Autotetraploidization Alters Morphology, Photosynthesis, Cytological Characteristics and Fruit Quality in Sour Jujube (Ziziphus acidojujuba Cheng et Liu)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Cultivating Conditions
2.2. Examination of Ploidy by Chromosome Counting
2.3. Evaluation of Tree Characteristics
2.4. Evaluation of Flower Characteristics
2.5. Evaluation of Leaf Characteristics
2.6. Evaluation of Fruit Quality
2.7. Statistical Analysis
3. Results
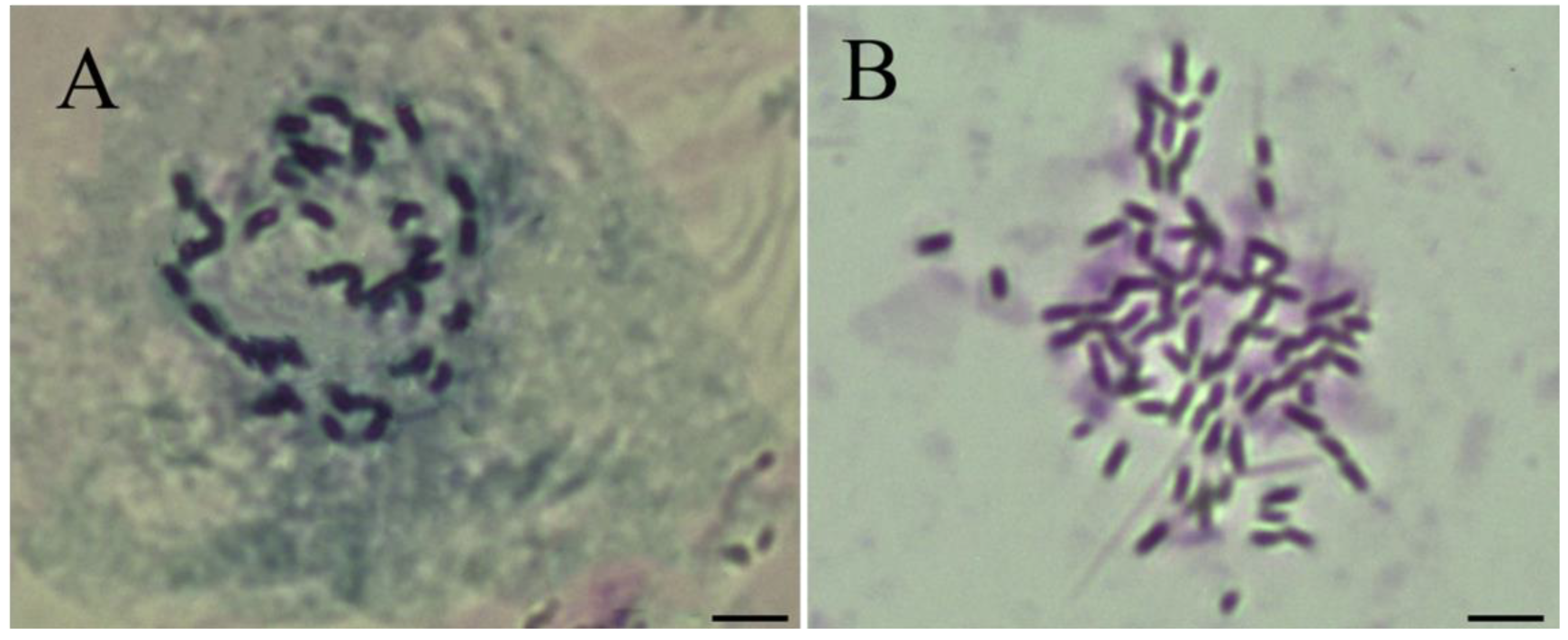
3.1. Karyotypic Evidence Showed ‘Zhuguang’ Was Autotetraploid
3.2. Evaluation of Tree Characteristics
3.3. Evaluation of Flower Characteristics
3.4. Evaluation of Leaf Characteristics
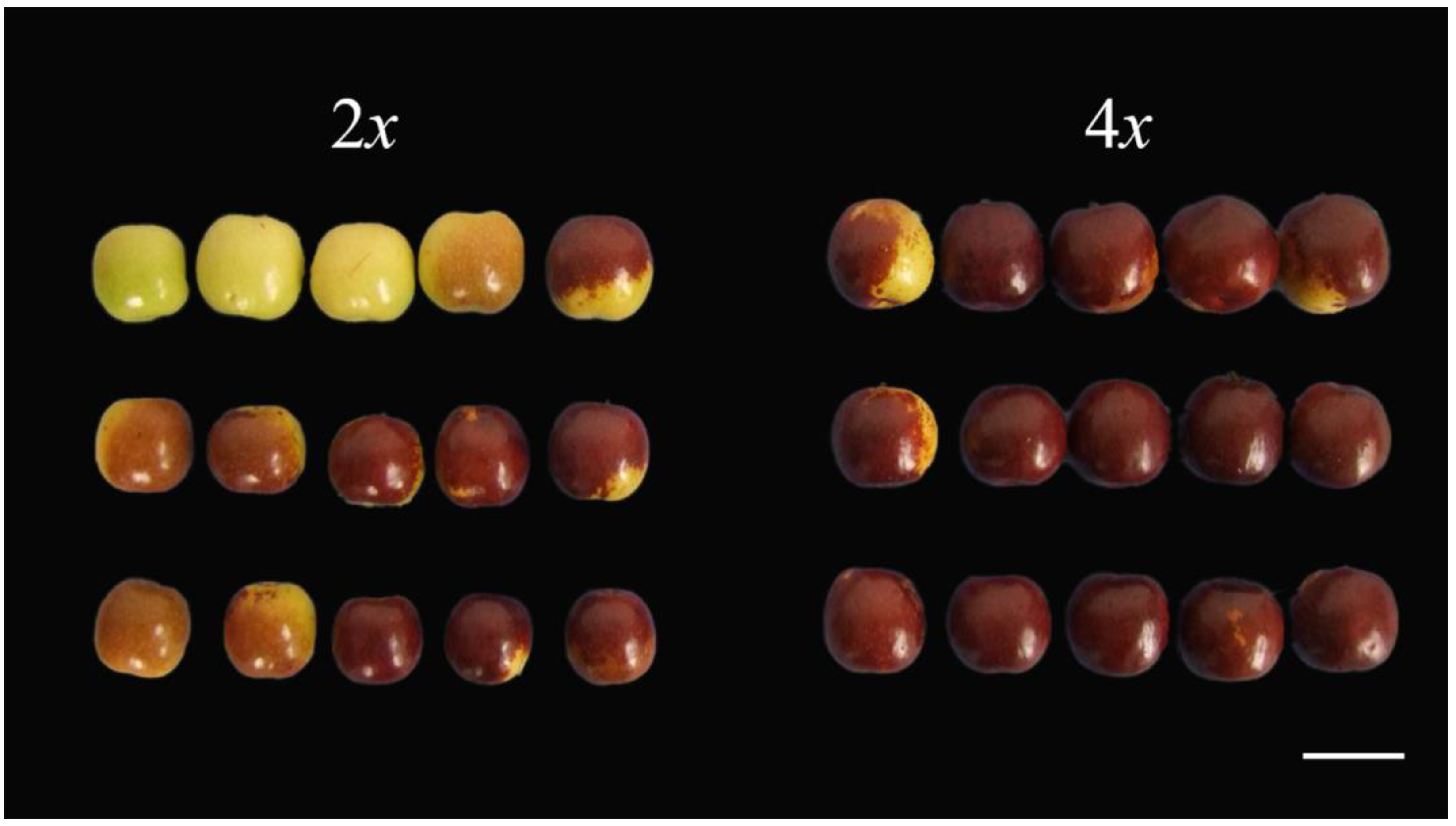
3.5. Autotetraploid Fruit Showed Changed of Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marfil, C.F.; Duarte, P.F.; Masuelli, R.W. Phenotypic and epigenetic variation induced in newly synthesized allopolyploids and autopolyploids of potato. Sci. Hortic. 2018, 234, 101–109. [Google Scholar] [CrossRef]
- Tokumoto, Y.; Kajiura, H.; Takeno, S.; Harada, Y.; Suzuki, N.; Hosaka, T.; Gyokusen, K.; Nakazawa, Y. Induction of tetraploid hardy rubber tree, Eucommia ulmoides, and phenotypic differences from diploid. Plant Biotechnol. 2016, 33, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Spoelhof, J.P.; Soltis, P.S.; Soltis, D.E. Pure polyploidy: Closing the gaps in autopolyploid research. J. Syst. Evol. 2017, 55, 340–352. [Google Scholar] [CrossRef] [Green Version]
- Jaillon, O.; Aury, J.M.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C.; et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 2007, 449, 463–467. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.; Yin, H.; Li, L.; Wang, R.; Wu, J.; Wu, J.; Zhang, S. Different modes of gene duplication show divergent evolutionary patterns and contribute differently to the expansion of gene families involved in Important fruit traits in pear (Pyrus bretschneideri). Front. Plant Sci. 2018, 9, 161. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Wang, Z.; Shi, Z.; Zhang, S.; Ming, R.; Zhu, S.; Khan, M.A.; Tao, S.; Korban, S.S.; Wang, H.; et al. The genome of the pear (Pyrus bretschneideri Rehd.). Genome Res. 2013, 23, 396–408. [Google Scholar] [CrossRef] [Green Version]
- Parisod, C.; Holderegger, R.; Brochmann, C. Evolutionary consequences of autopolyploidy. New Phytol. 2010, 186, 5–17. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Hao, H.; Ma, L.Y.; Zhao, C.; Yu, X.Y. Tetraploid muskmelon alters morphological characteristics and improves fruit quality. Sci. Hortic. 2010, 125, 396–400. [Google Scholar] [CrossRef]
- Wu, J.H.; Ferguson, A.R.; Murray, B.G.; Jia, Y.; Datson, P.M.; Zhang, J. Induced polyploidy dramatically increases the size and alters the shape of fruit in Actinidia chinensis. Ann. Bot. 2012, 109, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Q.; Wang, H.H.; Shi, C.H.; Zhang, X.Y.; Duan, K.; Luo, J. Morphological, cytological and fertility consequences of a spontaneous tetraploid of the diploid pear (Pyrus pyrifolia Nakai) cultivar ‘Cuiguan’. Sci. Hortic. 2015, 189, 59–65. [Google Scholar] [CrossRef]
- Sanwal, S.K.; Rai, N.; Singh, J.; Buragohain, J. Antioxidant phytochemicals and gingerol content in diploid and tetraploid clones of ginger (Zingiber officinale Roscoe). Sci. Hortic. 2010, 124, 280–285. [Google Scholar] [CrossRef]
- Wu, J.H.; Ferguson, A.R.; Murray, B.G.; Duffy, A.M.; Jia, Y.; Cheng, C.; Martin, P.J. Fruit quality in induced polyploids of Actinidia chinensis. HortScience 2013, 48, 701–707. [Google Scholar] [CrossRef]
- Hannweg, K.; Visser, G.; de Jager, K.; Bertling, I. In vitro-induced polyploidy and its effect on horticultural characteristics, essential oil composition and bioactivity of Tetradenia riparia. S. Afr. J. Bot. 2016, 106, 186–191. [Google Scholar] [CrossRef]
- Corrêa, J.P.O.; Vital, C.E.; Pinheiro, M.V.M.; Batista, D.S.; Saldanha, C.W.; da Cruz, A.C.F.; Notini, M.M.; Freitas, D.M.S.; DaMatta, F.M.; Otoni, W.C. Induced polyploidization increases 20-hydroxyecdysone content, in vitro photoautotrophic growth, and ex vitro biomass accumulation in Pfaffia glomerata (Spreng.) Pedersen. In Vitro Cell. Dev. Biol.-Plant 2016, 52, 45–55. [Google Scholar] [CrossRef]
- Xia, J.; Ma, Y.J.; Wang, Y.; Wang, J.W. Deciphering transcriptome profiles of tetraploid Artemisia annua plants with high artemisinin content. Plant Physiol. Biochem. 2018, 130, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xue, H.; Lu, X.J.; Zhang, B.; Wang, F.; Ma, Y.; Zhang, Z.H. Autotetraploidization enhances drought stress tolerance in two apple cultivars. Trees 2015, 29, 1773–1780. [Google Scholar] [CrossRef]
- Xue, H.; Zhang, F.; Zhang, Z.-H.; Fu, J.-F.; Wang, F.; Zhang, B.; Ma, Y. Differences in salt tolerance between diploid and autotetraploid apple seedlings exposed to salt stress. Sci. Hortic. 2015, 190, 24–30. [Google Scholar] [CrossRef]
- Hannweg, K.; Steyn, W.; Bertling, I. In vitro-induced tetraploids of Plectranthus esculentus are nematode-tolerant and have enhanced nutritional value. Euphytica 2015, 207, 343–351. [Google Scholar] [CrossRef]
- Nukaya, T.; Sudo, M.; Yahata, M.; Nakajo, Y.; Ohta, T.; Yasuda, K.; Tominaga, A.; Mukai, H.; Kunitake, H. Characteristics in autotetraploid kumquats (Fortunella spp.) induced by colchicine treatment to nucellar embryos and their utilization for triploid breeding. Sci. Hortic. 2019, 245, 210–217. [Google Scholar] [CrossRef]
- Liu, M.J.; Wang, M. Germplasm Resources of Chinese Jujube; China Forestry Publishing House: Beijing, China, 2009; ISBN 7503857420. [Google Scholar]
- Wang, Z.; An, X.; Chitrakar, B.; Li, J.; Yuan, Y.; Liu, K.; Nie, X.; Zhang, Y.; Zhao, X.; Zhao, Z. Spatial and temporal distribution of phenolic and flavonoid compounds in sour jujube (Ziziphus. acidojujuba Cheng et Liu) and their antioxidant activities. Plant Foods Hum. Nutr. 2023, 78, 46–51. [Google Scholar] [CrossRef]
- Xue, X.; Zhao, A.; Wang, Y.; Ren, H.; Li, Y.; Li, D.; Du, J. Metabolomics-based analysis of flavonoid metabolites in Chinese jujube and sour jujube fruits from different harvest periods. J. Food Sci. 2022, 87, 3752–3765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cheng, M.; Li, Z.; Guan, S.; Cai, B.; Li, Q.; Rong, S. Composition and biological activity of rose and jujube kernel after fermentation with kombucha SCOBY. J. Food Process. Preserv. 2020, 44, e14758. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Wang, L.; Liu, P.; Zhao, J.; Zhao, Z.; Yao, S.; Stănic, F.; Liu, Z.; Wang, L. The historical and current research progress on jujube—A superfruit for the future. Hortic. Res. 2020, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Xu, X.-X.; Guo, S.; Xie, H.; Yan, H.; Ma, X.-F.; Niu, Y.; Duan, J.-A. Wild jujube (Ziziphus jujuba var. spinosa): A review of its phytonutrients, health benefits, metabolism, and applications. J. Agric. Food Chem. 2022, 70, 7871–7886. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Li, Z.; Qi, A.; Yao, P.; Zhou, Z.; Dong, T.T.X.; Tsim, K.W.K. A review of dietary Ziziphus jujuba Fruit (Jujube): Developing health food supplements for brain protection. Evid.-Based Complement. Altern. Med. 2017, 2017, 3019568. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Liu, P.; Liu, M.; Wang, J.; Zhao, J.; Zhao, Z.; Dai, L. In Vivo fast induction of homogeneous autopolyploids via callus in sour jujube (Ziziphus acidojujuba Cheng et Liu). Hortic. Plant J. 2016, 2, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Liu, M.J.; Dai, L.; Qin, Z.Y. In Vitro tetraploid induction of Ziziphus jujuba ‘Dongzao’and Z. acidojujuba (Z. spinosa Hu) with colchicine. Acta Hortic. Sin. 2005, 32, 1008–1012. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Liu, P.; Zho, J.; Zhao, Z.; Dai, L.; Li, X.; Liu, Z. Historical achievements and frontier advances in the production and research of Chinese Jujube (Ziziphus jujubein) in China. Acta Hortic. Sin. 2015, 42, 1683–1698. [Google Scholar] [CrossRef]
- Shi, Q.H.; Liu, P.; Wang, J.R.; Xu, J.; Ning, Q.; Liu, M.J. A novel in vivo shoot regeneration system via callus in woody fruit tree Chinese jujube (Ziziphus jujuba Mill.). Sci. Hortic. 2015, 188, 30–35. [Google Scholar] [CrossRef]
- Shi, Q.H.; Liu, P.; Liu, M.J.; Wang, J.R.; Xu, J. A novel method for rapid in vivo induction of homogeneous polyploids via calluses in a woody fruit tree (Ziziphus jujuba Mill.). Plant Cell Tissue Organ Cult. 2015, 121, 423–433. [Google Scholar] [CrossRef]
- Liu, P.; Liu, M.; Shi, Q.; Wang, J.; Hu, L.; Wang, L. ‘Zhuguang’, a new excellent tetraploid table cultivar of sour jujube. Acta Hortic. Sin. 2017, 44, 195–196. [Google Scholar]
- Li, D.K. Descriptors and Data Standard for Jujube (Ziziphus jujuba Mill.); China Agriculture Press: Beijing, China, 2006. [Google Scholar]
- Xue, C.L.; Liu, Z.G.; Dai, L.; Bu, J.D.; Liu, M.J.; Zhao, Z.H.; Jiang, Z.H.; Gao, W.L.; Zhao, J. Changing Host Photosynthetic, Carbohydrate, and Energy Metabolisms Play Important Roles in Phytoplasma Infection. Phytopathology 2018, 108, 1067–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.Y.; Zhao, Z.H.; Zhao, J.; Liu, M.J. Expression profiles of genes and enzymes related to ascorbic acid metabolism in fruits of Ziziphus jujuba Mill. ‘Jinsixiaozao’. Front. Agric. Sci. Eng. 2016, 3, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Feng, C.F.; Yu, H.C.; Wang, J.R.; Liu, M.J. Discovery and evaluation of natural mixoploid (2x + 4x) variants in Ziziphus jujuba Mill.‘Dongzao’. Acta Hortic. Sin. 2016, 43, 966–974. [Google Scholar] [CrossRef]
- Wang, L.X. Methods analysing cAMP from Hetian jade jujuba. Food Sci. Technol. 2011, 36, 303–306. [Google Scholar] [CrossRef]
- do Amaral, C.M.; de Almeida dos Santos-Serejo, J.; de Oliveira e Silva, S.; da Silva Ledo, C.A.; Amorim, E.P. Agronomic characterization of autotetraploid banana plants derived from ‘Pisang Lilin’ (AA) obtained through chromosome doubling. Euphytica 2015, 202, 435–443. [Google Scholar] [CrossRef]
- Janečková, H.; Husičková, A.; Lazár, D.; Ferretti, U.; Pospíšil, P.; Špundová, M. Exogenous application of cytokinin during dark senescence eliminates the acceleration of photosystem II impairment caused by chlorophyll b deficiency in barley. Plant Physiol. Biochem. 2019, 136, 43–51. [Google Scholar] [CrossRef]
- Xue, H.; Zhang, B.; Tian, J.R.; Chen, M.M.; Zhang, Y.Y.; Zhang, Z.H.; Ma, Y. Comparison of the morphology, growth and development of diploid and autotetraploid ‘Hanfu’ apple trees. Sci. Hortic. 2017, 225, 277–285. [Google Scholar] [CrossRef]
- Wang, K.; He, L.; Yan, H.; Wei, X. Induction of tetraploidity with antimicrotubule agents in oriental melon (Cucumis melovar. Makuwa). Isr. J. Plant Sci. 2015, 62, 198–207. [Google Scholar] [CrossRef]
- Valente, A.; Albuquerque, T.G.; Sanches-Silva, A.; Costa, H.S. Ascorbic acid content in exotic fruits: A contribution to produce quality data for food composition databases. Food Res. Int. 2011, 44, 2237–2242. [Google Scholar] [CrossRef]
- Zhang, C.M.; Yin, X.; Li, X.G.; Huang, J.; Wang, C.Z.; Lian, C.L. Genetic diversity of sour jujube along the Yellow River. J. Northwest A F Univ. Nat. Sci. Ed. 2013, 12, 107–112. [Google Scholar] [CrossRef]
- Zhao, A.; Xue, X.; Wang, Y.; Sui, C.; Ren, H.; Li, D. Characteristic analysis of sugars and organic acids components and contents of Chinese jujube and wild jujube fruits. J. Tarim Univ. 2016, 28, 29–36. [Google Scholar]
- Huang, J.; Zhang, C.; Zhao, X.; Fei, Z.; Wan, K.; Zhang, Z.; Pang, X.; Yin, X.; Bai, Y.; Sun, X. The jujube genome provides insights into genome evolution and the domestication of sweetness/acidity taste in fruit trees. PLoS Genet. 2016, 12, e1006433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.J.; Zhao, J.; Cai, Q.L.; Liu, G.C.; Wang, J.R.; Zhao, Z.H.; Liu, P.; Dai, L.; Yan, G.; Wang, W.J.; et al. The complex jujube genome provides insights into fruit tree biology. Nat. Commun. 2014, 5, 5315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Y.; Xue, Z.H.; Wu, G.E.; Liu, P.; Liu, M.J. Abnormal meiosis behaviors of triploid and tetraploid Chinese Jujube. Acta Hortic. Sin. 2018, 45, 659–668. [Google Scholar] [CrossRef]
- Wu, J.; Shahid, M.Q.; Chen, L.; Chen, Z.; Wang, L.; Liu, X.; Lu, Y. Polyploidy enhances F1 pollen sterility loci interactions that increase meiosis abnormalities and pollen sterility in autotetraploid rice. Plant Physiol. 2015, 169, 2700–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nghiem, C.Q.; Griffin, R.; Harbard, J.; Harwood, C.; Le, S.; Nguyen, K.D.; Van Pham, B. Reduced fertility in triploids of acacia auriculiformis and its hybrid with A. mangium. Euphytica 2018, 214, 77. [Google Scholar] [CrossRef]
- Yan, F.; Wang, L.; Zheng, X.; Luo, Z.; Wang, J.; Liu, M. Acquisition of triploid germplasms by controlled hybridisation between diploid and tetraploid in Chinese jujube. J. Hortic. Sci. Biotechnol. 2018, 94, 123–129. [Google Scholar] [CrossRef]
- Liang, S.L.; Dang, J.B.; Liang, G.L.; Guo, Q.G. Meiosis observation and fertility analysis in natural tetraploid loquat of ‘B431’. Acta Hortic. Sin. 2018, 45, 1895–1904. [Google Scholar] [CrossRef]






| Tree Characteristics | Xingtai 0604 (2x) ((Mean ± SD) | Zhuguang (4x) (Mean ± SD) |
|---|---|---|
| Apical dominance | Obvious | Not obvious |
| Tree form | Circular cone shape | Globose shape |
| Tree vigor | Vigorous | Intermediate |
| Plant height (m) | 2.99 ± 0.12 * | 2.46 ± 0.09 |
| Annual growth of extension shoots (cm) | 51.93 ± 7.93 * | 40.48 ± 6.56 |
| Annual number of extension shoots | 13.33 ± 1.53 * | 5.67 ± 2.51 |
| Flower Characteristics | Xingtai 0604 (2x) (Mean ± SD) | Zhuguang (4x) (Mean ± SD) |
|---|---|---|
| Flower diameter (mm) | 2.88 ± 0.13 | 3.62 ± 0.24 ** |
| Flower bud diameter (mm) | 1.88 ± 0.05 | 2.38 ± 0.08 ** |
| Petal length (mm) | 1.05 ± 0.01 | 1.24 ± 0.03 ** |
| Sepal length (mm) | 1.46 ± 0.03 | 1.65 ± 0.02 ** |
| Sepal width (mm) | 1.18 ± 0.02 | 1.47 ± 0.04 ** |
| Anther length (mm) | 0.55 ± 0.02 | 0.75 ± 0.02 ** |
| Anther width (mm) | 0.35 ± 0.02 | 0.39 ± 0.03 ** |
| Pollen diameter (μm) | 25.67 ± 2.34 | 31.34 ± 3.07 ** |
| Pollen germination rate (%) | 53.01 ± 6.06 ** | 27.21 ± 7.20 |
| Leaf Characteristics | Xingtai 0604 (2x) (Mean ± SD) | Zhuguang (4x) (Mean ± SD) |
|---|---|---|
| Length of bearing shoots (cm) | 14.62 ± 1.60 | 16.02 ± 1.35 * |
| Number of leaves on fruit-bearing shoot | 10 | 10 |
| Leaf length (cm) | 4.20 ± 0.42 | 4.53 ± 0.47 * |
| Leaf width (cm) | 2.21 ± 0.28 | 3.46 ± 0.27 * |
| Stoma length (μm) | 26.68 ± 1.08 | 34.96 ± 1.17 ** |
| Stoma width (μm) | 18.54 ± 0.69 | 24.15 ± 0.95 ** |
| Stomatal density (mm−2) | 275.87 ± 8.66 ** | 162.67 ± 8.53 |
| Chlorophyll a (mg/g) | 2.14 ± 0.12 | 2.62 ± 0.06 * |
| Chlorophyll b (mg/g) | 0.62 ± 0.01 | 0.98 ± 0.05 ** |
| Chlorophyll (mg/g) | 2.76 ± 0.12 | 3.60 ± 0.08 ** |
| Fruit Quality | Xingtai 0604 (2x) (Mean ± SD) | Zhuguang (4x) (Mean ± SD) |
|---|---|---|
| Fruit length (mm) | 24.36 ± 0.60 | 28.61 ± 0.65 ** |
| Fruit width (mm) | 25.39 ± 0.77 | 30.61 ± 1.44 ** |
| Fruit index (length/width) | 0.96 | 0.93 |
| Fruit weight (g) | 5.99 ± 1.37 | 8.03 ± 0.78 ** |
| Vc content (mg/g) | 2.65 ± 0.06 ** | 1.75 ± 0.02 |
| cAMP content (mg/100g) | 11.21 ± 0.57 | 15.47 ± 0.25 * |
| Titratable acid content (%) | 0.49 ± 0.06 | 0.42 ± 0.02 |
| Soluble sugar content (%) | 25.78 ± 0.15 ** | 22.87 ± 0.11 |
| Soluble sugar/titratable acid | 52.61 | 54.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, L.; Ye, T.; Zhao, J.; Wang, L.; Wei, H.; Liu, P.; Liu, M. Autotetraploidization Alters Morphology, Photosynthesis, Cytological Characteristics and Fruit Quality in Sour Jujube (Ziziphus acidojujuba Cheng et Liu). Plants 2023, 12, 1106. https://doi.org/10.3390/plants12051106
Wang L, Wang L, Ye T, Zhao J, Wang L, Wei H, Liu P, Liu M. Autotetraploidization Alters Morphology, Photosynthesis, Cytological Characteristics and Fruit Quality in Sour Jujube (Ziziphus acidojujuba Cheng et Liu). Plants. 2023; 12(5):1106. https://doi.org/10.3390/plants12051106
Chicago/Turabian StyleWang, Lihu, Lixin Wang, Tingting Ye, Jin Zhao, Lili Wang, Hairong Wei, Ping Liu, and Mengjun Liu. 2023. "Autotetraploidization Alters Morphology, Photosynthesis, Cytological Characteristics and Fruit Quality in Sour Jujube (Ziziphus acidojujuba Cheng et Liu)" Plants 12, no. 5: 1106. https://doi.org/10.3390/plants12051106
APA StyleWang, L., Wang, L., Ye, T., Zhao, J., Wang, L., Wei, H., Liu, P., & Liu, M. (2023). Autotetraploidization Alters Morphology, Photosynthesis, Cytological Characteristics and Fruit Quality in Sour Jujube (Ziziphus acidojujuba Cheng et Liu). Plants, 12(5), 1106. https://doi.org/10.3390/plants12051106







