RsRbohD1 Plays a Significant Role in ROS Production during Radish Pithiness Development
Abstract
1. Introduction
2. Results
2.1. Observation of Pithiness Formation
2.2. Relation of Pithiness Formation with ROS
2.3. Identification and Characterization of Radish Rbohs
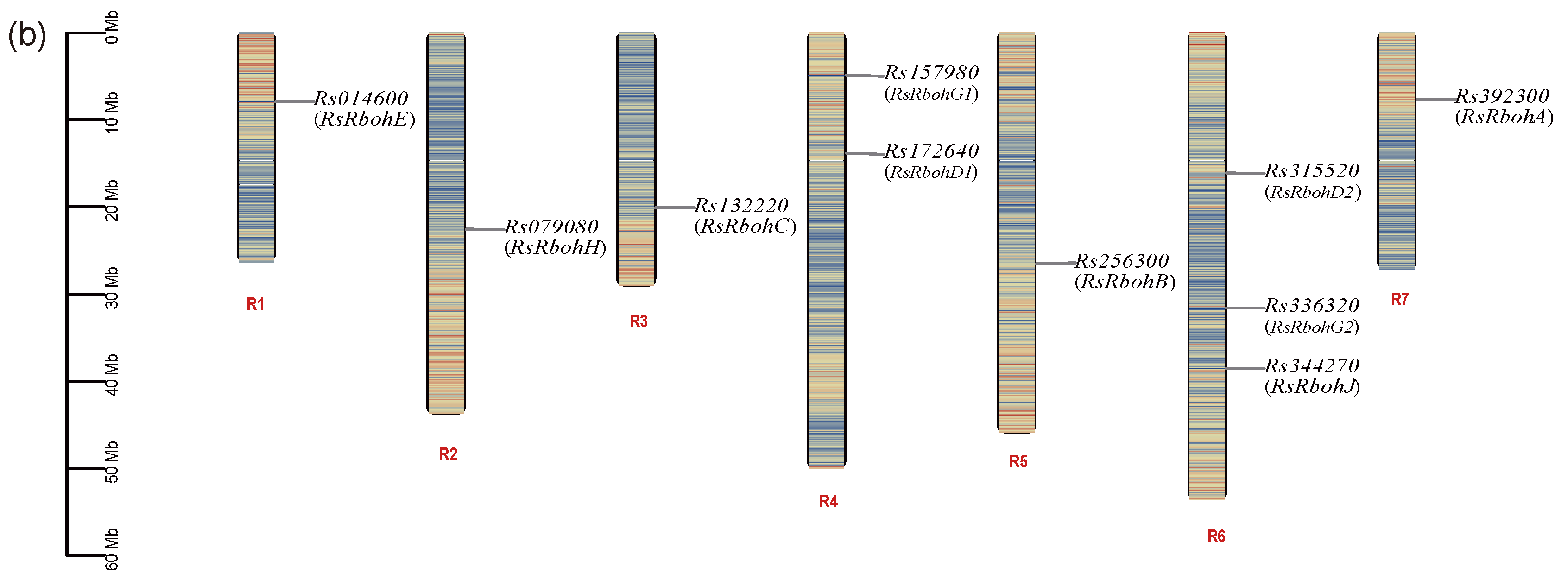
2.3.1. Rbohs’ Protein Information and Genes’ Chromosomal Localization
2.3.2. Gene Structure and Conserved Motif Analyses
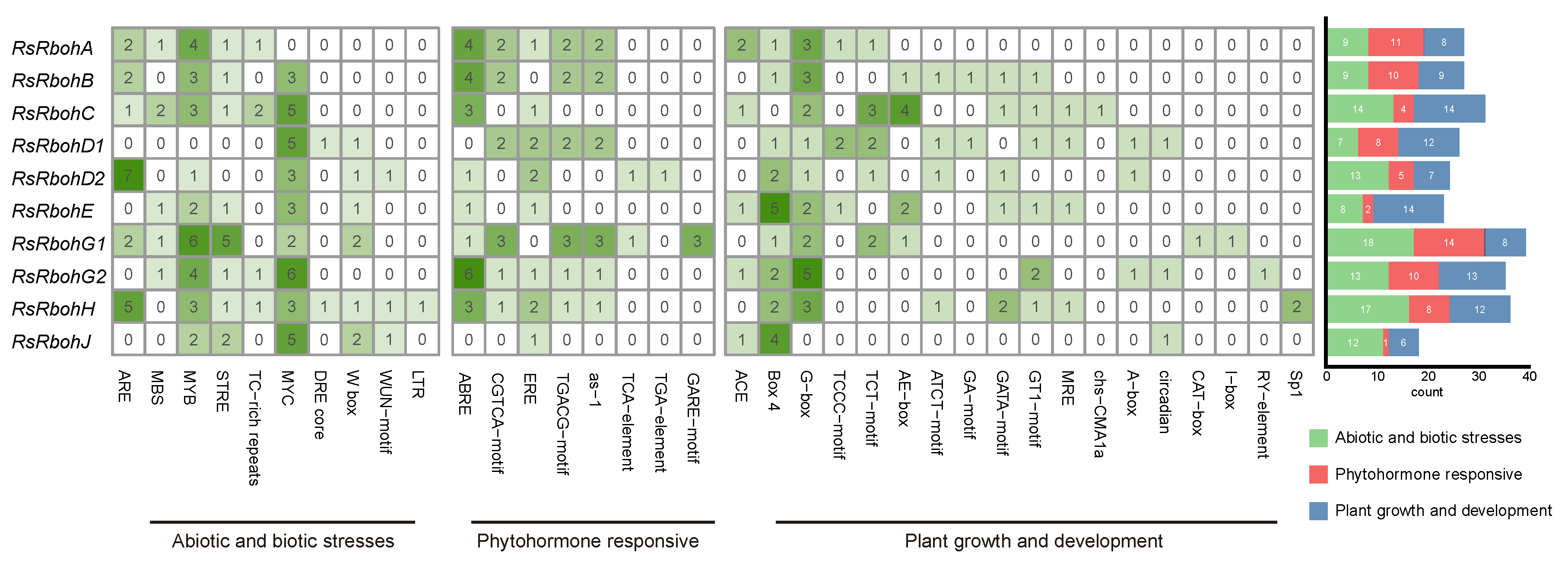
2.3.3. Cis-Acting Element Analysis of Radish Rboh Gene Promoters
2.4. Expression of RsRbohs in Different Pithiness Degrees of Fleshy Roots
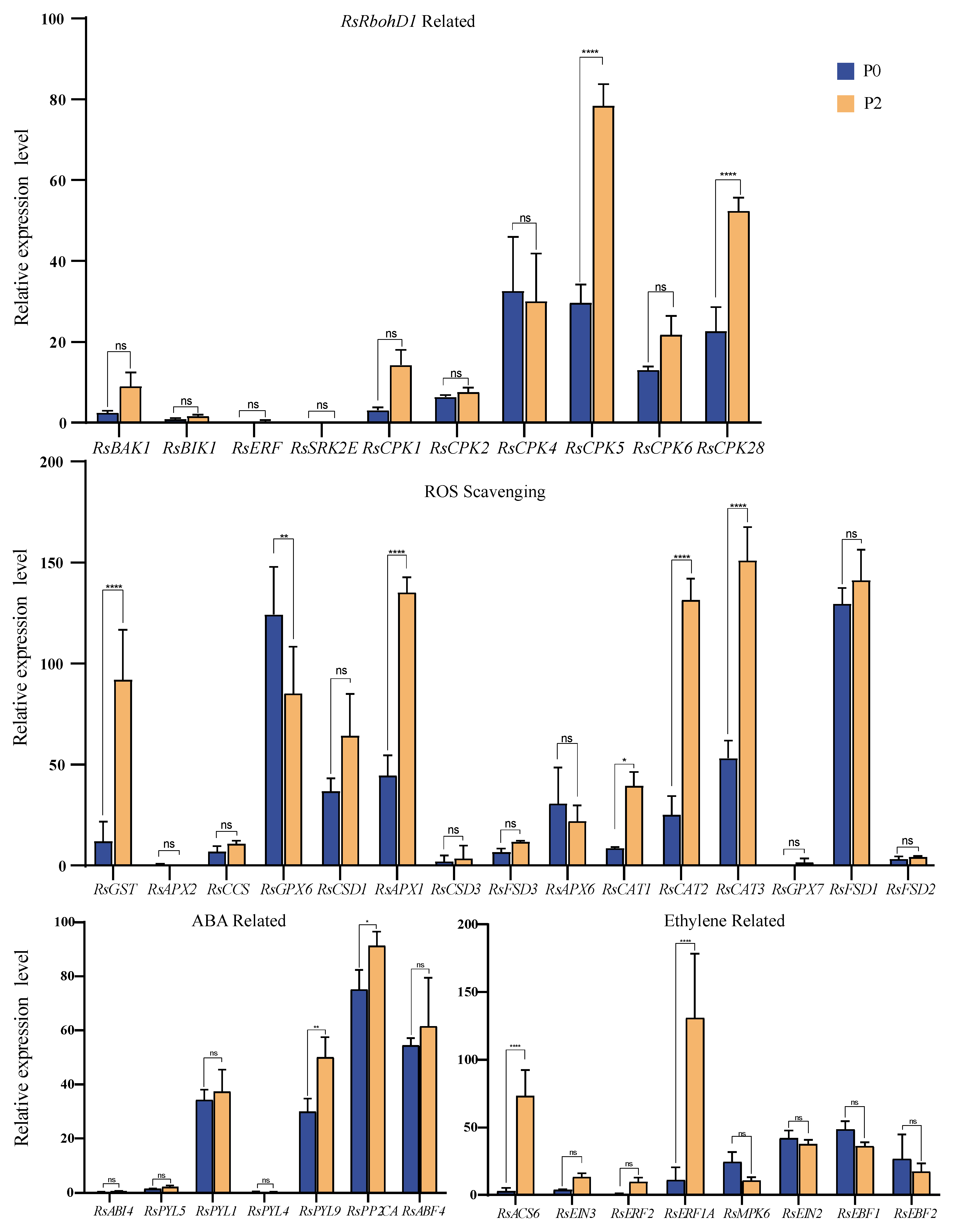
2.5. Comparison of the Expression of RbohD1-Related Genes between P0 and P2 Stages of Radish Roots
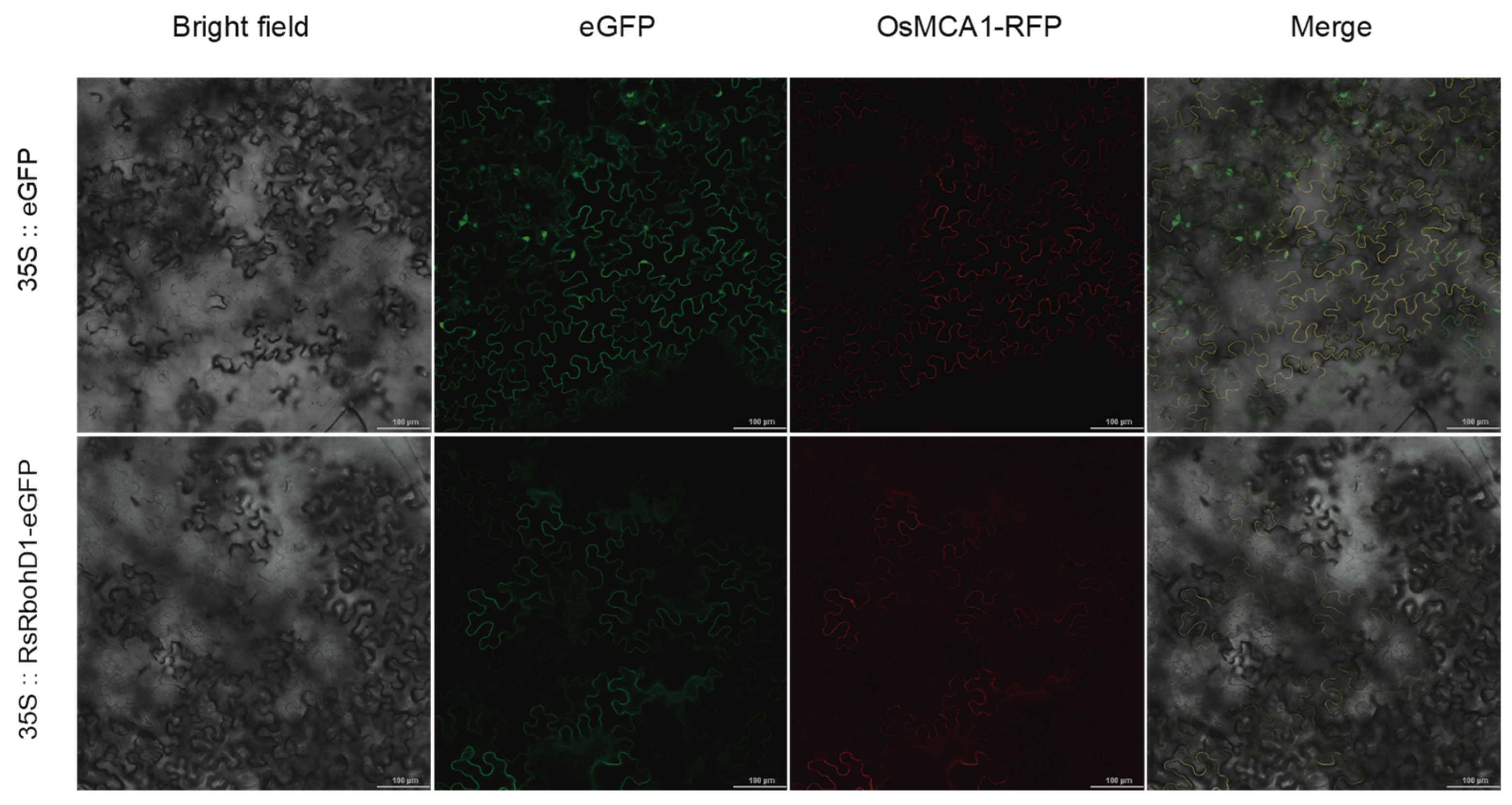
2.6. Subcellular Localization of RsRbohD1
3. Discussion
4. Materials and Methods
4.1. Plant MATERIALS Cultivation and Sampling
4.2. Histological Analysis
4.3. Identification of Radish Rboh Family Members
4.4. Sequence Features and Structural Characterization
4.5. Chromosomal Locations and Cis-Acting Element Prediction
4.6. Total RNA Extraction and Expression Analysis of RsRbohs
4.7. Subcellular Localization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asano, J. Effect of Organic Manures on Quality of Vegetables. Jpn. Agric. Res. Q. 1984, 18, 31–36. [Google Scholar]
- Harris, D.R.; Nguyen, V.Q.; Seberry, J.A.; Haigh, A.M.; McGlasson, W.B. Growth and postharvest performance of white radish (Raphanus sativus L.). Anim. Prod. Sci. 2000, 40, 879–888. [Google Scholar] [CrossRef]
- Manzoor, A.; Bashir, M.A.; Naveed, M.S.; Cheema, K.L.; Cardarelli, M. Role of Different Abiotic Factors in Inducing Pre-Harvest Physiological Disorders in Radish (Raphanus sativus). Plants 2021, 10, 2003. [Google Scholar] [CrossRef]
- Hoang, N.V.; Park, S.; Park, C.; Suh, H.; Kim, S.T.; Chae, E.; Kang, B.C.; Lee, J.Y. Oxidative stress response and programmed cell death guided by NAC013 modulate pithiness in radish taproots. Plant J. 2022, 109, 144–163. [Google Scholar] [CrossRef]
- Dietz, K.; Mittler, R.; Noctor, G. Recent progress in understanding the role of reactive oxygen species in plant cell signaling. Plant Physiol. 2016, 171, 1535–1539. [Google Scholar] [CrossRef]
- Gapper, C.; Dolan, L. Control of plant development by reactive oxygen species. Plant Physiol. 2006, 141, 341–345. [Google Scholar] [CrossRef]
- Wolf, S.; Höfte, H. Growth control: A saga of cell walls, ROS, and peptide receptors. Plant Cell 2014, 26, 1848–1856. [Google Scholar] [CrossRef]
- Del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef]
- Hirt, H. Aquaporins link ROS signaling to plant immunity. Plant Physiol. 2016, 171, 1540. [Google Scholar] [CrossRef]
- Kim, E.; Kim, Y.; Hong, W.; Lee, C.S.; Jeon, J.; Jung, K. Genome-wide Analysis of Root Hair Preferred RBOH Genes Suggests that Three RBOH Genes are Associated with Auxin-mediated Root Hair Development in Rice. J. Plant Biol. 2019, 62, 229–238. [Google Scholar] [CrossRef]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.F.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef]
- Kadota, Y.; Shirasu, K.; Zipfel, C. Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol. 2015, 56, 1472–1480. [Google Scholar] [CrossRef]
- Groom, Q.J.; Torres, M.A.; Fordham-Skelton, A.P.; Hammond-Kosack, K.E.; Robinson, N.J.; Jones, J.D. rbohA, a rice homologue of the mammalian gp91phox respiratory burst oxidase gene. Plant J. 1996, 10, 515–522. [Google Scholar] [CrossRef]
- Sagi, M.; Davydov, O.; Orazova, S.; Yesbergenova, Z.; Ophir, R.; Stratmann, J.W.; Fluhr, R. Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell 2004, 16, 616–628. [Google Scholar] [CrossRef]
- Takeda, S.; Gapper, C.; Kaya, H.; Bell, E.; Kuchitsu, K.; Dolan, L. Local positive feedback regulation determines cell shape in root hair cells. Science 2008, 319, 1241–1244. [Google Scholar] [CrossRef]
- Müller, K.; Linkies, A.; Leubner-Metzger, G.; Kermode, A.R. Role of a respiratory burst oxidase of Lepidium sativum (cress) seedlings in root development and auxin signalling. J. Exp. Bot. 2012, 63, 6325–6334. [Google Scholar] [CrossRef]
- Arthikala, M.K.; Quinto, C. RbohA coordinates lateral root emergence in common bean. Commun. Integr. Biol. 2018, 11, 1–5. [Google Scholar] [CrossRef]
- Scuffi, D.; Nietzel, T.; Fino, D.L.M.; Meyer, A.J.; Lamattina, L.; Schwarzländer, M.; Laxalt, A.M.; García-Mata, C. Hydrogen sulfide increases production of nadph oxidase-dependent hydrogen peroxide and phospholipase d-derived phosphatidic acid in guard cell signaling. Plant Physiol. 2018, 176, 2532–2542. [Google Scholar] [CrossRef]
- Gao, X.; He, P. Nuclear dynamics of Arabidopsis calcium-dependent protein kinases in effector-triggered immunity. Plant Signal. Behav. 2013, 8, e23868. [Google Scholar] [CrossRef]
- Dubiella, U.; Seybold, H.; Durian, G.; Komander, E.; Lassig, R.; Witte, C.P.; Schulze, W.X.; Romeis, T. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl. Acad. Sci. USA 2013, 110, 8744–8749. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Z.; Qin, A.; Zhou, Y.; Zhao, Z.; Yang, J.; Hu, M.; Liu, H.; Liu, Y.; Sun, S.; et al. FLS2-RBOHD module regulates changes in the metabolome of Arabidopsis in response to abiotic stress. Plant Environ. Interact. 2023, 4, 36–54. [Google Scholar] [CrossRef]
- Matschi, S.; Werner, S.; Schulze, W.X.; Legen, J.; Hilger, H.H.; Romeis, T. Function of calcium-dependent protein kinase CPK28 of Arabidopsis thaliana in plant stem elongation and vascular development. Plant J. 2013, 73, 883–896. [Google Scholar] [CrossRef]
- Otulak-Kozieł, K.; Kozieł, E.; Horváth, E.; Csiszár, J. AtGSTU19 and AtGSTU24 as Moderators of the Response of Arabidopsis thaliana to Turnip mosaic virus. Int. J. Mol. Sci. 2022, 23, 11531. [Google Scholar] [CrossRef]
- Passaia, G.; Queval, G.; Bai, J.; Margis-Pinheiro, M.; Foyer, C.H. The effects of redox controls mediated by glutathione peroxidases on root architecture in Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 1403–1413. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, W.; Chen, Z.; Gao, Q.; Xu, Q.; Cao, H. A role for APX1 gene in lead tolerance in Arabidopsis thaliana. Plant Sci. 2017, 256, 94–102. [Google Scholar] [CrossRef]
- Sultana, A.; Minami, I.; Matsushima, D.; Issak, M.; Nakamura, Y.; Todoriki, S.; Murata, Y. Catalases CAT1 and CAT3 are not key enzymes in alleviating gamma irradiation-induced DNA damage, H2O2 accumulation, or lipid peroxidation in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2013, 77, 1984–1987. [Google Scholar] [CrossRef]
- Giri, M.K.; Singh, N.; Banday, Z.Z.; Singh, V.; Ram, H.; Singh, D.; Chattopadhyay, S.; Nandi, A.K. GBF1 differentially regulates CAT2 and PAD4 transcription to promote pathogen defense in Arabidopsis thaliana. Plant J. 2017, 91, 802–815. [Google Scholar] [CrossRef]
- Murota, K.; Shimura, H.; Takeshita, M.; Masuta, C. Interaction between Cucumber mosaic virus 2b protein and plant catalase induces a specific necrosis in association with proteasome activity. Plant Cell Rep. 2017, 36, 37–47. [Google Scholar] [CrossRef]
- Zhao, Y.; Chan, Z.; Gao, J.; Xing, L.; Cao, M.; Yu, C.; Hu, Y.; You, J.; Shi, H.; Zhu, Y.; et al. ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc. Natl. Acad. Sci. USA 2016, 113, 1949–1954. [Google Scholar] [CrossRef]
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 2009, 324, 1064–1068. [Google Scholar] [CrossRef]
- Park, S.Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.F.; et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Schellingen, K.; Van Der Straeten, D.; Vandenbussche, F.; Prinsen, E.; Remans, T.; Vangronsveld, J.; Cuypers, A. Cadmium-induced ethylene production and responses in Arabidopsis thaliana rely on ACS2 and ACS6 gene expression. BMC Plant Biol. 2014, 14, 214. [Google Scholar] [CrossRef]
- Wang, X.; Meng, H.; Tang, Y.; Zhang, Y.; He, Y.; Zhou, J.; Meng, X. Phosphorylation of an ethylene response factor by MPK3/MPK6 mediates negative feedback regulation of pathogen-induced ethylene biosynthesis in Arabidopsis. J. Genet. Genom. 2022, 49, 810–822. [Google Scholar] [CrossRef]
- Fan, W.; Liao, X.; Tan, Y.; Wang, X.; Schroeder, J.I.; Li, Z. Arabidopsis PLANT U-BOX44 down-regulates osmotic stress signaling by mediating Ca2+-DEPENDENT PROTEIN KINASE4 degradation. Plant Cell 2023, 35, 3870–3888. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, D.; Yang, B.; Liu, W.Z.; Mu, B.; Song, H.; Chen, B.; Li, Y.; Ren, D.; Deng, H.; et al. Arabidopsis CPK6 positively regulates ABA signaling and drought tolerance through phosphorylating ABA-responsive element-binding factors. J. Exp. Bot. 2020, 71, 188–203. [Google Scholar] [CrossRef]
- Chen, C.; Galon, Y.; Rahmati Ishka, M.; Malihi, S.; Shimanovsky, V.; Twito, S.; Rath, A.; Vatamaniuk, O.K.; Miller, G. ASCORBATE PEROXIDASE6 delays the onset of age-dependent leaf senescence. Plant Physiol. 2021, 185, 441–456. [Google Scholar] [CrossRef]
- Touyz, R.M.; Schiffrin, E.L. Reactive oxygen species in vascular biology: Implications in hypertension. Histochem. Cell Biol. 2004, 122, 339–352. [Google Scholar] [CrossRef]
- Sonneveld, C.; Van den Bos, A.L. Effects of nutrient levels on growth and quality of radish (Raphanus sativus L.) grown on different substrates. J. Plant Nutr. 1995, 18, 501–513. [Google Scholar] [CrossRef]
- Thomas, T.H.; Rubatzky, V.E.; Yamaguchi, M. World Vegetables: Principles, Production and Nutritive Values. Plant Growth Regul. 2000, 30, 276. [Google Scholar] [CrossRef]
- Bonasia, A.; Gonnella, M.; Santamaria, P.; Elia, A. Substrate re-use affects yield and quality of seven radish cultivars grown in a closed soilless system. Acta Hortic. 2001, 548, 367–376. [Google Scholar] [CrossRef]
- Sadana, A.; Raju, S.S.; Kumar, P.V.; Sunitha, C. Effect of spacing and seed soaking with GA3 on growth, yield and quality of radish (Raphanus sativus L.). Andhra Pradesh J. Agric. Sci. 2015, 1, 80–84. [Google Scholar]
- Abdel, C.G. Physiological disorders of four radish (Raphanus sativus L. var. sativus) cultivars storage roots grown in controlled cabinets under varying temperatures and irrigation levels. Int. J. Farm. Allied Sci. 2016, 5, 185–198. [Google Scholar] [CrossRef]
- Mühlenbock, P.; Plaszczyca, M.; Plaszczyca, M.; Mellerowicz, E.; Karpinski, S. Lysigenous aerenchyma formation in Arabidopsis is controlled by LESION SIMULATING DISEASE1. Plant Cell 2007, 19, 3819–3830. [Google Scholar] [CrossRef]
- Yamauchi, T.; Yoshioka, M.; Fukazawa, A.; Mori, H.; Nishizawa, N.K.; Tsutsumi, N.; Yoshioka, H.; Nakazono, M. An NADPH Oxidase RBOH Functions in Rice Roots during Lysigenous Aerenchyma Formation under Oxygen-Deficient Conditions. Plant Cell 2017, 29, 775–790. [Google Scholar] [CrossRef]
- Fujimoto, M.; Sazuka, T.; Oda, Y.; Kawahigashi, H.; Wu, J.; Takanashi, H.; Ohnishi, T.; Yoneda, J.I.; Ishimori, M. Transcriptional switch for programmed cell death in pith parenchyma of sorghum stems. Proc. Natl. Acad. Sci. USA 2018, 115, E8783–E8792. [Google Scholar] [CrossRef]
- Luo, X.; Dai, Y.; Zheng, C.; Yang, Y.; Chen, W.; Wang, Q.; Chandrasekaran, U.; Du, J.; Liu, W.; Shu, K. The ABI4-RbohD/VTC2 regulatory module promotes reactive oxygen species (ROS) accumulation to decrease seed germination under salinity stress. New Phytol. 2021, 229, 950–962. [Google Scholar] [CrossRef]
- Hong, C.P.; Wang, M.C.; Yang, C.Y. NADPH Oxidase RbohD and Ethylene Signaling are Involved in Modulating Seedling Growth and Survival Under Submergence Stress. Plants 2020, 9, 471. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Q.; Sun, S.; Yang, J. Upregulated expression of RESPIRATORY BURST OXIDASE HOMOLOG D underlies lesion-mimic phenotype in dark-treated Arabidopsis pheide a oxygenase mutant leaves. Planta 2022, 255, 110. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, H.; Yang, L.; Chen, C.; Shabala, L.; Xiong, M.; Niu, M.; Liu, J.; Zheng, Z.; Zhou, L.; et al. Tissue-specific respiratory burst oxidase homolog-dependent H2O2 signaling to the plasma membrane H+-ATPase confers potassium uptake and salinity tolerance in Cucurbitaceae. J. Exp. Bot. 2019, 70, 5879–5893. [Google Scholar] [CrossRef]
- Xie, Y.; Mao, Y.; Duan, X.; Zhou, H.; Lai, D.; Zhang, Y.; Shen, W. Arabidopsis HY1-Modulated Stomatal Movement: An Integrative Hub Is Functionally Associated with ABI4 in Dehydration-Induced ABA Responsiveness. Plant Physiol. 2016, 170, 1699–1713. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidativestress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Bieker, S.; Potschin, M.; Zentgraf, U. Study of Hydrogen Peroxide as a Senescence-Inducing Signal. Methods Mol. Biol. 2018, 1744, 173–193. [Google Scholar] [CrossRef]
- Vanderauwera, S.; Zimmermann, P.; Rombauts, S.; Vandenabeele, S.; Langebartels, C.; Gruissem, W.; Inzé, D.; Van Breusegem, F. Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol. 2005, 139, 806–821. [Google Scholar] [CrossRef]
- Hu, C.-H.; Wang, P.-Q.; Zhang, P.-P.; Nie, X.-M.; Li, B.-B.; Tai, L.; Liu, W.-T.; Li, W.-Q.; Chen, K.-M. NADPH oxidases: The vital performers and center hubs during plant growth and signaling. Cells 2020, 9, 437. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Z.-H.; Liu, X.; Colmer, T.D.; Shabala, L.; Salih, A.; Zhou, M.; Shabala, S. Revealing the roles of GORK channels and NADPH oxidase in acclimation to hypoxia in Arabidopsis. J. Exp. Bot. 2017, 68, 3191–3204. [Google Scholar] [CrossRef]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Kaya, H.; Hiraoka, G.; Yumoto, F.; Kimura, S.; Kadota, Y.; Hishinuma, H.; Senzaki, E.; Yamagoe, S.; Nagata, K.; et al. Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J. Biol. Chem. 2008, 283, 8885–8892. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef]
- Sharma, Y.; Ishu Shumayla Dixit, S.; Singh, K.; Upadhyay, S.K. Decoding the features and potential roles of respiratory burst oxidase homologs in bread wheat. Curr. Plant Biol. 2023, 37, 100315. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, P.; Liang, W.; Cheng, Q.; Mu, B.; Niu, F.; Yan, J.; Liu, C.; Xie, H.; Kav, N.N.V.; et al. A Rapeseed WRKY Transcription Factor Phosphorylated by CPK Modulates Cell Death and Leaf Senescence by Regulating the Expression of ROS and SA-Synthesis-Related Genes. J. Agric. Food Chem. 2020, 68, 7348–7359. [Google Scholar] [CrossRef] [PubMed]
- Steffens, B.; Geske, T.; Sauter, M. Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol. 2011, 190, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, M.; Mueller, H.M.; Bauer, H.; Peirats-Llobet, M.; Rodriguez, P.L.; Geilfus, C.M.; Carpentier, S.C.; Al Rasheid, K.A.S.; Kollist, H.; Merilo, E.; et al. The role of Arabidopsis ABA receptors from the PYR/PYL/RCAR family in stomatal acclimation and closure signal integration. Nat. Plants 2019, 5, 1002–1011. [Google Scholar] [CrossRef]
- Guo, H.; Ecker, J.R. Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell 2003, 115, 667–677. [Google Scholar] [CrossRef]
- Gagne, J.M.; Smalle, J.; Gingerich, D.J.; Walker, J.M.; Yoo, S.D.; Yanagisawa, S.; Vierstra, R.D. Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proc. Natl. Acad. Sci. USA 2004, 101, 6803–6808. [Google Scholar] [CrossRef]
- Potuschak, T.; Lechner, E.; Parmentier, Y.; Yanagisawa, S.; Grava, S.; Koncz, C.; Genschik, P. EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F-box proteins: EBF1 and EBF2. Cell 2003, 115, 679–689. [Google Scholar] [CrossRef]
- Yanagisawa, S.; Yoo, S.D.; Sheen, J. Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 2003, 425, 521–525. [Google Scholar] [CrossRef]
- An, F.; Zhao, Q.; Ji, Y.; Li, W.; Jiang, Z.; Yu, X.; Zhang, C.; Han, Y.; He, W.; Liu, Y.; et al. Ethylene-induced stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 is mediated by proteasomal degradation of EIN3 binding F-box 1 and 2 that requires EIN2 in Arabidopsis. Plant Cell 2010, 22, 2384–2401. [Google Scholar] [CrossRef]
- Jeong, Y.M.; Kim, N.; Ahn, B.O.; Oh, M.; Chung, W.H.; Chung, H.; Jeong, S.; Lim, K.B.; Hwang, Y.J.; Kim, G.B.; et al. Elucidating the triplicated ancestral genome structure of radish based on chromosome-level comparison with the Brassica genomes. Theor. Appl. Genet. 2016, 129, 1357–1372. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Mengarelli, D.A.; Zanor, M.I. Genome-wide characterization and analysis of the CCT motif family genes in soybean (Glycine max). Planta 2021, 253, 15. [Google Scholar] [CrossRef]
- Kurusu, T.; Nishikawa, D.; Yamazaki, Y.; Gotoh, M.; Nakano, M.; Hamada, H.; Yamanaka, T.; Iida, K.; Nakagawa, Y.; Saji, H.; et al. Plasma membrane protein OsMCA1 is involved in regulation of hypo-osmotic shock-induced Ca2+ influx and modulates generation of reactive oxygen species in cultured rice cells. BMC Plant Biol. 2012, 12, 11. [Google Scholar] [CrossRef]

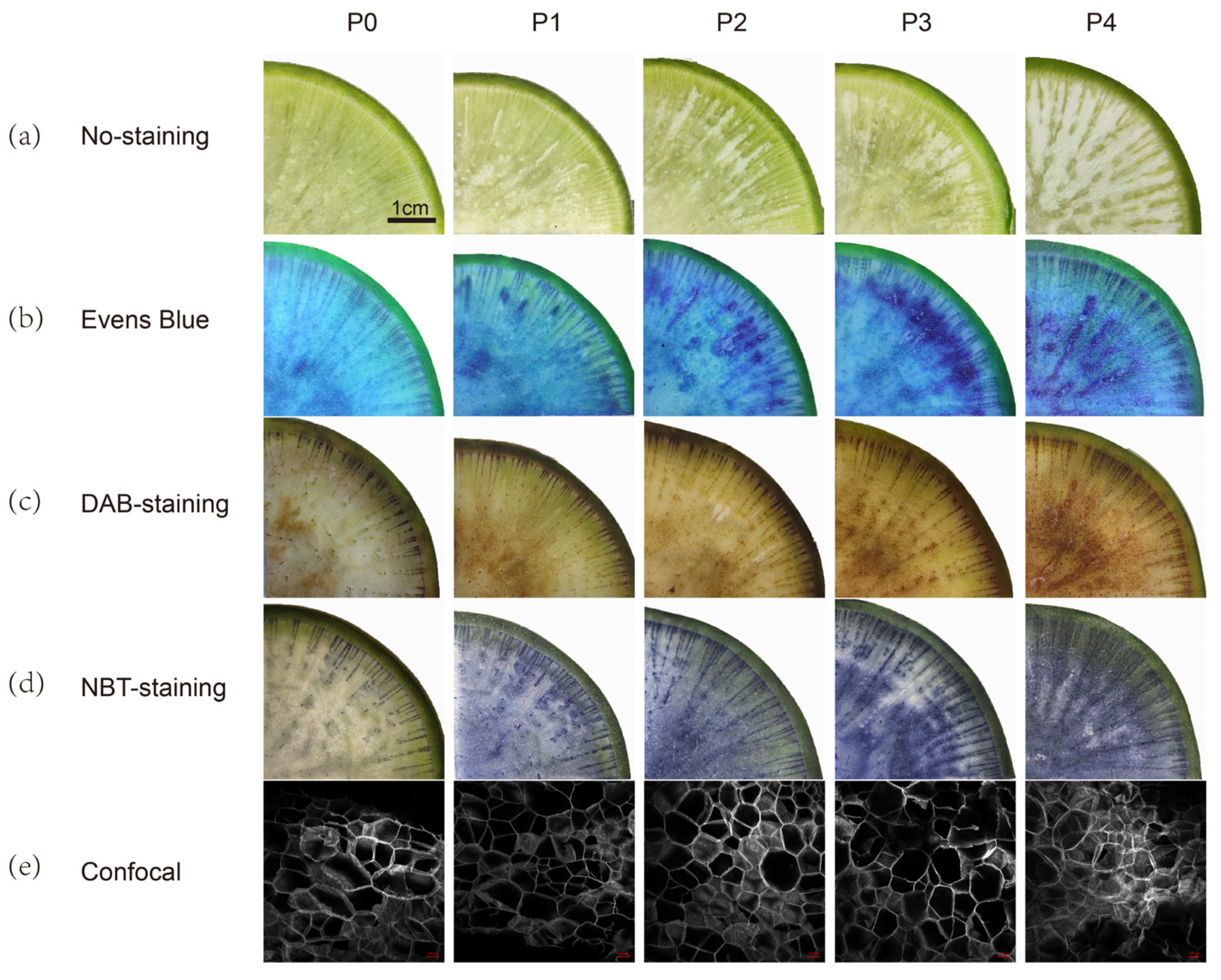


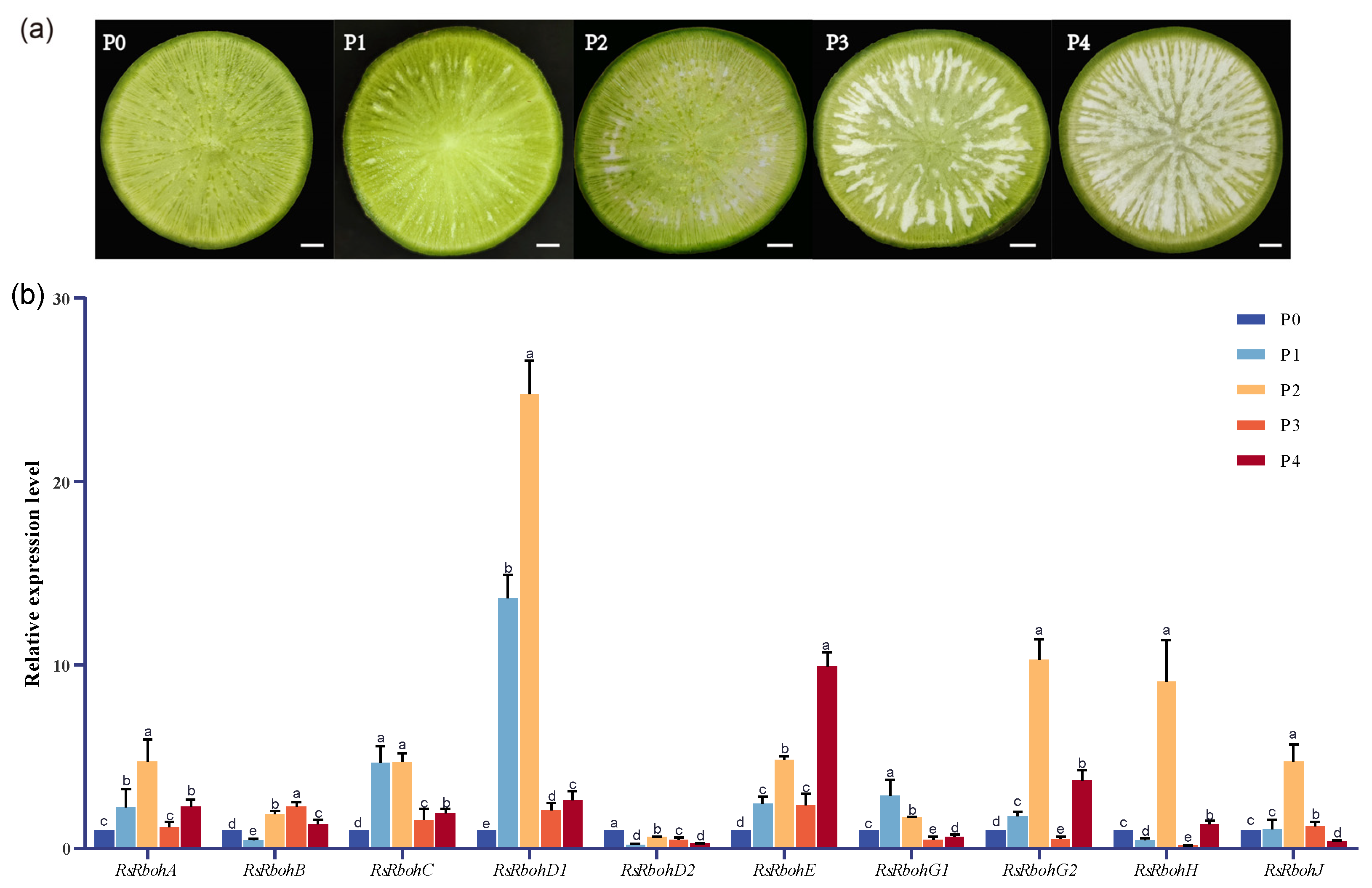
| Pithiness Grade | Parenchyma Tissue Characteristics of Radish Fleshy Roots |
|---|---|
| P0 | no spongy tissue |
| P1 | area of spongy tissue <5% |
| P2 | <20% of tissues are white spongy |
| P3 | >50% of tissues are white spongy |
| P4 | almost completely spongy |
| Gene ID | Gene | Chromosome No. | Protein Length (aa) | MW (kDa) | PI | Subcellular Localization |
|---|---|---|---|---|---|---|
| Rs392300 | RsRbohA | R7 | 906 | 103.1579 | 9.95 | Plasma membrane |
| Rs256300 | RsRbohB | R5 | 844 | 96.3529 | 8.7 | Plasma membrane |
| Rs132220 | RsRbohC | R3 | 917 | 103.6033 | 9.88 | Plasma membrane |
| Rs172640 | RsRbohD1 | R4 | 923 | 103.7353 | 9.63 | Plasma membrane |
| Rs315520 | RsRbohD2 | R6 | 923 | 104.2148 | 9.56 | Plasma membrane |
| Rs014600 | RsRbohE | R1 | 996 | 112.2343 | 9.28 | Plasma membrane and nucleus |
| Rs157980 | RsRbohG1 | R4 | 805 | 92.3545 | 9.76 | Plasma membrane |
| Rs336320 | RsRbohG2 | R6 | 611 | 69.9732 | 6.87 | Nucleus; cytoplasm and vacuole |
| Rs079080 | RsRbohH | R2 | 883 | 100.2423 | 9.56 | Plasma membrane |
| Rs344270 | RsRbohJ | R6 | 812 | 91.9631 | 9.41 | Plasma membrane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Q.; Wang, C.; Fan, W.; Li, S.; Zhang, H.; Huang, Z.; Liu, X.; Ma, Z.; Wang, Y.; Zhang, B. RsRbohD1 Plays a Significant Role in ROS Production during Radish Pithiness Development. Plants 2024, 13, 1386. https://doi.org/10.3390/plants13101386
Gong Q, Wang C, Fan W, Li S, Zhang H, Huang Z, Liu X, Ma Z, Wang Y, Zhang B. RsRbohD1 Plays a Significant Role in ROS Production during Radish Pithiness Development. Plants. 2024; 13(10):1386. https://doi.org/10.3390/plants13101386
Chicago/Turabian StyleGong, Qiong, Chaonan Wang, Weiqiang Fan, Shuiling Li, Hong Zhang, Zhiyin Huang, Xiaohui Liu, Ziyun Ma, Yong Wang, and Bin Zhang. 2024. "RsRbohD1 Plays a Significant Role in ROS Production during Radish Pithiness Development" Plants 13, no. 10: 1386. https://doi.org/10.3390/plants13101386
APA StyleGong, Q., Wang, C., Fan, W., Li, S., Zhang, H., Huang, Z., Liu, X., Ma, Z., Wang, Y., & Zhang, B. (2024). RsRbohD1 Plays a Significant Role in ROS Production during Radish Pithiness Development. Plants, 13(10), 1386. https://doi.org/10.3390/plants13101386







