Factors Influencing Seed Dormancy and Germination and Advances in Seed Priming Technology
Abstract
:1. Introduction
2. Factors Influencing Seed Dormancy and Germination
2.1. Seed Structure
2.2. Nutrient Change
2.2.1. Starch
2.2.2. Fat
2.2.3. Protein
2.3. Endogenous Hormones Signaling and Gene Regulation
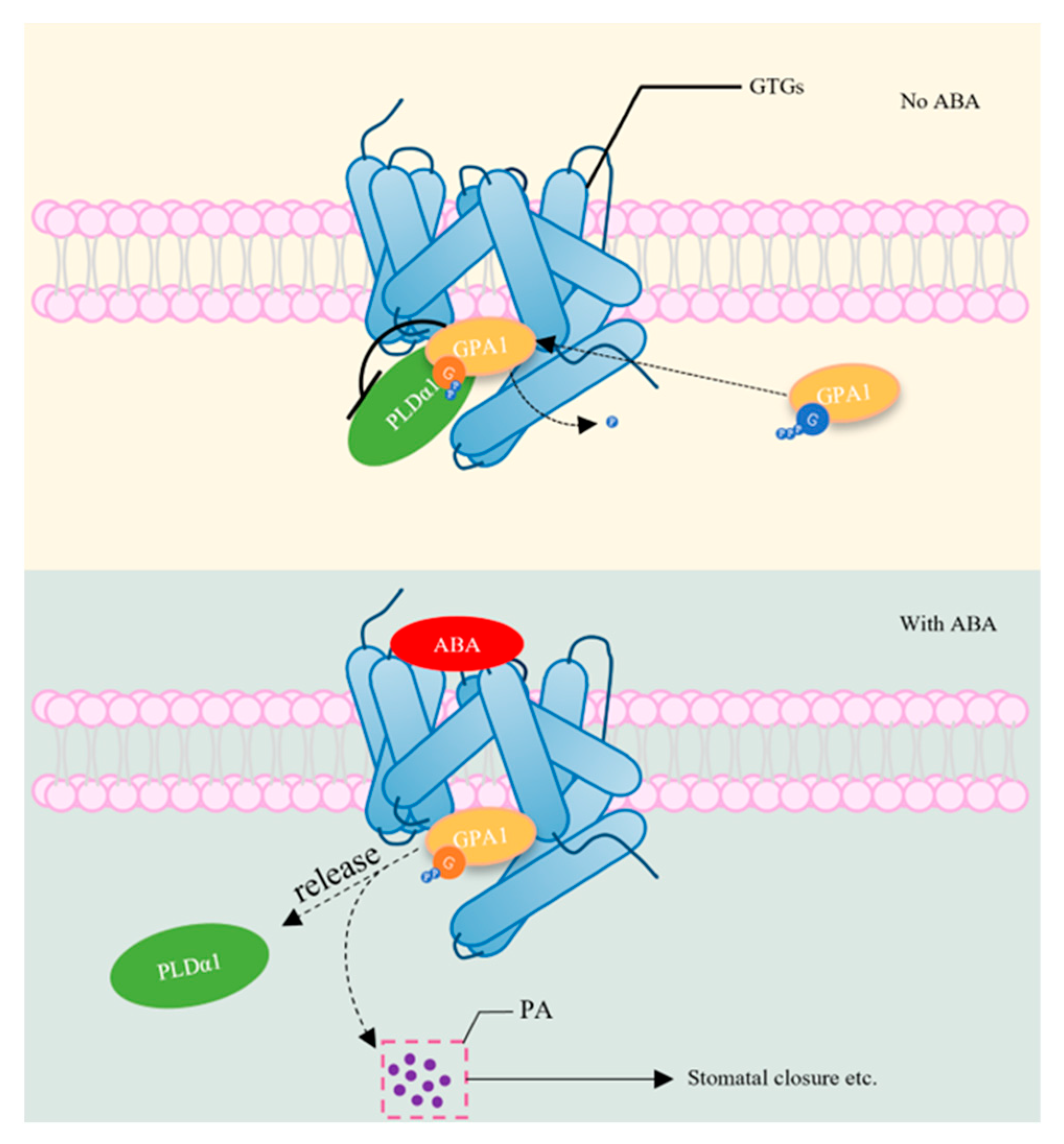
2.3.1. Abscisic Acid
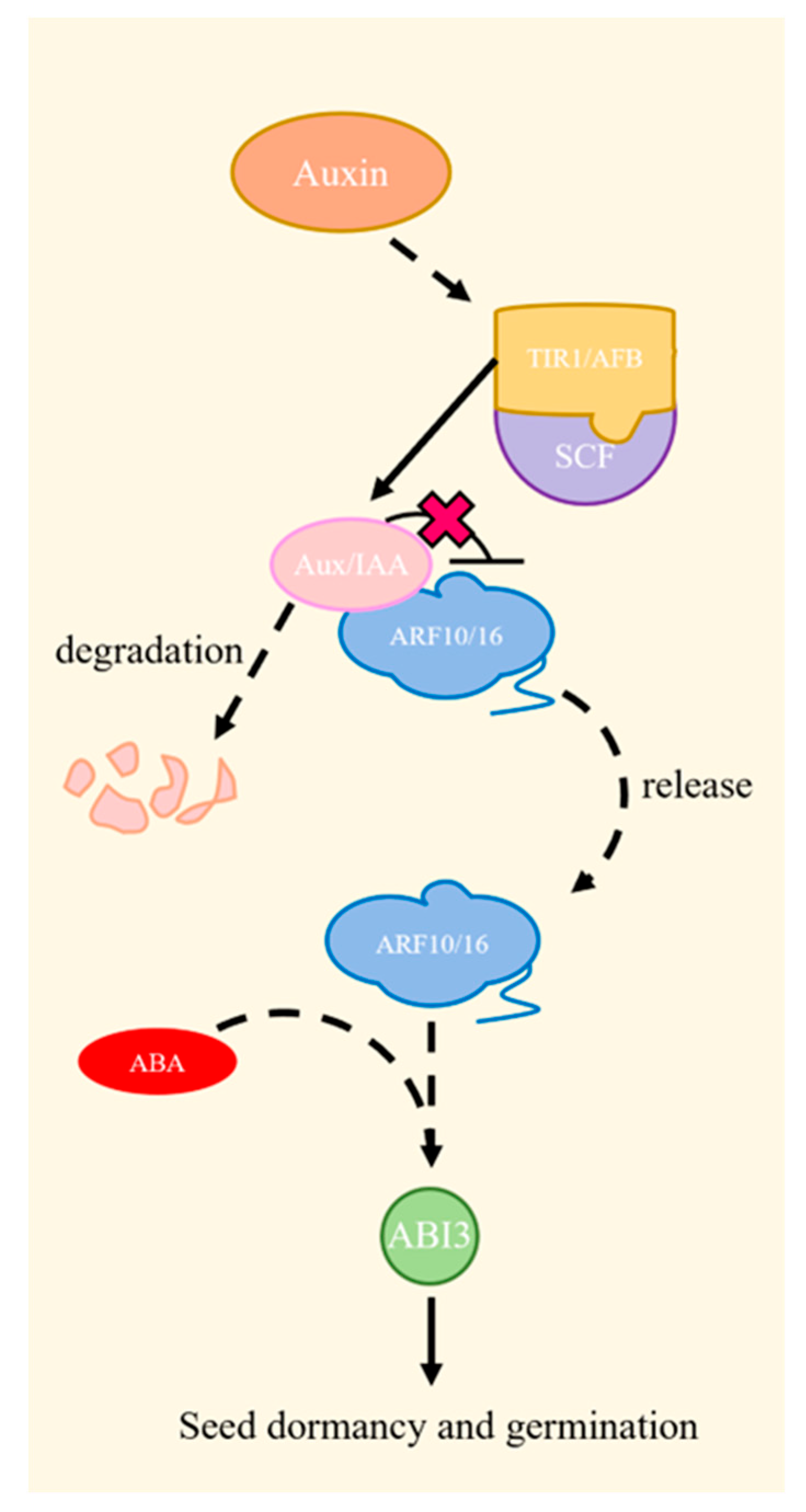
2.3.2. Auxins
2.3.3. Gibberellins Acid (GAs)
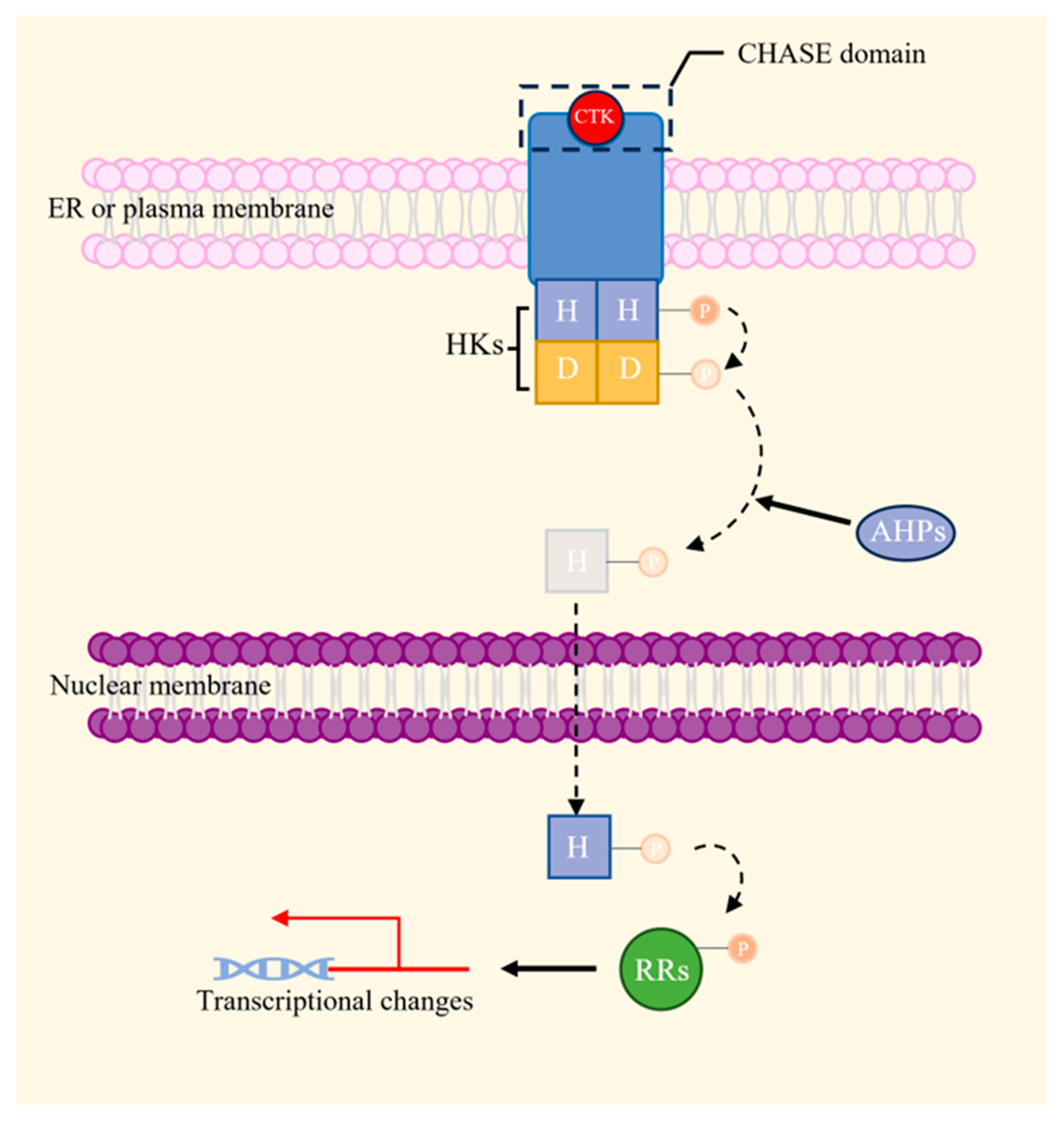
2.3.4. Cytokinins (CTKs)
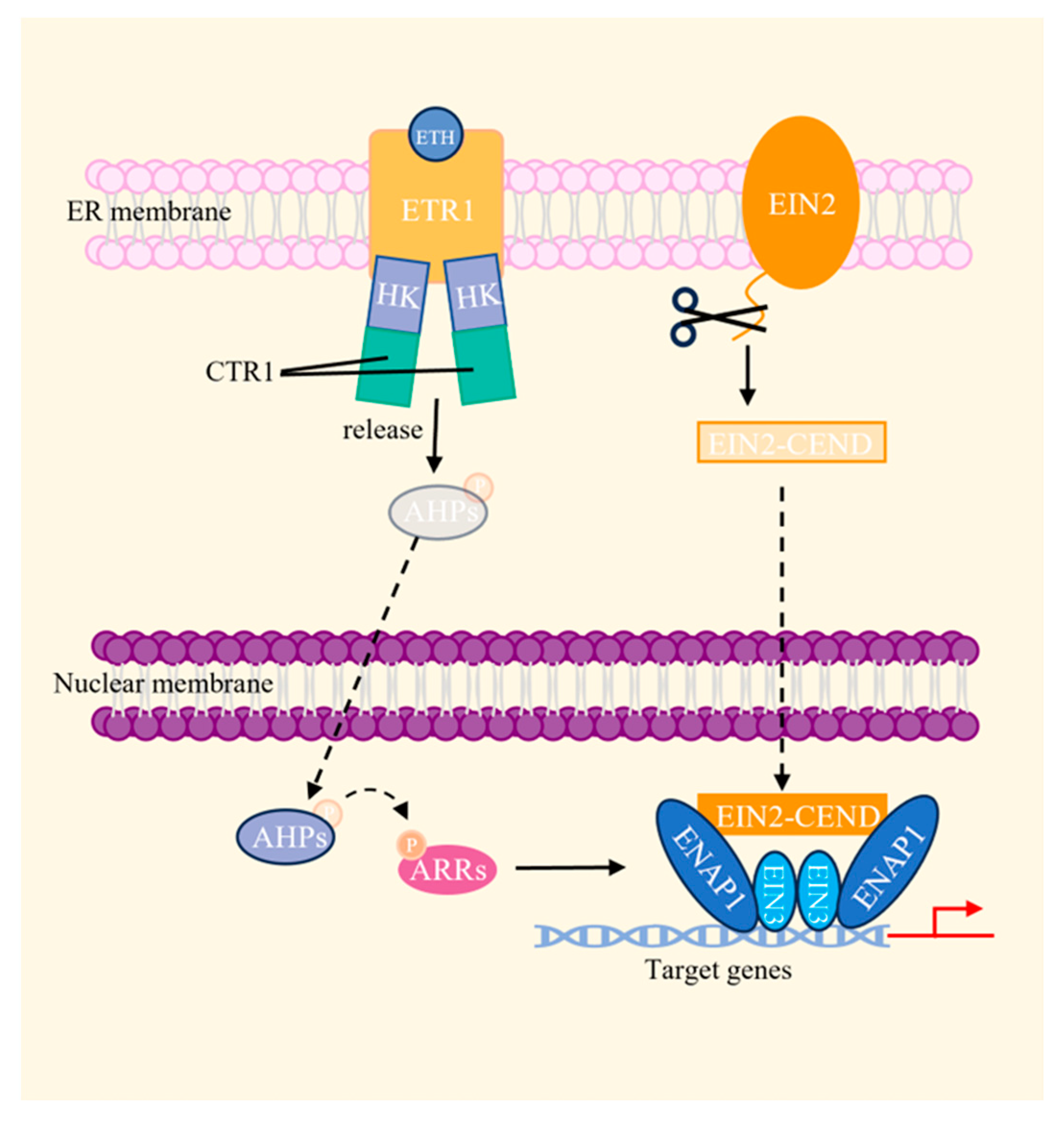
2.3.5. Ethylene (ETH)
3. Seed Priming Technologies
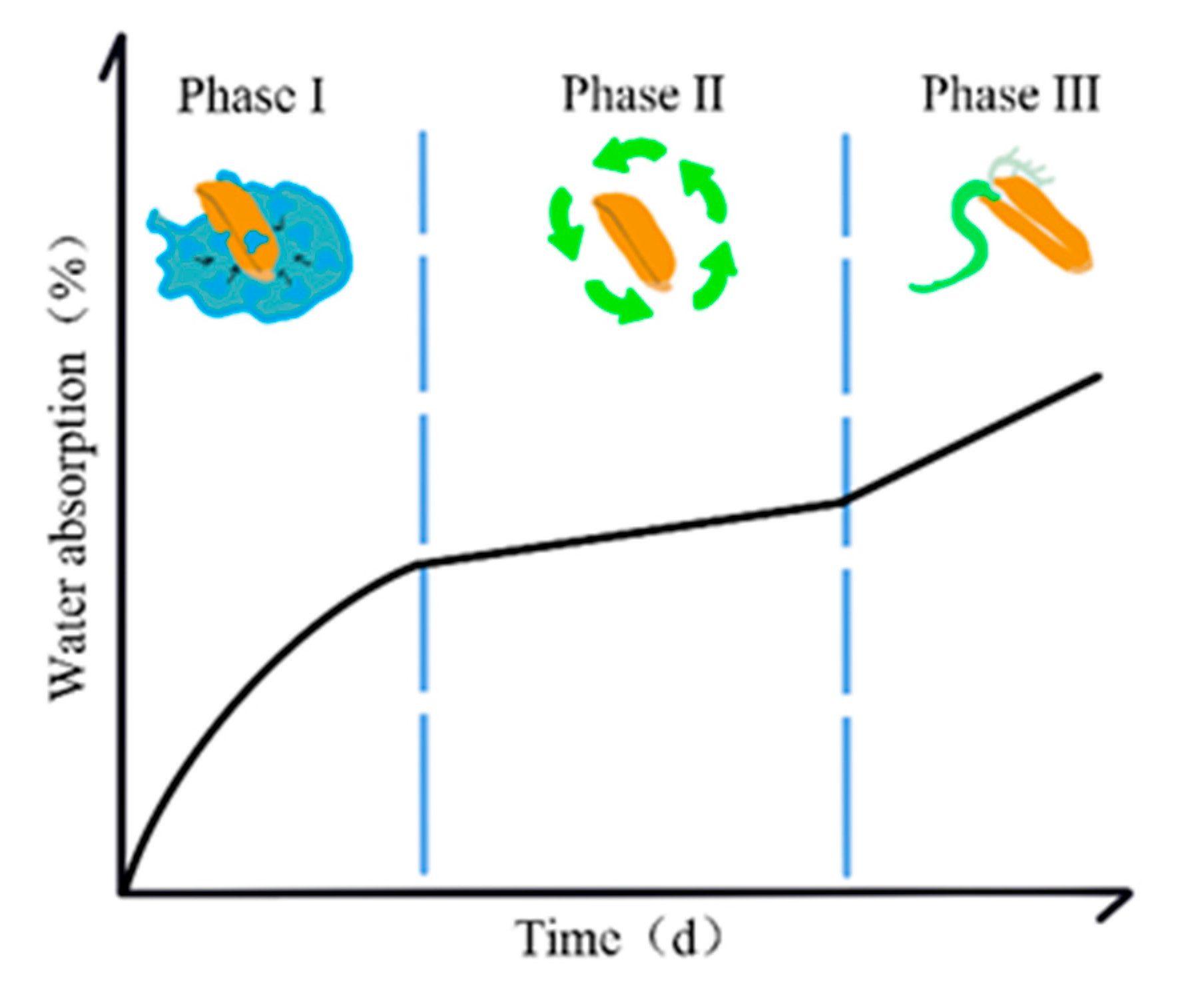
3.1. Hydropriming
3.2. Solid Matrix Priming (SMP)
3.3. Nano-Priming
3.4. Bio-Priming
3.5. Phytohormone Priming
3.6. Seed Priming and Stress Condition
4. Conclusions with Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miransari, M.; Smith, D.L. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- Finkelstein, R.; Reeves, W.; Ariizumi, T.; Steber, C. Molecular aspects of seed dormancy. Annu. Rev. Plant Biol. 2008, 59, 387–415. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Zhou, W.; Song, Z.P. Transcription dynamics of seed germination of Oryza rufipogon Griff. J. Fudan Univ. Nat. Sci. 2022, 62, 381–391. [Google Scholar] [CrossRef]
- Gregory, L.A.; Jack, E.D.; George, M.C.; Rebecca, D.L. Endo-, para-, and ecodormancy physiological terminology and classification for dormancy research. HortScience 1987, 22, 371–377. [Google Scholar]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Heydecker, W.; Higgins, J.; Gulliver, R. Accelerated germination by osmotic seed treatment. Nature 1973, 246, 42–46. [Google Scholar] [CrossRef]
- Heydecker, W.; Coolbear, P. Seed treatments for improved performance—Survey and attempted prognosis. Seed Sci. Technol. 1977, 5, 353–425. [Google Scholar]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Le, K.-A.; Van den Broeck, H.C.; Brouns, F.J.P.H.; De Brier, N.; et al. Impact of cereal seed sprouting on its nutritional and technological properties: A critical review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef] [PubMed]
- Piskurewicz, U.; Sentandreu, M.; Iwasaki, M.; Glauser, G.; Lopez-Molina, L. The Arabidopsis endosperm is a temperature-sensing tissue that implements seed thermoinhibition through phyB. Nat. Commun. 2023, 14, 1202. [Google Scholar] [CrossRef]
- Duan, X.H.; Luo, F.C.; Zhong, S.; Li, Q.X.; Xue, S.M.; Yang, G.R.; Huang, B.Z. A preliminary study on causes of seed dormancy of Brachiaria decumbens. Grassl. Turf 2015, 35, 49–52. [Google Scholar] [CrossRef]
- Altuner, F. Determination of the effect of salt (NaCl) stress on germination of oat (Avena sativa L.) seeds pretreated with gibberellic acid. Fresen Environ. Bull. 2020, 29, 11111–11118. [Google Scholar]
- Pan, W.; Liang, J.; Sui, J.; Li, J.; Liu, C.; Xin, Y.; Zhang, Y.; Wang, S.; Zhao, Y.; Zhang, J.; et al. ABA and bud dormancy in perennials: Current knowledge and future perspective. Genes 2021, 12, 1635. [Google Scholar] [CrossRef]
- Badenhuizen, N.P. Formation and distribution of amylose and amylopectin in starch granule. Nature 1963, 197, 464–467. [Google Scholar] [CrossRef]
- Tetlow, I.J. Starch biosynthesis in developing seeds. Seed Sci. Res. 2011, 21, 5–32. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S.; Singh, B. Germination behaviour, physico-nutritional properties, and diastase activity of brown rice influenced by germination time and temperature. Acta Aliment. 2018, 47, 70–79. [Google Scholar] [CrossRef]
- Lekjing, S.; Venkatachalam, K. Effects of germination time and kilning temperature on the malting characteristics, biochemical and structural properties of HomChaiya rice. Rsc Adv. 2020, 10, 16254–16265. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; An, H.-G.; Kang, S.-J.; Lee, Y.-T. Physicochemical and bioactive properties of a high ?-glucan barley variety ?Betaone? affected by germination processing. Int. J. Biol. Macromol. 2021, 177, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Graham, I.A. Seed storage oil mobilization. Annu. Rev. Plant Biol. 2008, 59, 115–142. [Google Scholar] [CrossRef] [PubMed]
- Pilco-Quesada, S.; Tian, Y.; Yang, B.; Repo-Carrasco-Valencia, R.; Suomela, J.-P. Effects of germination and kilning on the phenolic compounds and nutritional properties of quinoa (Chenopodium quinoa) and kiwicha (Amaranthus caudatus). J. Cereal Sci. 2020, 94, 102996. [Google Scholar] [CrossRef]
- Zhao, X.; Mao, K.; Xu, J.; Zheng, C.; Luo, X.; Shu, K. Protein phosphorylation and its regulatory roles in seed dormancy and germination. Chin. Bull. Bot. 2021, 56, 488–499. [Google Scholar]
- Khan, A.A. Primary, preventive and permissive roles of hormones in plant systems. Bot. Rev. 1975, 41, 391–420. [Google Scholar] [CrossRef]
- Chang, Y.; Geng, Z.; Wang, K.; Li, M.; Zhang, B.; Zhao, W. Mechanism study of OsBLR1 in promoting rice germination under hormones induction. J. Henan Agric. Univ. 2021, 55, 1058–1064. [Google Scholar]
- Wang, L.; Zhang, Z.; Zhang, F.; Shao, Z.; Zhao, B.; Huang, A.; Tran, J.; Hernandez, F.V.; Qiao, H. EIN2-directed histone acetylation requires EIN3-mediated positive feedback regulation in response to ethylene. Plant Cell 2021, 33, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Sirova, J.; Sedlarova, M.; Piterkova, J.; Luhova, L.; Petrivalsky, M. The role of nitric oxide in the germination of plant seeds and pollen. Plant Sci. 2011, 181, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, Y.; Zhang, B. Effects of endogenous hormone change and exogenous auxin on the germination process of Alfalfa seed. Acta Agrestia Sin. 2018, 26, 691–696. [Google Scholar]
- Pang, X.Y.; Wan, L.; Li, S.; Wang, Y.H.; Liu, C.; Xiao, X.L.; Li, X.H.; Ma, N. Effects of exogenous SLs and Nano-K2MoO4 on seed germination of Brassica napus L. under drought stress. Crops 2022, 4, 214–220. [Google Scholar] [CrossRef]
- Clemente, A.C.S.; Guimaraes, R.M.; Martins, D.C.; Gomes, L.A.A.; Caixeta, F.; Reis, R.G.E.; Rosa, S.D.V.F. Expression of genes associated with the biosynthetic pathways of abscisic acid, gibberellin, and ethylene during the germination of lettuce seeds. Genet. Mol. Res. 2015, 14, 4703–4715. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Shi, J.; Liang, W.; Zhang, D. Integrating GWAS and transcriptomics to identify genes involved in seed dormancy in rice. Theor. Appl. Genet. 2021, 134, 3553–3562. [Google Scholar] [CrossRef] [PubMed]
- Groot, S.P.C.; Karssen, C.M. Dormancy and germination of abscisic acid-deficient tomato seeds—Studies with the sitiens mutant. Plant Physiol. 1992, 99, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Koussevitzky, S.; Nott, A.; Mockler, T.C.; Hong, F.; Sachetto-Martins, G.; Surpin, M.; Lim, I.J.; Mittler, R.; Chory, J. Signals from chloroplasts converge to regulate nuclear gene expression. Science 2007, 316, 715–719. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.Y.; Zhang, J.Z.; Liu, T.J.; Hu, C.G. PtFCA from precocious trifoliate orange is regulated by alternative splicing and affects flowering time and root development in transgenic Arabidopsis. Tree Genet. Genomes 2016, 12, 85. [Google Scholar] [CrossRef]
- Johnston, C.A.; Taylor, J.P.; Gao, Y.; Kimple, A.J.; Grigston, J.C.; Chen, J.-G.; Siderovski, D.P.; Jones, A.M.; Willard, F.S. GTPase acceleration as the rate-limiting step in Arabidopsis G protein-coupled sugar signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 17317–17322. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-G. Heterotrimeric G-protein signaling in Arabidopsis. Plant Signal Behav. 2008, 3, 1042–1045. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.F.; et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.; Dupeux, F.; Round, A.; Antoni, R.; Park, S.Y.; Jamin, M.; Cutler, S.R.; Rodriguez, P.L.; Marquez, J.A. The abscisic acid receptor PYR1 in complex with abscisic acid. Nature 2009, 462, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Skubacz, A.; Daszkowska-Golec, A.; Szarejko, I. The role and regulation of ABI5 (ABA-Insensitive 5) in plant development, abiotic stress responses and phytohormone crosstalk. Front. Plant Sci. 2016, 7, 1884. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.Q.; Luan, S.; Li, J.; He, Z.H. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.J.; Estelle, M. Mechanism of auxin-regulated gene expression in plants. Annu. Rev. Genet. 2009, 43, 265–285. [Google Scholar] [CrossRef]
- Peer, W.A. From perception to attenuation: Auxin signalling and responses. Curr. Opin. Plant Biol. 2013, 16, 561–568. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, L.; Ding, Z. 26S proteasome: Hunter and prey in auxin signaling. Trends Plant Sci. 2016, 21, 546–548. [Google Scholar] [CrossRef] [PubMed]
- Groot, S.P.C.; Karssen, C.M. Gibberellins regulate seed-germination in tomato by endosperm weakening—A study with gibberellin-deficient mutants. Planta 1987, 171, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Li, T.; Li, Z.X.; Sun, B.J.; Li, Y.; Xu, X.W.; Wang, H.M. Effect of GA3 on seed germination and plant growth of Solanum melongena. Chin. Agric. Sci. Bull. 2018, 10, 48–54. [Google Scholar]
- Mao, J.Y.; Zhang, X.; Ning, F.S.X.; Zhao, H.T.; Mao, T.Y.; Cheng, Y.; Jiang, X.M. Effects of different gibberellin treatments on yield and quality of Heracleum moellendorffii Hance in winter greenhouse. China Veg. 2021, 11, 69–74. [Google Scholar] [CrossRef]
- Pan, X.Q.; Zhao, S.; Zhou, H.W.; Huang, D.F. Effect of low temperature immersion of different concentrations of gibberellin on germination of Perilla frutescens L. Shanghai Veg. 2021, 6, 72–75. [Google Scholar]
- Nguyen, T.N.; Tuan, P.A.; Ayele, B.T. Jasmonate regulates seed dormancy in wheat via modulating the balance between gibberellin and abscisic acid. J. Exp. Bot. 2022, 73, 2434–2453. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; He, F.; Liu, J.; Zhang, C.; Wang, Y.; Tian, S.; Sun, C.; Zhang, X. Understanding of hormonal regulation in rice seed germination. Life 2022, 12, 1021. [Google Scholar] [CrossRef] [PubMed]
- Daviere, J.M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef]
- Kim, D.H.; Yamaguchi, S.; Lim, S.; Oh, E.; Park, J.; Hanada, A.; Kamiya, Y.; Choi, G. SOMNUS, a CCCH-type zinc finger protein in Arabidopsis, negatively regulates light-dependent seed germination downstream of PIL5. Plant Cell 2008, 20, 1260–1277. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Park, J.; Lee, N.; Jeong, J.; Toh, S.; Watanabe, A.; Kim, J.; Kang, H.; Kim, D.H.; Kawakami, N.; et al. ABA-insensitive3, ABA-insensitive5, and DELLAs Interact to activate the expression of SOMNUS and other high-temperature-inducible genes in imbibed seeds in Arabidopsis. Plant Cell 2013, 25, 4863–4878. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Q.; He, D.; Zhou, Y.; Ni, H.; Tian, D.; Chang, G.; Jing, Y.; Lin, R.; Huang, J.; et al. AGAMOUS-LIKE67 cooperates with the Histone Mark Reader EBS to modulate seed germination under high temperature. Plant Physiol. 2020, 184, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Chiu, R.S.; Saleh, Y.; Gazzarrini, S. Inhibition of FUSCA3 degradation at high temperature is dependent on ABA signaling and is regulated by the ABA/GA ratio. Plant Signal Behav. 2016, 11, e1247137. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.F.; Huang, Z.H.; Tang, W.X.; Luo, W.H. Effect of 6-benzylaminopurine and gibberellin on seed germination of three Theaceae. Seed 2019, 2, 25–30. [Google Scholar] [CrossRef]
- Miller, C.O.; Skong, F.; Von Saltza, M.H.; Strong, F.M. Kinetin. A cell division factor from deoxyribonucleic acid. J. Am. Chem. Soc. 1955, 77, 1329–1334. [Google Scholar] [CrossRef]
- Stock, A.M.; Robinson, V.L.; Goudreau, P.N. Two-component sigtnal trusduction. Annu. Rev. Biochem. 2000, 69, 183–215. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Hendrickson, W.A. Sensor domains of two-component regulatory systems. Curr. Opin. Microbiol. 2010, 13, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Appleby, J.L.; Parkinson, J.S.; Bourret, R.B. Signal transduction via the multi-step phosphorelay not necessarily a road less traveled. Cell 1996, 86, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.E.; Shiu, S.H.; Armitage, J.P. Two-component systems and their co-option for eukaryotic signal transduction. Curr. Biol. 2011, 21, R320–R330. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Higuchi, M.; Hashimoto, Y.; Seki, M.; Kobayashi, M.; Kato, T.; Tabata, S.; Shinozaki, K.; Kakimoto, T. Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 2001, 409, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Pischke, M.S.; Mahonen, A.P.; Miyawaki, K.; Hashimoto, Y.; Seki, M.; Kobayashi, M.; Shinozaki, K.; Kato, T.; Tabata, S.; et al. In planta functions of the Arabidopsis cytokinin receptor family. Proc. Natl. Acad. Sci. USA 2004, 101, 8821–8826. [Google Scholar] [CrossRef]
- Nishimura, C.; Ohashi, Y.; Sato, S.; Kato, T.; Tabata, S.; Ueguchi, C. Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell 2004, 16, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Ueguchi, C.; Sato, S.; Kato, T.; Tabata, S. The AHK4 gene involved in the cytokinin-signaling pathway as a direct receptor molecule in Arabidopsis thaliana. Plant Cell Physiol. 2001, 42, s41. [Google Scholar] [CrossRef]
- Suzuki, T.; Miwa, K.; Ishikawa, K.; Yamada, H.; Aiba, H.; Mizuno, T. The Arabidopsis sensor His-kinase, AHK4, can respond to cytokinins. Plant Cell Physiol. 2001, 42, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Suzuki, T.; Terada, K.; Takei, K.; Ishikawa, K.; Miwa, K.; Yamashino, T.; Mizuno, T. The Arabidopsis AHK4 histidine kinase is a cytokinin-binding receptor that transduces cytokinin signals across the membrane. Plant Cell Physiol. 2001, 42, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Sheen, J. Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 2001, 413, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef] [PubMed]
- Li, H.P.; Sun, H.C.; Ping, W.C.; Liu, L.T.; Zhang, Y.J.; Zhang, K.; Bai, Z.Y.; Li, A.C.; Zhu, J.J.; Li, C.D. Exogenous ethylene promotes the germination of cotton seeds under salt stress. J. Plant Growth Regul. 2023, 42, 3923–3933. [Google Scholar] [CrossRef]
- Jiao, C.; Li, M.; Zhang, P. Effects of exogenous hormones soaking and osmotic treatment on thermal dormancy of seeds of Fraxinus mandshurica. Bull. Bot. Res. 2023, 43, 370–378. [Google Scholar] [CrossRef]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Mo, Y.; Zhang, P.; Zhu, H.; Deng, M.; Zhang, H.; Bao, J.; Wang, Z.; Zuo, Z.; Ma, Z.; Zhao, K. Effects of exogenous ethylene on the germination of different pepper varieties seeds. J. Yunnan Agric. Univ. 2016, 31, 268–273. [Google Scholar]
- Yuan, J.; Tan, X.F.; Luo, J.; Li, W.X. Effects of different treatments on the germination of Camellia oleifera seeds. China Seed Ind. 2009, 9, 50–51. [Google Scholar] [CrossRef]
- Corbineau, F.; Xia, Q.; Bailly, C.; El-Maarouf-Bouteau, H. Ethylene, a key factor in the regulation of seed dormancy. Front. Plant Sci. 2014, 5, 539. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, A.B.; Estelle, M.A.; Somerville, C.; Kende, H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 1988, 241, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Kwok, S.F.; Bleecker, A.B.; Meyerowitz, E.M. Arabidopsis ethylene-response gene ETR1—Similarity of product to 2-component regulators. Science 1993, 262, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Chang, C.; Sun, Q.; Meyerowitz, E.M. Ethylene insensitivity conferred by Arabidopsis ERS gene. Science 1995, 269, 1712–1714. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Meyerowitz, E.M. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 1998, 94, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Sakai, H.; Nourizadeh, S.; Chen, Q.H.G.; Bleecker, A.B.; Ecker, J.R.; Meyerowitz, E.M. EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell 1998, 10, 1321–1332. [Google Scholar] [CrossRef]
- Sakai, H.; Hua, J.; Chen, Q.H.G.; Chang, C.R.; Medrano, L.J.; Bleecker, A.B.; Meyerowitz, E.M. ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 1998, 95, 5812–5817. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.C.; Li, H.; Ecker, J.R. Ethylene biosynthesis and signaling networks. Plant Cell 2002, 14, S131–S151. [Google Scholar] [CrossRef]
- Huang, J.Y.; Zhao, X.B.; Buurger, M.; Chory, J.; Wang, X.C. The role of ethylene in plant temperature stress response. Trends Plant Sci. 2023, 28, 808–824. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Shen, Z.; Huang, S.-s.C.; Schmitz, R.J.; Urich, M.A.; Briggs, S.P.; Ecker, J.R. Processing and subcellular trafficking of ER-tethered EIN2 control response to ethylene gas. Science 2012, 338, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhang, C.; Ji, Y.; Zhao, Q.; He, W.; An, F.; Jiang, L.; Guo, H. Activation of ethylene signaling is mediated by nuclear translocation of the cleaved EIN2 carboxyl terminus. Cell Res. 2012, 22, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Qi, B.; Wang, L.; Zhao, B.; Rode, S.; Riggan, N.D.; Ecker, J.R.; Qiao, H. EIN2-dependent regulation of acetylation of histone H3K14 and non-canonical histone H3K23 in ethylene signalling. Nat. Commun. 2016, 7, 13018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, L.; Qi, B.; Zhao, B.; Ko, E.E.; Riggan, N.D.; Chin, K.; Qiao, H. EIN2 mediates direct regulation of histone acetylation in the ethylene response. Proc. Natl. Acad. Sci. USA 2017, 114, 10274–10279. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.C.; Wang, J.; Wang, B.K.; Gao, J.; Yu, Q.H. Effects of Exogenous MeJA and BR on tomato seed germination and root growth. Agric. Eng. 2022, 12, 138–143. [Google Scholar] [CrossRef]
- Kendall, S.L.; Hellwege, A.; Marriot, P.; Whalley, C.; Graham, I.A.; Penfield, S. Induction of dormancy in Arabidopsis summer annuals requires parallel regulation of DOG1 and hormone metabolism by low temperature and CBF transcription factors. Plant Cell 2011, 23, 2568–2580. [Google Scholar] [CrossRef] [PubMed]
- Chiang, G.C.; Bartsch, M.; Barua, D.; Nakabayashi, K.; Debieu, M.; Kronholm, I.; Koornneef, M.; Soppe, W.J.; Donohue, K.; De Meaux, J. DOG1 expression is predicted by the seed-maturation environment and contributes to geographical variation in germination in Arabidopsis thaliana. Mol. Ecol. 2011, 20, 3336–3349. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; MacGregor, D.R.; Dave, A.; Florance, H.; Moore, K.; Paszkiewicz, K.; Smirnoff, N.; Graham, I.A.; Penfield, S. Maternal temperature history activates Flowering Locus T in fruits to control progeny dormancy according to time of year. Proc. Natl. Acad. Sci. USA 2014, 111, 18787–18792. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.Z.; Li, C.Z.; Wang, F.; Zhang, L.H.; Su, W. The role of Hd3a florigen in ABA-mediated seed germination in rice. J. Fudan Univ. Nat. Sci. 2022, 62, 1–9. [Google Scholar] [CrossRef]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Bruggink, G.T.; Ooms, J.J.J.; Toorn, P.v.d. Induction of longevity in primed seeds. Seed Sci. Res. 1999, 9, 49–53. [Google Scholar] [CrossRef]
- Zheng, M.; Tao, Y.; Hussain, S.; Jiang, Q.; Peng, S.; Huang, J.; Cui, K.; Nie, L. Seed priming in dry direct-seeded rice: Consequences for emergence, seedling growth and associated metabolic events under drought stress. Plant Growth Regul. 2016, 78, 167–178. [Google Scholar] [CrossRef]
- Shanthala, J.; Siddaraju, R. Effect of hydro priming on biochemical properties sunflower hybrid and its parental line during seeds storage. Int. J. Plant Sci. 2013, 8, 221–229. [Google Scholar]
- Jiang, B.; Wang, L.; Xu, C.; Yan, M. Hydropriming enhances the germination of aged ultra-dry wheat seeds. Seed Sci. Technol. 2020, 48, 57–63. [Google Scholar] [CrossRef]
- Soniya, T.; Arivazhagan, E. Influence of hydropriming on seed germination and seedling growth of bitter gourd (Momordica charantia L.). Int. J. Bot. Stud. 2021, 6, 800–803. [Google Scholar]
- Pandita, V.K.; Anand, A.; Nagarajan, S.; Seth, R.; Sinha, S.N. Solid matrix priming improves seed emergence and crop performance in okra. Seed Sci. Technol. 2010, 38, 665–674. [Google Scholar] [CrossRef]
- Ermis, S.; Kara, F.; Özden, E.; Demir, I. Solid Matrix Priming of cabbage seed lots: Repair of ageing and increasing seed quality. J. Agric. Sci. 2016, 22, 588–595. [Google Scholar] [CrossRef]
- Lee, C.H. Solid matrix priming with hydrogels on Heteropappus arenarius seeds. Korean J. Hortic. Sci. 2013, 31, 700–705. [Google Scholar] [CrossRef]
- Ozden, E.; Ermis, S.; Sahin, O.; Taskin, M.B.; Demir, I. Solid matrix priming treatment with O2 enhanced quality of leek seed lots. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 371–375. [Google Scholar] [CrossRef]
- Khan, A.A. Preplant physiological seed conditioning. Hortic. Rev. 1992, 13, 131–181. [Google Scholar]
- Li, J.S.; Gao, Y.M.; Feng, Y. Effect of solid medium priming on onion seed’s germination rate. Nor. Horticul. 2006, 6, 16–17. [Google Scholar]
- Fraceto, L.F.; Grillo, R.; de Medeiros, G.A.; Scognamiglio, V.; Rea, G.; Bartolucci, C. Nanotechnology in agriculture: Which innovation potential does it have? Front. Environ. Sci. 2016, 4, 20. [Google Scholar] [CrossRef]
- Kottegoda, N.; Munaweera, I.; Madusanka, N.; Karunaratne, V. A green slow-release fertilizer composition based on urea-modified hydroxyapatite nanoparticles encapsulated wood. Curr. Sci. India 2011, 101, 73–78. [Google Scholar]
- Li, R.; He, J.; Xie, H.; Wang, W.; Bose, S.K.; Sun, Y.; Hu, J.; Yin, H. Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.). Int. J. Biol. Macromol. 2019, 126, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Kumar, V.; Lee, S.; Raza, N.; Kim, K.-H.; Ok, Y.S.; Tsang, D.C.W. Nanoparticle-plant interaction: Implications in energy, environment, and agriculture. Environ. Int. 2018, 119, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Raja, K.; Sowmya, R.; Sudhagar, R.; Moorthy, P.S.; Govindaraju, K.; Subramanian, K.S. Biogenic ZnO and Cu nanoparticles to improve seed germination quality in blackgram (Vigna mungo). Mater. Lett. 2019, 235, 164–167. [Google Scholar] [CrossRef]
- Zheng, L.; Hong, F.S.; Lu, S.P.; Liu, C. Effect of nano-TiO2 on strength of naturally and growth aged seeds of spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lu, L.; Wang, A.; Zhang, H.; Huang, M.; Wu, H.; Xing, B.; Wang, Z.; Ji, R. Nano-Biotechnology in agriculture: Use of nanomaterials to promote plant growth and stress tolerance. J. Agr. Food Chem. 2020, 68, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Tamindzic, G.; Azizbekian, S.; Miljakovic, D.; Turan, J.; Nikolic, Z.; Ignjatov, M.; Milosevic, D.; Vasiljevic, S. Comprehensive metal-based nanopriming for improving seed germination and initial growth of field pea (Pisum sativum L). Agronomy 2023, 13, 2932. [Google Scholar] [CrossRef]
- You, P.; He, X. Recent progress in seed nanopriming research. Pratacultural Sci. 2020, 37, 1548–1557. [Google Scholar]
- Bennett, A.J.; Whipps, J.M. Dual application of beneficial microorganisms to seed during drum priming. Appl. Soil. Ecol. 2008, 38, 83–89. [Google Scholar] [CrossRef]
- Singh, V.; Upadhyay, R.S.; Sarma, B.K.; Singh, H.B. Seed bio-priming with Trichoderma asperellum effectively modulate plant growth promotion in pea. Int. J. Agric. Environ. Biotechnol. 2016, 9, 361–365. [Google Scholar] [CrossRef]
- Muhammad, I.; Kolla, M.; Volker, R.; Günter, N. Impact of nutrient seed priming on germination, seedling development, nutritional status and grain yield of maize. J. Plant Nutr. 2015, 38, 1803–1821. [Google Scholar] [CrossRef]
- Shahzad, K.; Hussain, S.; Arfan, M.; Hussain, S.; Waraich, E.A.; Zamir, S.; Saddique, M.; Rauf, A.; Kamal, K.Y.; Hano, C.; et al. Exogenously applied gibberellic acid enhances growth and salinity stress tolerance of Maize through modulating the morpho-physiological, biochemical and molecular attributes. Biomolecules 2021, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-j.; Zhang, P.; Wang, R.-n.; Wang, P.; Huang, S.-b. Effects of variety and chemical regulators on cold tolerance during maize germination. J. Integr. Agric. 2018, 17, 2662–2669. [Google Scholar] [CrossRef]
- Zuo, B.; Xie, J.; Han, Q.; Cui, W.; Liu, T.; Wang, X.; Ding, R. Effect of gibberellin (GA_ (4+7)) priming treatment on photosynthesis and yield of maize. Chin. J. Pestic. Sci. 2017, 19, 331–340. [Google Scholar]
- Wei, T.-J.; Wang, M.-M.; Jin, Y.-Y.; Zhang, G.-H.; Liu, M.; Yang, H.-Y.; Jiang, C.-J.; Liang, Z.-W. Abscisic acid priming creates alkaline tolerance in alfalfa seedlings (Medicago sativa L.). Agriculture 2021, 11, 608. [Google Scholar] [CrossRef]
- Zhao, T.; Deng, X.; Xiao, Q.; Han, Y.; Zhu, S.; Chen, J. IAA priming improves the germination and seedling growth in cotton (Gossypium hirsutum L.) via regulating the endogenous phytohormones and enhancing the sucrose metabolism. Ind. Crops Prod. 2020, 155, 112788. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M.; Jamil, A. Seed enhancement with cytokinins: Changes in growth and grain yield in salt stressed wheat plants. Plant Growth Regul. 2006, 50, 29–39. [Google Scholar] [CrossRef]
- Mangena, P. Effect of hormonal seed priming on germination, growth, yiled, and biomass allocation in soybean grown under induced drought stress. Indian J. Agric. Res. 2020, 54, 441. [Google Scholar] [CrossRef]
- Gao, H.M.; Wen, S.; Zhang, X.L.; Zhang, Q. Effect of epibrassinolide soaking seed on maize seed germination and seedling growth under drought stress. J. Anhui Agric. Sci. 2006, 20, 5208–5209. [Google Scholar]
- Afzal, I.; Basra, S.M.; Farooq, M.; Nawaz, A. Alleviation of salinity stress in spring wheat by hormonal priming with ABA, salicylic acid and ascorbic acid. Int. J. Agric. Biol. 2006, 8, 23–28. [Google Scholar]
- Chakraborty, S.; Singh, A.; Roychoudhury, A. Extensive cross-talk among stress-regulated protective metabolites, biogenic-amines and phytohormone-signalling, co-ordinated by dopamine-mediated seed-priming, governs tolerance against fluoride stress in rice. Plant Cell Rep. 2022, 41, 2261–2278. [Google Scholar] [CrossRef] [PubMed]
- Faisal, S.; Muhammad, S.; Luqman, M.; Hasnain, M.; Rasool, A.; Awan, M.U.F.; Khan, Z.I.; Hussain, I. Effects of priming on seed germination, physico-chemistry and yield of late sown wheat crop (Triticum aestivum L.). Pol. J. Environ. Stud. 2023, 32, 1113–1124. [Google Scholar] [CrossRef]
- Hongna, C.; Leyuan, T.; Junmei, S.; Xiaori, H.; Xianguo, C. Exogenous salicylic acid signal reveals an osmotic regulatory role in priming the seed germination of Leymus chinensis under salt-alkali stress. Environ. Exp. Bot. 2021, 188, 104498. [Google Scholar] [CrossRef]
- Selovic, A.; Karalija, E.; Demir, A.; Paric, A.; Samec, D. The Effect of hydro-priming and proline priming of lettuce (Lactuca sativa L.) seeds on germination, photosynthetic pigments and metal metabolism under cadmium stress. Agriculture 2023, 13, 1472. [Google Scholar] [CrossRef]
- Li, Y.; Lin, H.; Liang, Q.; Wang, G. Effects of dopamine priming on seed germination and seedling growth of rice under salt stress. Chin. J. Rice Sci. 2021, 35, 487–494. [Google Scholar] [CrossRef]
- Zhang, R.D.; Liang, X.H.; Liu, J.; Nan, H.L.; Wang, S.Y.; Cao, X. Effects of seed priming on germination and physiological characteristics of sorghum seeds under drought stress. Crops 2022, 6, 234–240. [Google Scholar]
- Khampheng, B.; Shen, L.; Zhong, S.; Sun, Y.Z.; Yang, H.Q. Improving the antioxidant system and its stress resistance to tobacco seeds and seedling by proline priming. J. Shanxi Agric. Sci. 2019, 47, 39–48. [Google Scholar] [CrossRef]
- Jin, X.; Chen, H.T.; Shi, Y.; Bai, L.Q.; Hou, L.P.; Zhang, Y. Effect of citric acid seed priming on the growth and physiological characteristics of tomato seedlings under low phophorus stress. Chin. J. Eco-Agric. 2021, 29, 1159–1170. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, C.; Zheng, C.; Zhang, Y.; Fan, H.; Xia, F. Effects of melatonin priming on germination of Lespedeza davurica seeds under drought stress. Chin. J. Grassl. 2022, 44, 114–120. [Google Scholar]
- Shi, R.G.; Zhao, H.Y.; Wei, M.Y.; Dang, C.X.; Li, W.; Huang, H.Y.; Lin, H. Effects of exogenous choline chloride and calcium chloride on germination and physiological characteristics of wheat under salt stress. J. Anhui Agric. Sci. 2020, 14, 22–26. [Google Scholar]
- Hidayah, A.; Nisak, R.R.; Susanto, F.A.; Nuringtyas, T.R.; Yamaguchi, N.; Purwestri, Y.A. Seed Halopriming Improves Salinity Tolerance of Some Rice Cultivars During Seedling Stage. Bot. Stud. 2022, 63, 24. [Google Scholar] [CrossRef]
- Nawaz, A.; Amjad, M.; Pervez, M.A.; Afzal, I. Effect of halopriming on germination and seedling vigor of tomato. Afr. J. Agric. Res. 2011, 6, 3551–3559. [Google Scholar]
- Kaya, M.D.; Okcu, G.; Atak, M.; Cıkıhı, Y.; Kolsarıcı, O. Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). Eur. J. Agron. 2006, 24, 291–295. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef]
- Sadeghi, H.; Khazaei, F.; Yari, L.; Sheidaei, S. Effect of seed osmopriming on seed germination behavior and vigor of soybean (Glycine max L.). Agric. Biol. Sci. 2011, 6, 39–43. [Google Scholar]
- Damalas, C.A.; Koutroubas, S.D.; Fotiadis, S. Hydro-priming effects on seed germination and field performance of faba bean in spring sowing. Agriculture 2019, 9, 201. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Naveed, M.; Nawaz, A.; Shahzad, B. Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortication of bread wheat. Eur. J. Agron. 2018, 94, 98–107. [Google Scholar] [CrossRef]
- Ulfat, A.N.E.E.L.A.; Majid, S.A.; Hameed, A. Hormonal seed priming improves wheat (Triticum aestivum L.) field performance under drought and non-stress conditions. Pak. J. Bot. 2017, 49, 1239–1253. [Google Scholar]
- Wang, W.; He, A.; Peng, S.; Huang, J.; Cui, K.; Nie, L. The effect of storage condition and duration on the deterioration of primed rice seeds. Front. Plant Sci. 2018, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Sano, N.; Seo, M. Cell cycle inhibitors improve seed storability after priming treatments. J. Plant Res. 2019, 132, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Wang, H.; Tang, Z.H.; Zu, Y.G.; Liu, Y. High NaHCO3 stress causes direct injury to Nicotiana tabacum roots. J. Plant Interact. 2014, 9, 56–61. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, G.; Song, S.; Jin, Y.; Wang, X.; Yang, S.; Shen, X.; Gan, Y.; Wang, Y.; Li, R.; et al. A peroxisomal cinnamate:CoA ligase-dependent phytohormone metabolic cascade in submerged rice germination. Dev. Cell 2024. [Google Scholar] [CrossRef] [PubMed]
- Job, C.; Rajjou, L.; Lovigny, Y.; Belghazi, M.; Job, D. Patterns of protein oxidation in Arabidopsis seeds and during germination. Plant Physiol. 2005, 138, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M. Biochemical Aspects of protein changes in seed physiology and germination. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 885–898. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Ma, L.; Li, J.; Hou, D.; Zeng, B.; Zhang, L.; Liu, C.; Bi, Q.; Tan, J.; Yu, X.; et al. Factors Influencing Seed Dormancy and Germination and Advances in Seed Priming Technology. Plants 2024, 13, 1319. https://doi.org/10.3390/plants13101319
Fu Y, Ma L, Li J, Hou D, Zeng B, Zhang L, Liu C, Bi Q, Tan J, Yu X, et al. Factors Influencing Seed Dormancy and Germination and Advances in Seed Priming Technology. Plants. 2024; 13(10):1319. https://doi.org/10.3390/plants13101319
Chicago/Turabian StyleFu, Yanfeng, Li Ma, Juncai Li, Danping Hou, Bo Zeng, Like Zhang, Chunqing Liu, Qingyu Bi, Jinsong Tan, Xinqiao Yu, and et al. 2024. "Factors Influencing Seed Dormancy and Germination and Advances in Seed Priming Technology" Plants 13, no. 10: 1319. https://doi.org/10.3390/plants13101319






