Antioxidant Potential of Glutathione and Crosstalk with Phytohormones in Enhancing Abiotic Stress Tolerance in Crop Plants
Abstract
1. Introduction
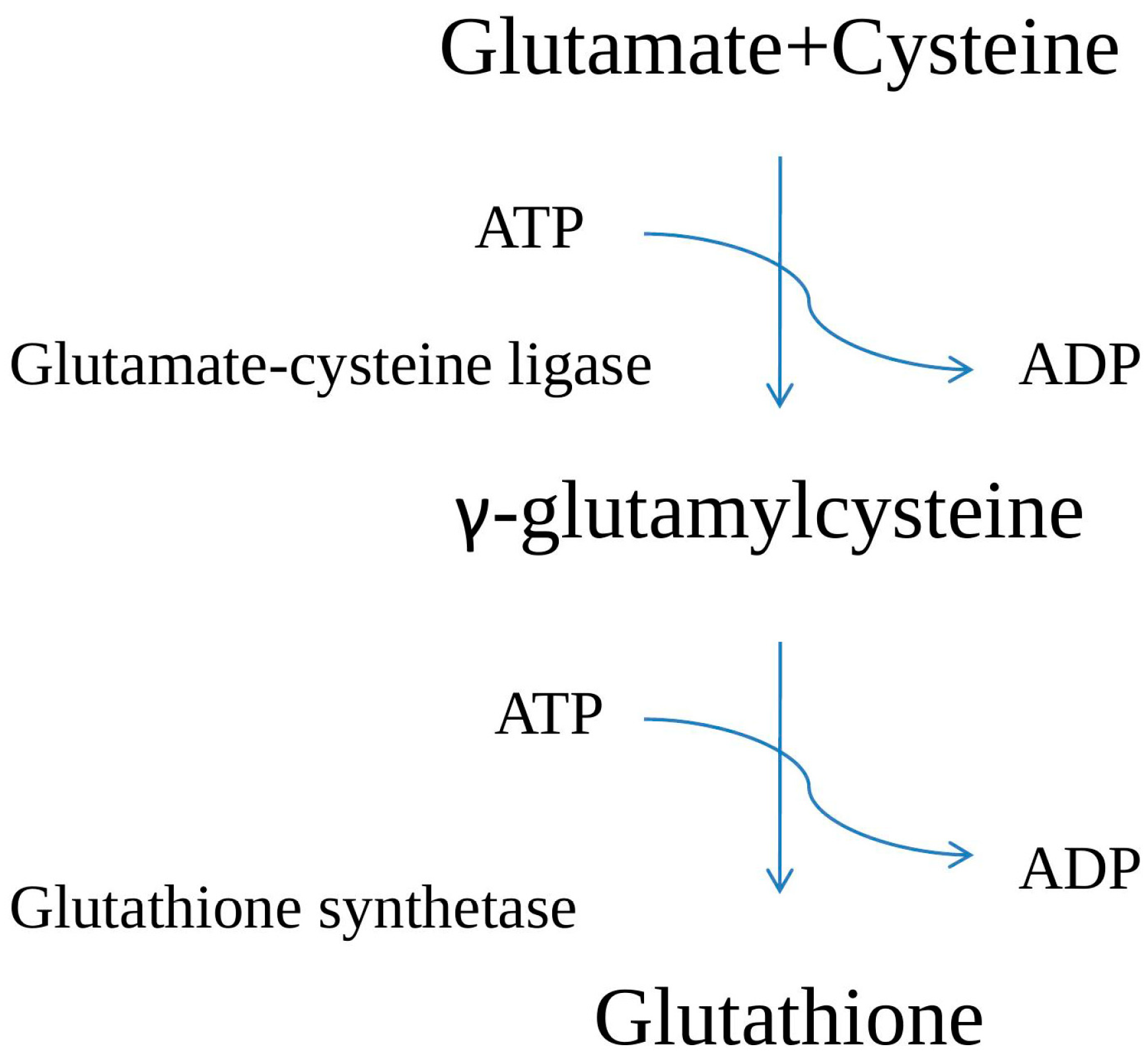
2. GSH Biosynthesis
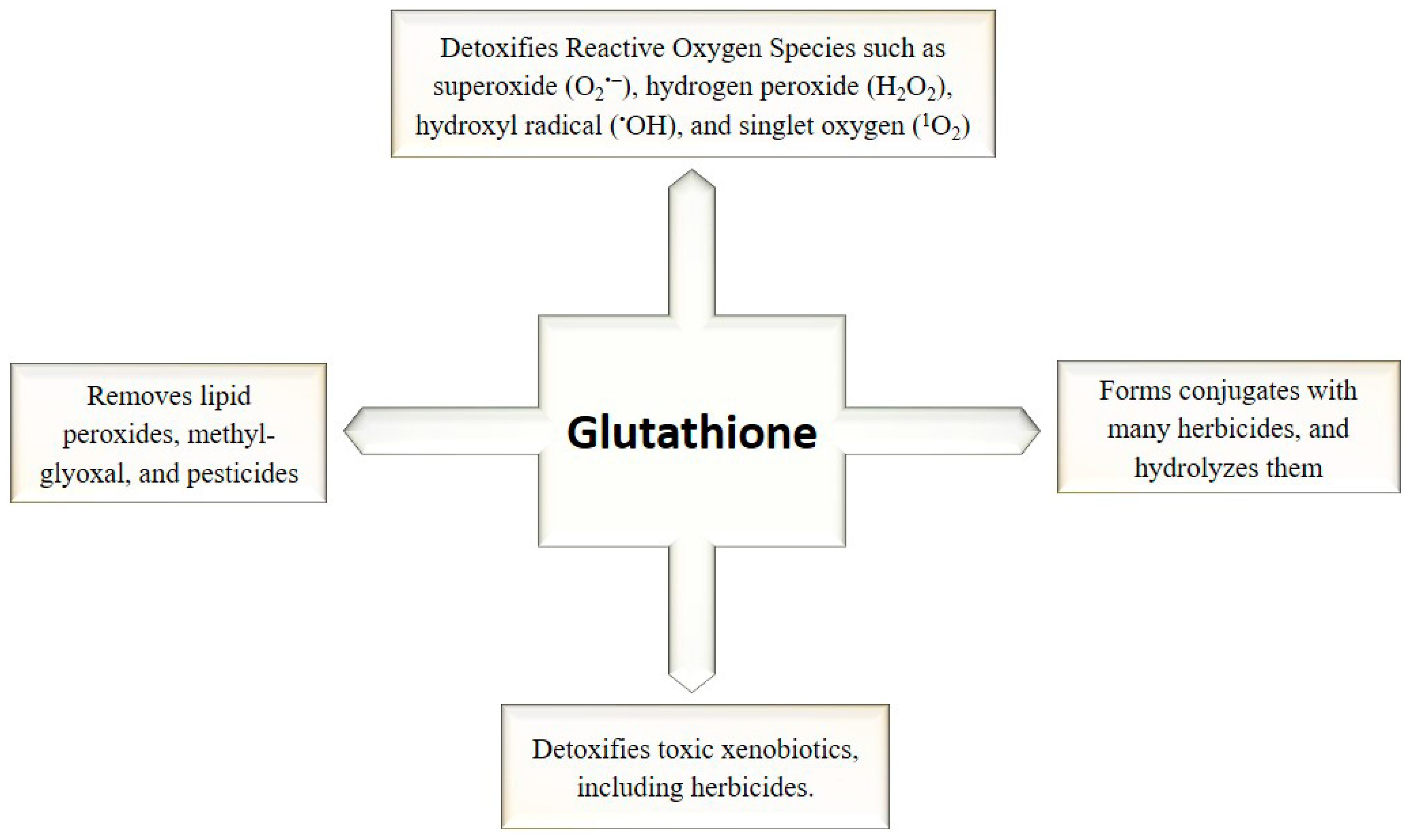
3. GSH as Regulatory and Antioxidant Molecule
4. Role of GSH under Abiotic Stress in Plant System
4.1. Oxidative Stress
4.2. Heat Stress
4.3. Cold Stress
4.4. Salinity Stress
4.5. Heavy Metal Stress
4.6. Drought Stress
| Abiotic Stress | Crop/Plant | Glutathione Application | Method | Result | Reference |
|---|---|---|---|---|---|
| Heat stress | Wheat | - | Endogenously enhanced (GR) | Enhanced tolerance to heat stress | [118] |
| Cucumber Mustard | N/A N/A | External application External application | Enhanced heat resistance, plant growth, chlorophyll content, and photosynthetic rate Improved osmoprotectants, ROS detoxification | [67] [119] | |
| Salinity stress | Pepper | 0.4 and 0.8 mM | Foliar spray | Enhanced growth and development by improving antioxidant defense system | [120] |
| Soybean | 1 mM | Foliar application | Increased photosynthesis and antioxidant enzyme activity | [121] | |
| Cold stress | Rice | 0.5 mM | GSH application via spraying | Improved growth and development by enhancing GSH level | [122] |
| Pepper | 0.5 mM | Spraying | Increased endogenous GSH content | [77] | |
| Drought stress | Mung bean | N/A | Exogenous application | Increased antioxidant system; decreased oxidative damage | [123] |
| Arabidopsis | N/A | Spraying | Improved drought tolerance | [115] | |
| Heavy metal stress | Maize | 30 µM | Foliar application | Enhanced Secondary metabolite and tolerance to cadmium metal stress, | [124] |
| Wheat | 20 µM | Foliar spray | tolerance to heavy metal stress; increased endogenous GSH content | [102] |
5. GSH Interaction with Phytohormones
6. Crosstalk between GSH and Jasmonate
7. GSH interaction with Salicylic Acid
8. GSH Defense Enzymes in Crops
9. GSH Role in Root Architecture and Root-Derived Changes in Shoots
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deneke, S.M. Thiol-based antioxidants. Curr. Top. Cell. Regul. 2001, 36, 151–180. [Google Scholar]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Canter, L.W. Environmental Impact of Agricultural Production Activities, 1st ed.; CRC Press: Boca Ration, FL, USA, 2018; p. 400. [Google Scholar] [CrossRef]
- Cook, D.; Fowler, S.; Fiehn, O.; Thomashow, M.F. A prominent role for the CBF cold response pathway in configuring the lowtemperature metabolome of Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 15243–15248. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Fatma, M.; Iqbal, N.; Sehar, Z.; Alyemeni, M.N.; Kaushik, P.; Khan, N.A.; Ahmad, P. Methyl jasmonate protects the PS II system by maintaining the stability of chloroplast D1 protein and accelerating enzymatic antioxidants in heat-stressed wheat plants. Antioxidants 2021, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defence in plants under abiotic stress: Revisiting the crucial role of a universal defence regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Mahmud, J.A.; Anee, T.I.; Nahar, K.; Islam, M.T. Drought stress tolerance in wheat: Omics approaches in understanding and enhancing antioxidant defense. In Abiotic Stress-Mediated Sensing and Signaling in Plants: An Omics Perspective; Zargar, S.M., Zargar, M.Y., Eds.; Springer: Singapore, 2018; pp. 267–307. [Google Scholar]
- Nahar, K.; Rhaman, M.S.; Parvin, K.; Bardhan, K.; Marques, D.N.; García-Caparrós, P.; Hasanuzzaman, M. Arsenic-induced oxidative stress and antioxidant defense in plants. Stresses 2022, 2, 179–209. [Google Scholar] [CrossRef]
- Leipner, J.; Fracheboud, Y.; Stamp, P. Effect of growing season on the photosynthetic apparatus and leaf antioxidative defenses in two maize genotypes of different chilling tolerance. Environ. Exp. Bot. 1999, 42, 129–139. [Google Scholar] [CrossRef]
- Davies, P.J. The Plant Hormones: Their nature, occurrence and functions. In Plant Hormones: Biosynthesis, Signal Transduction, Action! Davies, P.J., Ed.; Springer Science & Business Media Press: New York, NY, USA, 2004; pp. 1–15. [Google Scholar]
- Fleet, C.; Williams, M.E.; Gibberellins. Teaching Tools in plant biology: Lecture notes. Plant Cell 2011, 110. Available online: www.plantcell.org/cgi/doi/101105/tpc (accessed on 5 November 2022).
- Went, F. Auxin, the plant growth-hormone Avena sativa. Bot. Rev. 1935, 1, 162–182. [Google Scholar] [CrossRef]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.-P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.-C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Navarro, L.; Bari, R.; Achard, P.; Lison, P.; Nemri, A.; Harberd, N.P.; Jones, J. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr. Biol. 2008, 18, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Kumar, D.; Sultana, A.; Hazra, S.; Bhattacharyya, D.; Chattopadhyay, S. Glutathione regulates 1-aminocyclopropane-1-carboxylate synthase transcription via WRKY33 and 1-aminocyclopropane-1-carboxylate oxidase by modulating messenger RNA stability to induce ethylene synthesis during stress. Plant Physiol. 2015, 169, 2963–2981. [Google Scholar] [PubMed]
- Meister, A. Glutathione biosynthesis and its inhibition. In Biothiols Part B: Thiols in Signal Transduction and Gene Regulation; Packer, L., Ed.; Academic Press: San Diego, CA, USA, 1995; pp. 26–30. [Google Scholar] [CrossRef]
- Hell, R.; Bergmann, L. c-glutamylcysteine synthetase in higher plants: Catalytic properties and subcellular localization. Planta 1990, 180, 603–612. [Google Scholar] [CrossRef]
- Wachter, A.; Wolf, S.; Steininger, H.; Bogs, J.; Rausch, T. Differential targeting of GSH1 and GSH2 is achieved by multiple transcription initiation: Implications for the compartmentation of glutathione biosynthesis in the Brassicaceae. Plant J. 2005, 41, 15–30. [Google Scholar] [CrossRef]
- Galant, A.; Preuss, M.L.; Cameron, J.; Jez, J.M. Plant glutathione biosynthesis: Diversity in biochemical regulation and reaction products. Front. Plant Sci. 2011, 2, 45. [Google Scholar] [CrossRef]
- Hicks, L.M.; Cahoon, R.E.; Bonner, E.R.; Rivard, R.S.; Sheffield, J.; Jez, J.M. Thiol-based regulation of redox-active glutamate-cysteine ligase from Arabidopsis thaliana. Plant Cell 2007, 19, 2653–2661. [Google Scholar] [CrossRef]
- Meyer, A.J.; Fricker, M.D. Control of demand-driven biosynthesis of glutathione in green Arabidopsis suspension culture cells. Plant Physiol. 2002, 130, 1927–1937. [Google Scholar] [CrossRef]
- Jez, J.M.; Cahoon, R.E.; Chen, S. Arabidopsis thaliana glutamate-cysteine ligase: Functional properties, kinetic mechanism, and regulation of activity. J. Biol. Chem. 2004, 279, 33463–33470. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Zechmann, B.; Muller, M. Subcellular compartmentation of glutathione in dicotyledonous plants. Protoplasma 2010, 246, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, M.; Lim, B.; Wirtz, M.; Hell, R.; Cobbett, C.S.; Meyer, A.J. Restricting glutathione biosynthesis to the cytosol is sufficient for normal plant development. Plant J. Cell Mol. Biol. 2008, 53, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Oliver, D. Glutathione and its central role in mitigating plant stress. In Handbook of Plant and Crop Stress; Pessarakli, M., Ed.; Marcel Dekker: Basel, Switzerland, 1999; pp. 697–708. [Google Scholar]
- Mahmood, Q.; Ahmad, R.; Kwak, S.S.; Rashid, A.; Anjum, N.A. Ascorbate and glutathione: Pro-tectors of plants in oxidative stress. In Ascorbate–Glutathione Pathway and Stress Tolerance in Plants; Springer: New, York, NY, USA, 2010; pp. 209–229. [Google Scholar]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signalling: A metabolic link between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Navrot, N.; Collin, V.; Gualberto, J.; Gelhaye, E.; Hirasawa, M.; Rey, P.; Knaff, D.B.; Issakidis, E.; Jacquot, J.P.; Rouhier, N. Plant glutathione peroxidases are functional peroxiredoxins distributed in several subcellular compartments and regulated during biotic and abiotic stresses. Plant Physiol. 2006, 142, 1364–1379. [Google Scholar] [CrossRef]
- Rausch, T.; Gromes, R.; Liedschulle, V.; Muller, I.; Bogs, J.; Galovic, V.; Wachter, A. Novel insight into the regulation of GSH biosynthesis in higher plants. Plant Biol. 2007, 9, 565–572. [Google Scholar] [CrossRef]
- Yadav, S.K.; Singla-Pareek, S.L.; Sopory, S.K. An overview on the role of methylglyoxal and glyoxalases in plants. Drug Metab. Drug Interact 2008, 23, 51–68. [Google Scholar] [CrossRef]
- Lian, G.; Gnanaprakasam, J.R.; Wang, T.; Wu, R.; Chen, X.; Liu, L.; Shen, Y.; Yang, M.; Yang, J.; Chen, Y.; et al. Glutathione de novo synthesis but not recycling process coordinates with glutamine catabolism to control redox homeostasis and directs murine T cell differentiation. eLife 2018, 7, e36158. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Ruegsegger, A.; Brunold, C. Effect of cadmium on c-glutamylcysteine synthesis in maize seedlings. Plant Physiol. 1992, 99, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Klapheck, S. Homoglutathione: Isolation, quantification and occurrence in legumes. Physiol. Plant 1988, 74, 727–732. [Google Scholar] [CrossRef]
- Klapheck, S.; Chrost, B.; Starke, J.; Zimmermann, H. c-Glutamylcysteinylserine–a new homologue of glutathione in plants of the family Poaceae. Bot Acta 1991, 105, 174–179. [Google Scholar] [CrossRef]
- Kocsy, G.; Galiba, G.; Brunold, C. Role of glutathione in adaptation and signalling during chilling and cold acclimation in plants. Physiol. Plant 2001, 113, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Gómez, L.D.; Vanacker, H.; Buchner, P.; Noctor, G.; Foyer, C.H. Intercellular distribution of glutathione synthesis in maize leaves and its response to short-term chilling. Plant Physiol. 2004, 134, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Kocsy, G.; Szalai, G.; Galiba, G. Effect of osmotic stress on glutathione and hydroxymethylglutathione accumulation in wheat. J. Plant Physiol. 2004, 161, 785–794. [Google Scholar] [CrossRef]
- Kocsy, G.; Szalai, G.; Sutka, J.; Paldi, E.; Galiba, G. Heat tolerance together with heat stress-induced changes in glutathione and hydroxymethylglutathione levels is affected by chromosome 5A of wheat. Plant Sci. 2004, 166, 451–458. [Google Scholar] [CrossRef]
- Romano, A.H.; Nickerson, W.J. Cystine reductase of pea seeds and yeasts. J. Biol. Chem. 1954, 208, 409–416. [Google Scholar] [CrossRef]
- Creissen, G.; Firmin, J.; Fryer, M.; Kular, B.; Leykand, N.; Reynolds, H.; Pastori, G.; Wellburn, F.; Baker, N.; Wellburn, A.; et al. Elevated glutathione biosynthetic capacity in the chloroplasts of transgenic tobacco plants paradoxically causes increased oxidative stress. Plant Cell 1999, 11, 1277–1291, Erratum in Plant Cell 2000, 12, 301.. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Meyer, A.J. The integration of glutathione homeostasis and redox signalling. J. Plant Physiol. 2008, 165, 1390–1403. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, Y.; He, L.F. The central role of hydrogen sulfide in plant responses to toxic metal stress. Ecotoxicol. Environ. Saf. 2018, 157, 403–408. [Google Scholar] [CrossRef]
- Shivaraj, S.M.; Vats, S.; Bhat, J.A.; Dhakte, P.; Goyal, V.; Khatri, P.; Kumawat, S.; Singh, A.; Prasad, M.; Sonah, H.; et al. Nitric oxide and hydrogen sulfide crosstalk during heavy metal stress in plants. Physiol. Plant. 2020, 168, 437–455. [Google Scholar] [CrossRef]
- Huang, J.; Levine, A.; Wang, Z. Plant Abiotic Stress. Sci. World J. 2013, 2013, 432836. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Abiotic stress, generation of reactive oxygen species, and their consequences: An overview. In Revisiting the Role of Reactive Oxygen Species (ROS) in Plants: ROS Boon or Bane for Plants? Singh, V., Singh, S., Tripathi, D., Prasad, S.M., Chauhan, D.K., Eds.; Wiley: New York, NY, USA, 2018; pp. 23–50. [Google Scholar]
- Banerjee, A.; Roychoudhury, A. Effect of salinity stress on growth and physiology of medicinal plants. In Medicinal Plants and Environmental Challenges; Ghorbanpour, M., Varma, A., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 177–188. [Google Scholar]
- Schmitt, F.J.; Renger, G.; Friedrich, T.; Kreslavski, V. Reactive oxygen species: Re-evaluation of generation, monitoring and role in stress-signaling in phototrophic organisms. Biochim. Biophys. Acta (BBA)-Bioenerg. 2014, 1837, 385–848. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, M.; Bedhomme, M.; Lemaire, S.D.; Trost, P. The emerging roles of protein glutathionylation in chloroplasts. Plant Sci. 2012, 185, 86–96. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. The gymnastics of epigenomics in rice. Plant Cell Rep. 2018, 37, 25–49. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Abscisic-acid-dependent basic leucine zipper (bZIP) transcription factors in plant abiotic stress. Protoplasma 2017, 254, 3–16. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Role of Glutathione in Plant Abiotic Stress Tolerance. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; Wiley: New York, NY, USA, 2019; Volume I, pp. 159–172. [Google Scholar]
- Tausz, M.; Sircelj, H.; Grill, D. The glutathione system as a stress marker in plantecophysiology: Is a stress-response concept valid? J. Exp. Bot. Sulphur Metabol. Plant 2004, 55, 1955–1962. [Google Scholar] [CrossRef]
- Shao, H.B.; Chu, L.Y.; Shao, M.A.; Jaleel, C.A.; Mi, H.M. Higher plant antioxidants and redox signaling under environmental stresses. C R Biol. 2008, 331, 433–441. [Google Scholar] [CrossRef]
- Pastori, G.M.; Foyer, C.H. Common components, networks, and pathways of cross tolerance to stress. The central role of “redox” and abscisic acid-mediated controls. Plant Physiol. 2002, 129, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Kranner, I.; Colville, L. Metals and seeds: Biochemical and molecular implications and their significance for seed germination. Environ. Exp. Bot. 2010, 72, 93–105. [Google Scholar] [CrossRef]
- El-Shabrawi, H.; Kumar, B.; Kaul, T.; Reddy, M.K.; Singla-Pareek, S.L.; Sopory, S.K. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 2010, 245, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Zagorchev, L.; Seal, C.E.; Kranner, I.; Odjakova, M. Acentral role for thiols in plant tolerance to abiotic stress. Int. J. Mol. Sci. 2013, 14, 7405–7432. [Google Scholar] [CrossRef] [PubMed]
- Koffler, B.E.; Luschin-Ebengreuth, N.; Stabentheiner, E.; Singla, M. Compartment specific response of antioxidants to drought stress in Arabidopsis. Plant Sci. 2014, 227, 133–144. [Google Scholar] [CrossRef]
- Queval, G.; Jaillard, D.; Zechmann, B.; Noctor, G. Increased intra-cellular H2O2 availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant Cell Environ. 2011, 34, 21–32. [Google Scholar] [CrossRef]
- Zechmann, B. Compartment-specific importance of glutathione during abiotic and biotic stress. Front. Plant Sci. 2014, 5, 566. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, Y.; He, L.; Zhou, Q.; Yu, J.; Hui, D.; Huang, D. Exogenous Glutathione Improves High Root-Zone Temperature Tolerance by Modulating Photosynthesis, Antioxidant and Osmolytes Systems in Cucumber Seedlings. Sci. Rep. 2016, 6, 35424. [Google Scholar] [CrossRef]
- Szalai, G.; Kellos, T.; Galiba, G.; Kocsy, G. Glutathione as an antioxidant and regulatory molecule in plantsunder abiotic stress conditions. Plant Growth Regul. 2009, 28, 66–80. [Google Scholar] [CrossRef]
- Kocsy, G.; Szalai, G.; Galiba, G. Induction of glutathione synthesis and glutathione reductase activity by abiotic stresses in maize and wheat. Sci. World J. 2002, 2, 1726–1732. [Google Scholar] [CrossRef]
- Peltzer, D.; Dreyer, E.; Polle, A. Differential temperature dependencies of antioxidative enzymes in two contrasting species: Fagus sylvatica and Coleus blumei. Plant Physiol. Biochem. 2002, 40, 141–150. [Google Scholar] [CrossRef]
- Nieto-Sotelo, J.; Ho, T.H.D. Effect of heat shock on the metabolism of glutathione in maize roots. Plant Physiol. 1986, 82, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Mohanty, N. Response of seedlings to heat-stress in cultivars of wheat: Growth temperature-dependent differential modulation of photosystem 1 and 2 activity, and foliar antioxidant defense capacity. J. Plant Physiol. 2002, 159, 49–59. [Google Scholar] [CrossRef]
- Dat, J.F.; Foyer, C.H.; Scott, I.M. Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol. 1998, 118, 1455–1461. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Li, Y.; Di, B.; Zhang, J.; Liu, Y. Effect of high temperature and excessive light on glutathione content in apple peel. Front. Agric. China 2008, 2, 97–102. [Google Scholar] [CrossRef]
- Ma, Y.H.; Ma, F.W.; Zhang, J.K.; Li, M.J.; Wang, Y.H.; Liang, D. Effects of high temperature on activities and gene expression of enzymes involved in ascorbate–glutathione cycle in apple leaves. Plant Sci. 2008, 175, 761–766. [Google Scholar] [CrossRef]
- Laureaua, C.; Blignyb, R.; Streba, P. The significance of glutathione for photoprotection at contrasting temperatures in the alpine plant species Soldanella alpina and Ranunculus glacialis. Physiol. Plant 2011, 143, 246–260. [Google Scholar] [CrossRef]
- Yao, M.; Ge, W.; Zhou, Q.; Zhou, X.; Luo, M.; Zhao, Y.; Wei, B.; Ji, S. Exogenous Glutathione Alleviates Chilling Injury in Postharvest Bell Pepper by Modulating the Ascorbate-Glutathione (AsA-GSH) Cycle. Food Chem. 2021, 352, 129458. [Google Scholar] [CrossRef]
- Khapte, P.S.; Kumar, P.; Saxena, A.; Singh, A. Performance evaluation and character association studies in arid region greenhouse tomato hybrids. Indian J. Hortic. 2018, 75, 457–462. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, Y. Te ICE-CBF-COR pathway in cold acclimation and AFPs in plants. Middle East J. Sci. Res. 2011, 8, 493–498. [Google Scholar]
- Wolf, S.; Yakir, D.; Stevens, M.A.; Rudich, J. Cold temperature tolerance of wild tomato species. J. Am. Soc. Hort. Sci. 1986, 111, 960–964. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, P.; Chaudhary, S.; Edelstein, M. Tomato Grafting: A Global Perspective. HortSci 2017, 52, 1328–1336. [Google Scholar] [CrossRef]
- Ruelland, E.; Vaultier, M.N.; Zachowski, A.; Hurry, V. Cold signalling and cold acclimation in plants. Adv. Bot. Res. 2009, 49, 35–150. [Google Scholar] [CrossRef]
- Gul, N.; Ahmad, P.; Wani, T.A.; Tyagi, A.; Aslam, S. Glutathione improves low temperature stress tolerance in Pusa Sheetal cultivar of Solanum lycopersicum. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mauch, F.; Dudler, R. Differential induction of distinct glutathione-S-transferases of wheat by xenobiotics and by pathogen attack. Plant Physiol. 1993, 102, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.U.; Alharby, H.F.; Bamagoos, A.A.; Abdelhamid, M.T.; Rady, M.M. Sequenced Application of Glutathione as an Antioxidant with an Organic Biostimulant Improves Physiological and Metabolic Adaptation to Salinity in Wheat. Plant Physiol. Biochem. 2021, 158, 43–52. [Google Scholar] [CrossRef]
- Chawla, S.; Jain, S.; Jain, V. Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L.). J. Plant Biochem. Biotechnol. 2012, 22, 27–34. [Google Scholar] [CrossRef]
- Liu, Q.L.; Zhong, M.; Li, S.; Pan, Y.Z.; Jiang, B.B.; Jia, Y.; Zhang, H.Q. Overexpression of a chrysanthemum transcription factor gene, DgWRKY3, in tobacco enhances tolerance to salt stress. Plant Physiol. Biochem. 2013, 69, 27–33. [Google Scholar] [CrossRef]
- Zhou, Y.; Wen, Z.L.; Zhang, J.W.; Chen, X.J.; Cui, J.X.; Xu, W.; Liu, H.Y. Exogenous glutathione alleviates salt-induced oxidative stress in tomato seedlings by regulating glutathione metabolism, redox status, and the antioxidant system. Sci. Hortic. 2017, 220, 90–101. [Google Scholar] [CrossRef]
- Cheng, M.C.; Ko, K.; Chang, W.L.; Kuo, W.C.; Chen, G.H.; Lin, T.P. Increased glutathione contributes to stress tolerance and global translational changes in Arabidopsis. Plant J. 2015, 83, 926–939. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, W.; He, J.; Zhang, L.; Wei, Y.; Yang, M. Nitric oxide alleviates salt stress in seed germination and early seedling growth of pakchoi (Brassica chinensis L.) by enhancing physiological and biochemical parameters. Ecotoxicol. Environ. Saf. 2019, 187, 109785. [Google Scholar] [CrossRef] [PubMed]
- Delfina, A.R.; Brenda, R.; Raquel, L.; Bian, T.B.G. RNAi-mediated silencing of the HD-Zip gene HD20 in Nicotiana attenuata affects benzyl acetone emission from corollas via ABA levels and the expression of metabolic genes. BMC Plant Biol. 2012, 12, 1–15. [Google Scholar] [CrossRef]
- Nemat Alla, M.M.; Hassan, N.M. Alleviation of isoproturon toxicity to wheat by exogenous application of glutathione. Pestic Biochem. Physiol. 2014, 112, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Blumwald, E. Salinity-induced glutathione synthesis in Brassica napus. Planta 2002, 214, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.M.; Chen, L.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Considine, M.J.; Yu, J.Q.; Zhou, Y.H. Growth temperature-induced changes in biomass accumulation, photosynthesis and glutathione redox homeostasis as influenced by hydrogen peroxide in cucumber. Plant Physiol. Biochem. 2013, 71, 1–10. [Google Scholar] [CrossRef]
- Rady, M.M.; Hemida, K.A. Sequenced application of ascorbate proline-glutathione improves salt tolerance in maize seedlings. Ecotoxicol Environ. Saf. 2016, 133, 252–259. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Udechukwu, M.C.; Yiridoe, C.; Gibson, A.; Gong, M. Antioxidant mechanism of potato protein hydrolysates against in vitro oxidation of reduced glutathione. J. Funct. Foods 2016, 20, 195–203. [Google Scholar] [CrossRef]
- Uys, J.D.; Mulholland, P.J.; Townsend, D.M. Glutathione and redox signaling in substance abuse. Biomed. Pharm. 2014, 68, 799–807. [Google Scholar] [CrossRef]
- Zhang, H.; Forman, H.J. Glutathione synthesis and its role in redox signaling. Semin Cell Dev. Biol. 2012, 23, 722–728. [Google Scholar] [CrossRef]
- Anjum, N.A.; Ahmad, I.; Mohmood, I.; Pacheco, M.; Duarte, A.C.; Pereira, E.; Umar, S.; Ahmad, A.; Khan, N.A.; Iqbal, M.; et al. Modulation of glutathione and its related enzymes in plants’ responses to toxic metals and metalloids—A review. Environ. Exp. Bot. 2012, 75, 307–324. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhong, X.; Huo, J.; Zhang, W.; Deng, J.; Zhang, T.; Liu, K. Genome-wide analysis of tomato WIP family genes and their response to salt stress under glutathione treatment. Plant Biochem. Biotechnol. 2022, 31, 815–825. [Google Scholar] [CrossRef]
- Alamri, S.; Kushwaha, B.K.; Singh, V.P.; Siddiqui, M.H.; Al-Amri, A.A.; Alsubaie, Q.D.; Ali, H.M. Ascorbate and Glutathione Independently Alleviate Arsenate Toxicity in Brinjal but Both Require Endogenous Nitric Oxide. Physiol. Plant. 2021, 173, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Marrs, K.A. The functions and regulation of glutathione S transferases in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 127–158. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, P.; Long, S.; Minocha, R. Changes in phytochelatins and their biosynthetic intermediates in red spruce (Picea rubens Sarg.) cell suspension cultures under cadmium and zinc stress. Plant Cell Tissue Organ. Cult. 2007, 88, 201–216. [Google Scholar] [CrossRef]
- Schützendübel, A.; Schwanz, P.; Teichmann, T.; Gross, K.; Langenfeld-Heyser, R.; Godbold, D.L.; Polle, A. Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol. 2001, 127, 887–898. [Google Scholar] [CrossRef]
- Schutzendubel, A.; Polle, A. Plant responses to abiotic stresses: Heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 2002, 53, 1351–1365. [Google Scholar] [CrossRef]
- Bovet, L.; Feller, U.; Martinoia, E. Possible involvement of plant ABC transporters in cadmium detoxification: A cDNA sub-microarray approach. Environ. Int. 2005, 31, 263–267. [Google Scholar] [CrossRef]
- Rauser, W.E. Phytochelatins and related peptides-structure, biosynthesis, and function. Plant Physiol. 1995, 109, 1141–1149. [Google Scholar] [CrossRef]
- Miyadate, H.; Adachi, S.; Hiraizumi, A.; Tezuka, K.; Nakazawa, N.; Kawamoto, T.; Katou, K.; Kodama, I.; Sakurai, K.; Takahashi, H.; et al. OsHMA3, a P-1B-type of ATPase afects root-to-shoot cadmium translocation in rice by mediating efux into vacuoles. New Phytol. 2011, 189, 190–199. [Google Scholar] [CrossRef]
- Kim, D.Y.; Bovet, L.; Maeshima, M.; Martinoia, E.; Lee, Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J. 2007, 50, 207–218. [Google Scholar] [CrossRef]
- He, L.; Yuan, C.; Li, X.; Li, C.; Li, Y.; Chen, D.; Zhang, W.; Zheng, H.; Gao, J. Metabolomics analysis reveals diferent mechanisms of cadmium response and functions of reduced glutathione in cadmium detoxifcation in the Chinese cabbage. Plant Growth Regul. 2022, 98, 289–305. [Google Scholar] [CrossRef]
- Kumar, P.; Rouphael, Y.; Cardarelli, M.; Colla, G. Vegetable grafting as a tool to improve drought resistance and water use efficiency. Front. Plant Sci. 2017, 8, 1130. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.S.; Wong, S.K.; Ismail, N.H.; Zengin, G.; Duangjai, A.; Saokaew, S.; Phisalprapa, P.; Tan, K.W.; Goh, B.H.; Tang, S.Y. Mitigation of Environmental Stress-Impacts in Plants: Role of Sole and Combinatory Exogenous Application of Glutathione. Front. Plant Sci. 2021, 12, 791205. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Zhang, X.; Tang, W.; Oses-Prieto, J.A.; Suzuki, N.; Gendron, J.M.; Chen, H.; Guan, S.; Chalkley, R.J.; Peterman, T.K.; et al. A proteomics study of brassinosteroid response in Arabidopsis. Mol. Cell Proteom. 2007, 6, 2058–2071. [Google Scholar] [CrossRef]
- Chen, J.H.; Jiang, H.W.; Hsieh, E.J.; Chen, H.Y.; Chien, C.T.; Hsieh, H.L.; Lin, T.P. Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiol. 2012, 158, 340–351. [Google Scholar] [CrossRef]
- Saruhan, N.; Terzi, R.; Saglam, A.; Kadioglu, A. The relationship between leaf rolling and ascorbate-glutathione cycle enzymes in apoplastic and symplastic areas of Ctenanthe setosa subjected to drought Stress. Biol. Res. 2009, 42, 315–326. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol. Trace Elem. Res. 2011, 143, 1758–1776. [Google Scholar] [CrossRef]
- Almeselmani, M.; Deshmukh, P.; Sairam, R. High Temperature Stress Tolerance in Wheat Genotypes: Role of Antioxidant Defence Enzymes. Acta Agron. Hung. 2009, 57, 1–14. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Wollenweber, B.; Jiang, D. Multiple Heat Priming Enhances Thermo-Tolerance to a Later High Temperature Stress via Improving Subcellular Antioxidant Activities in Wheat Seedlings. Plant Physiol. Biochem. 2014, 74, 185–192. [Google Scholar] [CrossRef]
- Zou, M.; Yuan, L.; Zhu, S.; Liu, S.; Ge, J.; Wang, C. Response of Osmotic Adjustment and Ascorbate-Glutathione Cycle to Heat Stress in a Heat-Sensitive and a Heat-Tolerant Genotype of Wucai (Brassica campestris L.). Sci. Hortic. 2016, 211, 87–94. [Google Scholar] [CrossRef]
- Al-Elwany, O.A.; Mohamed, G.F.; Abdurrahman, H.A.; LATEF, A.A.A. Exogenous Glutathione-Mediated Tolerance to Deficit Irrigation in Salt-Affected Capsicum frutescence (L.) Plants Is Connected with Higher Antioxidant Content and Ionic Homeostasis. Not. Bot. Hortic. Agrobot. Cluj Napoca 2020, 48, 1957–1979. [Google Scholar] [CrossRef]
- Zaki, S.S.; Belal, E.E.E.; Mostafa, M.R. Cyanobacteria and Glutathione Applications Improve Productivity, Nutrient Contents, and Antioxidant Systems of Salt-Stressed Soybean Plant. Int. Lett. Nat. Sci. 2019, 76, 72–85. [Google Scholar] [CrossRef]
- Park, S.-I.; Kim, J.-J.; Kim, H.-S.; Kim, Y.-S.; Yoon, H.-S. Enhanced Glutathione Content Improves Lateral Root Development and Grain Yield in Rice Plants. Plant Mol. Biol. 2021, 105, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.; Fujita, M. Glutathione-Induced Drought Stress Tolerance in Mung Bean: Coordinated Roles of the Antioxidant Defence and Methylglyoxal Detoxification Systems. AoB Plants 2015, 7, plv069. [Google Scholar] [CrossRef]
- Wang, R.; Lin, K.; Chen, H.; Qi, Z.; Liu, B.; Cao, F.; Chen, H.; Wu, F. Metabolome Analysis Revealed the Mechanism of Exogenous Glutathione to Alleviate Cadmium Stress in Maize (Zea mays L.) Seedlings. Plants 2021, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.; Jones, J. Hormone (dis)harmony moulds plant health and disease. Science 2009, 324, 750–752. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Leon-Reyes, A.; Vander Ent, S.; Van Wees, S.C. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef]
- Tsuda, K.; Somssich, I.E. Transcriptional networks in plant immunity. New Phytol. 2015, 206, 932–947. [Google Scholar] [CrossRef]
- Ghanta, S.; Datta, R.; Bhattacharyya, D.; Kumar, D.; Sinha, R.; Hazra, S.; Mazumdar, A.B. Chattopadhyay, Multistep involvement of glutathione with salicylic acid and ethylene to combat environmental stress. J. Plant Physiol. 2014, 171, 940–950. [Google Scholar] [CrossRef]
- Kumar, D.; Datta, R.; Hazra, S.; Sultana, A.; Mukhopadhyay, R.; Chattopadhyay, S. Transcriptomic profling of Arabidopsis thaliana mutant pad2.1 in response to combined cold and osmotic stress. PLoS ONE 2015, 10, e0122690. [Google Scholar]
- Klessig, D.F.; Malamy, J. The salicylic acid signal in plants. Plant Mol. Biol. 1994, 26, 1439–1458. [Google Scholar] [CrossRef] [PubMed]
- Lawton, K.; Weymann, K.; Friedrich, L.; Vernooij, B.; Uknes, S.; Ryals, J. Systemic acquired resistance in Arabidopsis requires salicylic acid but not ethylene. Mol. Plant-Microbe Interact. 1995, 8, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Ghanta, S.; Bhattacharyya, D.; Sinha, R.; Banerjee, A.; Chattopadhyay, S. Nicotiana tabacum overexpressing γ-ECS exhibits biotic stress tolerance likely through NPR1-dependent salicylic acid-mediated pathway. Planta 2011, 233, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Riemann, M.; Haga, K.; Shimizu, T.; Okada, K.; Ando, S.; Mochizuki, S.; Nishizawa, Y.; Yamanouchi, U.; Nick, P.; Yano, M.; et al. Identification of rice Allene Oxide Cyclase mutants and the function of jasmonate for defence against Magnaporthe oryzae. Plant J. 2013, 74, 226–238. [Google Scholar] [CrossRef]
- Ahmad, P.; Rasool, S.; Gul, A.; Sheikh, S.A.; Akram, N.A.; Ashraf, M.; Kazi, A.M.; Gucel, S. Jasmonates: Multifunctional roles in stress tolerance. Front. Plant Sci. 2016, 7, 813. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.; Koornneef, A.; Claessens, S.M.; Korzelius, J.P.; Van Pelt, J.A.; Mueller, M.J.; Buchala, A.J.; Métraux, J.P.; Brown, R.; Kazan, K.; et al. NPR1 modulates crosstalk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 2003, 15, 760–770. [Google Scholar] [CrossRef]
- Theologis, A. One rotten apple spoils the whole bushel: The role of ethylene in fruit ripening. Cell 1992, 70, 181–184. [Google Scholar] [CrossRef]
- Yang, S.F.; Hofman, N.E. Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Díza, J.; Have, A.T.; Kan, J.A.L.V. The role of ethylene and wound signaling in resistance of tomato to Botrytis cinerea. Plant Physiol. 2002, 129, 1341–1351. [Google Scholar]
- Morgan, P.W.; Drew, M.C. Ethylene and plant responses to stress. Physiol. Plant. 1997, 100, 620–630. [Google Scholar] [CrossRef]
- Sharma, Y.K.; León, J.; Raskin, I.; Davis, K.R. Ozoneinduced responses in Arabidopsis thaliana—The role of salicylic acid in the accumulation of defense-related transcripts and induced resistance. Proc. Natl. Acad. Sci. USA 1996, 93, 5099–5104. [Google Scholar] [CrossRef] [PubMed]
- Yalpani, N.; Enyedi, A.J.; León, J.; Raskin, I. Ultraviolet light and ozone stimulate accumulation of salicylic acid, pathogenesis-related proteins and virus resistance in tobacco. Planta 1994, 193, 372–376. [Google Scholar] [CrossRef]
- Prasad, T.K.; Anderson, M.D.; Stewart, C.R. Localization and characterization of peroxidases in the mitochondria of chilling-acclimated maize seedlings. Plant Physiol. 1995, 108, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Lopez-Delgado, H.; Dat, J.F.; Scott, I.M. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol. Plant 1997, 100, 241–254. [Google Scholar] [CrossRef]
- Örvar, B.L.; McPherson, J.; Ellis, B.E. Pre-activating wounding response in tobacco prior to high-level ozone exposure prevents necrotic injury. Plant J. 1997, 11, 203–212. [Google Scholar] [CrossRef]
- Hausladen, A.; Alscher, R.G. Glutathione. In Antioxidants in Higher Plants; Alscher, R., Ed.; CRC Press: Boca Raton, FL, USA, 1993; pp. 1–30. [Google Scholar]
- Yang, j.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalk between Jasmonic acid and other plant hormone signalling. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Chattopadhyay, S. Interplay among glutathione, salicylic acid, and ethylene to combat environmental stresses. In Drought Stress Tolerance in Plants; Springer: Cham, Switzerland, 2016; Volume 1, pp. 145–161. [Google Scholar]
- Snyman, M.; Cronjé, M.J. Modulation of heat shock factors accompanies salicylic acid-mediated potentiation of Hsp70 in tomato seedlings. J. Exp. Bot. 2008, 59, 2125–2132. [Google Scholar] [CrossRef]
- Horváth, E.; Szalai, G.; Janda, T. Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Carillo, P.; Mastrolonardo, G.; Nacca, F.; Parisi, D.; Verlotta, A.; Fuggi, A. Nitrogen metabolism in durum wheat under salinity: Accumulation of proline and glycine betaine. Funct. Plant Biol. 2008, 35, 412–426. [Google Scholar] [CrossRef]
- Carillo, P.; Parisi, D.; Woodrow, P.; Pontecorvo, G.; Massaro, G.; Annunziata, M.G.; Fuggi, A.; Sulpice, R. Salt induced accumulation of glycine betaine is inhibited by high light in durum wheat. Funct. Plant Biol. 2011, 38, 139–150. [Google Scholar] [CrossRef]
- Kang, H.M.; Saltveit, M.E. Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiol. Plant. 2002, 115, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Z.; Cui, J.; Ding , J.; Xia, X.; Liu, D.; Yu, J. Alleviation of chilling-induced oxidative damage by salicylic acid pretreatment and related gene expression in eggplant seedlings. Plant Growth Regul. 2011, 65, 101–108. [Google Scholar] [CrossRef]
- Wang, G.; Hui, Z.; Li, F.; Zhao, M.; Zhang, J.; Wang, W. Improvement of heat and drought photosynthetic tolerance in wheat by over accumulation of glycine betaine. Plant Biotechnol. Rep. 2010, 4, 213–222. [Google Scholar] [CrossRef]
- Khan, N.A.; Syeed, S.; Masood, A.; Nazar, R.; Iqbal, N. Application of salicylic acid increases contents of nutrients and antioxidative metabolism in mungbean and alleviates adverse effects of salinity stress. Int. J. Plant Biol. 2010, 1, e1. [Google Scholar] [CrossRef]
- Syeed, S.; Anjum, N.A.; Nazar, R.; Iqbal, N.; Masood, A.; Khan, N.A. Salicylic acid-mediated changes in photosynthesis, nutrients content and antioxidant metabolism in two mustard (Brassica juncea L.) cultivars differing in salt tolerance. Acta Physiol. Plant. 2011, 33, 877–886. [Google Scholar] [CrossRef]
- Granroth, B. Biosynthesis and decomposition of cysteine derivatives in onion and other Allium species. Ann. Acad. Sci. Fenn. Chem. 1970, 154, 71. [Google Scholar]
- Coleman, J.O.D.; Randall, R.; Blakeklaff, M.M.A. Detoxification of xenobiotics in plant cells by glutathione conjugation and vacuolar compartmentalization: A fluorescent assay using monochlorobimane. Plant Cell Environ. 1997, 20, 449–460. [Google Scholar] [CrossRef]
- Kiddle, G.A.; Bennett, R.N.; Hick, A.J.; Wallsgrove, R.M. C-Slyase activities in leaves of crucifers and non-crucifers, and the characterization of three classes of C-S lyase activities from oil seed rape (Brassica napus L.). Plant Cell Environ. 1999, 22, 433–445. [Google Scholar] [CrossRef]
- Hoossain, M.D.; Rohman, M.M.; Fujita, M. Comparative investigation of glutathione S Transferase in different vegetable crops. Crop Sci. Biotechnol. 2007, 10, 19–26. [Google Scholar] [CrossRef]
- Azzeme, A.M.; Abdullah, S.N.A.; Aziz, M.A.; Wahab, P.E.M. Oil palm drought inducible DREB1 induced expression of DRE/CRT and non-DRE/CRT containing genes in lowland transgenic tomato under cold and PEG treatments. Plant Physiol. Biochem. 2017, 112, 129–151. [Google Scholar] [CrossRef]
- Le Martret, B.; Poage, M.; Shiel, K.; Nugent, G.D.; Dix, P.J. Tobacco chloroplast transformants expressing genes encoding dehydroascorbate reductase, glutathione reductase, and glutathione-S-transferase, exhibit altered anti-oxidant metabolism and improved abiotic stress tolerance. Plant Biotechnol. J. 2011, 9, 661–673. [Google Scholar] [CrossRef]
- Hatano-Iwasaki, A.; Ogawa, K. Overexpression of GSH1 gene mimics transcriptional response to low temperature during seed vernalization treatment of Arabidopsis. Plant Cell Physiol. 2012, 53, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylova, E.; Khusnutdinov, E.; Shein, M.Y.; Alekseev, V.Y.; Nikonorov, Y.; Kuluev, B. The Role of the GSTF11 gene in resistance to powdery mildew infection and cold stress. Plants 2021, 10, 2729. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Luo, Y.; Bai, H.; Li, X.; Tang, S.; Liao, X.; Zhang, L.; Liu, Q. DgMYB2 improves cold resistance in chrysanthemum by directly targeting DgGPX1. Hortic. Res. 2022, 9, uhab028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, M.; Teng, Y.; Jia, S.; Yu, D.; Wei, T.; Chen, C.; Song, W. Overexpression of the Glutathione Peroxidase 5 (RcGPX5) Gene from Rhodiola crenulata Increases Drought Tolerance in Salvia miltiorrhiza. Front Plant Sci. 2019, 9, 1950. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Asif, M.H.; Chakrabarty, D.; Tripathi, R.D.; Dubey, R.S.; Trivedi, P.K. Expression of a rice Lambda class of glutathione S-transferase, OsGSTL2, in Arabidopsis provides tolerance to heavy metal and other abiotic stresses. J. Hazard Mater. 2013, 248–249, 228–237. [Google Scholar] [CrossRef]
- Gao, H.; Yu, C.; Liu, R.; Li, X.; Huang, H.; Wang, X.; Zang, C.; Jiang, N.; Li, X.; Cheng, S.; et al. The Glutathione S-Transferase PtGSTF1 improve Biomass Production and Salt Tolerance through Regulating Xylem Cell Proliferation, Ion Homeostasis and Reactive Oxygen Species Scavenging in Poplar. Int. J. Mol. Sci. 2022, 23, 11288. [Google Scholar] [CrossRef]
- Pasternak, T.; Palme, K.; Paponov, I.A. Glutathione enhances auxin sensitivity in Arabidopsis roots. Biomolecules 2020, 10, 1550. [Google Scholar] [CrossRef]
- Marquez-Garcia, B.E.L.E.N.; Njo, M.; Beeckman, T.O.M.; Goormachtig, S.; Foyer, C.H. A new role for glutathione in the regulation of root architecture linked to strigolactones. Plant Cell Environ. 2014, 37, 488–498. [Google Scholar] [CrossRef]
- Nakamura, S.I.; Suzui, N.; Nagasaka, T.; Komatsu, F.; Ishioka, N.S.; Ito-Tanabata, S.; Fujimaki, S. Application of glutathione to roots selectively inhibits cadmium transport from roots to shoots in oilseed rape. J. Exp. Bot. 2013, 64, 1073–1081. [Google Scholar] [CrossRef]
- Salt, D.E.; Rauser, W.E. MgATP-dependent transport of phytochelatins across the tonoplast of oat roots. Plant Physiol. 1995, 107, 1293–1301. [Google Scholar] [CrossRef]
- Gong, J.M.; Lee, D.A.; Schroeder, J.I. Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 10118–10123. [Google Scholar] [CrossRef] [PubMed]


| Target Species | Common Name | Gene | Glutathione Content | Physiological Effect after Transgenic | Experimental Conditions in Pot or Soil Bed | References |
|---|---|---|---|---|---|---|
| Solanum lycopersicum | Tomato | DREB1 | Increase | Antioxidant machinery effectively activated | Greenhouse experiment | [161] |
| Nicotiana tobacum | Tobacco | DHAR:GR | Increase | Enhanced tolerance against cold stress | Pots for rooting then moved to soil for seed collection | [162] |
| Arabidopsis thaliana | Arabidopsis/thale cress | GSH1 | Increase (GSSG/GSH) | Vernalization | Pots experiment | [163] |
| Brassica napus | Rapeseed | GSTF11 | Decrease (GSH:GSSG) | Increased resistance to powdery mildew | Experimental plot and laboratory | [164] |
| Chrysanthemum | Crysanths | DgGPX1 | Increase Glutathione peroxidase | Reduced ROS generation, Cold tolerance enhanced. | Pots experiment | [165] |
| Salvia miltiorrhiza | Red sage/Danshen | RcGPX5 | GSH and total glutathione concentration higher | Increased tolerance to oxidative stress | Controlled conditions, and then introduced to field | [166] |
| Arabidopsis thaliana | Arabidopsis | OsGSTL2 | Overexpression of GSTs | Tolerance to heavy metal and abiotic stress | Under lab conditions | [167] |
| Populus trichocarpa | Poplar | PtGSTF1 | Overexpression of Glutathione S-transferase | Improved salt resistance, ROS scavenger | Pot experiments in greenhouse | [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai, G.K.; Kumar, P.; Choudhary, S.M.; Singh, H.; Adab, K.; Kosser, R.; Magotra, I.; Kumar, R.R.; Singh, M.; Sharma, R.; et al. Antioxidant Potential of Glutathione and Crosstalk with Phytohormones in Enhancing Abiotic Stress Tolerance in Crop Plants. Plants 2023, 12, 1133. https://doi.org/10.3390/plants12051133
Rai GK, Kumar P, Choudhary SM, Singh H, Adab K, Kosser R, Magotra I, Kumar RR, Singh M, Sharma R, et al. Antioxidant Potential of Glutathione and Crosstalk with Phytohormones in Enhancing Abiotic Stress Tolerance in Crop Plants. Plants. 2023; 12(5):1133. https://doi.org/10.3390/plants12051133
Chicago/Turabian StyleRai, Gyanendra Kumar, Pradeep Kumar, Sadiya M. Choudhary, Hira Singh, Komal Adab, Rafia Kosser, Isha Magotra, Ranjeet Ranjan Kumar, Monika Singh, Rajni Sharma, and et al. 2023. "Antioxidant Potential of Glutathione and Crosstalk with Phytohormones in Enhancing Abiotic Stress Tolerance in Crop Plants" Plants 12, no. 5: 1133. https://doi.org/10.3390/plants12051133
APA StyleRai, G. K., Kumar, P., Choudhary, S. M., Singh, H., Adab, K., Kosser, R., Magotra, I., Kumar, R. R., Singh, M., Sharma, R., Corrado, G., & Rouphael, Y. (2023). Antioxidant Potential of Glutathione and Crosstalk with Phytohormones in Enhancing Abiotic Stress Tolerance in Crop Plants. Plants, 12(5), 1133. https://doi.org/10.3390/plants12051133










