Adding New Scientific Evidences on the Pharmaceutical Properties of Pelargonium quercetorum Agnew Extracts by Using In Vitro and In Silico Approaches
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Bioactive Compounds
2.2. Antioxidant Properties
2.3. Enzyme Inhibitory Properties
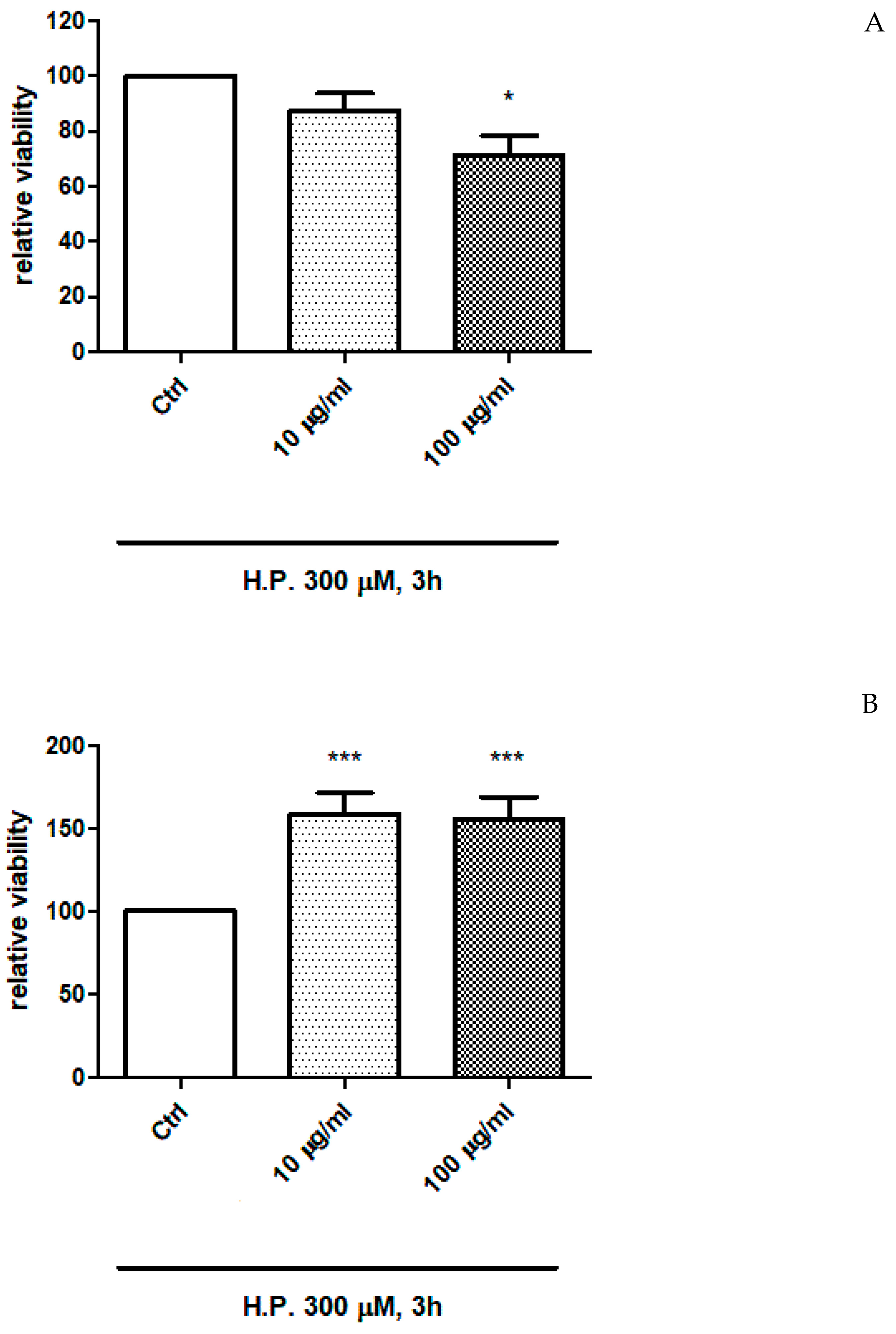
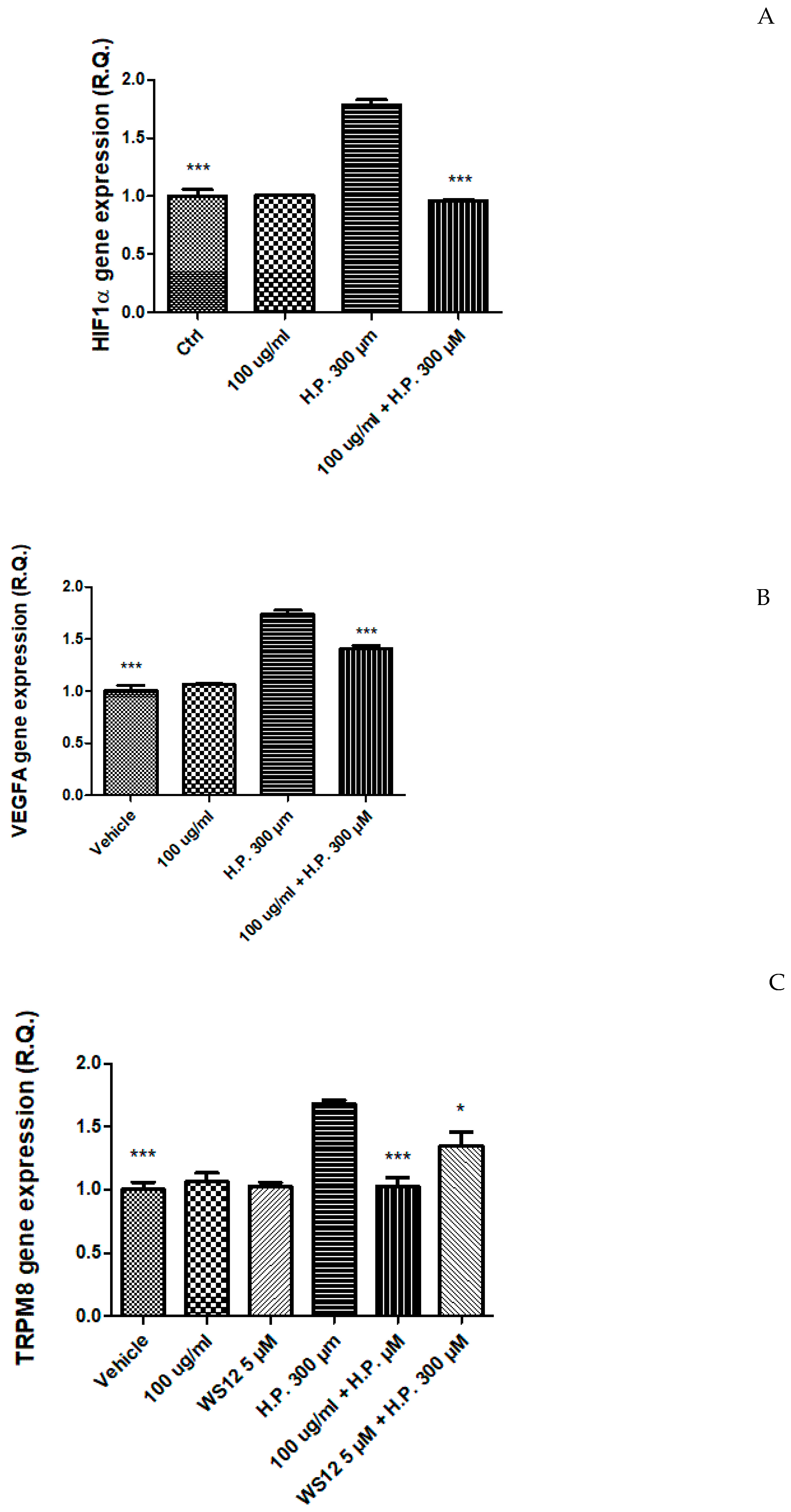
2.4. In Vitro Study
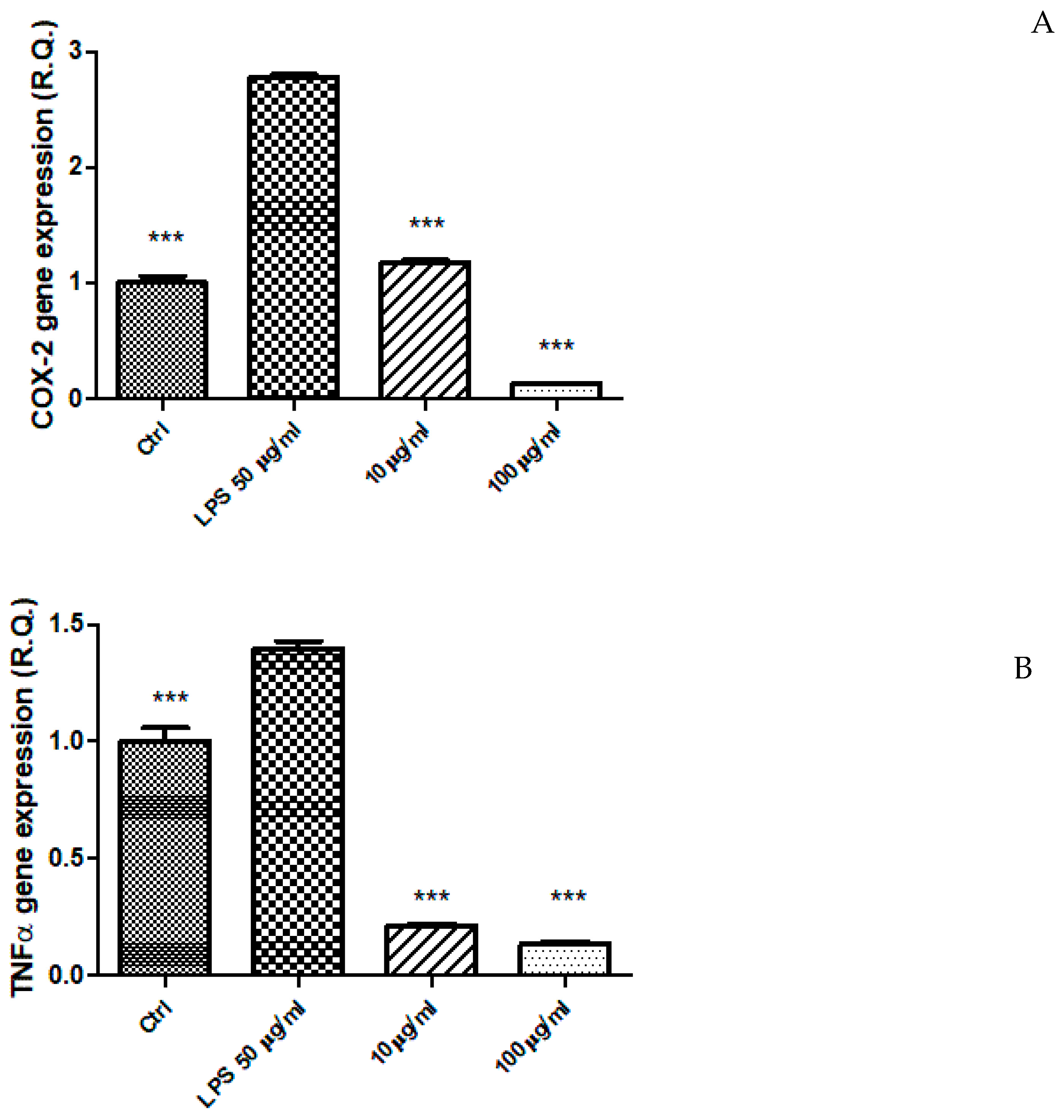
2.5. Ex Vivo Study
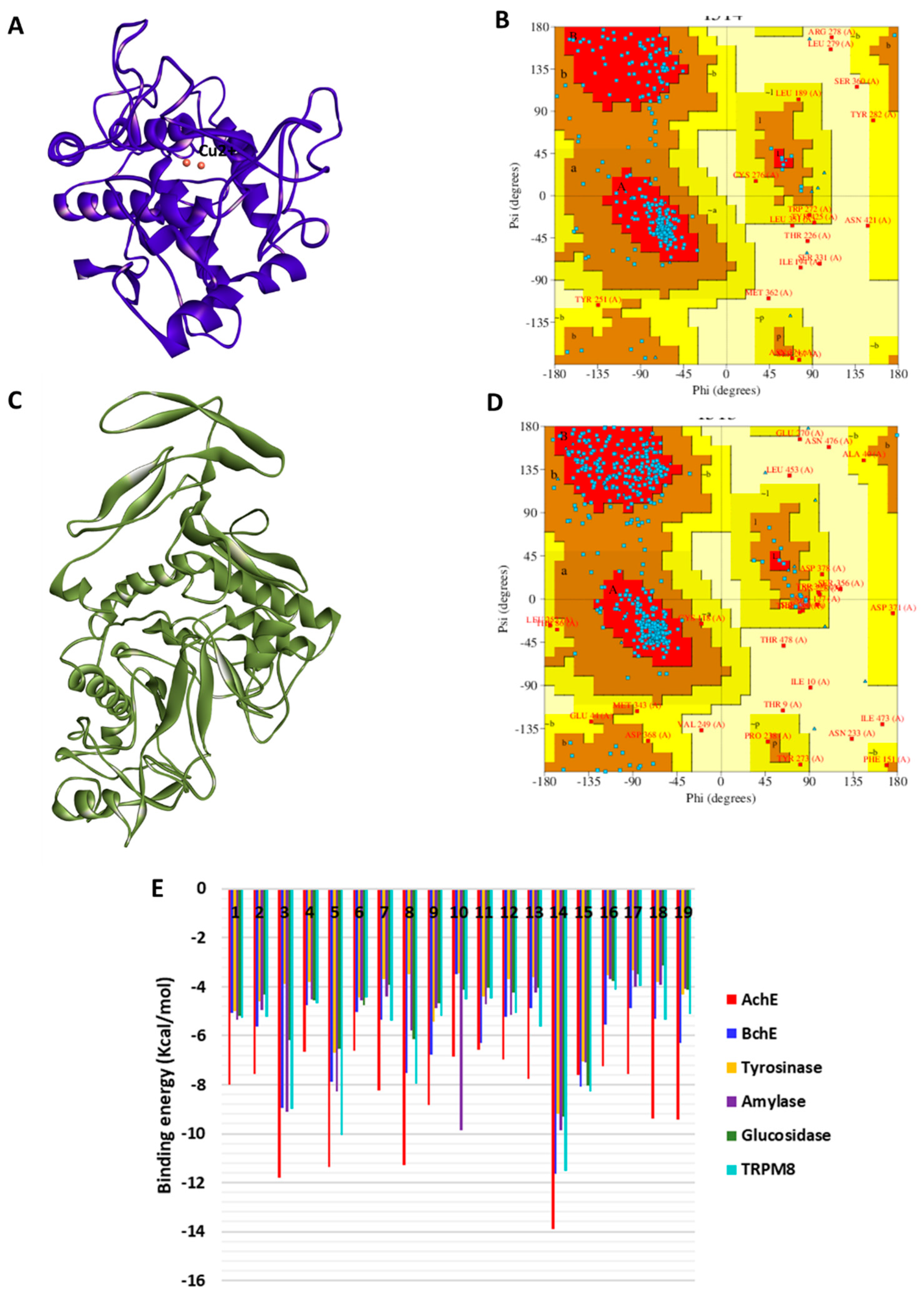
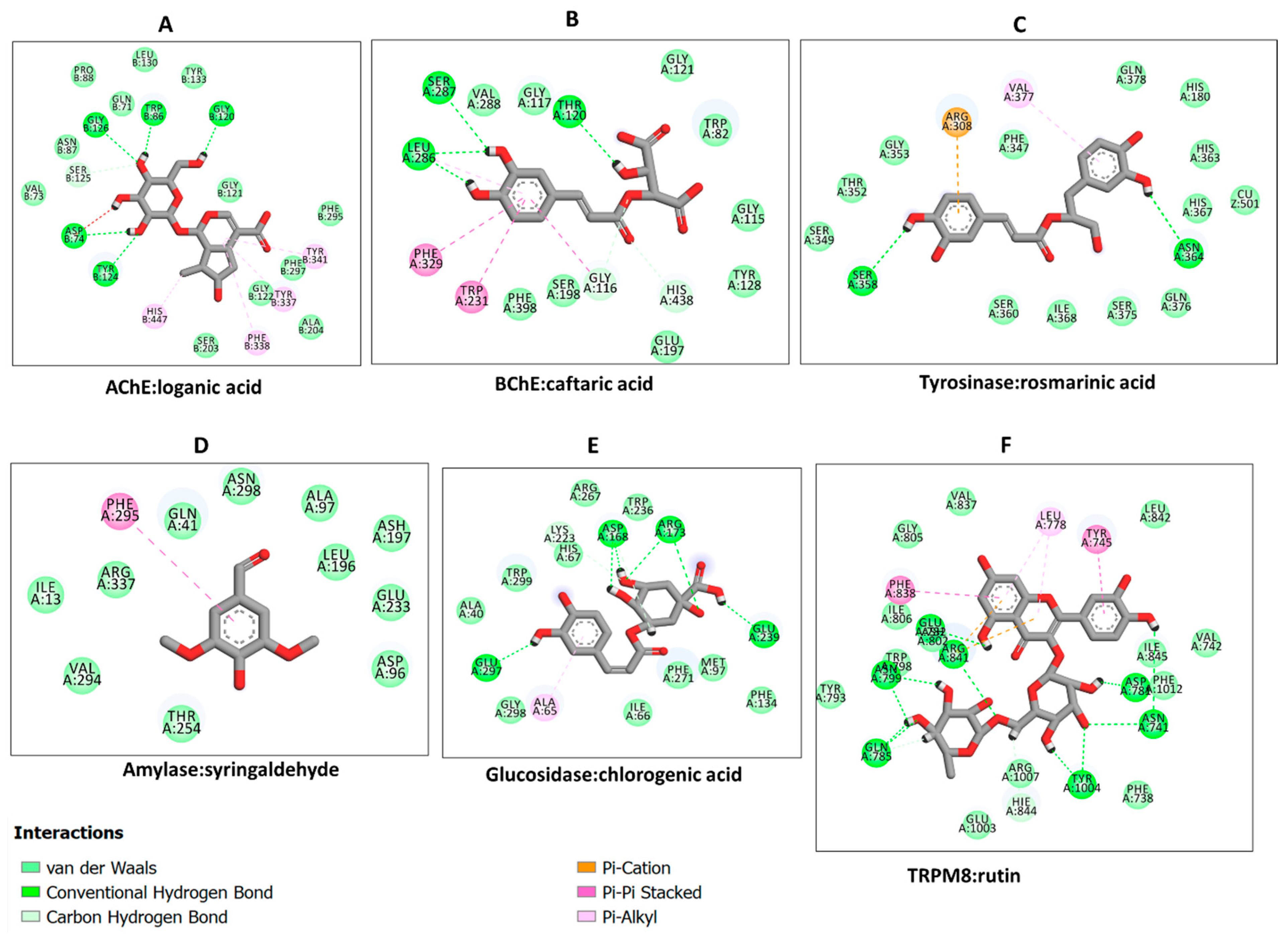
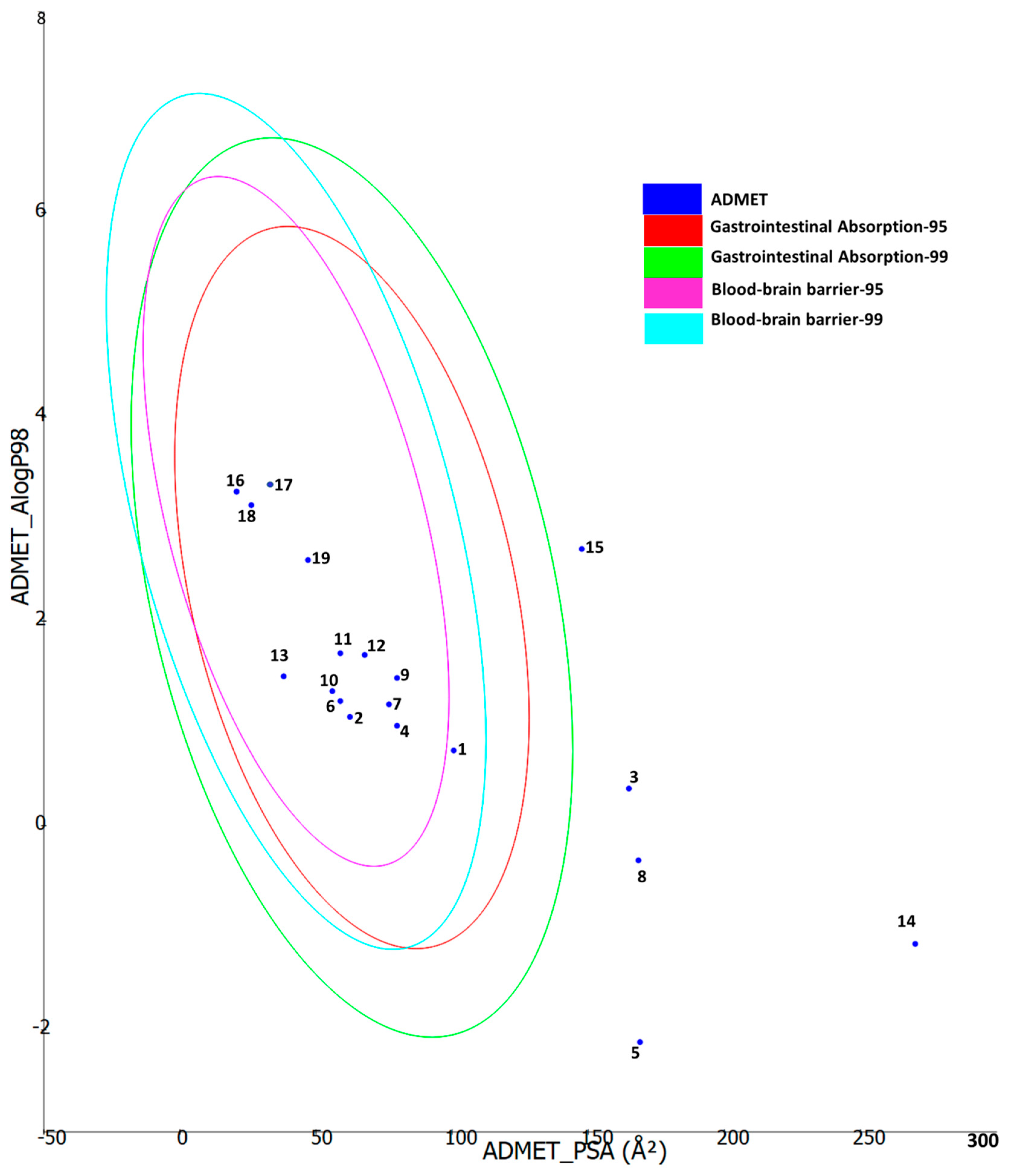
2.6. Molecular Docking
3. Materials and Methods
3.1. Plant Material and Preparation of Extracts
3.2. Chemicals and Reagents
3.3. Determination of Total Polyphenol and Flavonoids Contents
3.4. HPLC Determination of Phenolic Compounds
3.5. Antioxidant and Enzyme Inhibitory Assays
3.6. In Vitro Study
3.7. Ex Vivo Study
3.8. RNA Extraction, Reverse Transcription Andreal-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
3.9. Molecular Modelling
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taherpour, A.A.; Maroofi, H.; Kheradmand, K. Chemical composition of the essential oil of Pelargonium quercetorum Agnew. of Iran. Nat. Prod. Res. 2007, 21, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Agnew, A.D.Q. Contributions to the Flora of Iraq: IV: Notes on the Geraniaceae of Iraq, with a New Species of Pelargonium. Kew Bull. 1967, 21, 225–227. [Google Scholar] [CrossRef]
- Maroufi, Y.; Hoseini, S.R.; Alavi, M. Antiparasitic Effect of Leaf Extract and Major Metabolites of Pelargonium quercetorum Agnew. against Leishmania Major: In Vitro and In Silico Studies. J. Appl. Biotechnol. Rep. 2022, 9, 817–830. [Google Scholar] [CrossRef]
- Aztopal, N.; Cevatemre, B.; Sarimahmut, M.; Ari, F.; Dere, E.; Ozel, M.Z.; Firat, M.; Ulukaya, E. Pelargonium quercetorum Agnew induces apoptosis without PARP or cytokeratin 18 cleavage in non-small cell lung cancer cell lines. Oncol. Lett. 2016, 12, 1429–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumlupınar, B.; Karatoprak, G.Ş.; Fırat, M.; Akkol, E.K. Appraisal of the Antimicrobial and Cytotoxic Potentials of Nanoparticles Biosynthesized from the Extracts of Pelargonium quercetorum Agnew. Front. Biosci. 2021, 26, 1089–1096. [Google Scholar] [CrossRef]
- Kolodziej, H. Pelargonium reniforme and Pelargonium sidoides: Their botany, chemistry and medicinal use. In Geranium and Pelargonium; CRC Press: Boca Raton, FL, USA, 2002; pp. 274–302. [Google Scholar]
- Kolodziej, H. Fascinating Metabolic Pools of Pelargonium Sidoides and Pelargonium Reniforme, Traditional and Phytomedicinal Sources of the Herbal Medicine Umckaloabo. Phytomedicine 2007, 14 (Suppl. 6), 9–17. [Google Scholar] [CrossRef]
- Akkemik, E.; Fidan, M.; Balaban, M.; İnal, B. ICP-OES and LC-ESI-MS/MS analyses, enzyme inhibition and dna protection potential of Pelargonium quercetorum Agnew. Stud. Univ. Babes-Bolyai Chem. 2022, 67, 197–213. [Google Scholar] [CrossRef]
- Ferda, A.; Çelikler, S.; Karakaş, D. Total phenolic content, antioxidant and cyto-/genotoxic activities of Pelargonium quercetorum agnew in human breast cancer cells. J. Clin. Exp. Investig. 2017, 8, 22–30. [Google Scholar]
- Ferrante, C.; Recinella, L.; Ronci, M.; Orlando, G.; Di Simone, S.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Politi, M.; Tirillini, B. Protective effects induced by alcoholic Phlomis fruticosa and Phlomis herba-venti extracts in isolated rat colon: Focus on antioxidant, anti-inflammatory, and antimicrobial activities in vitro. Phytother. Res. 2019, 33, 2387–2400. [Google Scholar] [CrossRef]
- Locatelli, M.; Ferrante, C.; Carradori, S.; Secci, D.; Leporini, L.; Chiavaroli, A.; Leone, S.; Recinella, L.; Orlando, G.; Martinotti, S. Optimization of aqueous extraction and biological activity of Harpagophytum procumbens root on ex vivo rat colon inflammatory model. Phytother. Res. 2017, 31, 937–944. [Google Scholar] [CrossRef]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An hydroalcoholic chamomile extract modulates inflammatory and immune response in HT29 cells and isolated rat colon. Phytother. Res. 2016, 30, 1513–1518. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Ronci, M.; Menghini, L.; Brunetti, L.; Leone, S.; Tirillini, B.; Angelini, P.; Covino, S.; Venanzoni, R. Multidirectional pharma-toxicological study on Harpagophytum procumbens DC. ex Meisn.: An IBD-focused investigation. Antioxidants 2020, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Xu, C.; Hong, S.; Xia, X.; Cao, Y.; McDermott, J.; Mu, Y.; Han, J.-D.J. Immune cell types and secreted factors contributing to inflammation-to-cancer transition and immune therapy response. Cell Rep. 2019, 26, 1965–1977. [Google Scholar] [CrossRef] [Green Version]
- Borrelli, F.; Pagano, E.; Romano, B.; Panzera, S.; Maiello, F.; Coppola, D.; de Petrocellis, L.; Buono, L.; Orlando, P.; Izzo, A.A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis 2014, 35, 2787–2797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.J.; Li, L.Z.; Xu, P. Upregulation of TRPM8 can promote the colon cancer liver metastasis through mediating Akt/GSK-3 signal pathway. Biotechnol. Appl. Biochem. 2022, 69, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Karatoprak, G.Ş.; Fırat, M.; Koşar, M. Pelargonium quercetorum Agnew. bitkisinin antioksidan aktivitesinin belirlenmesi. Mersin Üniversitesi Sağlık Bilim. Derg. 2018, 11, 174–183. [Google Scholar] [CrossRef]
- El Aanachi, S.; Gali, L.; Nacer, S.N.; Bensouici, C.; Dari, K.; Aassila, H. Phenolic contents and in vitro investigation of the antioxidant, enzyme inhibitory, photoprotective, and antimicrobial effects of the organic extracts of Pelargonium graveolens growing in Morocco. Biocatal. Agric. Biotechnol. 2020, 29, 101819. [Google Scholar] [CrossRef]
- Said, E.; Hicham, E.; Naima, Z.; Naji, A.; Hafida, B.; Fatiha, B. Effect of Drying Techniques on the Moroccan Pelargonium graveolens L’Her. Leaves Essential Oil: Yield, Composition, Total Polyphenol Content, Antioxidant Activity, and Hygroscopic Parameters. J. Essent. Oil Bear. Plants 2022, 25, 508–523. [Google Scholar] [CrossRef]
- Ennaifer, M.; Bouzaiene, T.; Messaoud, C.; Hamdi, M. Phytochemicals, antioxidant, anti-acetyl-cholinesterase, and antimicrobial activities of decoction and infusion of Pelargonium graveolens. Nat. Prod. Res. 2020, 34, 2634–2638. [Google Scholar] [CrossRef]
- Chatatikun, M.; Supjaroen, P.; Promlat, P.; Chantarangkul, C.; Waranuntakul, S.; Nawarat, J.; Tangpong, J. Antioxidant and tyrosinase inhibitory properties of an aqueous extract of Garcinia atroviridis griff. ex. T. Anderson fruit pericarps. Pharmacogn. J. 2020, 12, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Meserole, L. Health foods in anti-aging therapy: Reducers of physiological decline and degenerative diseases. In Advances in Phytomedicine; Elsevier: Amsterdam, The Netherlands, 2002; Volume 1, pp. 173–180. [Google Scholar]
- Martemucci, G.; Portincasa, P.; Di Ciaula, A.; Mariano, M.; Centonze, V.; D’Alessandro, A.G. Oxidative stress, aging, antioxidant supplementation and their impact on human health: An overview. Mech. Ageing Dev. 2022, 206, 111707. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Nabavi, S.M. Antioxidants effects in health: Concluding remarks and future perspectives. In Antioxidants Effects in Health; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 6.3; pp. 851–858. [Google Scholar]
- Halim, M.A.; Kanan, K.A.; Nahar, T.; Rahman, M.J.; Ahmed, K.S.; Hossain, H.; Mozumder, N.H.M.R.; Ahmed, M. Metabolic profiling of phenolics of the extracts from the various parts of blackberry plant (Syzygium cumini L.) and their antioxidant activities. LWT 2022, 167, 113813. [Google Scholar] [CrossRef]
- Vo, G.T.; Liu, Z.; Chou, O.; Zhong, B.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Screening of phenolic compounds in australian grown grapes and their potential antioxidant activities. Food Biosci. 2022, 47, 101644. [Google Scholar] [CrossRef]
- Wei, J.; Li, S.; Su, T.; Zhao, J.; Jiang, Y.; Zubarev, Y.A.; Bi, Y. Phenolic compositions and antioxidant activities of Hippophae tibetana and H. rhamnoides ssp. sinensis berries produced in Qinghai-Tibet Plateau. Food Chem. X 2022, 15, 100397. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Koycheva, I.K.; Balcheva-Sivenova, Z.P.; Vasileva, S.M.; Georgiev, M.I. Rosmarinic acid—From bench to valuable applications in food industry. Trends Food Sci. Technol. 2021, 117, 182–193. [Google Scholar] [CrossRef]
- Ojeaburu, S.I.; Oriakhi, K. Hepatoprotective, antioxidant and, anti-inflammatory potentials of gallic acid in carbon tetrachloride-induced hepatic damage in Wistar rats. Toxicol. Rep. 2021, 8, 177–185. [Google Scholar] [CrossRef]
- Mahindrakar, K.V.; Rathod, V.K. Ultrasonic assisted aqueous extraction of catechin and gallic acid from Syzygium cumini seed kernel and evaluation of total phenolic, flavonoid contents and antioxidant activity. Chem. Eng. Process. Process Intensif. 2020, 149, 107841. [Google Scholar] [CrossRef]
- Spagnol, C.M.; Assis, R.P.; Brunetti, I.L.; Isaac, V.L.B.; Salgado, H.R.N.; Corrêa, M.A. In vitro methods to determine the antioxidant activity of caffeic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 358–366. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Zhou, Y.; Guo, R.; Fu, Z.-M.; Chen, D.-F. Structure-antioxidant activity relationship of ferulic acid derivatives: Effect of ester groups at the end of the carbon side chain. LWT 2020, 120, 108932. [Google Scholar] [CrossRef]
- Abdalla, A.A.A.; Yagi, S.; Abdallah, A.H.; Abdalla, M.; Sinan, K.I.; Zengin, G. Phenolic profile, antioxidant and enzyme inhibition properties of seed methanolic extract of seven new Sunflower lines: From fields to industrial applications. Process Biochem. 2021, 111, 53–61. [Google Scholar] [CrossRef]
- Benabderrahim, M.A.; Elfalleh, W.; Sarikurkcu, C.; Sarikurkcu, R.B. Biological activities and phytochemical composition of organs from Loranthus europaeus. Ind. Crops Prod. 2019, 141, 111772. [Google Scholar] [CrossRef]
- Iancu, C.; Cioancă, O.; Mircea, C.; Mocanu, M.; Hăncianu, M. Pelargonium sp.: Characterization of the polyphenols and their biological potential. Farmacia 2016, 64, 333–338. [Google Scholar]
- Fayoumi, L.; Khalil, M.; Ghareeb, D.; Chokr, A.; Bouaziz, M.; El-Dakdouki, M.H. Phytochemical constituents and therapeutic effects of the essential oil of rose geranium (Pelargonium hybrid) cultivated in Lebanon. S. Afr. J. Bot. 2022, 147, 894–902. [Google Scholar] [CrossRef]
- Panara, A.; Aalizadeh, R.; Thomaidis, N.S. Chemical characterisation of Pelargonium sidoides root based on LC-QToF-MS non-target screening strategies. Phytochem. Anal. 2022, 33, 40–56. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent research on the health benefits of blueberries and their anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef]
- Taqui, R.; Debnath, M.; Ahmed, S.; Ghosh, A. Advances on plant extracts and phytocompounds with acetylcholinesterase inhibition activity for possible treatment of Alzheimer’s disease. Phytomed. Plus 2022, 2, 100184. [Google Scholar] [CrossRef]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef]
- Nugroho, A.; Choi, J.S.; Hong, J.-P.; Park, H.-J. Anti-acetylcholinesterase activity of the aglycones of phenolic glycosides isolated from Leonurus japonicus. Asian Pac. J. Trop. Biomed. 2017, 7, 849–854. [Google Scholar] [CrossRef]
- Varela, M.T.; Ferrarini, M.; Mercaldi, V.G.; Sufi, B.d.S.; Padovani, G.; Nazato, L.I.S.; Fernandes, J.P.S. Coumaric acid derivatives as tyrosinase inhibitors: Efficacy studies through in silico, in vitro and ex vivo approaches. Bioorg. Chem. 2020, 103, 104108. [Google Scholar] [CrossRef]
- Ahammed, S.; Afrin, R.; Uddin, N.; Al-Amin, Y.; Hasan, K.; Haque, U.; Islam, K.M.M.; Alam, A.H.M.K.; Tanaka, T.; Sadik, G. Acetylcholinesterase Inhibitory and Antioxidant Activity of the Compounds Isolated from Vanda roxburghii. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 5569054. [Google Scholar] [CrossRef]
- Li, H.R.; Habasi, M.; Xie, L.Z.; Aisa, H.A. Effect of chlorogenic acid on melanogenesis of B16 melanoma cells. Molecules 2014, 19, 12940–12948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Iancu, C.; Mircea, C.; Petrariu, F.; Cioancă, O.; Stan, C.; Corciovă, A.; Murărașu, A.; Filip, N.; Hăncianu, M. The evaluation of normo-glycemic and cyto-regenerative effects of Pelargonium species extracts. FARMACIA 2020, 68, 135–141. [Google Scholar] [CrossRef]
- Ahamad, J.; Uthirapathy, S. Chemical characterization and antidiabetic activity of essential oils from Pelargonium graveolens leaves. ARO-Sci. J. Koya Univ. 2021, 9, 109–113. [Google Scholar]
- Seo, S.; Seo, K.; Ki, S.H.; Shin, S.M. Isorhamnetin inhibits reactive oxygen species-dependent hypoxia inducible factor (HIF)-1α accumulation. Biol. Pharm. Bull. 2016, 39, 1830–1838. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.-W.; Yu, B.-M.; Ji, Y.-B.; Zheng, M.-H.; Li, D.-H. Upregulation of vascular endothelial growth factor by hydrogen peroxide in human colon cancer. World J. Gastroenterol. 2002, 8, 153. [Google Scholar] [CrossRef]
- Gong, J.; Zhou, S.; Yang, S. Vanillic acid suppresses HIF-1α expression via inhibition of mTOR/p70S6K/4E-BP1 and Raf/MEK/ERK pathways in human colon cancer HCT116 cells. Int. J. Mol. Sci. 2019, 20, 465. [Google Scholar] [CrossRef] [Green Version]
- Taleb Agha, M.; Baharetha, H.M.; Al-Mansoub, M.A.; Tabana, Y.M.; Kaz Abdul Aziz, N.H.; Yam, M.F.; Abdul Majid, A.M.S. Proapoptotic and antiangiogenic activities of Arctium lappa L. on breast cancer cell lines. Scientifica 2020, 2020, 7286053. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Veschi, S.; Cama, A.; Acquaviva, A.; Libero, M.L.; Leone, S.; Di Simone, S.C.; Pagano, E.; Zengin, G. A grape (Vitis vinifera L.) pomace water extract modulates inflammatory and immune response in SW-480 cells and isolated mouse colon. Phytother. Res. 2022, 36, 4620–4630. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Zhao, Z.; Huang, X.; Xiong, H.; Mei, Z. Thymol activates TRPM8-mediated Ca2+ influx for its antipruritic effects and alleviates inflammatory response in Imiquimod-induced mice. Toxicol. Appl. Pharmacol. 2020, 407, 115247. [Google Scholar] [CrossRef]
- Sanechika, S.; Shimobori, C.; Ohbuchi, K. Identification of Herbal Components as TRPA1 Agonists and TRPM8 Antagonists. J. Nat. Med. 2021, 75, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Jiang, K.; Yin, N.; Ma, X.; Zhao, G.; Qiu, C.; Deng, G. Thymol mitigates lipopolysaccharide-induced endometritis by regulating the TLR4-and ROS-mediated NF-κB signaling pathways. Oncotarget 2017, 8, 20042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zengin, G.; Aktumsek, A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant to Turkey. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 481–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acquaviva, A.; Cristina Di Simone, S.; Canini, A.; Braglia, R.; Di Marco, G.; Campana, C.; Angelini, P.; Angeles Flores, G.; Venanzoni, R.; Loreta Libero, M.; et al. Phytochemical and Biological Investigations on the Pollen from Industrial Hemp Male Inflorescences. Food Res. Int. 2022, 161, 111883. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Zengin, G.; Uba, A.I.; Ocal, M.; Sharifi-Rad, M.; Caprioli, G.; Angeloni, S.; Altunoglu, Y.C.; Baloglu, M.C.; Yıldıztugay, E. Integration of in vitro and in silico approaches to assess three Astragalus species from Turkey flora: A novel spotlight from lab bench to functional applications. Food Biosci. 2022, 49, 101858. [Google Scholar] [CrossRef]
- Menghini, L.; Leporini, L.; Vecchiotti, G.; Locatelli, M.; Carradori, S.; Ferrante, C.; Zengin, G.; Recinella, L.; Chiavaroli, A.; Leone, S. Crocus sativus L. stigmas and byproducts: Qualitative fingerprint, antioxidant potentials and enzyme inhibitory activities. Food Res. Int. 2018, 109, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Orlando, G.; Leone, S.; Ferrante, C.; Chiavaroli, A.; Mollica, A.; Stefanucci, A.; Macedonio, G.; Dimmito, M.P.; Leporini, L.; Menghini, L. Effects of kisspeptin-10 on hypothalamic neuropeptides and neurotransmitters involved in appetite control. Molecules 2018, 23, 3071. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gerlits, O.; Ho, K.-Y.; Cheng, X.; Blumenthal, D.; Taylor, P.; Kovalevsky, A.; Radić, Z. A new crystal form of human acetylcholinesterase for exploratory room-temperature crystallography studies. Chem.-Biol. Interact. 2019, 309, 108698. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurt-Celep, I.; Zheleva-Dimitrova, D.; Gevrenova, R.; Uba, A.I.; Zengin, G.; Yıldıztugay, E.; Picot-Allain, C.M.N.; Lorenzo, J.M.; Mahomoodally, M.F.; Montesano, D. An In-Depth Study on the Metabolite Profile and Biological Properties of Primula auriculata Extracts: A Fascinating Sparkle on the Way from Nature to Functional Applications. Antioxidants 2022, 11, 1377. [Google Scholar] [CrossRef] [PubMed]
- Rosenberry, T.; Brazzolotto, X.; Macdonald, I.; Wandhammer, M.; Trovaslet-Leroy, M.; Darvesh, S.; Nachon, F. Comparison of the Binding of Reversible Inhibitors to Human Butyrylcholinesterase and Acetylcholinesterase: A Crystallographic, Kinetic and Calorimetric Study. Molecules 2017, 22, 2098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ielo, L.; Deri, B.; Germanò, M.P.; Vittorio, S.; Mirabile, S.; Gitto, R.; Rapisarda, A.; Ronsisvalle, S.; Floris, S.; Pazy, Y.; et al. Exploiting the 1-(4-fluorobenzyl)piperazine fragment for the development of novel tyrosinase inhibitors as anti-melanogenic agents: Design, synthesis, structural insights and biological profile. Eur. J. Med. Chem. 2019, 178, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Maurus, R.; Begum, A.; Williams, L.K.; Fredriksen, J.R.; Zhang, R.; Withers, S.G.; Brayer, G.D. Alternative Catalytic Anions Differentially Modulate Human α-Amylase Activity and Specificity. Biochemistry 2008, 47, 3332–3344. [Google Scholar] [CrossRef] [PubMed]
- Karade, S.S.; Hill, M.L.; Kiappes, J.L.; Manne, R.; Aakula, B.; Zitzmann, N.; Warfield, K.L.; Treston, A.M.; Mariuzza, R.A. N-Substituted Valiolamine Derivatives as Potent Inhibitors of Endoplasmic Reticulum α-Glucosidases I and II with Antiviral Activity. J. Med. Chem. 2021, 64, 18010–18024. [Google Scholar] [CrossRef]
- Yin, Y.; Le, S.C.; Hsu, A.L.; Borgnia, M.J.; Yang, H.; Lee, S.-Y. Structural basis of cooling agent and lipid sensing by the cold-activated TRPM8 channel. Science 2019, 363, eaav9334. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Martínez-Rosell, G.; Giorgino, T.; de Fabritiis, G. PlayMolecule ProteinPrepare: A Web Application for Protein Preparation for Molecular Dynamics Simulations. J. Chem. Inf. Model. 2017, 57, 1511–1516. [Google Scholar] [CrossRef]
- Miteva, M.A.; Guyon, F.; Tuffery, P. Frog2: Efficient 3D conformation ensemble generator for small compounds. Nucleic Acids Res. 2010, 38, W622–W627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
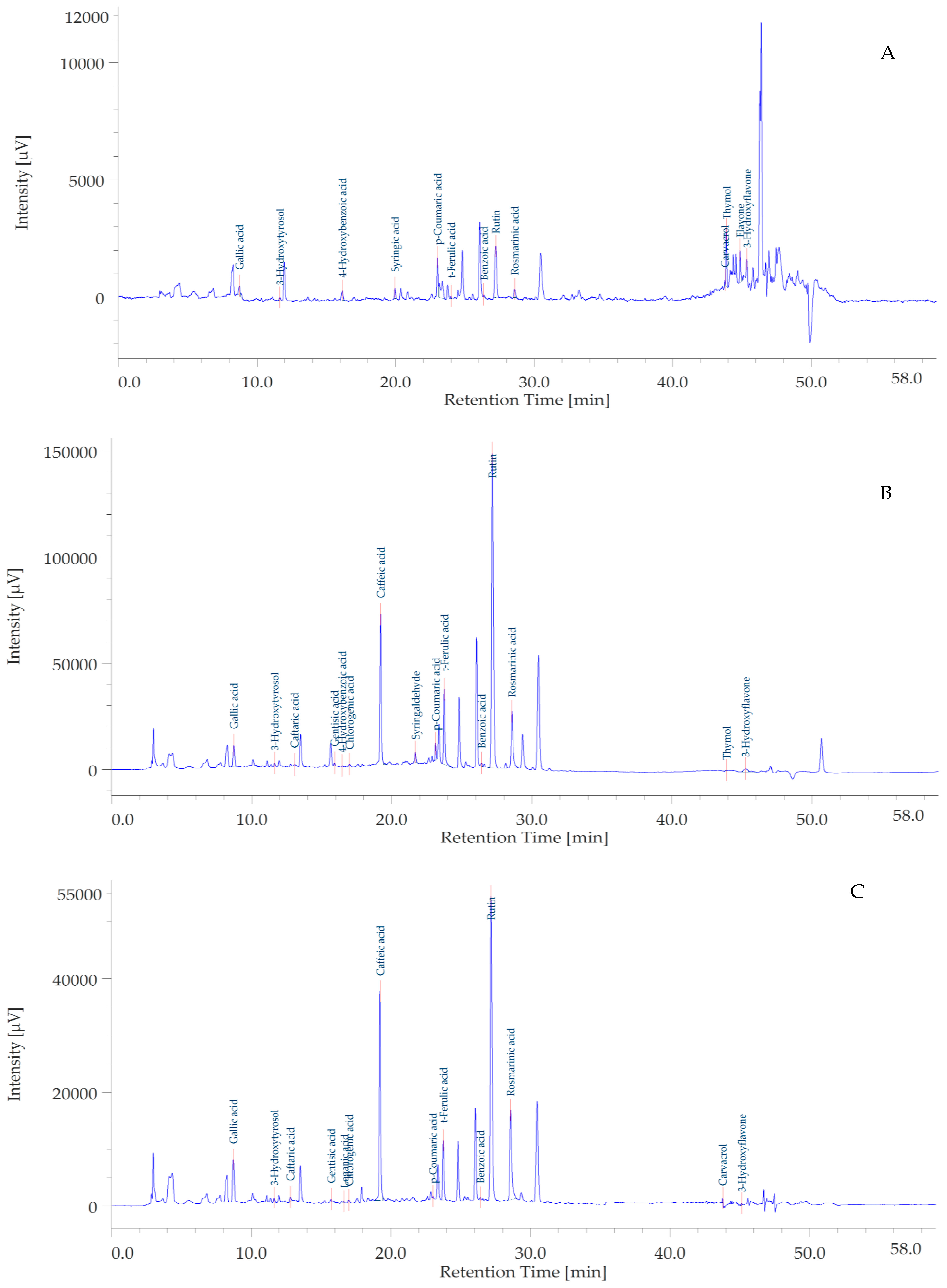
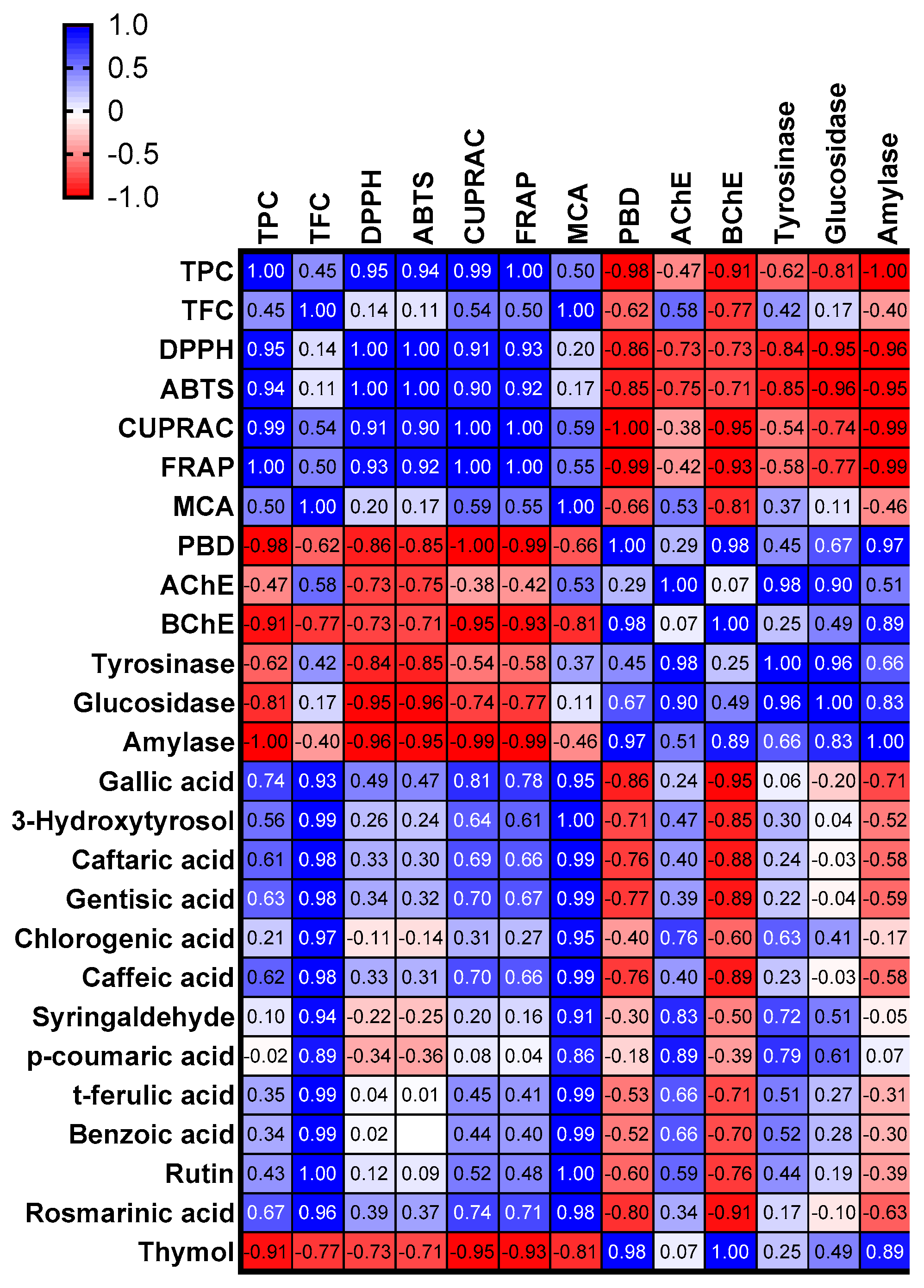
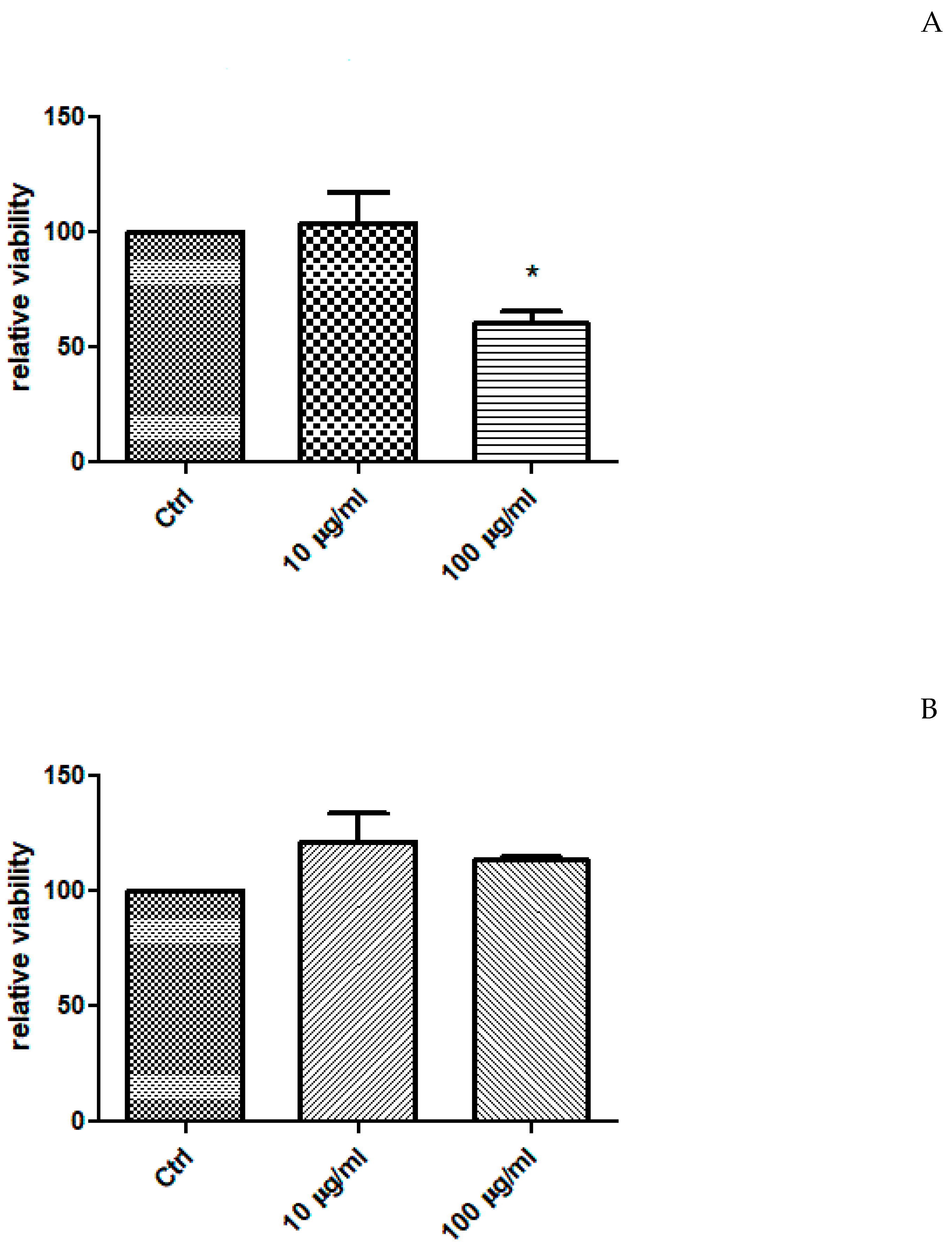
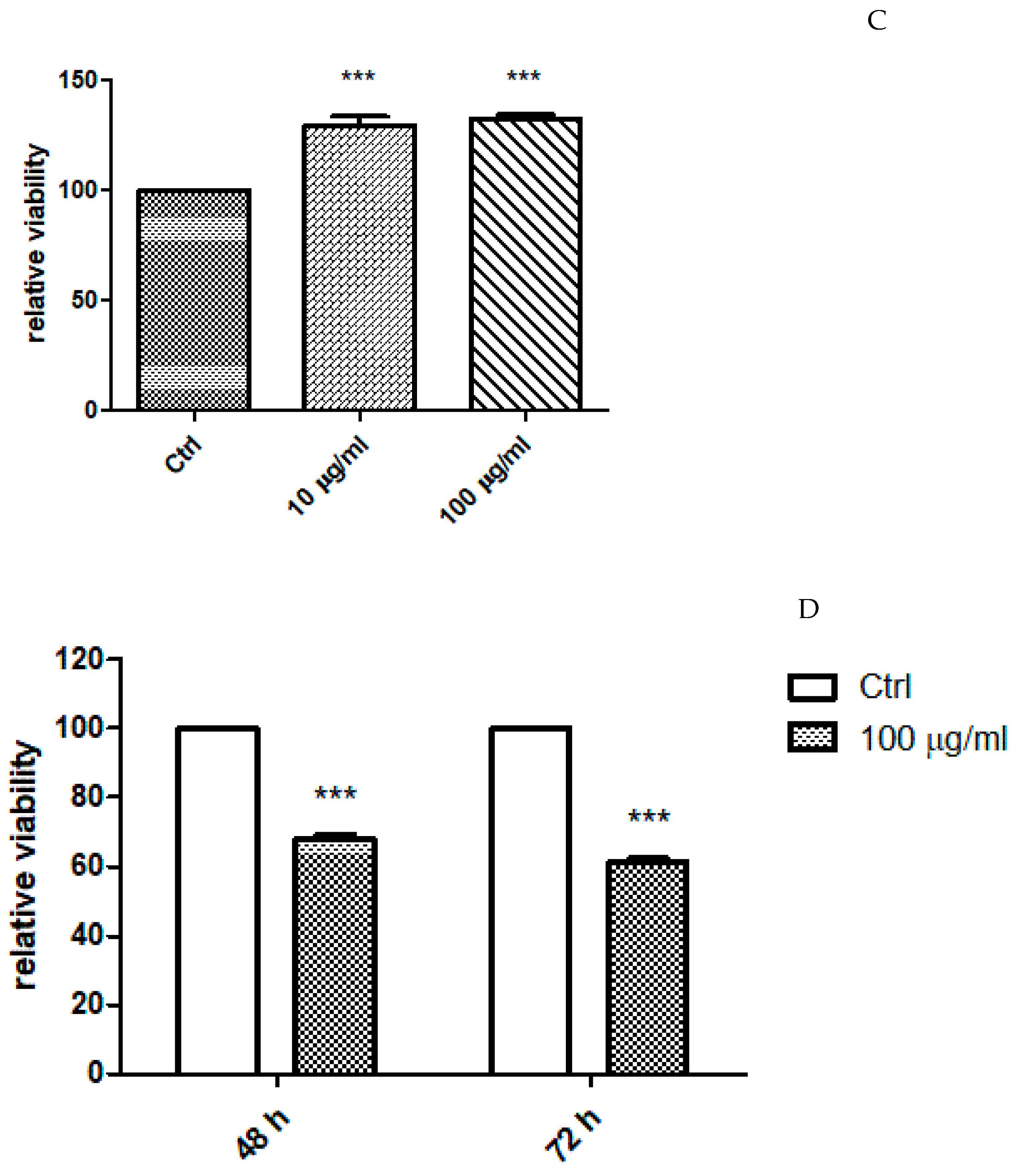

| Solvents | TPC (mg GAE/g Extract) | TFC (mg RE/g Extract) |
|---|---|---|
| Ethyl acetate | 21.94 ± 0.72 c | 2.78 ± 0.58 c |
| Methanol | 45.77 ± 1.02 b | 40.12 ± 0.20 a |
| Water | 62.52 ± 0.43 a | 16.15 ± 0.17 b |
| Components | Chemical Class | EA | MEOH | Water | Retention Time (Min) | Wavelenghts | |
|---|---|---|---|---|---|---|---|
| 1 | Gallic acid | Benzoic acids | nq | 12.55 ± 0.02 | 8.53 ± 0.03 | 8.723 | 271 |
| 2 | Hydroxytyrosol | Phenylethanoids | nq | 13.78 ± 0.03 | 6.18 ± 0.14 | 11.620 | 280 |
| 3 | Caftaric acid | Hydroxycinnamic acids | nq | 4.70 ± 0.15 | 3.20 ± 0.04 | 13.060 | 310 |
| 4 | Gentisic acid | Benzoic acids | nq | 5.03 ± 0.02 | 1.70 ± 0.21 | 15.907 | 325 |
| 5 | 4-Hydroxybenzoic acid | Benzoic acids | nq | nq | nq | 16.450 | 256 |
| 6 | Loganic acid | Iridoids | nq | nq | nq | 16.637 | |
| 7 | Chlorogenic acid | Hydroxycinnamic acids | nq | 1.70 ± 0.20 | 0.40 ± 0.03 | 16.960 | 325 |
| 8 | Caffeic acid | Hydroxycinnamic acids | nq | 51.75 ± 1.20 | 27.18 ± 0.44 | 19.207 | 325 |
| 9 | Syringic acid | Benzoic acids | nq | nq | nq | 19.960 | 274 |
| 10 | Syringaldehyde | Benzoic aldehydes | nq | 28.35 ± 0.25 | nq | 21.667 | 310 |
| 11 | p-Coumaric acid | Hydroxycinnamic acids | 1.43 ± 0.18 | 13.53 ± 0.35 | nq | 23.140 | 310 |
| 12 | t-Ferulic acid | Hydroxycinnamic acids | nq | 33.23 ± 0.21 | 8.80 ± 0.09 | 23.747 | 315 |
| 13 | Benzoic acid | Benzoic acids | 1.05 ± 0.02 | 8.35 ± 0.26 | 3.18 ± 0.05 | 26.400 | 275 |
| 14 | Rutin | Flavonol glycosides | 1.05 ± 0.01 | 190.33 ± 0.92 | 63.78 ± 0.98 | 27.170 | 254 |
| 15 | Rosmarinic acid | Hydroxycinnamic acids | nq | 45.28 ± 0.44 | 27.13 ± 0.38 | 28.570 | 325 |
| 16 | Carvacrol | Phenolic monoterpenes | nq | nq | nq | 43.757 | 275 |
| 17 | Thymol | Phenolic monoterpenes | 15.68 ± 0.02 | nq | nq | 43.873 | 275 |
| 18 | Flavone | Flavones | nq | nq | nq | 44.840 | 340 |
| 19 | 3-Hydroxyflavone | Flavones | nq | nq | nq | 45.327 | 340 |
| Solvents | DPPH (mg TE/g Extract) | ABTS (mg TE/g Extract) | CUPRAC (mg TE/g Extract) | FRAP (mg TE/g Extract) | MCA (mg EDTAE/g Extract) | PBD (mmol TE/g Extract) |
|---|---|---|---|---|---|---|
| Ethyl acetate | 9.28 ± 0.52 c | 27.17 ± 0.04 c | 96.07 ± 1.11 c | 32.65 ± 0.70 c | na | 1.64 ± 0.04 a |
| Methanol | 48.80 ± 0.01 b | 78.59 ± 0.10 b | 172.02 ± 6.72 b | 102.48 ± 6.20 b | 29.80 ± 0.79 a | 1.54 ± 0.07 b |
| Water | 140.40 ± 1.16 a | 212.31 ± 8.88 a | 207.83 ± 1.36 a | 142.01 ± 1.08 a | 12.28 ± 0.23 b | 1.51 ± 0.01 b |
| Solvents | AChE (mg GALAE/g Extract) | BChE (mg GALAE/g Extract) | Tyrosinase (mg KAE/g Extract) | α-Amylase (mmol ACAE/g Extract) | α-Glucosidase (mmol ACAE/g Extract) |
|---|---|---|---|---|---|
| Ethyl acetate | 1.63 ± 0.11 b | 2.59 ± 0.04 | 22.68 ± 1.26 b | 0.91 ± 0.02 a | 1.37 ± 0.07 a |
| Methanol | 2.31 ± 0.29 a | na | 31.24 ± 0.49 a | 0.45 ± 0.03 b | 1.38 ± 0.02 a |
| Water | 0.76 ± 0.08 c | na | 0.63 ± 0.11 c | 0.07 ± 0.01 c | 0.40 ± 0.01 b |
| Time (Min.) | Compasition A% (Water + Formic Acid 0.1%) | Compasition B% (Methanol + Formic Acid 0.1%) | Flow (mL/Min) |
|---|---|---|---|
| 1.00 | 97.0 | 3.0 | 0.600 |
| 5.00 | 77.0 | 23.0 | 0.600 |
| 12.00 | 73.0 | 27.0 | 0.600 |
| 18.00 | 57.0 | 43.0 | 0.600 |
| 25.00 | 52.0 | 48.0 | 0.600 |
| 32.00 | 50.0 | 50.0 | 0.600 |
| 34.00 | 50.0 | 50.0 | 0.600 |
| 37.00 | 35.0 | 65.0 | 0.600 |
| 40.00 | 5.0 | 95.0 | 0.600 |
| 47.00 | 5.0 | 95.0 | 0.600 |
| 48.00 | 97.0 | 3.0 | 0.600 |
| 60.00 | 97.0 | 3.0 | 0.600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiavaroli, A.; Libero, M.L.; Di Simone, S.C.; Acquaviva, A.; Nilofar; Recinella, L.; Leone, S.; Brunetti, L.; Cicia, D.; Izzo, A.A.; et al. Adding New Scientific Evidences on the Pharmaceutical Properties of Pelargonium quercetorum Agnew Extracts by Using In Vitro and In Silico Approaches. Plants 2023, 12, 1132. https://doi.org/10.3390/plants12051132
Chiavaroli A, Libero ML, Di Simone SC, Acquaviva A, Nilofar, Recinella L, Leone S, Brunetti L, Cicia D, Izzo AA, et al. Adding New Scientific Evidences on the Pharmaceutical Properties of Pelargonium quercetorum Agnew Extracts by Using In Vitro and In Silico Approaches. Plants. 2023; 12(5):1132. https://doi.org/10.3390/plants12051132
Chicago/Turabian StyleChiavaroli, Annalisa, Maria Loreta Libero, Simonetta Cristina Di Simone, Alessandra Acquaviva, Nilofar, Lucia Recinella, Sheila Leone, Luigi Brunetti, Donatella Cicia, Angelo Antonio Izzo, and et al. 2023. "Adding New Scientific Evidences on the Pharmaceutical Properties of Pelargonium quercetorum Agnew Extracts by Using In Vitro and In Silico Approaches" Plants 12, no. 5: 1132. https://doi.org/10.3390/plants12051132
APA StyleChiavaroli, A., Libero, M. L., Di Simone, S. C., Acquaviva, A., Nilofar, Recinella, L., Leone, S., Brunetti, L., Cicia, D., Izzo, A. A., Orlando, G., Zengin, G., Uba, A. I., Cakilcioğlu, U., Mukemre, M., Elkiran, O., Menghini, L., & Ferrante, C. (2023). Adding New Scientific Evidences on the Pharmaceutical Properties of Pelargonium quercetorum Agnew Extracts by Using In Vitro and In Silico Approaches. Plants, 12(5), 1132. https://doi.org/10.3390/plants12051132


















