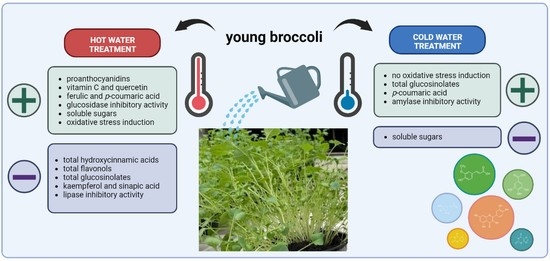
Effect of Hot- and Cold-Water Treatment on Broccoli Bioactive Compounds, Oxidative Stress Parameters and Biological Effects of Their Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Hot- and Cold-Water Stress on Different Groups of Phenolic Compounds, Soluble Sugars and Glucosinolates in Broccoli
2.2. Effect of Hot- and Cold-Water Stress on Parameters of Oxidative Stress and Pigments in Broccoli
2.3. Effect of Hot- and Cold-Water Stress on the Concentration of Individual Phenolic Compounds and Vitamin C in Broccoli
2.4. Concentration of Individual Phenolic Compounds and Vitamin C during In Vitro Simulated Digestion of Broccoli: Comparison of Control, Hot-Water- and Cold-Water-Stressed Plants
2.5. Effect of Hot- and Cold-Water Stress on Antioxidant Potential of Broccoli Extracts
2.6. Effect of Hot- and Cold-Water Stress on the Potential of Extracts of Young Broccoli Plants to Inhibit α-Amylase, α-Glucosidase and Lipase Enzyme
2.7. Representation and Distribution of Decreased, Unchanged and Increased Variables in Young Broccoli Plants Due to Hot/Cold-Water Treatment
2.8. Chemometric Data Analysis
2.8.1. Principal Component Analysis
2.8.2. Hierarchical Clustering
2.8.3. Pearson’s Correlation Coefficients
3. Materials and Methods
3.1. Plant Material
3.2. Determination of the Proportion of Total Phenols
3.3. Determination of the Proportion of Total Flavonoids
3.4. Determination of the Proportion of Total Proanthocyanins
3.5. Determination of the Proportion of Total Tannins
3.6. Determination of the Proportion of Total Phenolic Acids
3.7. Determination of the Proportion of Total Hydroxycinnamic Acids and Flavonols
3.8. Determination of the Proportion of Soluble Sugars
3.9. Determination of the Proportion of Total Intact Glucosinolates
3.10. Determination of Parameters of Oxidative Stress
3.11. Determination of the Proportion of Chlorophyll, Carotenoids and Porphyrins
3.12. In Vitro Simulated Human Digestion of Extracts
3.13. Separation, Identification and Quantification of Individual Compounds by the HPLC Method
3.14. Determination of Antioxidant Capacity
3.15. Determination of the Effect of Broccoli Extracts on α-Amylase, α-Glucosidase and Lipase Enzyme Activity
3.16. Statistical Data Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, V. Environmental Plant Physiology: Botanical Strategies for a Climate Smart Planet; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- He, M.; He, C.-Q.; Ding, N.-Z. Abiotic stresses: General defenses of land plants and chances for engineering multistres tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, M.R.; Knight, H. Low-temperature perception leading to gene expression and cold tolerance in higher plants. New Phytol. 2012, 195, 737–751. [Google Scholar] [CrossRef]
- Prinsloo, G.; Nogemane, N. The effects of season and water availability on chemical composition, secondary metabolites and biological activity in plants. Phytochem. Rev. 2018, 17, 889–902. [Google Scholar] [CrossRef]
- Farooq, M.A.; Kaya, C.; Riaz, A.; Farooq, M.; Nawaz, I.; Wilkes, A.; Li, Y. Potential mechanisms of abiotic stress tolerance in crop plants induced by thiourea. Front. Plant Sci. 2019, 10, 1336. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643. [Google Scholar] [CrossRef]
- Suwa, R.; Hakata, H.; Hara, H.; El-Shemy, H.A.; Adu-Gyamfi, J.J.; Nguyen, N.T.; Fujita, K. High temperature effects on photosynthate partitioning and sugar metabolism during ear expansion in maize (Zea mays L.) genotypes. Plant Physiol. Biochem. 2010, 48, 124–130. [Google Scholar] [CrossRef]
- Gunawardena, K.; Kumarendran, B.; Ebenezer, R.; Gunasingha, M.S.; Pathmeswaran, A.; De Silva, N. Soil-transmitted hel-minth infections among plantation sector schoolchildren in Sri Lanka: Prevalence after ten years of preventive chemo-therapy. PLoS Negl. Trop. Dis. 2011, 9, 1341. [Google Scholar]
- Edreira, J.I.R.; Otegui, M.E. Heat stress in temperate and tropical maize hybrids: Differences in crop growth, biomass partitioning and reserves use. Field Crop. Res. 2012, 130, 87–98. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory net-works. J. Exp. Bot. 2012, 4, 1593–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenov, M.A.; Halford, N.G. Identifying target traits and molecular mechanisms for wheat breeding under a changing climate. J. Exp. Bot. 2009, 10, 2791–2804. [Google Scholar] [CrossRef] [PubMed]
- Hama, J.R.; Omer, R.A.; Rashid, R.S.M.; Mohammad, N.E.A.M.; Thoss, V. The diversity of phenolic compounds along defatted kernel, green husk and leaves of walnut (Juglans regia L.). Anal. Chem. Lett. 2016, 6, 35–46. [Google Scholar] [CrossRef]
- Chen, S.S.; Spiro, M. Study of microwave extraction of essential oil constituents from plant materials. J. Microw. Power Electromagn. Energy 1994, 4, 231–241. [Google Scholar] [CrossRef]
- Los, D.A.; Mironov, K.S.; Allakhverdiev, S.I. Regulatory role of membrane fluidity in gene expression and physiological functions. Photosynth. Res. 2013, 116, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Rajashekar, C.B. Cold Response and Freezing Tolerance in Plants. In Plant Environment Interactions; CRC Press: Boca Raton, FL, USA, 2000; pp. 321–341. [Google Scholar]
- De Palma, M.; Grillo, S.; Massarelli, I.; Costa, A.; Balogh, G.; Vigh, L.; Leone, A. Regulation of desaturase gene expression, changes in membrane lipid composition and freezing tolerance in potato plants. Mol. Breed. 2008, 21, 15–26. [Google Scholar] [CrossRef]
- Janská, A.; Maršík, P.; Zelenková, S.; Ovesná, J. Cold stress and acclimation—What is important for metabolic adjustment? Plant Biol. 2010, 12, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, R.; Popova, L. Effect of exogenous treatment with salicylic acid on photosynthetic activity and antioxidant capacity of chilled wheat plants. Gen. Appl. Plant Physiol. 2007, 33, 155–170. [Google Scholar]
- Hassan, M.A.; Xiang, C.; Farooq, M.; Muhammad, N.; Yan, Z.; Yuanyuan, K.; Bruno, A.K.; Lele, Z.; Jincal, L. Cold stress in wheat: Plant acclimation responses and management strategies. Front. Plant Sci. 2021, 12, 676884. [Google Scholar] [CrossRef]
- Paśko, P.; Tyszka-Czochara, M.; Galanty, A.; Gdula-Argasińska, J.; Żmudzki, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S. Comparative study of predominant phytochemical compounds and proapoptotic potential of broccoli sprouts and florets. Plant Foods Hum. Nutr. 2018, 73, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Razis, A.F.A.; Noor, N.M. Cruciferous vegetables: Dietary phytochemicals for cancer prevention. Asian Pac. J. Cancer Prev. 2013, 14, 1565–1570. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, S.R.; Kwak, J.H. Chemical composition and antioxidant activity in different tissues of brassica vegetables. Molecules 2015, 20, 1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachiega, P.; Salgado, J.M.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Schwarz, K.; Tezotto, T.; Morzelle, M.C. Antioxidant and an-tiproliferative activities in different maturation stages of broccoli (Brassica oleracea italica) biofortified with selenium. Food Chem. 2016, 190, 771–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglesias–Acosta, M.; Martinez–Bellesta, C.; Teruel, J.A.; Carvajal, M. The response of broccoli plants to high temperature and possible role of root aquaporins. Environ. Exp. Bot. 2010, 68, 83–90. [Google Scholar] [CrossRef]
- Liebelt, D.J.; Jordan, J.T.; Doherty, C.J. Only a matter of time: The impact of daily and seasonal rhythms on phytochemicals. Phytochem. Rev. 2019, 18, 1409–1433. [Google Scholar] [CrossRef]
- Šola, I.; Vujčić Bok, V.; Dujmović, M.; Rusak, G. Developmentally-related changes in phenolic and L-ascorbic acid content and antioxidant capacity of Chinese cabbage sprouts. Food Sci. Technol. 2020, 57, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Yadav, A.; Yadav, M.; Yadav, J.P. Effect of climate change on phytochemical diversity, total phenolic content and in vitro antioxidant activity of Aloe vera (L.) Burm. f. BMC Res. Notes 2017, 10, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.; Ayugase, J. Effect of low temperature on flavonoids, oxygen radical absorbance capacity values, and major components of winter sweet spinach (Spinacia oleracea L.). J. Sci. Food Agric. 2014, 95, 2095–2104. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Patel, S.; Pan, X.; Naz, S.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Harding, S.A. Condensed tannins: Arbiters of abiotic stress tolerance? Tree Physiol. 2019, 39, 341–344. [Google Scholar] [CrossRef]
- Gourlay, G.; Hawkins, B.J.; Albert, A.; Schnitzler, J.-P.; Constabel, C.P. Condensed tannins as antioxidants that protect poplar against oxidative stress from drought and UV-B. Plant Cell Environ. 2022, 45, 362–377. [Google Scholar] [CrossRef]
- Top, S.M.; Preston, C.M.; Dukes, J.S.; Tharayil, N. Climate influences the content and chemical composition of foliar tannins in green and senesced tissues of Quercus rubra. Front. Plant Sci. 2017, 8, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poudel, P.R.; Koyama, K.; Goto-Yamamoto, N. Evaluating the influence of temperature on proanthocyanidin biosynthesis in developing grape berries (Vitis vinifera L.). Mol. Biol. Rep. 2020, 47, 3501–3510. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cuin, T.A.; Zhou, M.; Twomey, A.; Naidu, B.P.; Shabala, S. Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. J. Exp. Bot. 2007, 58, 4245–4255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars—Metabolism, sensing and abiotic stress. A complex network in the life of plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harsh, A.; Sharma, Y.K.; Joshi, U.; Rampuria, S.; Singh, G.; Kumar, S.; Sharma, R. Effect of short-term heat stress on total sugars, proline and some antioxidant enzymes in moth bean (Vigna aconitifolia). Ann. Agric. Sci. 2016, 61, 57–64. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, M.; Devaraj, V.R. Induction of thermotolerance through heat acclimation in lablab bean (Dolichos lablab). Afr. J. Biotechnol. 2013, 12, 5695–5704. [Google Scholar]
- Tarkowski, L.P.; Van den Ende, W. Cold tolerance triggered by soluble sugars: A multifaceted countermeasure. Front. Plant Sci. 2015, 6, 203. [Google Scholar] [CrossRef] [Green Version]
- Yuanyuan, M.; Yali, Z.; Jiang, L.; Hongbo, S. Roles of plant soluble sugars and their responses to plant cold stress. Afr. J. Biotechnol. 2009, 8, 2004–2010. [Google Scholar]
- Becker, T.M.; Juvik, J.A. The role of glucosinolate hydrolysis products from Brassica vegetable consumption in inducing antioxidant activity and reducing cancer incidence. Diseases 2016, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Ballesta, M.d.C.; Moreno, D.A.; Carvajal, M. The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhile, L.; Kuanhong, W.; Jing, Y.; Biao, Z.; Zhujun, Z. The importance of glucosinolates on plant response to abiotic stress in Brassicaceae family. Acta Hortic. Sin. 2022, 49, 200–220. [Google Scholar]
- Oerlemans, K.; Barrett, D.M.; Suades, C.B.; Verkerk, R.; Dekker, M. Thermal degradation of glucosinolates in red cabbage. Food Chem. 2006, 95, 19–29. [Google Scholar] [CrossRef]
- Steindal, A.L.H.; Rødven, R.; Hansen, E.; Mølmann, J. Effects of photoperiod, growth temperature and cold acclimatisation on glucosinolates, sugars and fatty acids in kale. Food Chem. 2015, 174, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ljubej, V.; Karalija, E.; Salopek-Sondi, B.; Šamec, D. Effects of short-term exposure to low temperatures on proline, pigments, and phytochemicals level in kale (Brassica oleracea var. acephala). Horticulturae 2021, 7, 341. [Google Scholar] [CrossRef]
- Awasthi, R.; Bhandari, K.; Nayyar, H. Temperature stress and redox homestasis in agricultural crops. Front. Environ. Sci. 2015, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Šola, I.; Stić, P.; Dujmović, M.; Rusak, G. Effect of flooding and drought on the content of phenolics, sugars, photosynthetic pigments and vitamin C, and antioxidant potential of young Chinese cabbage. Eur. Food Res. Technol. 2021, 247, 1913–1920. [Google Scholar] [CrossRef]
- Zafar, S.A.; Hameed, A.; Khan, S.; Ashraf, M. Heat shock induced morpho-physiological response in indica rice (Oryza sativa L.) at early seedling stage. Pak. J. Bot. 2017, 49, 453–463. [Google Scholar]
- Kumar Tewari, A.; Tripathy, B.C. Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiol. 1998, 17, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Efeoğlu, B.; Terzioğlu, S. Photosynthetic responses of two wheat varieties to high temperature. EurAsian J. Biosci. 2009, 3, 97–106. [Google Scholar] [CrossRef]
- Martinez, M.A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.; Pozo, M.J.; Conrath, U. Recognizing plant defense priming. Trends Plant Sci. 2016, 10, 818–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, P.S.; Victorelli, F.D.; Fonseca-Santos, B.; Chorilli, M. A review of analytical methods for p-coumaric acid in plant-based products, beverages, and biological matrices. Crit. Rev. Anal. Chem. 2019, 49, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.H.; Ahn, M.A.; Kim, E.; Lee, H.J.; Lee, J.H.; Wi, S.H.; Hyun, T.K. Plant response to cold stress: Cold stress changes antioxidant metabolism in heading type Kimchi cabbage (Brassica rapa L. ssp. pekinensis). Antioxidants 2022, 4, 700. [Google Scholar] [CrossRef] [PubMed]
- Laddomada, B.; Blanco, A.; Mita, G.; D’Amico, L.; Singh, R.P.; Ammar, K.; Guzmán, C. Drought and heat stress impacts on phenolic acids accumulation in durum wheat cultivars. Foods 2021, 9, 2142. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.; Galal, F.H.; Seufi, A.M. Effect of extreme temperature changes on phenolic, flavonoid contents and antioxidant activity of tomato seedlings (Solanum lycopersicum L.). Peer J. 2021, 9, 11193. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, B.; Huang, B. Differential heat-induced changes in phenolic acids associated with genotypic variations in heat tolerance for hard fescue. Crop Sci. 2019, 59, 667–674. [Google Scholar] [CrossRef]
- Król, A.; Amarowicz, R.; Weidner, S. Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous of long-term drought stress. Acta Physiol. Plant. 2014, 36, 1491–1499. [Google Scholar] [CrossRef] [Green Version]
- Adisakwattana, S.; Sookkongwaree, K.; Roengsumran, S.; Petsom, A.; Ngamrojnavanich, N.; Chavasiri, W.; Deesamer, S.; Yibchok-anuna, S. Structure–activity relationships of trans-cinnamic acid derivatives on α-glucosidase inhibition. Bioorganic Med. Chem. Lett. 2004, 14, 2893–2896. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, D.-S.; Park, S.-H.; Shin, J.-W.; Youn, S.-W.; Park, K.-C. Inhibitory effect of p-coumaric acid by Rhodiola sachalinensis on melanin synthesis in B16F10 cells. Pharmazie 2008, 63, 290–295. [Google Scholar]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Šola, I.; Stipaničev, M.; Vujčić, V.; Mitić, B.; Huđek, A.; Rusak, G. Comparative analysis of native Crocus taxa as a great source of flavonoids with high antioxidant activity. Plant Foods Hum. Nutr. 2018, 73, 189–195. [Google Scholar] [CrossRef]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in disease prevention and cure: An overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef] [Green Version]
- Figueroa-Méndez, R.; Rivas-Arancibia, S. Vitamin C in health and disease: Its role in the metabolism of cells and redox state in the brain. Front. Physiol. 2015, 6, 397. [Google Scholar] [CrossRef] [Green Version]
- Gegotek, A.; Skrzydlewska, E. Antioxidative and anti-Inflammatory activity of ascorbic acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, M.; Zhu, H.; Huang, M.; Zhu, Q.; Tang, D.; Han, X.; Li, J.; Sun, J.; Fu, J. Ascorbic acid alleviates damage from heat stress in the photosystem II of tall fescue in both the photochemical and thermal phases. Front. Plant Sci. 2017, 8, 1373. [Google Scholar] [CrossRef] [Green Version]
- Njoku, P.C.; Ayuk, A.A.; Okoye, C.V. Temperature effects on vitamin C content in citrus fruits. Pak. J. Nutr. 2011, 10, 1168–1169. [Google Scholar] [CrossRef]
- Cuvas-Limon, R.B.; Ferreira-Santos, P.; Cruz, M.; Teixeira, J.A.; Belmares, R.; Nobre, C. Effect of gastrointestinal digestion on the bioaccessibility of phenolic compounds and antioxidant activity of fermented Aloe vera juices. Antioxidants 2022, 11, 2479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xing, B.; Sun, M.; Zhou, B.; Ren, G.; Qin, P. Changes in bio-accessibility, polyphenol profile and antioxidants of quinoa and djulis sprouts during in vitro simulated gastrointestinal digestion. Food Sci. Nutr. 2020, 8, 4232–4241. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J.-G. Role of reactive oxygen species and hormones in plant responses to temperature changes. Int. J. Mol. Sci. 2021, 22, 8843. [Google Scholar] [CrossRef] [PubMed]
- Pucciariello, C.; Banti, V.; Perata, P. ROS signaling as common element in low oxygen and heat stresses. Plant Physiol. Biochem. 2012, 59, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Shah, P.; Modi, H.A. Comparative study of DPPH, ABTS and FRAP assays for determination of antioxidant activity. Int. J. Res. Appl. Sci. Eng. Technol. 2015, 3, 2321–9653. [Google Scholar]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Soengas, P.; Rodríguez, V.M.; Velasco, P.; Cartea, M.E. Effect of temperature stress on antioxidant defenses in Brassica oleracea. ACS Omega 2019, 5, 5237–5243. [Google Scholar] [CrossRef]
- Mugaranja, K.P.; Kulal, A. Investigation of effective natural inhibitors for starch hydrolysing enzymes from Simaroubaceae plants by molecular docking analysis and comparison with in-vitro studies. Heliyon 2022, 8, e09360. [Google Scholar] [CrossRef] [PubMed]
- Kicel, A.; Magiera, A.; Skrzywanek, M.; Malczuk, M.; Olszewska, M.A. The inhibition of α-glucosidase, α-amylase and protein glycation by phenolic extracts of Cotoneaster bullatus, Cotoneaster zabelii, and Cotoneaster integerrimus leaves and fruits: Focus on anti-hyperglycemic activity and kinetic parameters. Molecules 2022, 27, 7081. [Google Scholar] [CrossRef] [PubMed]
- Gironés-Vilaplana, A.; Baenas, N.; Villaño, D.; Speisky, H.; Garcia-Viguera, C.; Moreno, A.A. Evaluation of Latin-American fruits rich in phytochemicals with biological effects. J. Funct. Foods 2014, 7, 599–608. [Google Scholar] [CrossRef]
- Podsędek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziołkiewicz, M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Zakłos-Szyda, M.; Majewska, I.; Redzynia, M.; Koziołkiewicz, M. Antidiabetic effect of polyphenolic extracts from selected edible plants as α-amylase, α-glucosidase and PTP1B Inhibitors, and β pancreatic cells cytoprotective agents—A comparative study. Curr. Top. Med. Chem. 2015, 15, 2431–2444. [Google Scholar] [CrossRef]
- Kawada, Y.; Miura, M.; Gomyo, T. Inhibitory effect of vegetables, fruits and herbs on α-glucosidase in an immobilized enzyme assay system. Food Sci. Technol. 2006, 12, 275–277. [Google Scholar] [CrossRef] [Green Version]
- Fabroni, S.; Ballistreri, G.; Amenta, M.; Romeo, F.V.; Rapisarda, P. Screening of the anthocyanin profile and in vitro pancreatic lipase inhibition by anthocyanin-containing extracts of fruits, vegetables, legumes and cereals. J. Sci. Food Agric. 2016, 14, 4713–4723. [Google Scholar] [CrossRef] [PubMed]
- de Souza, P.M.; de Sales, P.M.; Simeoni, L.A.; Silva, E.C.; Silveira, D.; Magalhães, P.D. Inhibitory activity of α-amylase and α-glucosidase by plant extracts from the Brazilian Cerrado. Planta Med. 2012, 78, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Silveira, D. α-Amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: An update. Mini-Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Baenas, N.; Piegholdt, S.; Schloesser, A.; Moreno, D.A.; García-Viguera, C.; Rimbach, G.; Wagner, A.E. Metabolic activity of radish sprouts derived isothiocyanates in Drosophila melanogaster. Int. J. Mol. Sci. 2016, 17, 251. [Google Scholar] [CrossRef] [Green Version]
- Mollica, A.; Stefanucci, A.; Zengin, G.; Locatelli, M.; Macedonio, G.; Orlando, G.; Ferrante, C.; Menghini, L.; Recinella, L.; Leone, S.; et al. Polyphenolic composition, enzyme inhibitory effects ex-vivo and in-vivo studies on two Brassicaceae of north-central Italy. Biomed. Pharmacother. 2018, 107, 129–138. [Google Scholar] [CrossRef]
- Assefa, S.T.; Yang, E.Y.; Chae, S.Y.; Song, M.; Lee, J.; Cho, M.C.; Jang, S. Alpha glucosidase inhibitory activities of plants with focus on common vegetables. Plants 2020, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Akil, L.; Ahmad, H.A. Relationships between obesity and cardiovascular diseases in four southern states and Colorado. J. Health Care Poor Underserved 2017, 22, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Ballinger, A.; Peikin, S. Orlistat: Its current status as an anti-obesity drug. Eur. J. Pharmacol. 2002, 440, 109–117. [Google Scholar] [CrossRef]
- Seyedan, A.; Alshawsh, M.A.; Alshagga, M.A.; Koosha, S.; Mohamed, Z. Medicinal plants and their inhibitory activities against pancreatic lipase: A review. Evid.-Based Complement. Altern. Med. 2015, 2015, 973143. [Google Scholar]
- Elsbaey, M.; Amen, Y.; Nakagawa, T.; Shimizu, K. White poplar: Targeted isolation of pancreatic lipase inhibitors. Ind. Crop. Prod. 2019, 141, 111778. [Google Scholar] [CrossRef]
- Verrillo, M.; Savy, D.; Cangemi, S.; Savarese, C.; Cozzolino, V.; Piccolo, A. Valorization of lignins from energy crops and agro-industrial byproducts as antioxidant and antibacterial materials. J. Sci. Food Agric. 2021, 102, 2885–2892. [Google Scholar] [CrossRef] [PubMed]
- Šola, I.; Vujčić Bok, V.; Pinterić, M.; Auer, S.; Ludwig-Müller, J.; Rusak, G. Improving the phytochemical profile and bi-oactivity of Chinese cabbage sprouts by interspecific transfer of metabolites. Food Res. Int. 2020, 137, 109726. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Šamec, D.; Bogović, M.; Vincek, D.; Martinčić, J.; Salopek-Sondi, B. Assessing the authenticity of the white cabbage (Brassica oleracea var. capitata f. alba) cv. ‘Varaždinski’ by molecular and phytochemical markers. Food Res. Int. 2014, 60, 266–272. [Google Scholar] [CrossRef]
- Mawlong, I.; Sujith Kumar, M.S.; Gurung, B.; Singh, K.H.; Singh, D. A simple spectrophotometric method for estimating total glucosinolates in mustard de-oiled cake. Int. J. Food Prop. 2017, 20, 3274–3281. [Google Scholar] [CrossRef] [Green Version]
- Linić, I.; Mlinarić, S.; Brkljačić, L.; Pavlović, I.; Smolko, A.; Salopek-Sondi, B. Ferulic acid and salicylic acid foliar treatments reduce short-term salt stress in chinese cabbage by increasing phenolic compounds accumulation and photosynthetic performance. Plants 2021, 11, 2346. [Google Scholar] [CrossRef]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am. J. Anal. Chem. 2014, 5, 730–736. [Google Scholar] [CrossRef] [Green Version]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophills and carotenoids from commonly grown Fren species by using various extracting solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Rusak, G.; Šola, I.; Vujčić Bok, V. Matcha and Sencha green tea extracts with regard to their phenolics pattern and antioxidant and antidiabetic activity during in vitro digestion. J. Food Sci. Technol. 2021, 58, 3568–3578. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Llorent-Martínez, E.J.; Castilho, P.C. Inhibition of α-amylase, α-glucosidase and pancreatic lipase by phenolic compounds of Rumex maderensis (Madeira sorrel). Influence of simulated gastrointestinal digestion on hyper-glycaemia-related damage linked with aldose reductase activity and protein glycation. LWT 2020, 118, 108727. [Google Scholar]
- Wuensch, K.L.; Evans, J.D. Straightforward statistics for the behavioral sciences. J. Am. Stat. Assoc. 1996, 91, 1750. [Google Scholar] [CrossRef]




| Con | HT | CT | |
|---|---|---|---|
| TP (mg GAE/g dw) | 10.56 ± 0.55 a | 10.05 ± 0.98 a | 10.20 ± 1.36 a |
| TF (mg QE/g dw) | 16.31 ± 0.90 a | 17.01 ± 0.87 a | 16.56 ± 0.73 a |
| TFlo (mg QE/g dw) | 0.14 ± 0.01 a | 0.12 ± 0.01 b | 0.14 ± 0.00 a |
| TPAN (mg CatE/g dw) | 0.78 ± 0.05 b | 1.02 ± 0.06 a | 0.76 ± 0.09 b |
| TT (mg CatE/g dw) | 5.05 ± 0.12 a | 4.62 ± 0.20 a | 4.70 ± 0.78 a |
| TPA (mg CAE/g dw) | 2.23 ± 0.36 a | 2.32 ± 0.26 a | 2.08 ± 0.30 a |
| THCA (mg CAE/g dw) | 0.13 ± 0.02 a | 0.10 ± 0.01 b | 0.14 ± 0.01 a |
| SS (mg SucE/g dw) | 18.06 ± 0.75 b | 31.14 ± 1.47 a | 15.07 ± 1.61 c |
| GLS (mg SinE/g dw) | 28.42 ± 2.76 b | 24.07 ± 1.34 c | 34.95 ± 2.37 a |
| Con | HT | CT | |
|---|---|---|---|
| Hydrogen peroxide (μM/g dw) | 2.33 ± 0.13 b | 2.56 ± 0.30 a | 1.93 ± 0.12 c |
| Proline (mg/g dw) | 1.34 ± 0.17 b | 3.31 ± 0.09 a | 1.15 ± 0.12 c |
| Lipid peroxidation (μM MDA/g dw) | 23.92 ± 0.74 b | 30.62 ± 1.39 a | 23.53 ± 1.91 b |
| Chlorophyll a (mg/g dw) | 5.06 ± 0.24 a | 5.14 ± 0.23 a | 5.07 ± 0.28 a |
| Chlorophyll b (mg/g dw) | 4.55 ± 0.22 b | 5.35 ± 0.34 a | 4.80 ± 0.63 b |
| Chlorophyll a + b (mg/g dw) | 9.61 ± 0.28 b | 10.49 ± 0.37 a | 9.87 ± 0.75 b |
| Lycopene (mg/g dw) | 0.26 ± 0.01 b | 0.28 ± 0.02 a | 0.26 ± 0.02 b |
| Carotenoids (mg/g dw) | 0.63 ± 0.10 a | 0.40 ± 0.19 b | 0.60 ± 0.22 a |
| Porphyrins (mg/g dw) | 25.37 ± 0.90 b | 28.27 ± 1.09 a | 25.71 ± 2.80 b |
| Con | HT | CT | |
|---|---|---|---|
| p-Coumaric acid | 14.22 ± 0.68 c | 19.04 ± 0.29 a | 16.72 ± 0.51 b |
| Ferulic acid | 49.41 ± 4.81 b | 64.99 ± 2.62 a | 43.18 ± 3.28 b |
| Sinapic acid | 627.59 ± 37.52 a | 399.80 ± 37.67 b | 599.33 ± 10.14 a |
| Total identified phenolic acids | 691.22 ± 42.02 a | 423.83 ± 35.48 b | 659.24 ± 7.85 a |
| Kaempferol | 155.82 ± 2.24 a | 140.72 ± 3.81 b | 166.71 ± 6.38 a |
| Quercetin | 32.93 ± 0.49 b | 35.11 ± 0.73 a | 33.70 ± 0.13 b |
| Isorhamnetin | 38.54 ± 5.45 a | 38.14 ± 2.64 a | 38.06 ± 5.52 a |
| Total identified flavonols | 227.29 ± 5.66 ab | 213.98 ± 6.44 b | 238.47 ± 7.52 a |
| Luteolin | 27.13 ± 0.56 a | 27.26 ± 0.12 a | 26.54 ± 0.20 a |
| Total identified flavonoids | 254.41 ± 5.11 ab | 241.23 ± 6.41 b | 265.00 ± 7.56 a |
| Total identified phenolics | 945.63 ± 39.85 a | 665.07 ± 29.74 b | 924.24 ± 8.27 a |
| L-Ascorbic acid | 1144.43 ± 66.64 b | 1523.00 ± 70.30 a | 1143.09 ± 33.32 b |
| Total identified compounds | 2090.06 ± 103.54 a | 2188.06 ± 88.29 a | 2067.34 ± 39.40 a |
| Con | HT | CT | |
|---|---|---|---|
| ABTS | 9.56 ± 0.72 a | 8.72 ± 0.85 a | 8.60 ± 0.83 a |
| FRAP | 12.74 ± 0.61 a | 12.50 ± 0.46 a | 12.35 ± 0.62 a |
| DPPH | 4.72 ± 0.40 a | 3.69 ± 0.46 b | 4.13 ± 0.51 b |
| TP | TF | TFlo | TT | TPAN | TPA | THCA | SS | GLS | Chl a | Chl b | Chl a + b | Lycop | Carot | Porph | Vit C | p-Coumar | Ferul | Sinap | Q | K | ABTS | FRAP | DPPH | H2O2 | Prol | MDA | α-amylase | α-glucos | Lipase | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | 1.00 | |||||||||||||||||||||||||||||
| TF | −0.92 | 1.00 | ||||||||||||||||||||||||||||
| TFlo | 0.62 | −0.88 | 1.00 | |||||||||||||||||||||||||||
| TT | 0.99 | −0.87 | 0.53 | 1.00 | ||||||||||||||||||||||||||
| TPAN | −0.68 | 0.91 | −1.00 | −0.59 | 1.00 | |||||||||||||||||||||||||
| TPA | −0.15 | 0.52 | −0.86 | −0.03 | 0.83 | 1.00 | ||||||||||||||||||||||||
| THCA | 0.54 | −0.82 | 0.99 | 0.44 | −0.99 | −0.91 | 1.00 | |||||||||||||||||||||||
| SS | −0.60 | 0.86 | −1.00 | −0.50 | 0.99 | 0.88 | −1.00 | 1.00 | ||||||||||||||||||||||
| GLS | 0.17 | −0.54 | 0.88 | 0.06 | −0.84 | −1.00 | 0.92 | −0.89 | 1.00 | |||||||||||||||||||||
| Chl a | −0.80 | 0.97 | −0.97 | −0.73 | 0.98 | 0.71 | −0.94 | 0.96 | −0.73 | 1.00 | ||||||||||||||||||||
| Chl b | −0.90 | 1.00 | −0.90 | −0.85 | 0.93 | 0.56 | −0.85 | 0.88 | −0.58 | 0.98 | 1.00 | |||||||||||||||||||
| Chl a + b | −0.89 | 1.00 | −0.91 | −0.84 | 0.94 | 0.57 | −0.86 | 0.89 | −0.60 | 0.98 | 1.00 | 1.00 | ||||||||||||||||||
| Lycop | −0.82 | 0.98 | −0.96 | −0.75 | 0.98 | 0.69 | −0.92 | 0.95 | −0.71 | 1.00 | 0.99 | 0.99 | 1.00 | |||||||||||||||||
| Carot | 0.80 | −0.97 | 0.97 | 0.73 | −0.98 | −0.71 | 0.93 | −0.96 | 0.73 | −1.00 | −0.98 | −0.99 | −1.00 | 1.00 | ||||||||||||||||
| Porph | −0.80 | 0.97 | −0.97 | −0.72 | 0.98 | 0.71 | −0.94 | 0.96 | −0.73 | 1.00 | 0.98 | 0.98 | 1.00 | −1.00 | 1.00 | |||||||||||||||
| Vit C | −0.72 | 0.93 | −0.99 | −0.64 | 1.00 | 0.79 | −0.97 | 0.99 | −0.80 | 0.99 | 0.95 | 0.96 | 0.99 | −0.99 | 0.99 | 1.00 | ||||||||||||||
| p-Coumar | −0.98 | 0.98 | −0.77 | −0.95 | 0.82 | 0.35 | −0.71 | 0.75 | −0.38 | 0.91 | 0.97 | 0.97 | 0.92 | −0.91 | 0.91 | 0.85 | 1.00 | |||||||||||||
| Ferul | −0.51 | 0.80 | −0.99 | −0.41 | 0.98 | 0.93 | −1.00 | 0.99 | −0.94 | 0.92 | 0.83 | 0.84 | 0.91 | −0.92 | 0.93 | 0.96 | 0.68 | 1.00 | ||||||||||||
| Sinap | 0.79 | −0.96 | 0.97 | 0.71 | −0.99 | −0.73 | 0.95 | −0.97 | 0.75 | −1.00 | −0.98 | −0.98 | −1.00 | 1.00 | −1.00 | −1.00 | −0.90 | −0.93 | 1.00 | |||||||||||
| Q | −0.92 | 1.00 | −0.88 | −0.87 | 0.91 | 0.52 | −0.83 | 0.86 | −0.54 | 0.97 | 1.00 | 1.00 | 0.98 | −0.97 | 0.97 | 0.94 | 0.98 | 0.80 | −0.96 | 1.00 | ||||||||||
| K | 0.37 | −0.70 | 0.96 | 0.27 | −0.94 | −0.97 | 0.98 | −0.97 | 0.98 | −0.85 | −0.74 | −0.75 | −0.84 | 0.85 | −0.86 | −0.91 | −0.56 | −0.99 | 0.87 | −0.71 | 1.00 | |||||||||
| ABTS | 0.92 | −0.70 | 0.27 | 0.96 | −0.33 | 0.26 | 0.17 | −0.23 | −0.23 | −0.50 | −0.66 | −0.64 | −0.53 | 0.50 | −0.49 | −0.39 | −0.82 | −0.13 | 0.48 | −0.69 | −0.02 | 1.00 | ||||||||
| FRAP | 0.78 | −0.47 | −0.01 | 0.84 | −0.06 | 0.51 | −0.11 | 0.04 | −0.48 | −0.24 | −0.43 | −0.41 | −0.28 | 0.25 | −0.24 | −0.13 | −0.63 | 0.15 | 0.22 | −0.47 | −0.29 | 0.96 | 1.00 | |||||||
| DPPH | 0.99 | −0.97 | 0.73 | 0.97 | −0.78 | −0.29 | 0.66 | −0.71 | 0.32 | −0.88 | −0.96 | −0.95 | −0.90 | 0.88 | −0.88 | −0.82 | −1.00 | −0.63 | 0.87 | −0.97 | 0.51 | 0.85 | 0.67 | 1.00 | ||||||
| H2O2 | −0.13 | 0.51 | −0.86 | −0.02 | 0.82 | 1.00 | −0.91 | 0.88 | −1.00 | 0.70 | 0.55 | 0.57 | 0.68 | −0.70 | 0.71 | 0.78 | 0.34 | 0.92 | −0.72 | 0.51 | −0.97 | 0.27 | 0.52 | −0.28 | 1.00 | |||||
| Prol | −0.67 | 0.90 | −1.00 | −0.58 | 1.00 | 0.83 | −0.99 | 1.00 | −0.85 | 0.98 | 0.92 | 0.93 | 0.97 | −0.98 | 0.98 | 1.00 | 0.81 | 0.98 | −0.99 | 0.91 | −0.94 | −0.32 | −0.05 | −0.77 | 0.83 | 1.00 | ||||
| MDA | −0.69 | 0.92 | −1.00 | −0.61 | 1.00 | 0.82 | −0.98 | 0.99 | −0.83 | 0.99 | 0.94 | 0.94 | 0.98 | −0.99 | 0.99 | 1.00 | 0.83 | 0.97 | −0.99 | 0.92 | −0.93 | −0.35 | −0.08 | −0.79 | 0.81 | 1.00 | 1.00 | |||
| α-amylase | −0.10 | −0.30 | 0.72 | −0.21 | −0.67 | −0.97 | 0.79 | −0.74 | 0.96 | −0.52 | −0.34 | −0.36 | −0.49 | 0.51 | −0.53 | −0.62 | −0.12 | −0.81 | 0.54 | −0.30 | 0.89 | −0.48 | −0.70 | 0.05 | −0.97 | −0.68 | −0.65 | 1.00 | ||
| α-glucos | −0.82 | 0.98 | −0.96 | −0.75 | 0.98 | 0.68 | −0.92 | 0.95 | −0.70 | 1.00 | 0.99 | 0.99 | 1.00 | −1.00 | 1.00 | 0.99 | 0.92 | 0.91 | −1.00 | 0.98 | −0.83 | −0.53 | −0.28 | −0.90 | 0.67 | 0.97 | 0.98 | −0.49 | 1.00 | |
| lipase | 0.62 | −0.88 | 1.00 | 0.53 | −1.00 | −0.87 | 0.99 | −1.00 | 0.88 | −0.97 | −0.90 | −0.91 | −0.96 | 0.97 | −0.97 | −0.99 | −0.77 | −0.99 | 0.97 | −0.88 | 0.96 | 0.26 | −0.01 | 0.73 | −0.86 | −1.00 | −1.00 | 0.72 | −0.96 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šola, I.; Davosir, D.; Kokić, E.; Zekirovski, J. Effect of Hot- and Cold-Water Treatment on Broccoli Bioactive Compounds, Oxidative Stress Parameters and Biological Effects of Their Extracts. Plants 2023, 12, 1135. https://doi.org/10.3390/plants12051135
Šola I, Davosir D, Kokić E, Zekirovski J. Effect of Hot- and Cold-Water Treatment on Broccoli Bioactive Compounds, Oxidative Stress Parameters and Biological Effects of Their Extracts. Plants. 2023; 12(5):1135. https://doi.org/10.3390/plants12051135
Chicago/Turabian StyleŠola, Ivana, Dino Davosir, Emilie Kokić, and Jana Zekirovski. 2023. "Effect of Hot- and Cold-Water Treatment on Broccoli Bioactive Compounds, Oxidative Stress Parameters and Biological Effects of Their Extracts" Plants 12, no. 5: 1135. https://doi.org/10.3390/plants12051135
APA StyleŠola, I., Davosir, D., Kokić, E., & Zekirovski, J. (2023). Effect of Hot- and Cold-Water Treatment on Broccoli Bioactive Compounds, Oxidative Stress Parameters and Biological Effects of Their Extracts. Plants, 12(5), 1135. https://doi.org/10.3390/plants12051135