Do We Need to Breed for Regional Adaptation in Soybean?—Evaluation of Genotype-by-Location Interaction and Trait Stability of Soybean in Germany
Abstract
:1. Introduction
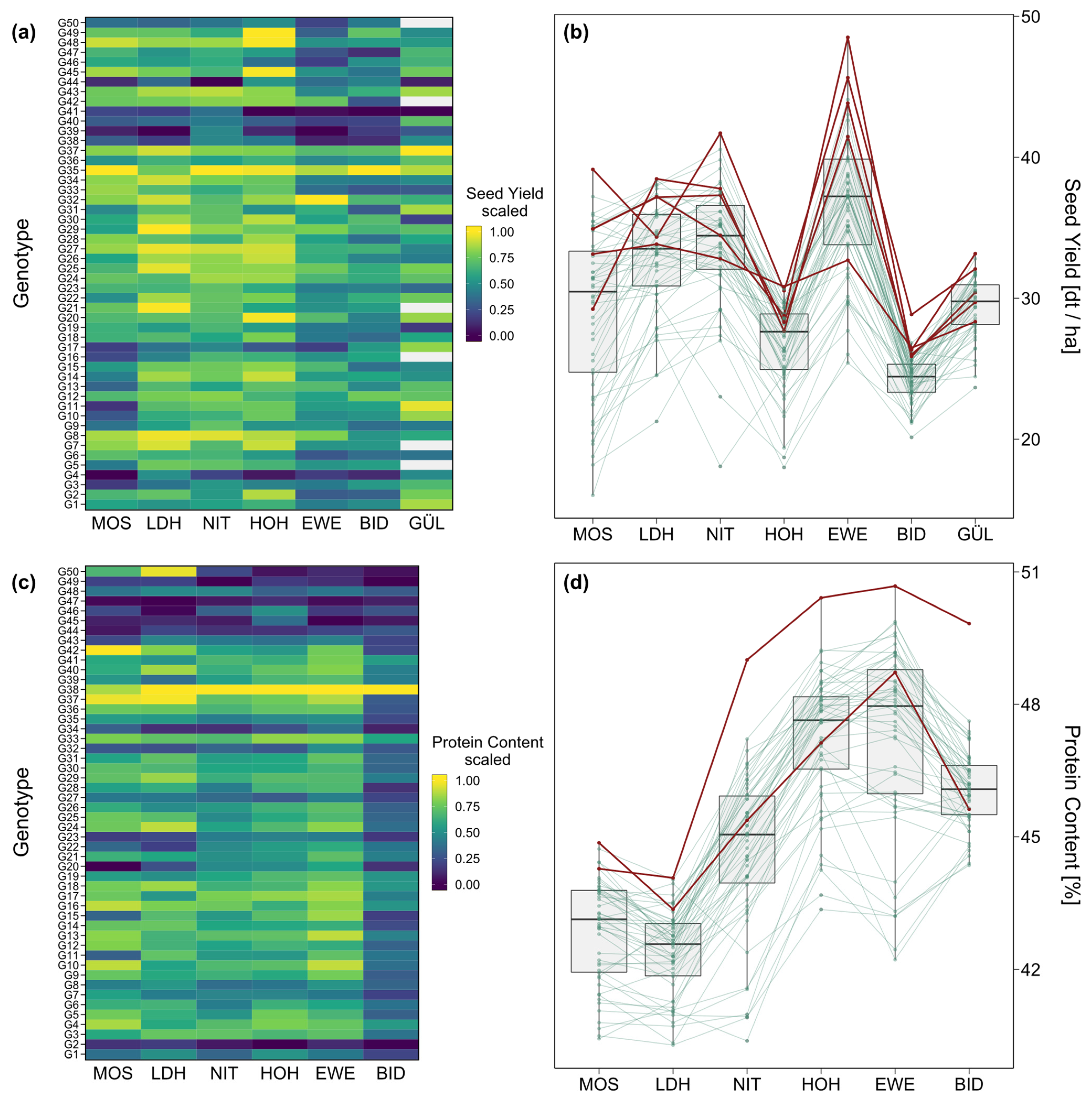
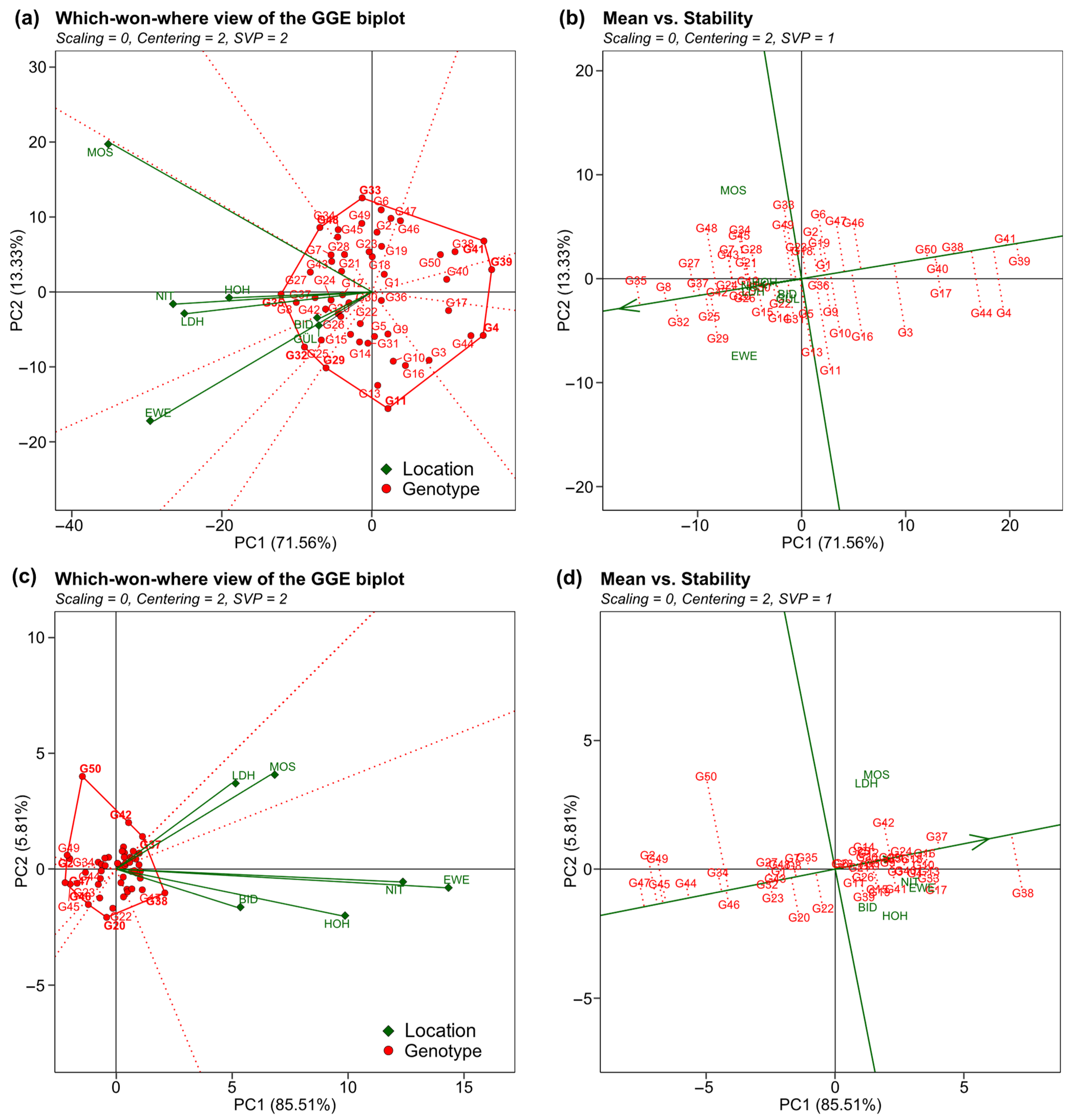
2. Results
3. Discussion
3.1. Phenotypic Performance and Variance Components
3.2. Performance and Trait Stability of Genotypes
3.3. Expansion of Soybean Cultivation to Higher Latitudes Is Possible
3.4. Conclusions for Soybean Breeding at Higher Latitudes
4. Materials and Methods
4.1. Plant Material and Field Trials
4.2. Phenotypic Analysis
4.3. Genotype-by-Location Interaction and Stability Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nielsen, Z. Lebensmittel Zeitung. 2021, p. 41. Available online: www.lebensmittelzeitung.net (accessed on 19 March 2021).
- Ahrens, S. Umsatz mit Milchalternativen im Lebensmitteleinzelhandel nach Produktgruppe 2020. Available online: https://de.statista.com/statistik/daten/studie/1222972/umfrage/umsatz-mit-veganen-alternativen-im-lebensmitteleinzelhandel-nach-produktgruppe/ (accessed on 22 August 2022).
- The Vegan Society. A Growing Plant Milk Market. Available online: https://www.vegansociety.com/news/market-insights/plant-milk-market (accessed on 22 August 2022).
- Qiu, L.J.; Chang, R.Z. The origin and history of soybean. In The Soybean: Botany, Production and Uses; Guriqbal Singh, G.S., Ed.; CABI: Wallingford, UK, 2010; pp. 1–23. ISBN 9781845936440. [Google Scholar]
- Roßberg, D.; Recknagel, J. Untersuchungen zur Anbaueignung von Sojabohnen in Deutschland. J. Cultiv. Plants 2017, 69, 137–145. [Google Scholar] [CrossRef]
- Wilcox, J.R. World Distribution and Trade of Soybean. In Soybeans: Improvement, Production, and Uses, 3rd ed.; Shibles, R.M., Harper, J.E., Wilson, R.F., Shoemaker, R.C., Eds.; American Socienty of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 2004; pp. 1–14. [Google Scholar]
- Kumudini, S. Soybean Growth and Development. In The Soybean: Botany, Production and Uses; Guriqbal Singh, G.S., Ed.; CABI: Wallingford, UK, 2010; pp. 48–73. ISBN 9781845936440. [Google Scholar]
- Panthee, D.R. Varietal Improvement in Soybean. In The Soybean: Botany, Production and Uses; Guriqbal Singh, G.S., Ed.; CABI: Wallingford, UK, 2010; pp. 92–112. ISBN 9781845936440. [Google Scholar]
- Statistisches Bundesamt. Landwirtschaftliche Betriebe, Fläche: Bundesländer, Jahre, Bodennutzungsarten. Available online: https://www-genesis.destatis.de/genesis/online?operation=abruftabelleBearbeiten&levelindex=2&levelid=1656687314161&auswahloperation=abruftabelleAuspraegungAuswaehlen&auswahlverzeichnis=ordnungsstruktur&auswahlziel=werteabruf&code=41271-0012&auswahltext=&wertauswahl=823&wertauswahl=275&werteabruf=Werteabruf#abreadcrumb (accessed on 1 July 2022).
- Hamner, K.C. Interrelation of Light and Darkness in Photoperiodic Induction. Bot. Gaz. 1940, 101, 658–687. [Google Scholar]
- Zhang, L.-X.; Liu, W.; Tsegaw, M.; Xu, X.; Qi, Y.-P.; Sapey, E.; Liu, L.-P.; Wu, T.-T.; Sun, S.; Han, T.-F. Principles and practices of the photo-thermal adaptability improvement in soybean. J. Integr. Agric. 2020, 19, 295–310. [Google Scholar] [CrossRef]
- Food and Agricultural Organization of the United Nations. FAOSTAT Statistical Database. Available online: https://www.fao.org/faostat/en/#data/TCL (accessed on 23 August 2022).
- Fearnside, P.M. Soybean cultivation as a threat to the environment in Brazil. Environ. Conserv. 2001, 28, 23–38. [Google Scholar] [CrossRef]
- Bundesanstalt für Landwirtschaft und Ernährung. Ackerbohne, Erbse & Co.: Die Eiweißpflanzenstrategie des Bundesministeriums für Ernährung und Landwirtschaft zur Förderung des Leguminosenanbaus in Deutschland; Bundesministerium für Ernährung und Landwirtschaft: Berlin, Germany, 2020.
- Lin, C.S.; Binns, M.R.; Lefkovitch, L.P. Stability Analysis: Where Do We Stand? 1. Crop Sci. 1986, 26, 894–900. [Google Scholar] [CrossRef]
- Becker, H.C.; Léon, J. Stability Analysis in Plant Breeding. Plant Breed. 1988, 101, 1–23. [Google Scholar] [CrossRef]
- Annicchiarico, P. Genotype x Environment Interaction: Challenges and Opportunities for Plant Breeding and Cultivar Recommendations; Food and Agriculture Organization of the United Nations: Rome, Italy, 2002; ISBN 92-5-104870-3. [Google Scholar]
- Miedaner, T. Grundlagen der Pflanzenzüchtung; DLG-Verlag: Frankfurt am Main, Germany, 2010; ISBN 978-3-7690-0752-7. [Google Scholar]
- Becker, H. Pflanzenzüchtung, 2. Aufl.; UTB GmbH; Ulmer: Stuttgart, Germany, 2011; ISBN 978-3-8252-3558-1. [Google Scholar]
- Matus-Cádiz, M.A.; Hucl, P.; Perron, C.E.; Tyler, R.T. Genotype × Environment Interaction for Grain Color in Hard White Spring Wheat. Crop Sci. 2003, 43, 219. [Google Scholar] [CrossRef]
- Fan, X.-M.; Kang, M.S.; Chen, H.; Zhang, Y.; Tan, J.; Xu, C. Yield Stability of Maize Hybrids Evaluated in Multi-Environment Trials in Yunnan, China. Agron. J. 2007, 99, 220–228. [Google Scholar] [CrossRef]
- Olivoto, T.; L’ucio, A.D. ’C. metan: An R package for multi-environment trial analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Yan, W. Analysis and Handling of G × E in a Practical Breeding Program. Crop Sci. 2016, 56, 2106–2118. [Google Scholar] [CrossRef]
- Atlin, G.N.; Baker, R.J.; McRae, K.B.; Lu, X. Selection Response in Subdivided Target Regions. Crop Sci. 2000, 40, 7–13. [Google Scholar] [CrossRef]
- Yan, W.; Tinker, N.A. Biplot analysis of multi-environment trial data: Principles and applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S. GGE Biplot Analysis: A Graphical Tool for Breeders, Geneticists, and Agronomists; CRC Press: Boca Raton, FL, USA, 2003; ISBN 0-8493-1338-4. [Google Scholar]
- Balko, C.; Hahn, V.; Ordon, F. Kühletoleranz bei der Sojabohne (Glycine max (L.) Merr.)–Voraussetzung für die Ausweitung des Sojaanbaus in Deutschland. J. Für Kult. 2014, 66, 378–388. [Google Scholar] [CrossRef]
- Jähne, F.; Balko, C.; Hahn, V.; Würschum, T.; Leiser, W.L. Cold stress tolerance of soybeans during flowering: QTL mapping and efficient selection strategies under controlled conditions. Plant Breed. 2019, 138, 708–720. [Google Scholar] [CrossRef]
- Karges, K.; Bellingrath-Kimura, S.D.; Watson, C.A.; Stoddard, F.L.; Halwani, M.; Reckling, M. Agro-economic prospects for expanding soybean production beyond its current northerly limit in Europe. Eur. J. Agron. 2022, 133, 126415. [Google Scholar] [CrossRef]
- Kurasch, A.K.; Hahn, V.; Leiser, W.L.; Vollmann, J.; Schori, A.; Bétrix, C.-A.; Mayr, B.; Winkler, J.; Mechtler, K.; Aper, J.; et al. Identification of mega-environments in Europe and effect of allelic variation at maturity E loci on adaptation of European soybean. Plant Cell Environ. 2017, 40, 765–778. [Google Scholar] [CrossRef]
- Sobko, O.; Stahl, A.; Hahn, V.; Zikeli, S.; Claupein, W.; Gruber, S. Environmental Effects on Soybean (Glycine max (L.) Merr) Production in Central and South Germany. Agronomy 2020, 10, 1847. [Google Scholar] [CrossRef]
- Toleikiene, M.; Slepetys, J.; Sarunaite, L.; Lazauskas, S.; Deveikyte, I.; Kadziuliene, Z. Soybean Development and Productivity in Response to Organic Management above the Northern Boundary of Soybean Distribution in Europe. Agronomy 2021, 11, 214. [Google Scholar] [CrossRef]
- Würschum, T.; Leiser, W.L.; Jähne, F.; Bachteler, K.; Miersch, M.; Hahn, V. The soybean experiment ‘1000 Gardens’: A case study of citizen science for research, education, and beyond. Theor. Appl. Genet. 2019, 132, 617–626. [Google Scholar] [CrossRef]
- Agrarmeteorologie Bayern. Available online: www.wetter-by.de (accessed on 30 March 2022).
- Agrarmeteorologie Baden-Württemberg. Available online: www.wetter-bw.de (accessed on 30 March 2022).
- Climate Data Center. Available online: https://opendata.dwd.de/climate_environment/CDC/observations_germany/climate/daily/kl/historical/ (accessed on 30 March 2022).
- Cullis, B.R.; Smith, A.B.; Coombes, N.E. On the design of early generation variety trials with correlated data. JABES 2006, 11, 381–393. [Google Scholar] [CrossRef]
- Piepho, H.-P.; Möhring, J. Computing heritability and selection response from unbalanced plant breeding trials. Genetics 2007, 177, 1881–1888. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Hunt, L.A.; Sheng, Q.; Szlavnics, Z. Cultivar Evaluation and Mega-Environment Investigation Based on the GGE Biplot. Crop Sci. 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Shukla, G.K. Some Statistical Aspects of Partitioning Genotype-Environmental Components of Variability. Heredity 1972, 29, 237–345. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Butler, D. Asreml: Fits the Linear Mixed Model; VSN International: Hemel Hempstead, UK, 2020. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Jun, C.; Granville, M.; Samson, L.D. Ggcorrplot2: Visualize a Correlation Matrix using Ggplot2. R Package Version 0.1.2. Available online: https://github.com/caijun/ggcorrplot2 (accessed on 1 September 2022).



| EWE | HOH | MOS | NIT | LDH | BID | GÜL | ||
|---|---|---|---|---|---|---|---|---|
| LAT | N 48°31′ 17.1876 | N 48°43′ 18.048 | N 48°26′ 35.2536 | N 48°57′ 35.4564 | N 48°32′ 38.4252 | N 51°45′ 7.1568 | N 53°49′ 12.504 | |
| ALT | 141 m | 400 m | 440 m | 334 m | 403 m | 80 m | 14 m | |
| TEMP | Apr–Oct | 14.96 °C | 13.63 °C | 14.02 °C | 14.04 °C | 13.82 °C | 14.74 °C | 14.19 °C |
| Apr | 8.19 °C | 6.95 °C | 7.05 °C | 7.11 °C | 6.87 °C | 6.73 °C | 6.21 °C | |
| May | 12.17 °C | 10.77 °C | 11.75 °C | 11.29 °C | 10.75 °C | 12.13 °C | 11.00 °C | |
| Jun | 20.55 °C | 19.22 °C | 19.72 °C | 19.86 °C | 19.53 °C | 20.21 °C | 19.65 °C | |
| Jul | 19.34 °C | 18.13 °C | 18.97 °C | 19.00 °C | 18.45 °C | 19.74 °C | 19.64 °C | |
| Aug | 18.10 °C | 16.51 °C | 17.17 °C | 17.14 °C | 16.75 °C | 17.59 °C | 16.72 °C | |
| Sep | 16.33 °C | 15.10 °C | 15.20 °C | 15.50 °C | 15.86 °C | 16.29 °C | 15.42 °C | |
| Oct | 10.05 °C | 8.75 °C | 8.26 °C | 8.38 °C | 8.56 °C | 10.50 °C | 10.67 °C | |
| PCPN | Apr–Oct | 481.8 mm | 432.5 mm | 821.7 mm | 424.5 mm | 724.2 mm | 373.8 mm | 460.9 mm |
| Apr | 44.8 mm | 36.2 mm | 51.0 mm | 14.1 mm | 21.1 mm | 23.7 mm | 41.8 mm | |
| May | 114.4 mm | 72.0 mm | 97.6 mm | 90.6 mm | 155.4 mm | 45.8 mm | 80.1 mm | |
| Jun | 103.7 mm | 89.4 mm | 126.8 mm | 99.8 mm | 230.6 mm | 91.9 mm | 47.6 mm | |
| Jul | 105.1 mm | 69.3 mm | 252.6 mm | 60.4 mm | 123.3 mm | 56.4 mm | 69.9 mm | |
| Aug | 77.7 mm | 103.3 mm | 233.2 mm | 124.4 mm | 146.9 mm | 89.0 mm | 96.5 mm | |
| Sep | 22.2 mm | 28.7 mm | 37.0 mm | 18.9 mm | 35.1 mm | 39.8 mm | 74.0 mm | |
| Oct | 13.9 mm | 33.6 mm | 23.5 mm | 16.3 mm | 11.8 mm | 27.2 mm | 51.0 mm | |
| SG | Pseudo-gley | Haplic luvisol | Luvisol | Luvisol | Marsh | Cambisol | Haplic luvisol | |
| ST | Loamy Sand | Silty clay | Sandy loam | Silty clay | Loamy sand | Clay loam | Loamy sand | |
| SD | 21.04.2021 | 25.05.2021 | 17.04.2021 | 30.04.2021 | 17.04.2021 | 05.05.2021 | 10.05.2021 |
| Trait | Location § | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SY | Across | 30.37 | 21.07 | 36.83 | 14.17 *** | 17.78 *** | 5.69 *** | 9.60 | 0.90 | - | 0.40 |
| NTH | 26.83 | 21.60 | 30.47 | 5.18 ** | 12.11 ns | 1.47 ns | 9.35 | 0.55 | - | 0.28 | |
| STH | 31.82 | 21.54 | 38.77 | 19.66 *** | 14.69 *** | 4.23 *** | 10.01 | 0.91 | - | 0.22 | |
| EWE | 36.57 | 25.41 | 48.51 | 30.88 *** | - | - | 13.63 | - | 0.82 | ||
| HOH | 26.74 | 18.00 | 30.82 | 13.91 *** | - | - | 11.45 | - | 0.71 | ||
| MOS | 29.06 | 16.02 | 39.14 | 36.64 *** | - | - | 6.60 | - | 0.92 | ||
| NIT | 33.81 | 18.07 | 41.72 | 21.47 *** | - | - | 4.86 | - | 0.88 | ||
| LDH | 32.92 | 21.26 | 38.47 | 19.75 *** | - | - | 8.80 | - | 0.79 | ||
| BID | 24.26 | 20.13 | 28.84 | 5.09 ** | - | - | 6.05 | - | 0.54 | ||
| GÜL | 29.35 | 23.67 | 33.16 | 9.45 * | - | - | 12.58 | - | 0.53 | ||
| PC | Across | 45.09 | 41.81 | 48.51 | 2.21 *** | 4.45 *** | 0.41 *** | 0.73 | 0.94 | - | 0.19 |
| EWE | 47.29 | 42.22 | 50.68 | 4.74 *** | - | - | 0.51 | - | 0.94 | ||
| HOH | 47.22 | 43.35 | 50.42 | 2.72 *** | - | - | 0.79 | - | 0.85 | ||
| MOS | 42.87 | 40.44 | 44.86 | 1.58 *** | - | - | 0.34 | - | 0.89 | ||
| NIT | 44.69 | 40.39 | 49.01 | 3.50 *** | - | - | 0.41 | - | 0.94 | ||
| LDH | 42.38 | 40.30 | 44.06 | 1.14 *** | - | - | 0.39 | - | 0.82 | ||
| BID | 46.09 | 44.35 | 49.83 | 1.69 ** | - | - | 2.28 | - | 0.55 | ||
| OC | Across | 17.47 | 16.48 | 18.7 | 0.31 *** | 6.25 *** | 0.16 *** | 0.44 | 0.81 | - | 0.50 |
| PH | Across | 101.16 | 89.66 | 113.91 | 31.78 *** | 75.48 *** | 19.56 *** | 46.15 | 0.79 | - | 0.62 |
| KDM | Across | 80.98 | 77.14 | 82.80 | 1.00 *** | 3.36 *** | 0.90 *** | 0.98 | 0.82 | - | 0.90 |
| DTM | Across | 150.45 | 139.10 | 154.20 | 9.19 *** | 88.41 *** | 5.64 *** | 3.36 | 0.87 | - | 0.62 |
| Genotype | Stability Rank STH | STH | Rank SY STH | Mean SY STH | Stability Rank NTH § | NTH $ | Rank SY NTH | Mean SY NTH |
|---|---|---|---|---|---|---|---|---|
| G1 | 7 | 1.97 | 37 | 30.55 | 25 | 9.64 | 8 | 28.37 |
| G2 | 42 | 15.18 | 31 | 31.56 | 26 | 10.56 | 22 | 27.17 |
| G3 | 33 | 9.93 | 42 | 27.40 | 1 | 0 | 17 | 27.41 |
| G4 | 43 | 15.67 | 48 | 22.95 | 17 | 4.95 | 42 | 24.07 |
| G5 | 22 | 6.68 | 27 | 32.04 | - | - | - | - |
| G6 | 38 | 12.86 | 35 | 30.69 | 11 | 2.45 | 35 | 25.26 |
| G7 | 26 | 7.95 | 9 | 35.47 | - | - | - | - |
| G8 | 16 | 4.81 | 2 | 38.04 | 1 | 0 | 20 | 27.29 |
| G9 | 24 | 7.07 | 36 | 30.63 | 5 | 0.56 | 36 | 25.24 |
| G10 | 36 | 11.88 | 38 | 30.25 | 20 | 6.91 | 9 | 28.19 |
| G11 | 48 | 27.77 | 34 | 30.78 | 29 | 15.82 | 5 | 28.96 |
| G12 | 5 | 1.75 | 21 | 33.69 | 12 | 2.57 | 3 | 29.19 |
| G13 | 47 | 19.33 | 30 | 31.68 | 10 | 2.31 | 18 | 27.38 |
| G14 | 28 | 8.50 | 22 | 33.38 | 18 | 4.98 | 21 | 27.20 |
| G15 | 15 | 4.43 | 19 | 34.13 | 1 | 0 | 32 | 25.84 |
| G16 | 44 | 15.94 | 40 | 29.27 | - | - | - | - |
| G17 | 6 | 1.80 | 45 | 24.94 | 22 | 7.91 | 10 | 28.16 |
| G18 | 13 | 3.70 | 28 | 32.00 | 23 | 8.32 | 28 | 26.28 |
| G19 | 10 | 3.05 | 33 | 31.02 | 37 | 50.89 | 38 | 24.88 |
| G20 | 21 | 5.85 | 15 | 34.82 | 33 | 23.15 | 12 | 28.07 |
| G21 | 35 | 11.16 | 16 | 34.33 | - | - | - | - |
| G22 | 14 | 3.95 | 24 | 32.97 | 6 | 0.61 | 13 | 28.06 |
| G23 | 17 | 4.85 | 26 | 32.12 | 15 | 3.33 | 37 | 25.20 |
| G24 | 9 | 3.05 | 12 | 35.11 | 1 | 0 | 11 | 28.08 |
| G25 | 18 | 5.42 | 7 | 36.15 | 1 | 0 | 7 | 28.48 |
| G26 | 8 | 2.18 | 13 | 34.94 | 11 | 2.45 | 31 | 25.97 |
| G27 | 4 | 1.23 | 4 | 36.86 | 21 | 7.74 | 26 | 26.68 |
| G28 | 23 | 6.91 | 20 | 34.03 | 24 | 8.75 | 27 | 26.40 |
| G29 | 39 | 13.27 | 8 | 35.58 | 7 | 0.76 | 6 | 28.59 |
| G30 | 2 | 0.85 | 18 | 34.18 | 35 | 37.47 | 33 | 25.80 |
| G31 | 25 | 7.87 | 29 | 31.71 | 34 | 33.10 | 24 | 26.99 |
| G32 | 49 | 30.04 | 3 | 36.99 | 16 | 4.66 | 14 | 28.06 |
| G33 | 45 | 16.16 | 25 | 32.48 | 27 | 13.64 | 39 | 24.81 |
| G34 | 30 | 0.85 | 17 | 34.31 | 2 | 0.14 | 29 | 26.20 |
| G35 | 41 | 7.87 | 1 | 38.77 | 13 | 2.69 | 1 | 30.47 |
| G36 | 3 | 30.04 | 32 | 31.25 | 4 | 0.32 | 15 | 27.92 |
| G37 | 11 | 3.11 | 5 | 36.35 | 14 | 3.18 | 2 | 29.90 |
| G38 | 34 | 10.24 | 46 | 24.61 | 19 | 5.46 | 41 | 24.51 |
| G39 | 40 | 13.51 | 50 | 21.54 | 3 | 0.27 | 43 | 23.71 |
| G40 | 1 | 0.40 | 44 | 25.41 | 32 | 21.10 | 30 | 26.06 |
| G41 | 19 | 5.56 | 49 | 22.14 | 30 | 16.16 | 44 | 21.60 |
| G42 | 20 | 5.68 | 10 | 35.33 | - | - | - | - |
| G43 | 27 | 8.07 | 11 | 35.15 | 9 | 1.67 | 4 | 29.02 |
| G44 | 50 | 38.57 | 47 | 24.16 | 38 | 83.21 | 40 | 24.70 |
| G45 | 32 | 9.76 | 14 | 34.84 | 28 | 15.53 | 19 | 27.35 |
| G46 | 37 | 12.84 | 41 | 28.56 | 1 | 0 | 23 | 27.14 |
| G47 | 31 | 9.75 | 39 | 29.93 | 31 | 17.29 | 34 | 25.57 |
| G48 | 29 | 8.61 | 6 | 36.20 | 8 | 1.59 | 25 | 26.85 |
| G49 | 46 | 17.5 | 23 | 33.22 | 36 | 47.85 | 16 | 27.78 |
| G50 | 12 | 3.23 | 43 | 26.52 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Döttinger, C.A.; Hahn, V.; Leiser, W.L.; Würschum, T. Do We Need to Breed for Regional Adaptation in Soybean?—Evaluation of Genotype-by-Location Interaction and Trait Stability of Soybean in Germany. Plants 2023, 12, 756. https://doi.org/10.3390/plants12040756
Döttinger CA, Hahn V, Leiser WL, Würschum T. Do We Need to Breed for Regional Adaptation in Soybean?—Evaluation of Genotype-by-Location Interaction and Trait Stability of Soybean in Germany. Plants. 2023; 12(4):756. https://doi.org/10.3390/plants12040756
Chicago/Turabian StyleDöttinger, Cleo A., Volker Hahn, Willmar L. Leiser, and Tobias Würschum. 2023. "Do We Need to Breed for Regional Adaptation in Soybean?—Evaluation of Genotype-by-Location Interaction and Trait Stability of Soybean in Germany" Plants 12, no. 4: 756. https://doi.org/10.3390/plants12040756
APA StyleDöttinger, C. A., Hahn, V., Leiser, W. L., & Würschum, T. (2023). Do We Need to Breed for Regional Adaptation in Soybean?—Evaluation of Genotype-by-Location Interaction and Trait Stability of Soybean in Germany. Plants, 12(4), 756. https://doi.org/10.3390/plants12040756







