Cobena, A.; Misselbrook, T.; Vallejo, A. Effectiveness of urease inhibition on the abatement of ammonia, nitrous oxide and nitric oxide emissions in a non-irrigated Mediterranean barley field. Chemosphere 2012, 89, 310–318. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Alva, A.K. High Water Regime Can Reduce Ammonia Volatilization from Soils under Potato Production. Commun. Soil Sci. Plant Anal. 2007, 38, 1203–1220. [Google Scholar] [CrossRef]
- Souza, T.L.; Guelfi, D.R.; Silva, A.L.; Andrade, A.B.; Chagas, W.F.T.; Cancellier, E.L. Ammonia and carbon dioxide emissions by stabilized conventional nitrogen fertilizers and controlled release in corn crop. Cienc. Agrotec. 2017, 41, 494–510. [Google Scholar] [CrossRef]
- Corrêa, D.; Cardoso, A.S.; Ferreira, M.R.; Siniscalchi, D.; Gonçalves, P.H.D.A.; Lumasini, R.N.; Reis, R.A.; Ruggieri, A.C. Ammonia Volatilization, Forage Accumulation, and Nutritive Value of Marandu Palisade Grass Pastures in Different N Sources and Doses. Atmosphere 2021, 12, 1179. [Google Scholar] [CrossRef]
- He, Z.L.; Alva, A.K.; Calvert, D.V.; Banks, D.J. Ammonia volatilization from different fertilizer sources and effect of temperature and soil pH. Soil Sci. 1999, 164, 750–758. [Google Scholar] [CrossRef]
- Parvathy, P.C.; Jyothi, A.N.; John, K.S.; Sreekumar, J. Cassava Starch Based Superabsorbent Polymer as Soil Conditioner: Impact on Soil Physico-Chemical and Biological Properties and Plant Growth. Clean-Soil Air Water 2014, 42, 1610–1617. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K.; Scherer, H.W. Fixation and defixation of ammonium in soils: A review. Biol. Fertil. Soils 2011, 47, 1–14. [Google Scholar] [CrossRef]
- Chin, S.; Schmidt, S.; Buckley, S.; Pirie, R.; Redding, M.; Laycock, B.; Luckman, P.; Batstone, D.J.; Robinson, N.; Brackin, R. Sorbents can tailor nitrogen release from organic wastes to match the uptake capacity of crops. Sci. Total Environ. 2018, 645, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Khodadi Dehkordi, D. Effects of hydrophillic polymers on soil, water, wheat plants and microorganisms. Appl. Ecol. Environ. Res. 2018, 16, 1711–1724. [Google Scholar] [CrossRef]
- Oksińska, M.P.; Magnucka, E.G.; Jelcuś, K.; Jakubiak-Marcinkowska, A.; Ronka, S.; Trochimczuk, A.W.; Pietr, S.W. Colonization and biodegradation of the cross-linked potassium polyacrylate component of water absorbing geocomposite by soil microorganisms. Appl. Soil Ecol. 2019, 133, 114–123. [Google Scholar] [CrossRef]
- Powlson, D.S.; Dawson, C.J. Use of ammonium sulphate as a sulphur fertilizer: Implications for ammonia volatilization. Soil Use Manag. 2021, 38, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Martens, D.A.; Bremner, J.M. Soil properties affecting volatilization of ammonia from soils treated with urea. Commun. Soil Sci. Plant Anal. 1989, 20, 1645–1657. [Google Scholar] [CrossRef]
- Kawakami, E.M.; Oosterhius, D.M.; Snider, J.L.; Mozaffari, M. Physiological and yield responses of field-grown cotton to application of urea with the urease inhibitor NBPT and the nitrification inhibitor DCD. Eur. J. Agron. 2012, 43, 147–154. [Google Scholar] [CrossRef]
- Kawakami, E.M.; Oosterhius, D.M.; Snider, J.L. Influence of high temperature and urea fertilization with N-(N-Butyl) thiophosphoric triamide and dicyandiaminde on cotton growth and physiology. J. Plant Nutr. 2013, 36, 1615–1639. [Google Scholar] [CrossRef]
- Dewi, F.C.; Putra, E.T.S.; Wulandari, C. The Effect of Urease Inhibitors Coated Urea on the Growth, Physiological Activities and Yield of Maize (Zea mays L.) in Inceptisol Jogonalan, Klaten. Ilmu Pertan. (Agric. Sci.) 2018, 3, 160–165. [Google Scholar] [CrossRef]
- Marchesan, E.; Grohs, M.; Walter, M.; da Silva, L.S.; Formentini, T.C. Agronomic performance of rice to the use of urease inhibitor in two cropping systems. Rev. Cienc. Agron. 2013, 44, 594–603. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Yin, X.; Savoy, H.J.; McClure, A.; Essington, M.E. Ammonia Volatilization Loss and Corn Nitrogen Nutrition and Productivity with Efficiency Enhanced UAN and Urea under No-tillage. Sci. Rep. 2019, 9, 6610. [Google Scholar] [CrossRef]
- Dewes, T. Effect of pH, temperature, amount of litter and storage density on ammonia emissions from stable manure. J. Agric. Sci. 1996, 127, 501–509. [Google Scholar] [CrossRef]
- Rochette, P.; Angers, D.A.; Chantigny, M.H.; Gasser, M.O.; Macdonald, J.D.; Pelster, D.E.; Bertrand, N. NH3 volatilization, soil concentration and soil pH following subsurface banding of urea at increasing rates. Can. J. Soil Sci. 2013, 93, 261–268. [Google Scholar] [CrossRef]
- Fan, X.H.; Song, Y.S.; Lin, D.X.; Yang, L.Z.; Zhou, J.M. Ammonia volatilization losses from urea applied to wheat on a paddy soil in Taihu region China. Pedosphere 2005, 15, 59–65. [Google Scholar]
- Yang, F.; Cen, R.; Feng, W.; Liu, J.; Qu, Z.; Miao, Q. Effects of Super-Absorbent Polymer on Soil Remediation and Crop Growth in Arid and Semi-Arid Areas. Sustainability 2020, 12, 7825. [Google Scholar] [CrossRef]
- Gunes, A.; Kitir, N.; Turan, M.; Elkoca, E.; Yildirim, E.; Avci, N. Evaluation of effects of water-saving superabsorbent polymer on maize (Zea mays L.) yield and phosphorus fertilizer efficiency. Turk. J. Agric. For. 2016, 40, 365–378. [Google Scholar] [CrossRef]
- Islam, M.R.; Hu, Y.; Mao, S.; Mao, J.; Eneji, A.E.; Xue, X. Effectiveness of a water-saving super-absorbent polymer in soil water conservation for corn (Zea mays L.) based on ecophysiological parameters. J. Sci. Food Agric. 2011, 91, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Maldonado, D.; Vega Erramuspe, I.B.; Peresin, M.S. Natural polymers as alternative adsorbents and treatment agents for water remediation. BioResources 2019, 14, 10093–10160. [Google Scholar] [CrossRef]
- Grant, C.A.; Derksen, D.A.; McLaren, D.; Irvine, R.B. Nitrogen Fertilizer and Urease Inhibitor Effects on Canola Emergence and Yield in a One-Pass Seeding and Fertilizing System. Agron. J. 2010, 102, 875–884. [Google Scholar] [CrossRef]
- Chloupek, O.; Dostál, V.; Středa, T.; Psota, V.; Dvořáčková, O. Drought tolerance of barley varieties in relation to their root system size. Plant Breed. 2010, 129, 630–636. [Google Scholar] [CrossRef]
- Zanin, L.; Tomasi, N.; Zamboni, A.; Varanini, Z.; Pinton, R. The Urease Inhibitor NBPT Negatively Affects DUR3-mediated Uptake and Assimilation of Urea in Maize Roots. Front. Plant Sci. 2015, 6, 1007. [Google Scholar] [CrossRef]
- Habibullah, H.; Nelson, K.A.; Motavalli, P.P. Management of Nitrapyrin and Pronitridine Nitrification Inhibitors with Urea Ammonium Nitrate for Winter Wheat Production. Agronomy 2018, 8, 204. [Google Scholar] [CrossRef]
- Qi, X.; Wu, W.; Shah, F.; Peng, S.; Huang, J.; Cui, K.; Liu, H.; Nie, L. Ammonia volatilization from urea-application influenced germination and early seedling growth of dry direct-seeded rice. Sci. World J. 2012, 2012, 857472. [Google Scholar] [CrossRef]
- Gagnon, B.; Ziad, N. Grain corn and soil nitrogen responses to sidedress nitrogen sources and application. Agron. J. 2010, 103, 1014–1022. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Yao, J.; He, A.L.; Du, J.; Zheng, C.F.; Zhang, J.M. Effects of the reducing and efficiency-increasing application of urea ammonium nitrate solution on the yield and nitrogen uptake and utilization of wheat. J. Henan Agric. Sci. 2017, 46, 6–12. [Google Scholar]
- Ren, B.; Guo, Y.; Liu, P.; Zhao, B.; Zhang, J. Effects of Urea-Ammonium Nitrate Solution on Yield, N2O Emission, and Nitrogen Efficiency of Summer Maize Under Integration of Water and Fertilizer. Front. Plant Sci. 2021, 12, 700331. [Google Scholar] [CrossRef]
- Liu, G.; Yang, Z.; Du, J.; He, A.; Yang, H.; Xue, G.; Yu, C.; Zhang, Y. Adding NBPT to urea increases N use efficiency of maize and decreases the abundance of N-cycling soil microbes under reduced fertilizer-N rate on the North China Plain. PLoS ONE 2020, 15, e0240925. [Google Scholar] [CrossRef] [PubMed]
- Drulis, P.; Kriaučiūnienė, Z.; Liakas, V. The Influence of Different Nitrogen Fertilizer Rates, Urease Inhibitors and Biological Preparations on Maize Grain Yield and Yield Structure Elements. Agronomy 2022, 12, 741. [Google Scholar] [CrossRef]
- Ferreira, L.A.R.; Silva, S.R.; Kölln, O.T. Wheat Yield and Nitrogen Utilization Efficiency Affected by Urea Coated with NBPT Urease Inhibitor and Environmental Conditions in Brazilian Rhodic Oxisols. Int. J. Plant Prod. 2022, 16, 313–328. [Google Scholar] [CrossRef]
- Guardia, G.; García-Gutiérrez, S.; Rodríguez-Pérez, R.; Recio, J.; Vallejo, A. Increasing N use efficiency while decreasing gaseous N losses in a non-tilled wheat (Triticum aestivum L.) crop using a double inhibitor. Agric. Ecosyst. Environ. 2021, 319, 107546. [Google Scholar] [CrossRef]
- Linquist, B.A.; Liu, L.; van Kessel, C.; van Groenigen, K.J. Enhanced efficiency nitrogen fertilizers for rice systems: Meta-analysis of yield and nitrogen uptake. Field Crops Res. 2013, 154, 246–254. [Google Scholar] [CrossRef]
- Riache, M.; Revilla, P.; Maafi, O.; Malvar, R.A.; Djemel, A. Combining Ability and Heterosis of Algerian Saharan Maize Populations (Zea mays L.) for Tolerance to No-Nitrogen Fertilization and Drought. Agronomy 2021, 11, 492. [Google Scholar] [CrossRef]
- Rimski-Korsakov, H.; Rubio, G.; Lavado, R.S. Effect of Water Stress in Maize Crop Production and Nitrogen Fertilizer Fate. J. Plant Nutr. 2009, 32, 565–578. [Google Scholar] [CrossRef]
- Mao, L.; Zha, R.; Chen, S.; Zhang, J.; Jie, L.; Zha, X. Mixture Compound Fertilizer and Super Absorbent Polymer Application Significantly Promoted Growth and Increased Nutrient Levels in Pinus massoniana Seedlings and Soil in Seriously Eroded Degradation Region of Southern China. Front. Plant Sci. 2021, 12, 763175. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.F.; Silva Aragão, O.O.; Silva, R.G.; Jesus, E.C.; Chagas, W.F.T.; Correia, P.S.; Souza Moreira, F.M. Environmentally friendly urea produced from the association of N-(n-butyl) thiophosphoric triamide with biodegradable polymer coating obtained from a soybean processing byproduct. J. Clean. Prod. 2020, 276, 123014. [Google Scholar] [CrossRef]
- Zbíral, J.; Malý, S.; Váňa, M. Soil Analysis, 3rd ed.; Central Institute for Supervising and Testing in Agriculture: Brno, Czech Republic, 2011; pp. 18–52. (In Czech) [Google Scholar]
- Nachabe, M. Refining the definition of field capacity in the literature. J. Irrig. Drain. Eng. 1998, 124, 230–232. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Netto, A.L.; Campostrini, E.; Goncalves de Oliverira, J.; Bressan-Smith, R.E. Photosynthetic pigments, nitrogen, chlorophyll a fluorescence and SPAD-502 readings in coffee leaves. Sci. Hortic. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Škarpa, P.; Klofáč, D.; Krčma, F.; Šimečková, J.; Kozáková, Z. Effect of Plasma Activated Water Foliar Application on Selected Growth Parameters of Maize (Zea mays L.). Water 2020, 12, 3545. [Google Scholar] [CrossRef]
- Zbíral, J.; Čižmárová, E.; Dočkalová, R.; Fojtlová, E.; Hájková, H.; Holcová, H.; Kabátová, N.; Niedobová, E.; Rychlý, M.; Staňková, K.; et al. Analysis of Plant Material, 3rd ed.; Central Institute for Supervising and Testing in Agriculture: Brno, Czech Republic, 2014; pp. 18–38. (In Czech) [Google Scholar]
- StatSoft, Inc. STATISTICA (Data Analysis Software System), version 12; StatSoft, Inc.: Tulsa, OK, USA, 2013; Available online: www.statsoft.com (accessed on 13 October 2022).



| Tr. | Additive | UAN | AS | Urea | AN | |
|---|---|---|---|---|---|---|
| 1 | - | - | 7.70 | 5.18 | 8.82 | 4.58 |
| 2 | acid NHA | 100:1 | 7.26 | 3.25 | 3.74 | 2.34 |
| 3 | neutral NHA | 100:1 | 7.60 | 4.65 | 5.39 | 4.35 |
| 4 | acid NHA | 100:2 | 5.29 | 3.06 | 3.52 | 2.18 |
| 5 | neutral NHA | 100:2 | 7.58 | 4.60 | 5.30 | 4.38 |
| Treatment | N Content % DM | AN (N Uptake) mg/pot | FNRE % |
|---|---|---|---|
| UAN | 1.17 b ± 0.04 | 87.0 b ± 5.0 | 21.8 b ± 1.3 |
| UANNHA-A | 1.19 ab ± 0.03 | 95.0 a ± 3.2 | 23.8 a ± 0.8 |
| UANNHA-N | 1.21 ab ± 0.03 | 95.2 a ± 4.0 | 23.8 a ± 1.0 |
| UANNBPT | 1.25 b ± 0.07 | 98.3 a ± 6.6 | 24.6 a ± 1.6 |
| Fertilizer | N-NH4+ | N-NO3− | N-NH2 | N Total |
|---|---|---|---|---|
| UAN | 19.5 | 19.5 | 39.0 | 78.0 |
| Urea solution | 0.0 | 0.0 | 39.0 | 39.0 |
| Ammonium sulphate solution | 19.5 | 0.0 | 0.0 | 19.5 |
| Ammonium nitrate solution | 19.5 | 19.5 | 0.0 | 39.0 |
| Treatment | Additives | Fertilizer (Fertilizer Solution): Additive Ratio |
|---|---|---|
| 1 | - | - |
| 2 | acid NHA | 100:1 |
| 3 | neutral NHA | 100:1 |
| 4 | acid NHA | 100:2 |
| 5 | neutral NHA | 100:2 |
| 6 | NBPT | 100:0.3 |
| 7 | StabilureN 30 | 100:0.1 |
| Soil Parameter | Value |
|---|---|
| pHCaCl2 | 6.09 |
| Soil oxidizable carbon (Cox) | 0.80% |
| Clay | 20% |
| Silt | 27% |
| Sand | 53% |
| Cation exchange capacity | 164 mmol/kg |
| N total | 0.19% |
| N-NH4+ (K2SO4) | 1.48 mg/kg |
| N-NO3− (K2SO4) | 17.2 mg/kg |
| P (Mehlich 3) | 36.4 mg/kg |
| K (Mehlich 3) | 400 mg/kg |
| Ca (Mehlich 3) | 2720 mg/kg |
| Mg (Mehlich 3) | 214 mg/kg |
| Treatment | Additives | Fertilizer (Fertilizer Solution): Additive Ratio |
|---|---|---|
| UAN | - | - |
| UANNHA-A | acid NHA | 100:2 |
| UANNHA-N | neutral NHA | 100:2 |
| UANNBPT | NBPT | 100:0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kriška, T.; Škarpa, P.; Antošovský, J. Effect of Natural Liquid Hydroabsorbents on Ammonia Emission from Liquid Nitrogen Fertilizers and Plant Growth of Maize (Zea Mays L.) under Drought Conditions. Plants 2023, 12, 728. https://doi.org/10.3390/plants12040728
Kriška T, Škarpa P, Antošovský J. Effect of Natural Liquid Hydroabsorbents on Ammonia Emission from Liquid Nitrogen Fertilizers and Plant Growth of Maize (Zea Mays L.) under Drought Conditions. Plants. 2023; 12(4):728. https://doi.org/10.3390/plants12040728
Chicago/Turabian StyleKriška, Tomáš, Petr Škarpa, and Jiří Antošovský. 2023. "Effect of Natural Liquid Hydroabsorbents on Ammonia Emission from Liquid Nitrogen Fertilizers and Plant Growth of Maize (Zea Mays L.) under Drought Conditions" Plants 12, no. 4: 728. https://doi.org/10.3390/plants12040728
APA StyleKriška, T., Škarpa, P., & Antošovský, J. (2023). Effect of Natural Liquid Hydroabsorbents on Ammonia Emission from Liquid Nitrogen Fertilizers and Plant Growth of Maize (Zea Mays L.) under Drought Conditions. Plants, 12(4), 728. https://doi.org/10.3390/plants12040728






