The Influence of Zinc Oxide Nanoparticles and Salt Stress on the Morphological and Some Biochemical Characteristics of Solanum lycopersicum L. Plants
Abstract
:1. Introduction
2. Results
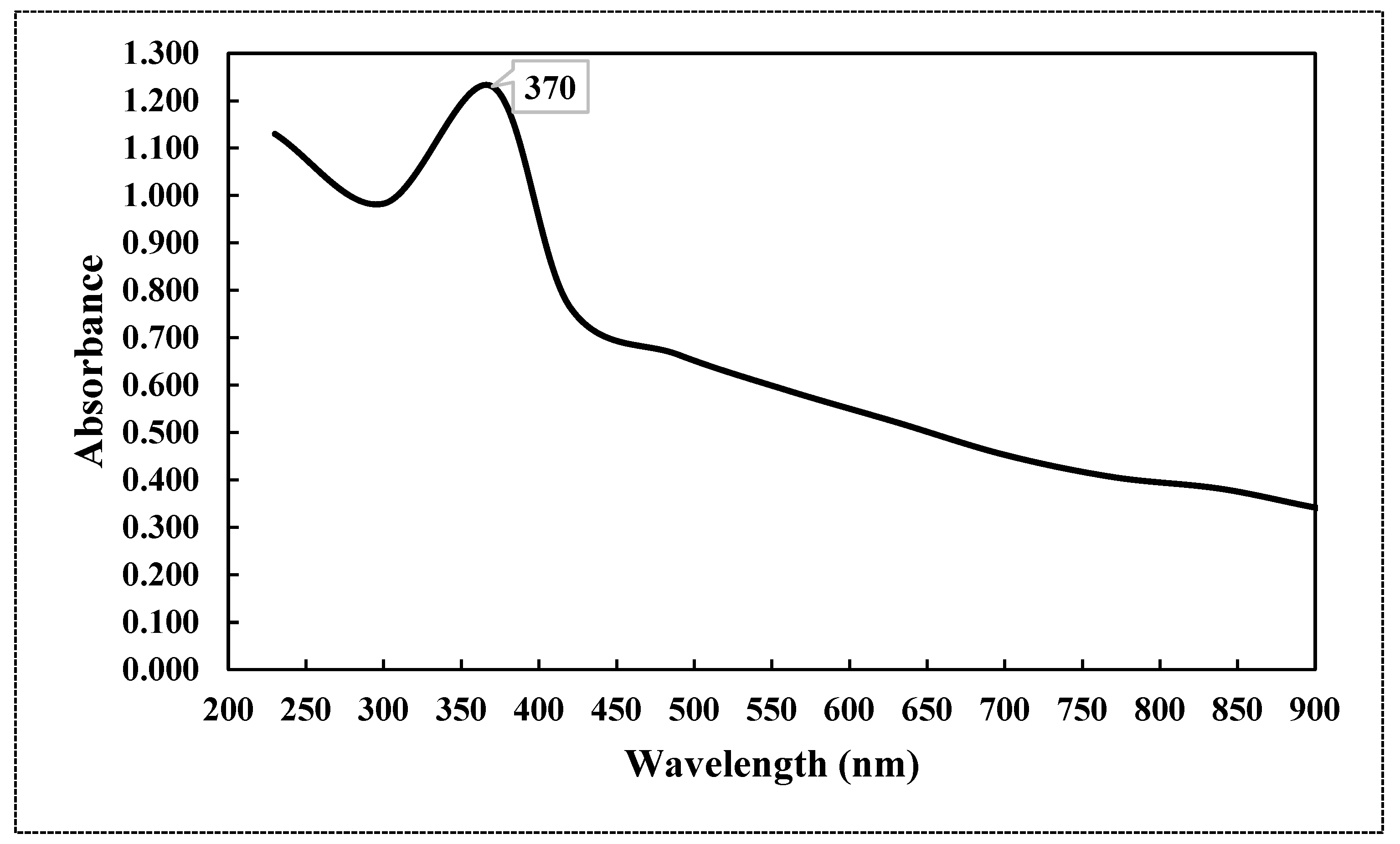
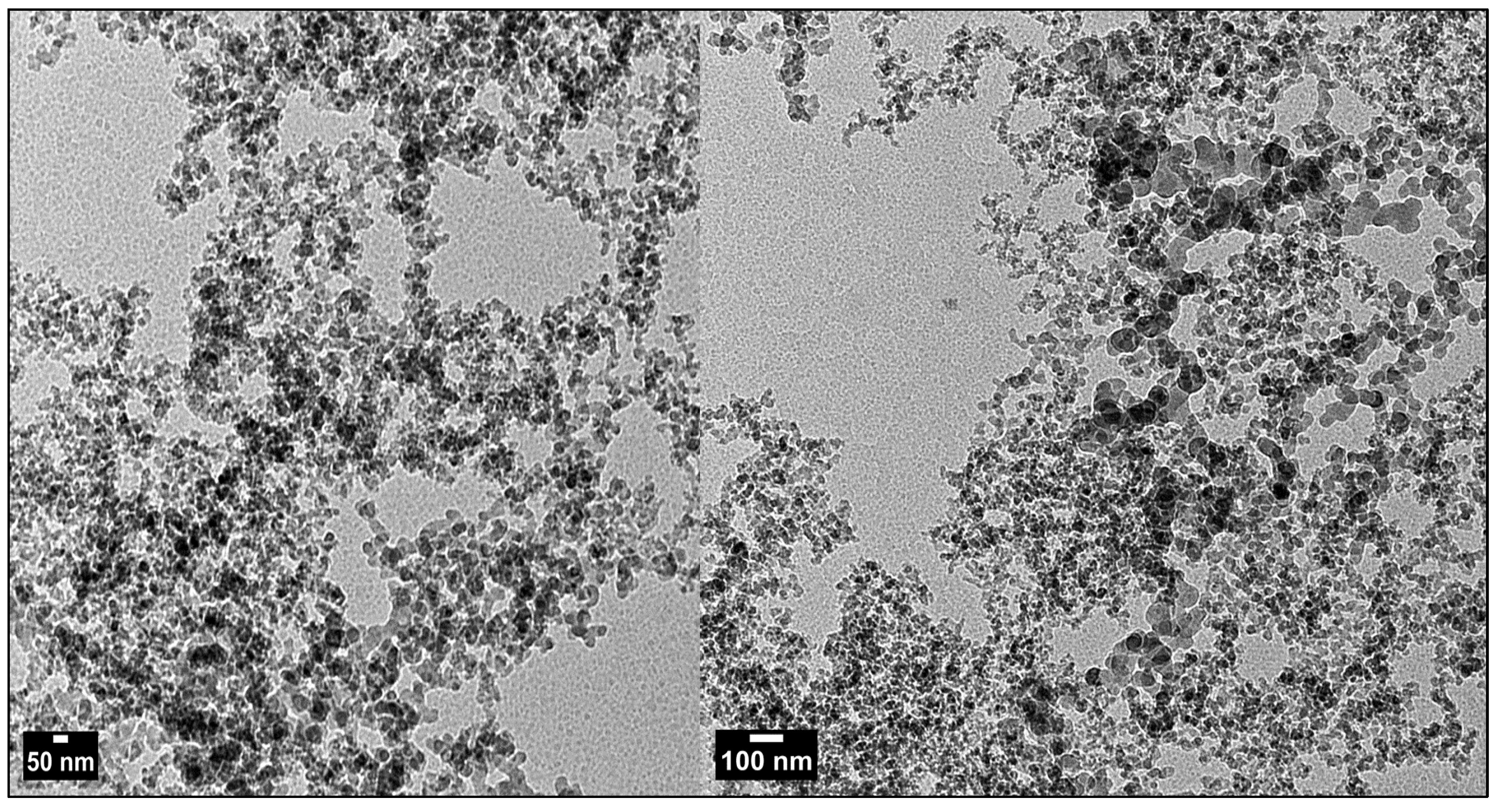
2.1. Characterization of Chemically Synthesized ZnO-NPs
2.2. Assessment of the Elements, Growth, and Chlorophyll Attributes in Different Treatments from Tomato
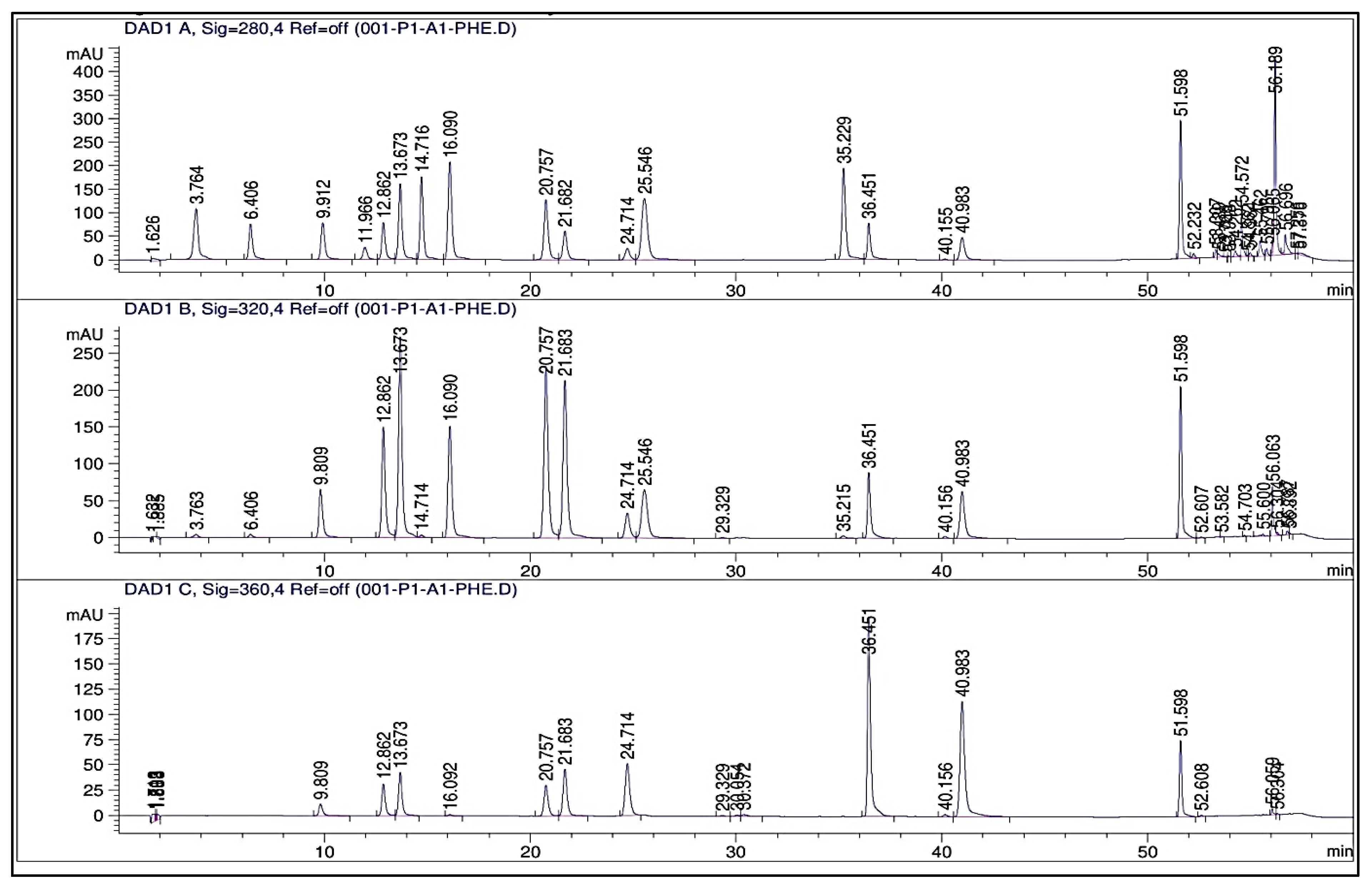
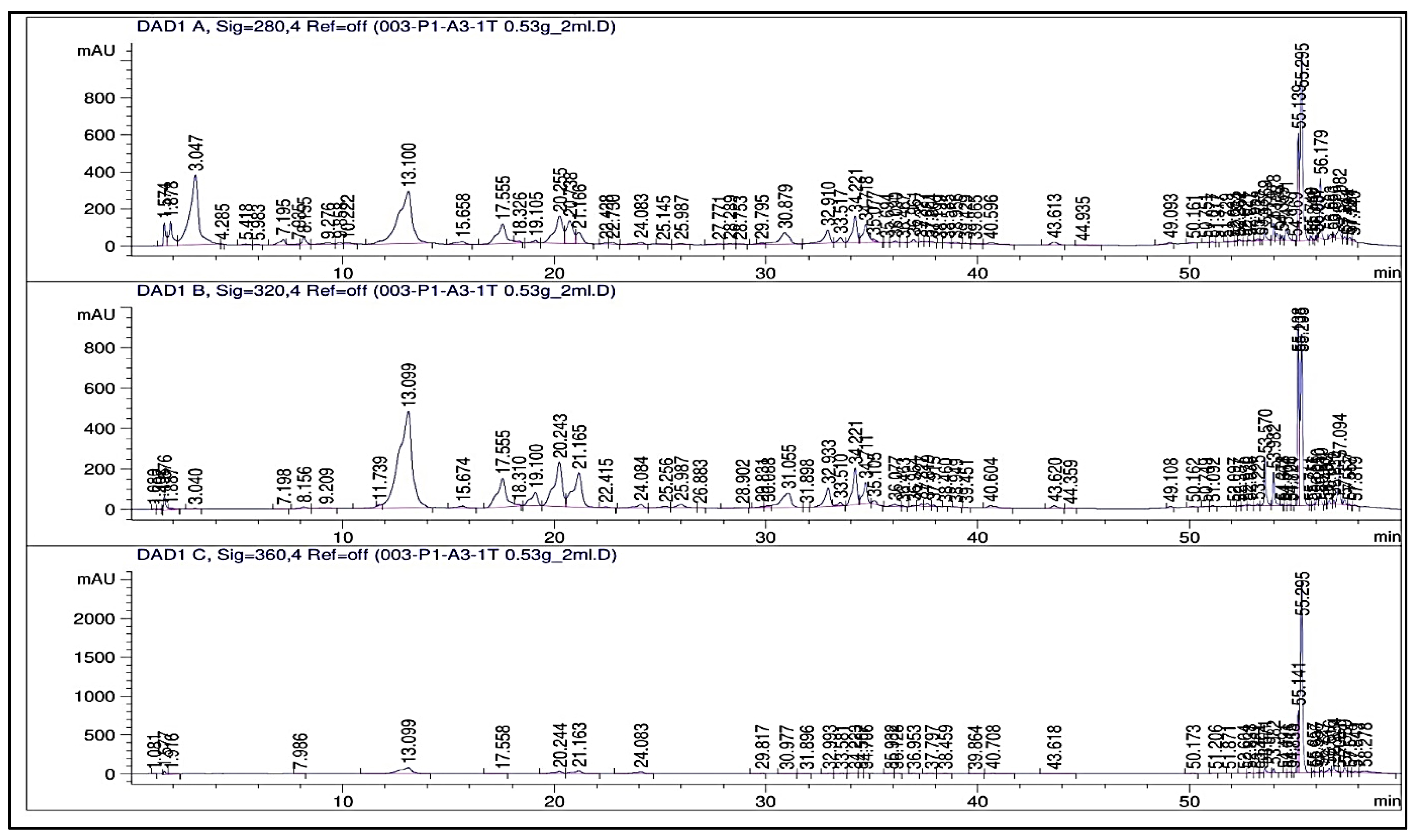
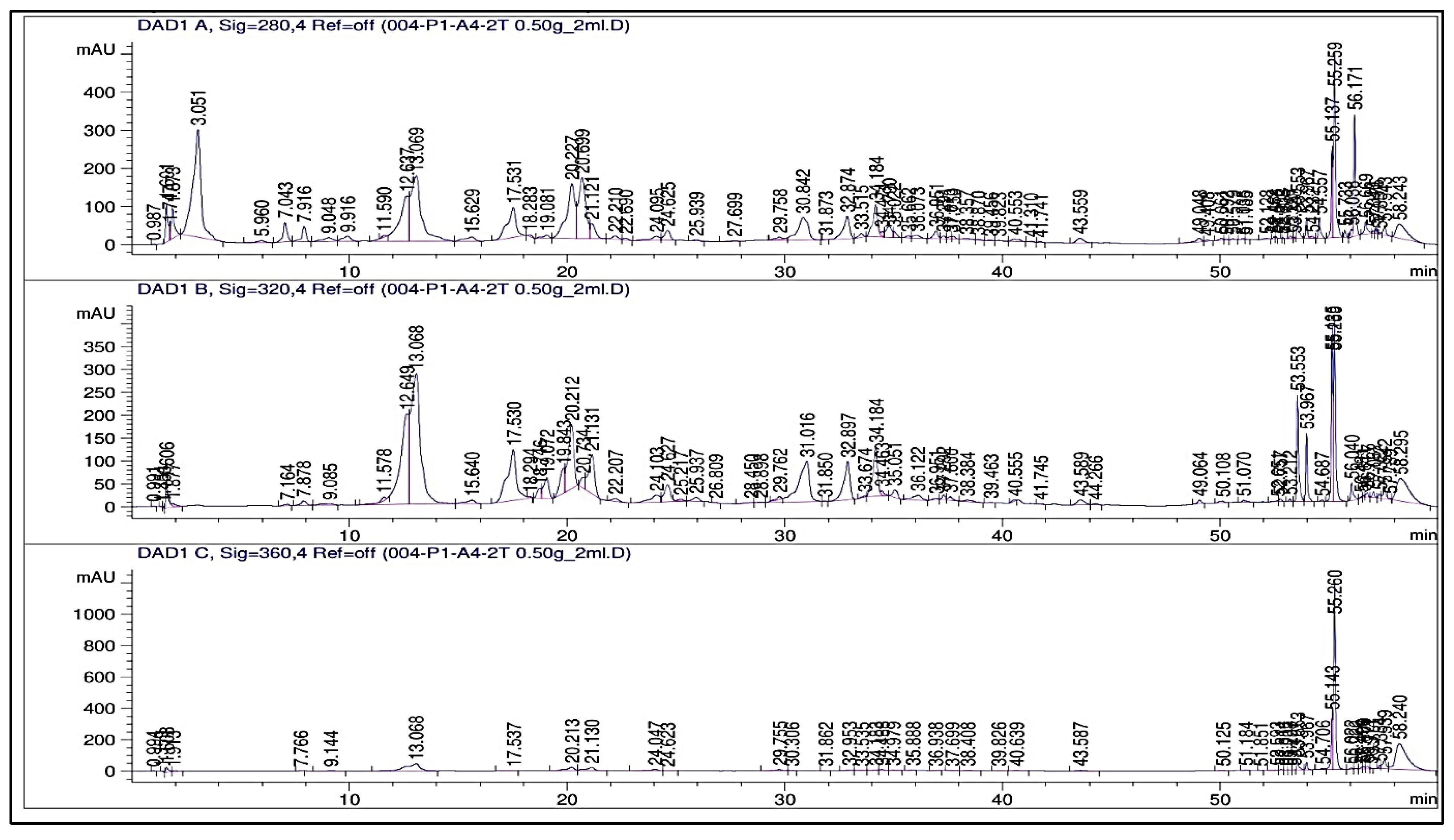
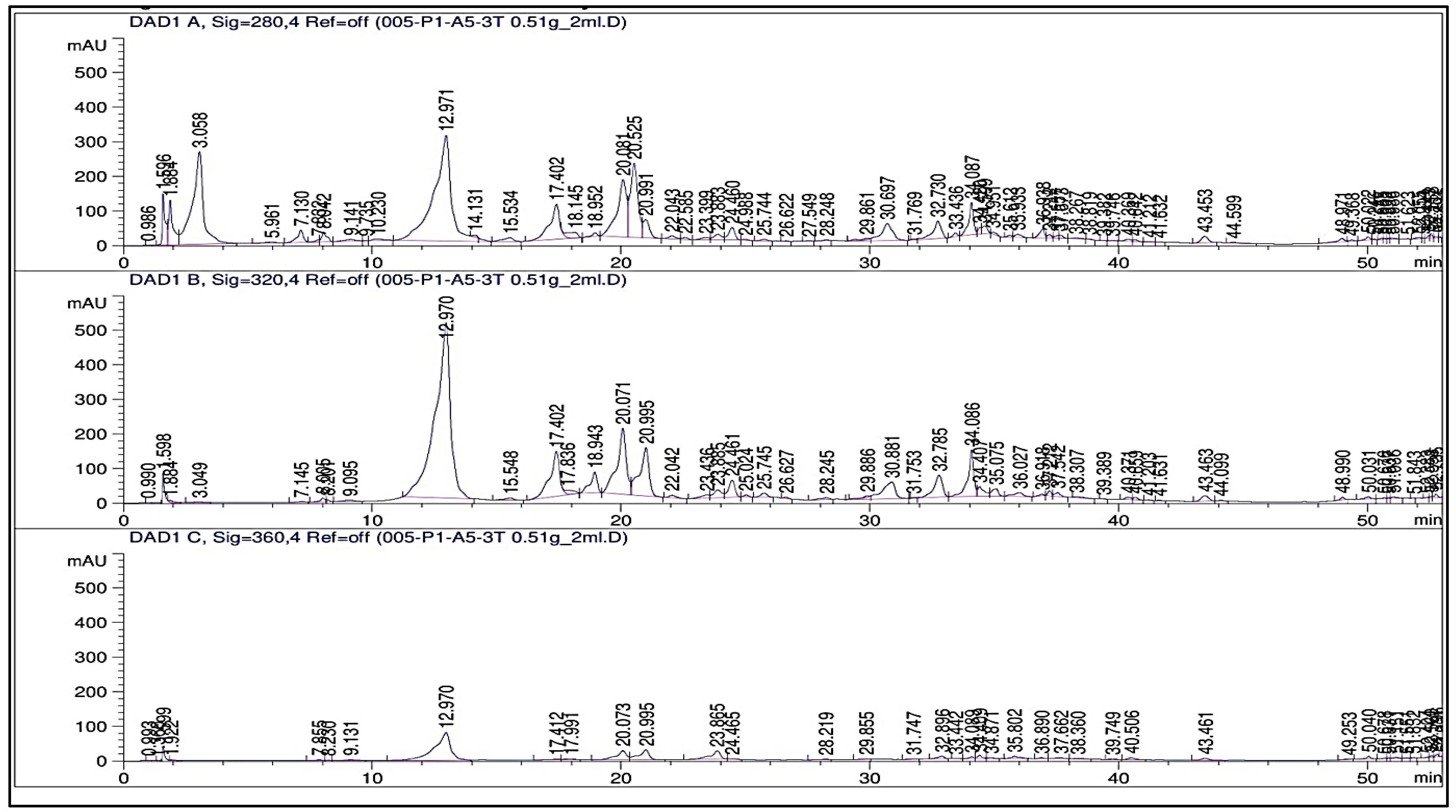
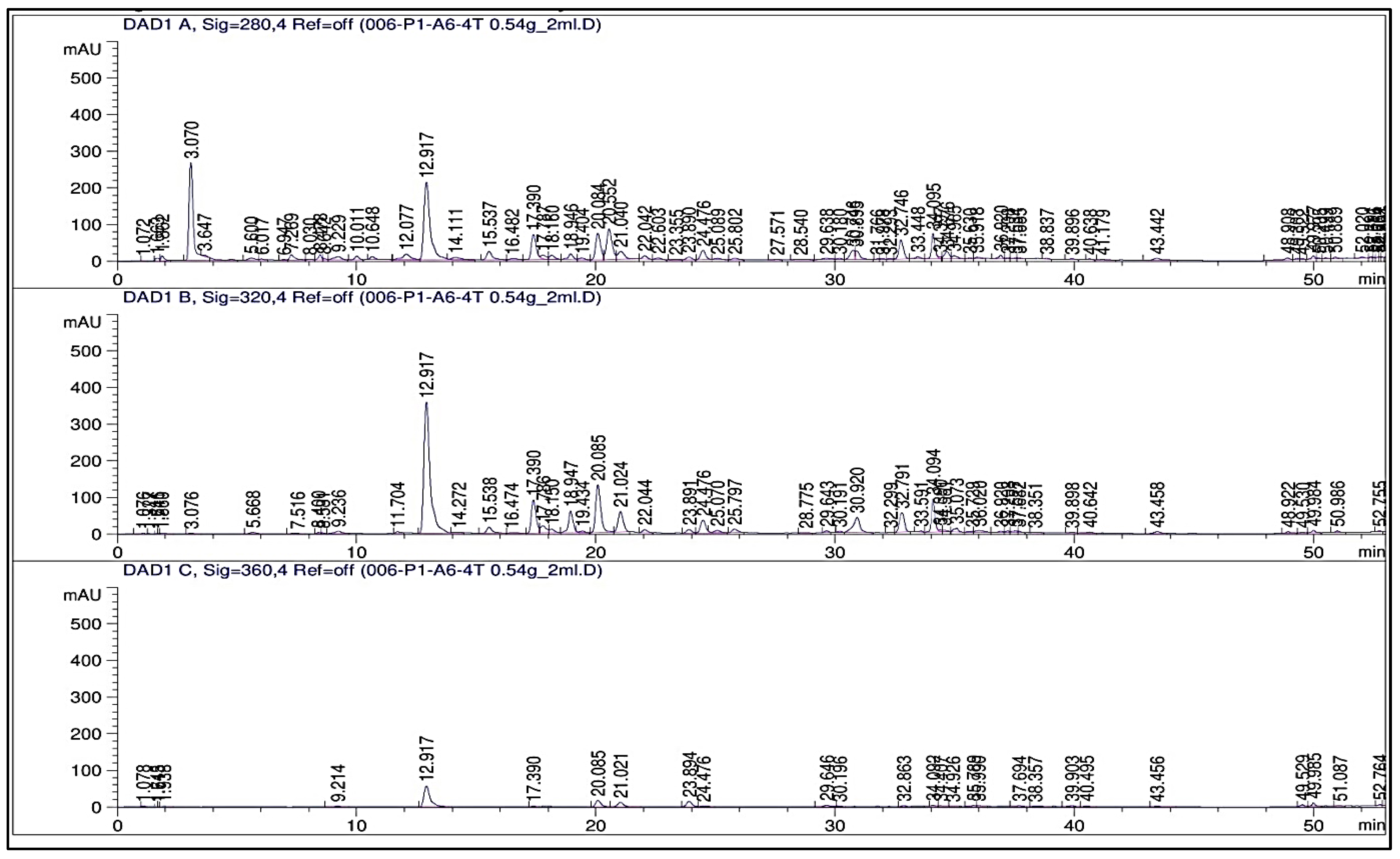
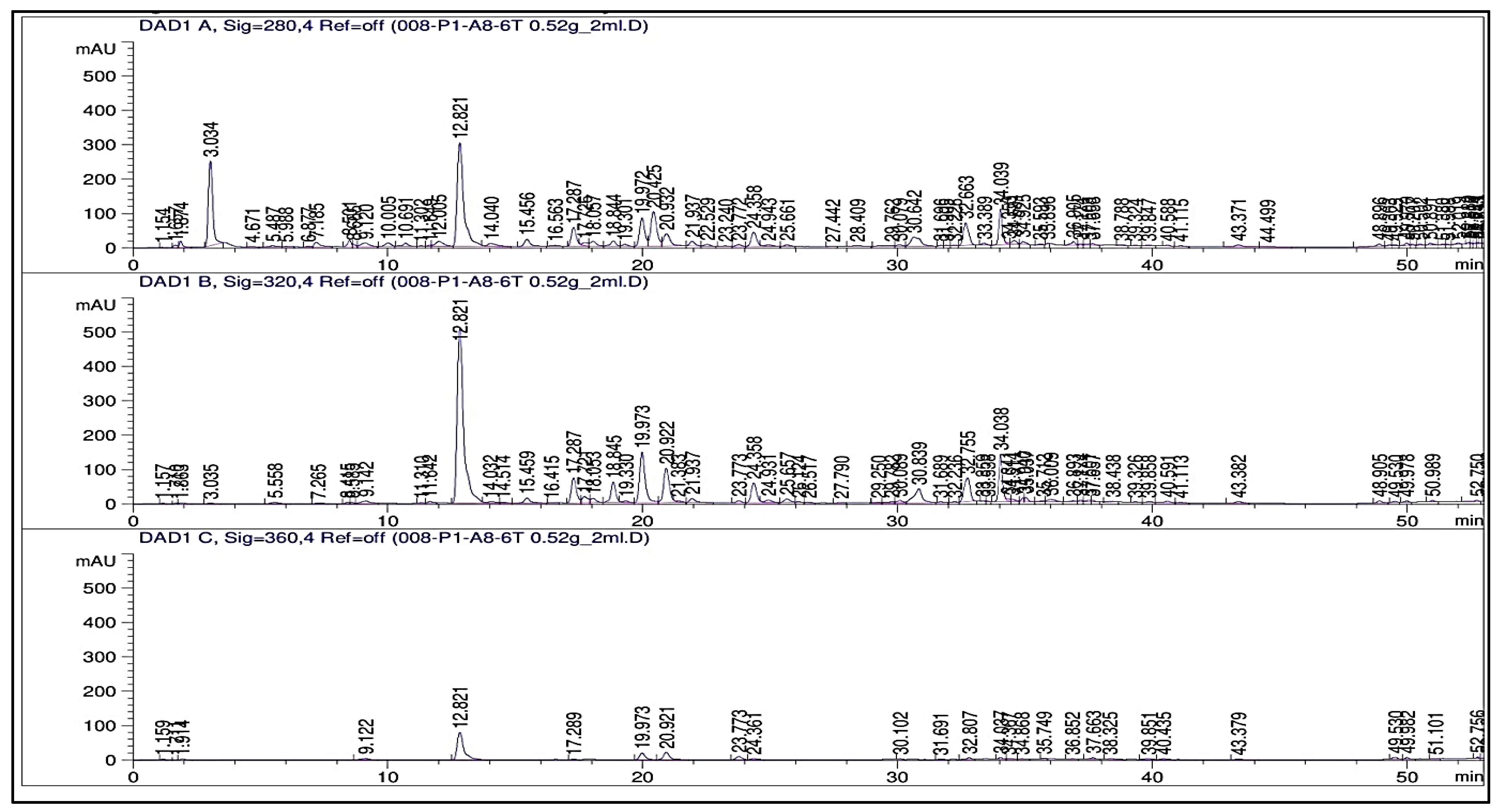
2.3. Phenolic Profile
2.4. Determination of Different Biochemical and Stress Markers in the Leaves from Different Treatments of Tomato
3. Discussion
3.1. Characterization of Chemically Synthesized ZnO-NPs
3.2. Assessment of the Elements, Growth, and Chlorophyll Attributes in Different Treatments from Tomato
3.3. Phenolic Profile (HPLC, TPCs, and TFCs)
3.4. Determination of Some Biochemical and Stress Markers in the Leaves from Different Treatments of Tomato
4. Materials and Methods
4.1. Design, Preparations, and Equipment for Tomato Planting
4.2. Determining the Moisture Content of the Potting Soil
4.3. Determining the Amount of Air-Dried Soil to Place in Each Pot
4.4. Determination of the Macro and Microelements in the Leaves of Tomato Plants
4.5. Determination of Chlorophyll and Growth Attributes
4.6. Determination of Proline and Protein Contents
4.7. Determination of Total Free Amino Acids
4.8. Determination of Total Hydrolyzable Sugars
4.9. Determination of Lipid Peroxidation
4.10. Determination of Hydrogen Peroxide (H2O2)
4.11. Determination of Total Flavonoid Compounds (TFCs)
4.12. Determination of Total Phenolic Compounds (TPCs)
4.13. Determination of Phenolic Compounds; High-Performance Liquid Chromatography (HPLC) Analysis
4.14. Chemical Synthesis of Zinc Oxide Nanoparticles
4.15. Characterization of Chemically Synthesized Zinc Oxide Nanoparticles
4.16. Statistical Analyzsis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | One-way analysis of variance |
| Cu/Cu2+ | Copper/copper ion |
| DAS | Days after sowing |
| DAT | Day after transplanting |
| DLS | Dynamic light scattering |
| DW | Distilled water |
| EC | Electric conductivity |
| EDAX | Energy-dispersive X-ray spectroscopy |
| FTIR | Fourier transform infrared spectroscopy |
| FW | Fresh weight |
| GAE | Gallic acid |
| H2O2 | Hydrogen peroxide |
| HPLC | High-performance liquid chromatography |
| JCPDS | Joint committee on powder diffraction standards |
| K/K+ | Potassium/potassium ion |
| L | Length |
| LDV | Laser doppler velocimetry |
| LSD | Least significant difference |
| MDA | Malondialdehyde |
| Mg/Mg2+ | Magnesium/magnesium ion |
| Milli Q | Ultra-pure water |
| Mn/Mn+2 | Manganese/manganese ion |
| N and M | Normal and molar concentrations |
| Na/Na+ | Sodium/sodium ion |
| OH− | Hydroxyl ion |
| PC | Pot capacity |
| PMSF | Phenyl methane sulfonyl fluoride |
| QE | Quercetin |
| ROS | Reactive oxygen species |
| SPAD | Soil plant analysis development |
| STEM | Scanning electron microscopy |
| TDR | Time domain reflectometer |
| TEM | Transmission electron microscopy |
| TFCs | Total flavonoid content |
| TPCs | Total phenolic content |
| W | Width |
| WASP | Web Agri Stat Package |
| XRD | X-ray diffraction |
| ZarxJos | Tomato drought-tolerant cv. Zarina-like rootstocks |
| Zn/Zn2+ | Zinc/zinc ion |
| ZnO | Zinc oxide |
| ZnO-NPs | Zinc oxide nanoparticles |
Appendix A



References
- Chabi, I.B.; Zannou, O.; Dedehou, E.S.C.A.; Ayegnon, B.P.; Oscar Odouaro, O.B.; Maqsood, S.; Galanakis, C.M.; Pierre Polycarpe Kayodé, A. Tomato Pomace as a Source of Valuable Functional Ingredients for Improving Physicochemical and Sensory Properties and Extending the Shelf Life of Foods: A Review. Heliyon 2024, 10, e25261. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and Lycopene and Multiple Health Outcomes: Umbrella Review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.M.; Romdhane, W.B.; Tarroum, M.; Al-Dakhil, M.; Al-Doss, A.; Alsadon, A.A.; Hassairi, A. Analysis of Salinity Tolerance in Tomato Introgression Lines Based on Morpho-Physiological and Molecular Traits. Plants 2021, 10, 2594. [Google Scholar] [CrossRef] [PubMed]
- De Sio, F.; Rapacciuolo, M.; De Giorgi, A.; Sandei, L.; Giuliano, B.; Tallarita, A.; Golubkina, N.; Sekara, A.; Stoleru, V.; Cuciniello, A.; et al. Industrial Processing Affects Product Yield and Quality of Diced Tomato. Agriculture 2021, 11, 230. [Google Scholar] [CrossRef]
- Inculet, C.-S.; Mihalache, G.; Sellitto, V.M.; Hlihor, R.-M.; Stoleru, V. The Effects of a Microorganisms-Based Commercial Product on the Morphological, Biochemical and Yield of Tomato Plants under Two Different Water Regimes. Microorganisms 2019, 7, 706. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Kumar, R. Soil Salinity: A Serious Environmental Issue and Plant Growth Promoting Bacteria as One of the Tools for Its Alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Ahmed, M.; Tóth, Z.; Decsi, K. The Impact of Salinity on Crop Yields and the Confrontational Behavior of Transcriptional Regulators, Nanoparticles, and Antioxidant Defensive Mechanisms under Stressful Conditions: A Review. Int. J. Mol. Sci. 2024, 25, 2654. [Google Scholar] [CrossRef] [PubMed]
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water Scarcity in Agriculture: An Overview of Causes, Impacts and Approaches for Reducing the Risks. Heliyon 2023, 9, e18507. [Google Scholar] [CrossRef] [PubMed]
- Nabati, J.; Kafi, M.; Nezami, A.; Moghaddam, P.R.; Ali, M.; Mehrjerdi, M.Z. Effect of Salinity on Biomass Production and Activities of Some Key Enzymatic Antioxidants in Kochia (Kochia scoparia). Pak. J. Bot 2011, 43, 539–548. [Google Scholar]
- Tester, M.; Langridge, P. Breeding Technologies to Increase Crop Production in a Changing World. Science 2010, 327, 818–822. [Google Scholar] [CrossRef]
- Norbert, M. Kecskeméti 549 Paradicsom Termesztése. Kertészkedj, 2023. [Google Scholar]
- Ahmed, M.; Decsi, K.; Tóth, Z. Different Tactics of Synthesized Zinc Oxide Nanoparticles, Homeostasis Ions, and Phytohormones as Regulators and Adaptatively Parameters to Alleviate the Adverse Effects of Salinity Stress on Plants. Life 2023, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Bhat, J.A.; Chen, C.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.; Yu, F. Zinc Oxide Nanoparticles (ZnO-NPs) Induce Salt Tolerance by Improving the Antioxidant System and Photosynthetic Machinery in Tomato. Plant Physiol. Biochem. 2021, 161, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Gaafar, R.; Diab, R.; Halawa, M.; Elshanshory, A.; El-Shaer, A.; Hamouda, M. Role of Zinc Oxide Nanoparticles in Ameliorating Salt Tolerance in Soybean. Egypt. J. Bot. 2020, 60, 733–747. [Google Scholar] [CrossRef]
- Natasha, N.; Shahid, M.; Bibi, I.; Iqbal, J.; Khalid, S.; Murtaza, B.; Bakhat, H.F.; Farooq, A.B.U.; Amjad, M.; Hammad, H.M.; et al. Zinc in Soil-Plant-Human System: A Data-Analysis Review. Sci. Total Environ. 2022, 808, 152024. [Google Scholar] [CrossRef] [PubMed]
- Cuajungco, M.P.; Ramirez, M.S.; Tolmasky, M.E. Zinc: Multidimensional Effects on Living Organisms. Biomedicines 2021, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Malik, A.; Alwarthan, A.; Shaik, M.R. The Enormity of the Zinc Deficiency Problem and Available Solutions; an Overview. Arab. J. Chem. 2022, 15, 103668. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO Nanostructured Materials and Their Potential Applications: Progress, Challenges and Perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterial 2022, 12, 3066. [Google Scholar] [CrossRef] [PubMed]
- Perumal, V.; Priyanka, N.; Kathiravan, M.; Irulappan, G.; Indiraarulselvi, P.; Geetha, N.; Koramutla, M.; Bhattacharya, R.; Tiwari, M.; Sharma, N.; et al. Enhanced Plant Growth Promoting Role of Phycomolecules Coated Zinc Oxide Nanoparticles with P Supplementation in Cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2016, 110, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Smaoui, S.; Chérif, I.; Ben Hlima, H.; Khan, M.U.; Rebezov, M.; Thiruvengadam, M.; Sarkar, T.; Shariati, M.A.; Lorenzo, J.M. Zinc Oxide Nanoparticles in Meat Packaging: A Systematic Review of Recent Literature. Food Packag. Shelf Life 2023, 36, 101045. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Li, R.; Serov, D.A.; Burmistrov, D.E.; Baimler, I.V.; Baryshev, A.S.; Simakin, A.V.; Uvarov, O.V.; Astashev, M.E.; Nefedova, N.B.; et al. Fluoroplast Doped by Ag2O Nanoparticles as New Repairing Non-Cytotoxic Antibacterial Coating for Meat Industry. Int. J. Mol. Sci. 2023, 24, 869. [Google Scholar] [CrossRef] [PubMed]
- Burmistrov, D.E.; Serov, D.A.; Simakin, A.V.; Baimler, I.V.; Uvarov, O.V.; Gudkov, S.V. A Polytetrafluoroethylene (PTFE) and Nano-Al2O3 Based Composite Coating with a Bacteriostatic Effect against E. coli and Low Cytotoxicity. Polymers 2022, 14, 4764. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Sarimov, R.M.; Astashev, M.E.; Pishchalnikov, R.Y.; Yanykin, D.V.; Simakin, A.V.; Shkirin, A.V.; Serov, D.A.; Konchekov, E.M.; Ogly, G.N.G.; et al. Modern Physical Methods and Technologies in Agriculture. Phys. Uspekhi 2024, 67, 194–210. [Google Scholar] [CrossRef]
- Serov, D.A.; Burmistrov, D.E.; Simakin, A.V.; Astashev, M.E.; Uvarov, O.V.; Tolordava, E.R.; Semenova, A.A.; Lisitsyn, A.B.; Gudkov, S.V. Composite Coating for the Food Industry Based on Fluoroplast and ZnO-NPs: Physical and Chemical Properties, Antibacterial and Antibiofilm Activity, Cytotoxicity. Nanomaterials 2022, 12, 4158. [Google Scholar] [CrossRef] [PubMed]
- Serov, D.A.; Baimler, I.V.; Burmistrov, D.E.; Baryshev, A.S.; Yanykin, D.V.; Astashev, M.E.; Simakin, A.V.; Gudkov, S.V. The Development of New Nanocomposite Polytetrafluoroethylene/Fe2O3 NPs to Prevent Bacterial Contamination in Meat Industry. Polymers 2022, 14, 4880. [Google Scholar] [CrossRef] [PubMed]
- Rasha, E.; Monerah, A.; Manal, A.; Rehab, A.; Mohammed, D.; Doaa, E. Biosynthesis of Zinc Oxide Nanoparticles from Acacia nilotica (L.) Extract to Overcome Carbapenem-Resistant Klebsiella Pneumoniae. Molecules 2021, 26, 1919. [Google Scholar] [CrossRef] [PubMed]
- Zak, A.K.; Razali, R.; Majid, W.A.; Darroudi, M. Synthesis and Characterization of a Narrow Size Distribution of Zinc Oxide Nanoparticles. Int. J. Nanomed. 2011, 6, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Zak, A.K.; Abrishami, M.E.; Majid, W.H.A.; Yousefi, R.; Hosseini, S.M. Effects of Annealing Temperature on Some Structural and Optical Properties of ZnO Nanoparticles Prepared by a Modified Sol–Gel Combustion Method. Ceram. Int. 2011, 37, 393–398. [Google Scholar] [CrossRef]
- Zak, A.K.; Majid, W.H.A.; Darroudi, M.; Yousefi, R. Synthesis and Characterization of ZnO Nanoparticles Prepared in Gelatin Media. Mater. Lett. 2011, 65, 70–73. [Google Scholar] [CrossRef]
- Yedurkar, S.; Maurya, C.; Mahanwar, P. Biosynthesis of Zinc Oxide Nanoparticles Using Ixora Coccinea Leaf Extract—A Green Approach. Open J. Synth. Theory Appl. 2016, 5, 1–14. [Google Scholar] [CrossRef]
- Jayarambabu, N. Germination and Growth Characteristics of Mungbean Seeds (Vigna radiata L.) Affected by Synthesized Zinc Oxide Nanoparticles. Int. J. Curr. Eng. Technol. 2014, 4, 5. [Google Scholar]
- Zafar, S.; Hasnain, Z.; Aslam, N.; Mumtaz, S.; Jaafar, H.Z.; Wahab, P.E.M.; Qayum, M.; Ormenisan, A.N. Impact of Zn Nanoparticles Synthesized via Green and Chemical Approach on Okra (Abelmoschus esculentus L.) Growth under Salt Stress. Sustainability 2021, 13, 3694. [Google Scholar] [CrossRef]
- Mahalakshmi, H. Vijaya In Vitro Biocompatibility and Antimicrobial Activities of Zinc Oxide Nanoparticles (ZnO NPs) Prepared by Chemical and Green Synthetic Route— A Comparative Study. BioNanoScience 2020, 10, 112–121. [Google Scholar] [CrossRef]
- Modena, M.M.; Rühle, B.; Burg, T.P.; Wuttke, S. Nanoparticle Characterization: What to Measure? Adv. Mater. 2019, 31, 1901556. [Google Scholar] [CrossRef]
- Salih, A.M.; Al-Qurainy, F.; Khan, S.; Tarroum, M.; Nadeem, M.; Shaikhaldein, H.O.; Gaafar, A.-R.Z.; Alfarraj, N.S. Biosynthesis of Zinc Oxide Nanoparticles Using Phoenix dactylifera and Their Effect on Biomass and Phytochemical Compounds in Juniperus procera. Sci. Rep. 2021, 11, 19136. [Google Scholar] [CrossRef] [PubMed]
- Antony Lilly Grace, M.; Veerabhadra Rao, K.; Anuradha, K.; Judith Jayarani, A.; Arun kumar, A.; Rathika, A. X-Ray Analysis and Size-Strain Plot of Zinc Oxide Nanoparticles by Williamson-Hall. Mater. Today Proc. 2023, 92, 1334–1339. [Google Scholar] [CrossRef]
- García, A.B.; Cuesta, A.; Montes-Morán, M.A.; Martínez-Alonso, A.; Tascón, J.M.D. Zeta Potential as a Tool to Characterize Plasma Oxidation of Carbon Fibers. J. Colloid Interface Sci. 1997, 192, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Shivani; Bhojiya, A.A.; Singh, H.; Daima, H.K.; Singh, M.; Mohanty, S.R.; Stephen, B.J.; Singh, A. Microbial Fabrication of Zinc Oxide Nanoparticles and Evaluation of Their Antimicrobial and Photocatalytic Properties. Front. Chem. 2020, 8, 778. [Google Scholar] [CrossRef] [PubMed]
- Shebl, A.; Hassan, A.A.; Salama, D.M.; Abd El-Aziz, M.E.; Abd Elwahed, M.S.A. Green Synthesis of Nanofertilizers and Their Application as a Foliar for Cucurbita pepo L. J. Nanomater. 2019, 2019, 3476347. [Google Scholar] [CrossRef]
- Bihmidine, S.; Hunter, C.T.; Johns, C.E.; Koch, K.E.; Braun, D.M. Regulation of Assimilate Import into Sink Organs: Update on Molecular Drivers of Sink Strength. Front. Plant Sci. 2013, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Kinraide, T.B. Interactions among Ca2+, Na+ and K+ in Salinity Toxicity: Quantitative Resolution of Multiple Toxic and Ameliorative Effects. J. Exp. Bot. 1999, 50, 1495–1505. [Google Scholar] [CrossRef]
- Lefoulon, C. The Bare Necessities of Plant K+ Channel Regulation. Plant Physiol. 2021, 187, 2092–2109. [Google Scholar] [CrossRef] [PubMed]
- Pardo, J.M.; Rubio, F. Na+ and K+ Transporters in Plant Signaling. In Transporters and Pumps in Plant Signaling; Geisler, M., Venema, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 65–98. ISBN 978-3-642-14369-4. [Google Scholar]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in Plants: Growth Regulation, Signaling, and Environmental Stress Tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Rozov, A.; Khusainov, I.; El Omari, K.; Duman, R.; Mykhaylyk, V.; Yusupov, M.; Westhof, E.; Wagner, A.; Yusupova, G. Importance of Potassium Ions for Ribosome Structure and Function Revealed by Long-Wavelength X-Ray Diffraction. Nat. Commun. 2019, 10, 2519. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J. Potassium Control of Plant Functions: Ecological and Agricultural Implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Ali, B.; Saleem, M.H.; Ali, S.; Shahid, M.; Sagir, M.; Tahir, M.B.; Qureshi, K.A.; Jaremko, M.; Selim, S.; Hussain, A.; et al. Mitigation of Salinity Stress in Barley Genotypes with Variable Salt Tolerance by Application of Zinc Oxide Nanoparticles. Front. Plant Sci. 2022, 13, 973782. [Google Scholar] [CrossRef]
- Cohu, C.M.; Abdel-Ghany, S.E.; Gogolin Reynolds, K.A.; Onofrio, A.M.; Bodecker, J.R.; Kimbrel, J.A.; Niyogi, K.K.; Pilon, M. Copper Delivery by the Copper Chaperone for Chloroplast and Cytosolic Copper/Zinc-Superoxide Dismutases: Regulation and Unexpected Phenotypes in an Arabidopsis Mutant. Mol. Plant 2009, 2, 1336–1350. [Google Scholar] [CrossRef] [PubMed]
- Uresti-Porras, J.-G.; Cabrera-De-La Fuente, M.; Benavides-Mendoza, A.; Olivares-Sáenz, E.; Cabrera, R.I.; Juárez-Maldonado, A. Effect of Graft and Nano ZnO on Nutraceutical and Mineral Content in Bell Pepper. Plants 2021, 10, 2793. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, S.; Zhong, L.; Zhao, X.; Wang, L. Effects of Zinc Oxide Nanoparticles on Growth, Development, and Flavonoid Synthesis in Ginkgo Biloba. Int. J. Mol. Sci. 2023, 24, 15775. [Google Scholar] [CrossRef] [PubMed]
- Salama, D.M.; Osman, S.A.; Abd El-Aziz, M.E.; Abd Elwahed, M.S.A.; Shaaban, E.A. Effect of Zinc Oxide Nanoparticles on the Growth, Genomic DNA, Production and the Quality of Common Dry Bean (Phaseolus vulgaris). Biocatal. Agric. Biotechnol. 2019, 18, 101083. [Google Scholar] [CrossRef]
- Mogazy, A.M.; Hanafy, R.S. Foliar Spray of Biosynthesized Zinc Oxide Nanoparticles Alleviate Salinity Stress Effect on Vicia Faba Plants. J. Soil Sci. Plant Nutr. 2022, 22, 2647–2662. [Google Scholar] [CrossRef]
- El-Badri, A.M.A.; Batool, M.; Mohamed, I.A.A.; Khatab, A.; Sherif, A.; Wang, Z.; Salah, A.; Nishawy, E.; Ayaad, M.; Kuai, J.; et al. Modulation of Salinity Impact on Early Seedling Stage via Nano-Priming Application of Zinc Oxide on Rapeseed (Brassica napus L.). Plant Physiol. Biochem. 2021, 166, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Ekinci, M.; Turan, M.; Ors, S.; Dursun, A. Physiological, Morphological and Biochemical Responses of Exogenous Hydrogen Sulfide in Salt-Stressed Tomato Seedlings. Sustainability 2023, 15, 1098. [Google Scholar] [CrossRef]
- Mahawar, L.; Živčák, M.; Barboricova, M.; Kovár, M.; Filaček, A.; Ferencova, J.; Vysoká, D.M.; Brestič, M. Effect of Copper Oxide and Zinc Oxide Nanoparticles on Photosynthesis and Physiology of Raphanus sativus L. under Salinity Stress. Plant Physiol. Biochem. 2024, 206, 108281. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Bhandari, S.R.; Cho, M.C.; Lee, J.G. Evaluation of Chlorophyll Fluorescence Parameters and Proline Content in Tomato Seedlings Grown under Different Salt Stress Conditions. Hortic. Environ. Biotechnol. 2020, 61, 433–443. [Google Scholar] [CrossRef]
- Sarwar, M.; Anjum, S.; Ali, Q.; Alam, M.W.; Haider, M.S.; Mehboob, W. Triacontanol Modulates Salt Stress Tolerance in Cucumber by Altering the Physiological and Biochemical Status of Plant Cells. Sci. Rep. 2021, 11, 24504. [Google Scholar] [CrossRef] [PubMed]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The Critical Role of Zinc in Plants Facing the Drought Stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Montalvo, D.; Degryse, F.; da Silva, R.C.; Baird, R.; McLaughlin, M.J. Chapter Five—Agronomic Effectiveness of Zinc Sources as Micronutrient Fertilizer. In Advances in Agronomy; Sparks, D.L., Ed.; Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2016; Volume 139, pp. 215–267. [Google Scholar]
- Chen, Z.; Yuan, Y.; Fu, D.; Shen, C.; Yang, Y. Identification and Expression Profiling of the Auxin Response Factors in Dendrobium Officinale under Abiotic Stresses. Int. J. Mol. Sci. 2017, 18, 927. [Google Scholar] [CrossRef] [PubMed]
- Hamzah Saleem, M.; Usman, K.; Rizwan, M.; Al Jabri, H.; Alsafran, M. Functions and Strategies for Enhancing Zinc Availability in Plants for Sustainable Agriculture. Front. Plant Sci. 2022, 13, 1033092. [Google Scholar] [CrossRef] [PubMed]
- Fageria, V.D. Nutrient Interactions in Crop Plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Chanu Thounaojam, T.; Thounaojam, T.M.; Upadhyaya, H. Chapter 16—Role of Zinc Oxide Nanoparticles in Mediating Abiotic Stress Responses in Plant. In Zinc-Based Nanostructures for Environmental and Agricultural Applications; Abd-Elsalam, K.A., Ed.; Nanobiotechnology for Plant Protection; Elsevier: Amsterdam, The Netherlands, 2021; pp. 323–337. ISBN 978-0-12-822836-4. [Google Scholar]
- Khanm, H.; Vaishnavi, B.A.; Shankar, A.G. Raise of Nano-Fertilizer Era: Effect of Nano Scale Zinc Oxide Particles on the Germination, Growth and Yield of Tomato (Solanum lycopersicum). Int. J. Curr. Microbiol. App. Sci. 2018, 7, 1861–1871. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Wang, R.; Wang, R.; Zhang, P.; Ju, Q.; Xu, J. Physiological, Transcriptomic, and Metabolomic Analyses Reveal Zinc Oxide Nanoparticles Modulate Plant Growth in Tomato. Environ. Sci. Nano 2020, 7, 3587–3604. [Google Scholar] [CrossRef]
- Faizan, M.; Hayat, S. Effect of Foliar Spray of ZnO-NPs on the Physiological Parameters and Antioxidant Systems of Lycopersicon esculentum. Pol. J. Nat. Sci. 2019, 34, 87–105. [Google Scholar]
- Bahr, G.; Tomatis, P.E.; Vila, A.J. 2.10—The Biochemistry and Enzymology of Zinc Enzymes. In Comprehensive Inorganic Chemistry III, 3rd ed.; Reedijk, J., Poeppelmeier, K.R., Eds.; Elsevier: Oxford, UK, 2023; pp. 231–267. ISBN 978-0-12-823153-1. [Google Scholar]
- Ahmed, R.; Uddin, M.K.; Quddus, M.A.; Samad, M.Y.A.; Hossain, M.A.M.; Haque, A.N.A. Impact of Foliar Application of Zinc and Zinc Oxide Nanoparticles on Growth, Yield, Nutrient Uptake and Quality of Tomato. Horticulturae 2023, 9, 162. [Google Scholar] [CrossRef]
- Gerona, M.E.B.; Deocampo, M.P.; Egdane, J.A.; Ismail, A.M.; Dionisio-Sese, M.L. Physiological Responses of Contrasting Rice Genotypes to Salt Stress at Reproductive Stage. Rice Sci. 2019, 26, 207–219. [Google Scholar] [CrossRef]
- Singh, A. Soil Salinization Management for Sustainable Development: A Review. J. Environ. Manag. 2021, 277, 111383. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Soil Salinity: A Global Threat to Sustainable Development. Soil Use Manag. 2022, 38, 39–67. [Google Scholar] [CrossRef]
- Shahzad, R.; Harlina, P.W.; Ewas, M.; Zhenyuan, P.; Nie, X.; Gallego, P.P.; Ullah Khan, S.; Nishawy, E.; Khan, A.H.; Jia, H. Foliar Applied 24-Epibrassinolide Alleviates Salt Stress in Rice (Oryza sativa L.) by Suppression of ABA Levels and Upregulation of Secondary Metabolites. J. Plant Interact. 2021, 16, 533–549. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Ahmad, A.; Alhammad, B.A.; Tola, E. Exogenous Application of Zinc Oxide Nanoparticles Improved Antioxidants, Photosynthetic, and Yield Traits in Salt-Stressed Maize. Agronomy 2023, 13, 2645. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of Cereal Grains with Zinc: Agronomic or Genetic Biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Nanoparticles Potentially Mediate Salt Stress Tolerance in Plants. Plant Physiol. Biochem. 2021, 160, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Jethva, J.; Schmidt, R.R.; Sauter, M.; Selinski, J. Try or Die: Dynamics of Plant Respiration and How to Survive Low Oxygen Conditions. Plants 2022, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity Effects on Polyphenol Content and Antioxidant Activities in Leaves of the Halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of Preharvest Abiotic Stresses on the Accumulation of Bioactive Compounds in Horticultural Produce. Front. Plant Sci. 2019, 10, 01212. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.J.; Bozonnet, S.; Mullen, W.; Jenkins, G.I.; Lean, M.E.J.; Crozier, A. Occurrence of Flavonols in Tomatoes and Tomato-Based Products. J. Agric. Food Chem. 2000, 48, 2663–2669. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic Compounds and Related Enzymes as Determinants of Quality in Fruits and Vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Krauss, S.; Schnitzler, W.H.; Grassmann, J.; Woitke, M. The Influence of Different Electrical Conductivity Values in a Simplified Recirculating Soilless System on Inner and Outer Fruit Quality Characteristics of Tomato. J. Agric. Food Chem. 2006, 54, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Sgherri, C.; Navari-Izzo, F.; Pardossi, A.; Soressi, G.P.; Izzo, R. The Influence of Diluted Seawater and Ripening Stage on the Content of Antioxidants in Fruits of Different Tomato Genotypes. J. Agric. Food Chem. 2007, 55, 2452–2458. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fuentes, A.D.; López-Vargas, E.R.; Pinedo-Espinoza, J.M.; Campos-Montiel, R.G.; Valdés-Reyna, J.; Juárez-Maldonado, A. Postharvest Behavior of Bioactive Compounds in Tomato Fruits Treated with Cu Nanoparticles and NaCl Stress. Appl. Sci. 2017, 7, 980. [Google Scholar] [CrossRef]
- Kim, H.-J.; Fonseca, J.M.; Kubota, C.; Kroggel, M.; Choi, J.-H. Quality of Fresh-Cut Tomatoes as Affected by Salt Content in Irrigation Water and Post-Processing Ultraviolet-C Treatment. J. Sci. Food Agric. 2008, 88, 1969–1974. [Google Scholar] [CrossRef]
- Moles, T.M.; de Brito Francisco, R.; Mariotti, L.; Pompeiano, A.; Lupini, A.; Incrocci, L.; Carmassi, G.; Scartazza, A.; Pistelli, L.; Guglielminetti, L.; et al. Salinity in Autumn-Winter Season and Fruit Quality of Tomato Landraces. Front. Plant Sci. 2019, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Ihme, N.; Kiesewetter, H.; Jung, F.; Hoffmann, K.H.; Birk, A.; Müller, A.; Grützner, K.I. Leg Oedema Protection from a Buckwheat Herb Tea in Patients with Chronic Venous Insufficiency: A Single-Centre, Randomised, Double-Blind, Placebo Controlled Clinical Trial. Eur. J. Clin. Pharmacol. 1996, 50, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, E.; Ruiz, J.M.; Ferreres, F.; Moreno, D.A. Phenolic Profiles of Cherry Tomatoes as Influenced by Hydric Stress and Rootstock Technique. Food Chem. 2012, 134, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.; Gill, S.; Corpas, F.; Ortega Villasante, C.; Hernández, L.E.; Tuteja, N.; Sofo, A.; Hasanuzzaman, M.; Fujita, M. Editorial: Recent Insights Into the Double Role of Hydrogen Peroxide in Plants. Front. Plant Sci. 2022, 13, 843274. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Physiological Responses of Tomato Seedlings (Lycopersicon esculentum) to Salt Stress. Mod. Appl. Sci. 2009, 3, 171–176. [Google Scholar] [CrossRef]
- Zahra, S.; Baghizadeh, A.; Mohamadali, V.; Yazdanpanah, A.; Yousefi, M. The Salicylic Acid Effect on the Tomato (Lycopersicum esculentum Mill) Sugar, Protein and Proline Contents under Salinity Stress (Nacl). J. Biophys. Struct. Biol. 2010, 2, 35–41. [Google Scholar]
- Tavallali, V.; Rahemi, M.; Eshghi, S.; Kholdebarin, B.; Ramezanian, A. Zinc Alleviates Salt Stress and Increases Antioxidant Enzyme Activity in the Leaves of Pistachio (Pistacia vera L. ’Badami’) Seedlings. Turk. J. Agric. For. 2010, 34, 349–359. [Google Scholar] [CrossRef]
- Tavallali, V.; Rahemi, M.; Maftoun, M.; Panahi, B.; Karimi, S.; Ramezanian, A.; Vaezpour, M. Zinc Influence and Salt Stress on Photosynthesis, Water Relations, and Carbonic Anhydrase Activity in Pistachio. Sci. Hortic. 2009, 123, 272–279. [Google Scholar] [CrossRef]
- Cakmak, I. Tansley Review No. 111. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Imakumbili, M.L.E.; Semu, E.; Semoka, J.M.R.; Abass, A.; Mkamilo, G. Managing Cassava Growth on Nutrient Poor Soils under Different Water Stress Conditions. Heliyon 2021, 7, e07331. [Google Scholar] [CrossRef] [PubMed]
- Barbes, L.; Barbulescu, A.; Radulescu, C.; Stihi, C.; Chelarescu, E. Determination of Heavy Metals in Leaves and Bark of Populus nigra L. by Atomic Absorption Spectrometry. Rom. Rep. Phys. 2014, 66, 877–886. [Google Scholar]
- Carmassi, G.; Incrocci, L.; Incrocci, G.; Pardossi, A. Non-destructive estimation of leaf area in (Solanum lycopersicum L.) and gerbera (Gerbera jamesonii H. Bolus). Agric. Med. 2007, 137, 172–176. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Perveen, S.; Kamran Khan, M.; Shaheen, M.R.; Hussain, R.; Sarwar, N.; Rashid, S.; Nafees, M.; Farid, G.; Alamri, S.; et al. Effect of Zinc Nanoparticles Seed Priming and Foliar Application on the Growth and Physio-Biochemical Indices of Spinach (Spinacia oleracea L.) under Salt Stress. PLoS ONE 2022, 17, e0263194. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, M.-P.; Gonzalez, B.; Billard, J.-P.; Boucaud, J. Carbohydrate Content, Fructan and Sucrose Enzyme Activities in Roots, Stubble and Leaves of Ryegrass (Lolium perenne L.) as Affected by Source/Sink Modification after Cutting. J. Plant Physiol. 1992, 140, 282–291. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The Effect of Drought and Ultraviolet Radiation on Growth and Stress Markers in Pea and Wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Ahmed, M.; Marrez, D.A.; Abdelmoeen, N.M.; Mahmoud, E.A.; Abdel-Shakur Ali, M.; Decsi, K.; Tóth, Z. Proximate Analysis of Moringa oleifera Leaves and the Antimicrobial Activities of Successive Leaf Ethanolic and Aqueous Extracts Compared with Green Chemically Synthesized Ag-NPs and Crude Aqueous Extract against Some Pathogens. Int. J. Mol. Sci. 2023, 24, 3529. [Google Scholar] [CrossRef]
- Kim, K.-H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic Acid Profiles and Antioxidant Activities of Wheat Bran Extracts and the Effect of Hydrolysis Conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Nejati, K.; Rezvani, Z.; Pakizevand, R. Synthesis of ZnO Nanoparticles and Investigation of the Ionic Template Effect on Their Size and Shape. Int. Nano Lett. 2011, 1, 75–81. [Google Scholar]
- Solaiman, M.A.; Ali, M.A.; Abdel-Moein, N.M.; Mahmoud, E.A. Synthesis of Ag-NPs Developed by Green-Chemically Method and Evaluation of Antioxidant Activities and Anti-Inflammatory of Synthesized Nanoparticles against LPS-Induced NO in RAW 264.7 Macrophages. Biocatal. Agric. Biotechnol. 2020, 29, 101832. [Google Scholar] [CrossRef]
- Murdock, R.C.; Braydich-Stolle, L.; Schrand, A.M.; Schlager, J.J.; Hussain, S.M. Characterization of Nanomaterial Dispersion in Solution Prior to In Vitro Exposure Using Dynamic Light Scattering Technique. Toxicol. Sci 2008, 101, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Marrez, D.A.; Mohamed Abdelmoeen, N.; Abdelmoneem Mahmoud, E.; Ali, M.A.-S.; Decsi, K.; Tóth, Z. Studying the Antioxidant and the Antimicrobial Activities of Leaf Successive Extracts Compared to the Green-Chemically Synthesized Silver Nanoparticles and the Crude Aqueous Extract from Azadirachta indica. Processes 2023, 11, 1644. [Google Scholar] [CrossRef]

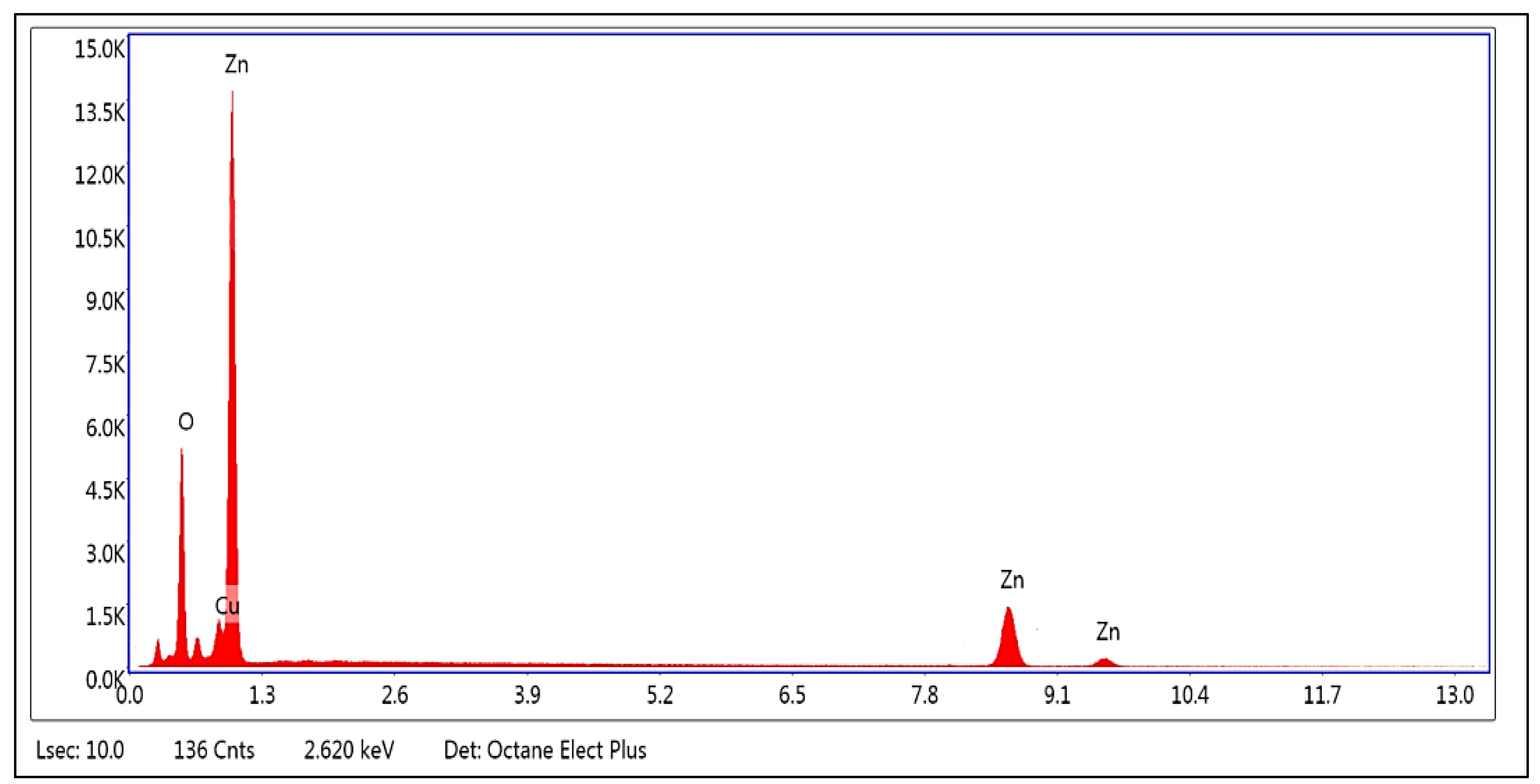
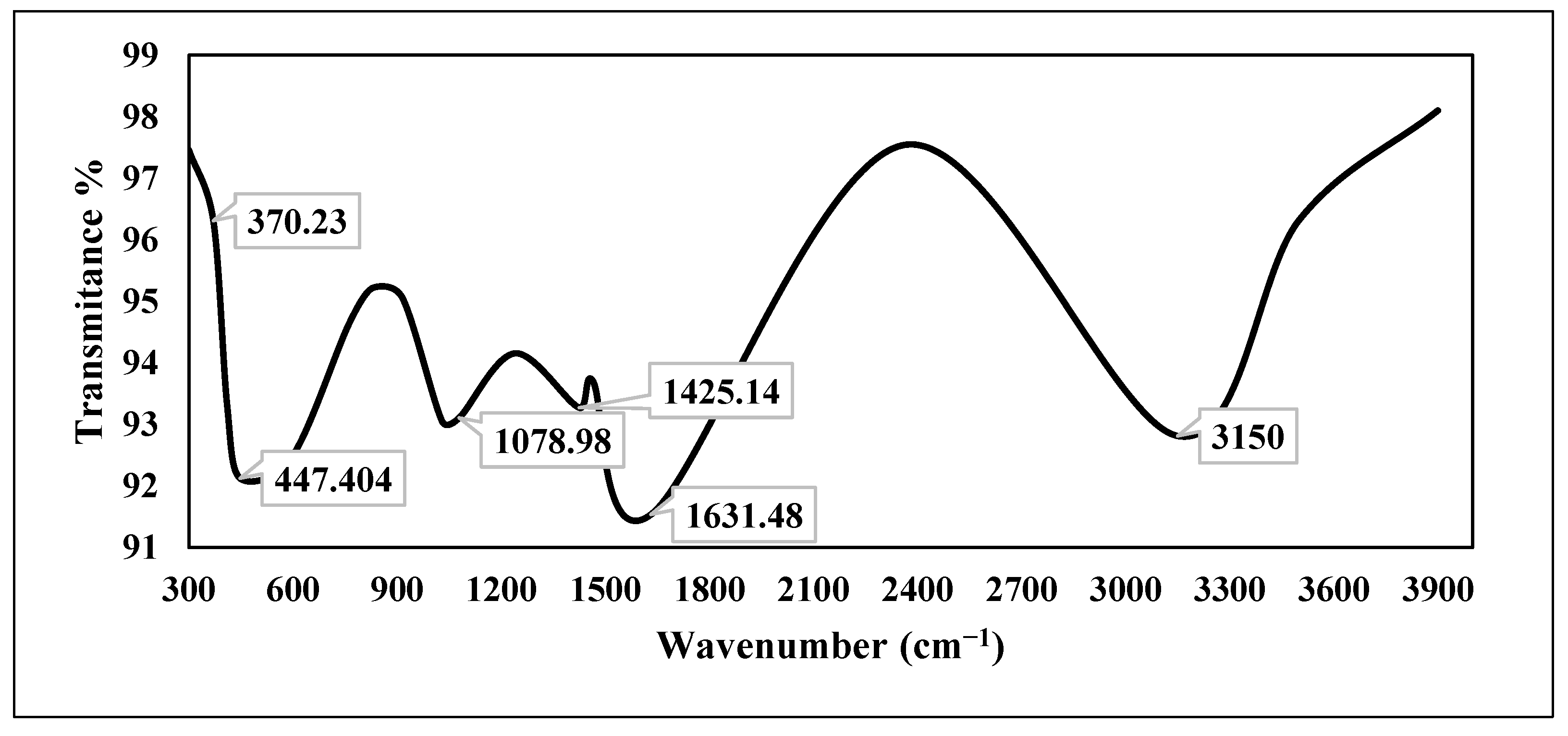

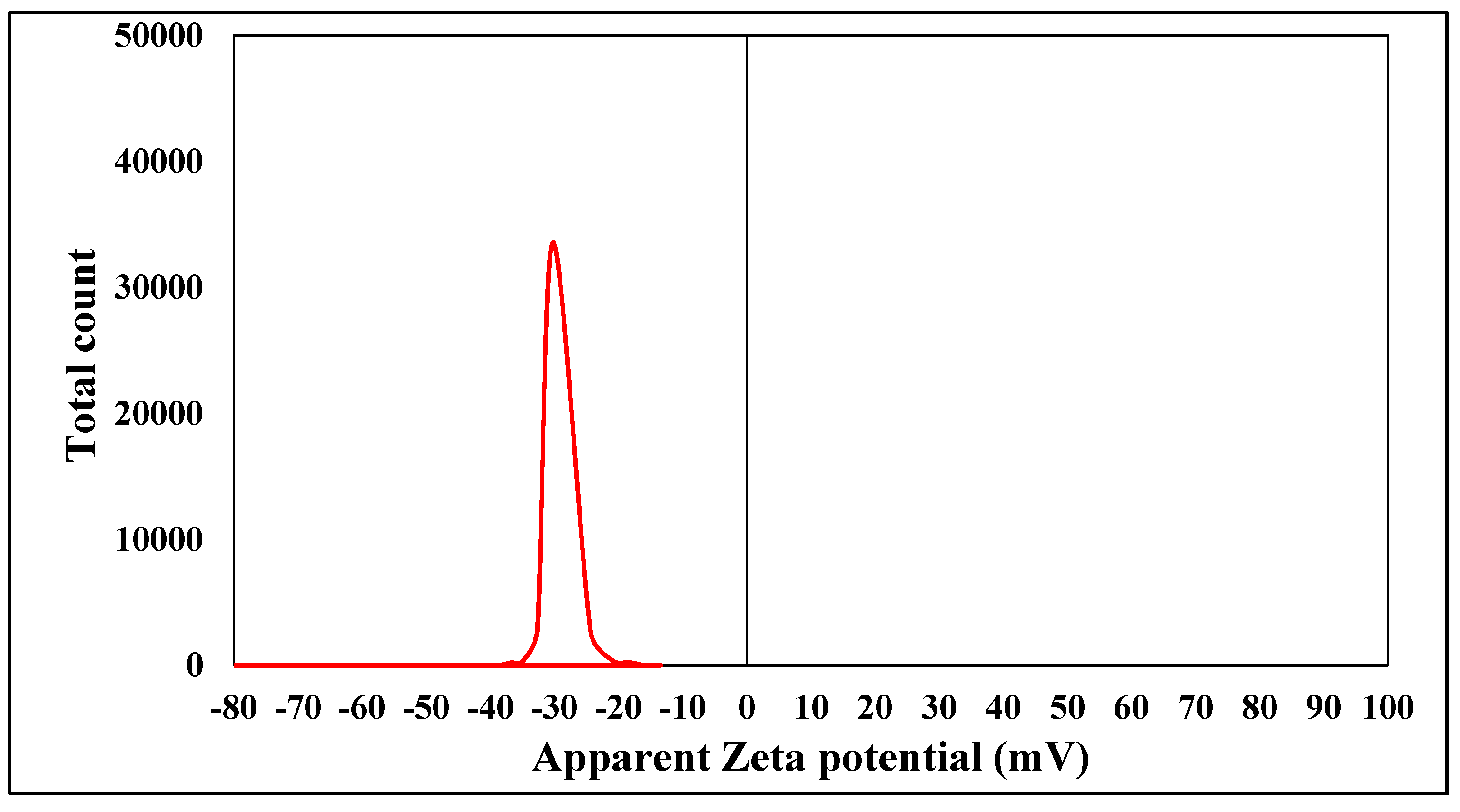
| Element | Weight % | Atomic % |
|---|---|---|
| ZnK | 67.55 | 42.95 |
| OxygenK | 32.02 | 56.81 |
| CuK | 0.43 | 0.23 |
| Treatments | Concentrations (µg/g) | |||||
|---|---|---|---|---|---|---|
| Na | K | Mg | Zn | Cu | Mn | |
| T1 Control (dw) | 1.47 ± 0.13 c | 29.20 ± 0.22 a | 5.57 ± 0.02 ns | 0.48 ± 0.11 c | 0.01 ± 0.04 c | 0.22 ± 0.01 ns |
| T2 (dw+ZnO-NPs 75 mg/L) | 1.31 ± 0.10 c | 29.02 ± 0.02 a | 5.56 ± 0.02 ns | 2.72 ± 0.37 b | 0.02 ± 0.03 c | 0.22 ± 0.00 ns |
| T3 (dw+ZnO-NPs 150 mg/L) | 1.49 ± 0.06 c | 29.10 ± 0.23 a | 5.59 ± 0.02 ns | 3.06 ± 0.58 ab | 0.01 ± 0.01 c | 0.21 ± 0.01 ns |
| T4 NaCl (150 mM) | 26.48 ± 0.21 a | 13.56 ± 0.38 c | 5.63 ± 0.03 ns | 0.47 ± 0.08 c | 0.02 ± 0.03 b | 0.63 ± 0.00 ns |
| T5 (150 mM NaCl+ZnO-NPs 75 mg/L) | 13.51 ± 0.06 b | 26.66 ± 0.40 b | 5.63 ± 0.01 ns | 2.44 ± 0.18 b | 0.004 ± 0.09 ab | 0.77 ± 0.00 ns |
| T6 (150 mM NaCl+ZnO-NPs 150 mg/L) | 13.27 ± 0.23 b | 26.34 ± 0.42 b | 5.62 ± 0.01 ns | 4.13 ± 0.38 a | 0.012 ± 0.05 a | 0.78 ± 0.00 ns |
| LSD (0.01) | 0.606 | 1.267 | ND | 1.692 | 0.204 | ND |
| LSD (0.05) | 0.442 | 0.925 | ND | 1.235 | 0.149 | ND |
| Coefficient of variation | 4.010 | 2.431 | 0.684 | 40.099 | 21.315 | 59.121 |
| Treatments | Readings of Chlorophyll Content in Different Stages | |||
|---|---|---|---|---|
| 5 July 2023 | 19 July 2023 | 28 July 2023 | 11 August 2023 | |
| T1 Control (dw) | 48.50 ± 1.89 ns | 51.98 ± 2.82 ns | 60.43 ± 1.24 ab | 63.13 ± 1.97 a |
| T2 (dw+ZnO-NPs 75 mg/L) | 46.05 ± 0.42 ns | 57.78 ± 1.65 ns | 60.13 ± 2.55 b | 61.25 ± 2.25 ab |
| T3 (dw+ZnO-NPs 150 mg/L) | 44.60 ± 1.06 ns | 56.83 ± 1.05 ns | 62.75 ± 2.09 a | 63.75 ± 2.43 a |
| T4 NaCl (150 mM) | 46.93 ± 0.93 ns | 54.88 ± 1.14 ns | 52.65 ± 0.44 cd | 55.25 ± 0.85 c |
| T5 (150 mM NaCl+ZnO-NPs 75 mg/L) | 49.20 ± 0.71 ns | 55.78 ± 1.05 ns | 53.18 ± 2.22 c | 60.75 ± 1.11 abc |
| T6 (150 mM NaCl+ZnO-NPs 150 mg/L) | 48.68 ± 1.08 ns | 54.68 ± 2.75 ns | 52.48 ± 1.26 d | 61.68 ± 2.47 ab |
| LSD (0.01) | ND | ND | ND | ND |
| LSD (0.05) | ND | ND | 5.404 | 5.805 |
| Coefficient of variation | 4.696 | 6.482 | 6.444 | 6.531 |
| Treatments | Readings of Plant Height (cm) in Different Stages | |||
|---|---|---|---|---|
| 5 July 2023 | 19 July 2023 | 28 July 2023 | 11 August 2023 | |
| T1 Control (dw) | 24.80 ± 2.16 ns | 47.50 ± 1.19 ns | 57.75 ± 1.93 b | 60.75 ± 2.01 c |
| T2 (dw+ZnO-NPs 75 mg/L) | 28.38 ± 2.51 ns | 50.75 ± 0.85 ns | 63.50 ± 2.72 a | 67.00 ± 2.16 b |
| T3 (dw+ZnO-NPs 150 mg/L) | 28.38 ± 2.50 ns | 50.75 ± 3.48 ns | 63.50 ± 1.80 a | 75.25 ± 2.10 a |
| T4 NaCl (150 mM) | 25.50 ± 2.25 ns | 47.00 ± 0.71 ns | 54.75 ± 2.25 c | 57.00 ± 2.42 d |
| T5 (150 mM NaCl+ZnO-NPs 75 mg/L) | 25.75 ± 1.65 ns | 49.50 ± 1.19 ns | 57.75 ± 2.98 b | 60.00 ± 1.68 cd |
| T6 (150 mM NaCl+ZnO-NPs 150 mg/L) | 24.13 ± 1.68 ns | 47.00 ± 0.58 ns | 57.25 ± 2.75 bc | 61.13 ± 2.09 bc |
| LSD (0.01) | ND | ND | 10.062 | 8.369 |
| LSD (0.05) | ND | ND | 7.345 | 6.110 |
| Coefficient of variation | 16.804 | 6.796 | 8.116 | 6.530 |
| Treatments | Readings of Stem Width (cm) in Different Stages | |||
|---|---|---|---|---|
| 5 July 2023 | 19 July 2023 | 28 July 2023 | 11 August 2023 | |
| T1 Control (dw) | 0.48 ± 0.03 ns | 0.73 ± 0.73 abc | 0.95 ± 0.03 b | 1.13 ± 0.01 ab |
| T2 (dw+ZnO-NPs 75 mg/L) | 0.50 ± 0.00 ns | 0.86 ± 0.00 a | 1.09 ± 0.08 a | 1.20 ± 0.02 a |
| T3 (dw+ZnO-NPs 150 mg/L) | 0.50 ± 0.03 ns | 0.86 ± 0.05 ab | 1.08 ± 0.03 ab | 1.16 ± 0.04 ab |
| T4 NaCl (150 mM) | 0.50 ± 0.00 ns | 0.64 ± 0.02 bc | 0.75 ± 0.04 d | 0.78 ± 0.03 d |
| T5 (150 mM NaCl+ZnO-NPs 75 mg/L) | 0.48 ± 0.03 ns | 0.59 ± 0.01 c | 0.81 ± 0.02 c | 0.90 ± 0.02 c |
| T6 (150 mM NaCl+ZnO-NPs 150 mg/L) | 0.46 ± 0.02 ns | 0.66 ± 0.02 bc | 0.94 ± 0.04 b | 1.09 ± 0.05 b |
| LSD (0.01) | ND | 0.190 | 0.139 | 0.126 |
| LSD (0.05) | ND | 0.139 | 0.101 | 0.092 |
| Coefficient of variation | 8.394 | 13.192 | 7.244 | 5.794 |
| Treatments | Readings of Leaf Area (cm2) in Different Stages | |||
|---|---|---|---|---|
| 5 July 2023 | 19 July 2023 | 28 July 2023 | 11 August 2023 | |
| T1 Control (dw) | 119.56 ± 3.94 c | 448.15 ± 15.85 a | 510.75 ± 0.75 b | 596.10 ± 15.90 ab |
| T2 (dw+ZnO-NPs 75 mg/L) | 182.63 ± 1.38 a | 434.73 ± 0.78 a | 482.13 ± 3.63 bc | 638.10 ± 5.40 a |
| T3 (dw+ZnO-NPs 150 mg/L) | 136.38 ± 1.38 bc | 414.63 ± 5.13 a | 594.00 ± 16.50 a | 632.15 ± 17.15 a |
| T4 NaCl (150 mM) | 154.875 ± 18.38 ab | 351.50 ± 26.50 b | 392.25 ± 20.25 d | 452.75 ± 40.25 c |
| T5 (150 mM NaCl+ZnO-NPs 75 mg/L) | 131.63 ± 1.63 bc | 360.88 ± 3.13 b | 442.25 ± 7.25 cd | 510.80 ± 58.20 bc |
| T6 (150 mM NaCl+ZnO-NPs 150 mg/L) | 136.13 ± 16.13 bc | 435.19 ± 14.81 a | 479.75 ± 31.75 bc | 484.50 ± 34.50 bc |
| LSD (0.01) | ND | ND | 89.702 | ND |
| LSD (0.05) | 35.166 | 49.134 | 59.213 | 116.275 |
| Coefficient of variation | 10.012 | 4.927 | 5.005 | 8.602 |
| Compounds to Be Detected | RT (min) | Concentration (μg/g)/Treatment | |||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | ||
| Gallic acid | 3.7 | 122.91 | 149.63 | 141.73 | 206.62 | 192.26 | 203.61 |
| Protocatechuic acid | 6.4 | 1.38 | 1.74 | 18.07 | 20.26 | 21.04 | 24.13 |
| Gentisic acid | 9.7 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| p-hydroxybenzoic acid | 9.8 | 10.19 | 12.96 | 15.21 | 19.03 | 22.25 | 25.36 |
| Catechin | 11.8 | 25.14 | 25.23 | 31.37 | 95.52 | 91.60 | 99.66 |
| Chlorogenic acid | 12.7 | 154.08 | 239.25 | 355.54 | 359.51 | 617.96 | 603.38 |
| Caffeic acid | 13.5 | 0.63 | 2.32 | 1.59 | 7.90 | 5.05 | 8.69 |
| Syringic acid | 14.6 | 8.10 | 4.66 | 44.27 | 49.33 | 46.87 | 46.72 |
| Vanillic acid | 16.0 | 4.36 | 4.49 | 4.79 | 7.74 | 10.47 | 9.20 |
| Ferulic acid | 20.6 | 37.35 | 41.80 | 44.71 | 58.35 | 67.70 | 80.53 |
| Sinapic acid | 21.5 | 18.88 | 25.99 | 36.12 | 27.67 | 65.09 | 60.21 |
| Rutin | 24.5 | 5.58 | 17.72 | 1.03 | 2.11 | 3.14 | 5.27 |
| p-coumaric acid | 25.4 | 13.22 | 11.35 | 0.86 | 1.13 | 2.97 | 27.21 |
| Apigenin-7-glucoside | 27.5 | 5.69 | 15.31 | 36.98 | 52.80 | 39.21 | 69.89 |
| Rosmarinic acid | 29 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cinnamic acid | 35.1 | 1.61 | 1.26 | 7.31 | 13.80 | 15.03 | 23.22 |
| Quercetin | 36.3 | 1.10 | 2.93 | 1.02 | 1.40 | 2.04 | 2.71 |
| Apigenin | 39.2 | 0.19 | 1.19 | 5.50 | 4.62 | 6.79 | 5.20 |
| Kaempferol | 40.8 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Chrysin | 51.5 | 4.00 | 4.19 | 6.92 | 9.83 | 14.31 | 14.79 |
| Total | 414.41 | 562.02 | 753.02 | 937.62 | 1223.78 | 1309.78 | |
| Treatments | Concentrations | |||||||
|---|---|---|---|---|---|---|---|---|
| TPCs (µg/g) | TFCs (µg/g) | Total Hydrolazable Sugars (µg/g) | Total Free Amino Acids (µg/g) | Protein Content (µg/g) | Proline Content (µg/g) | H2O2 (µg/g) | MDA (mmols/mL) | |
| T1 Control (dw) | 2096 ± 0.10 d | 401 ± 3.39 b | 84.58 ± 4.10 d | 163.73 ± 2.92 f | 81.28 ± 1.16 a | 15.23 ± 0.64 f | 418.76 ± 1.78 e | 1.56 ± 0.077 e |
| T2 (dw+ZnO-NPs 75 mg/L) | 2829 ± 0.39 bc | 356.56 ± 37.23 b | 107.25 ± 3.43 c | 265.09 ± 5.63 e | 79.11 ± 0.50 ab | 26.90 ± 0.42 e | 571.14 ± 13.33 d | 2.00 ± 0.75 d |
| T3 (dw+ZnO-NPs 150 mg/L) | 3628 ± 0.50 ab | 362.48 ± 25.98 b | 121.78 ± 4.70 b | 352.59 ± 2.84 d | 78.61 ± 1.13 b | 30.71 ± 1.99 d | 744.00 ± 2.33 b | 2.41 ± 0.094 c |
| T4 NaCl (150 mM) | 4043 ± 0.44 a | 1191.83 ± 16.66 a | 155.97 ± 0.90 a | 986.68 ± 8.61 a | 26.11 ± 0.33 d | 71.54 ± 2.60 a | 1158.76 ± 11.40 a | 11.77 ± 0.24 a |
| T5 (150 mM NaCl+ZnO-NPs 75 mg/L) | 3730 ± 0.32 ab | 1222.39 ± 62.84 a | 103.22 ± 4.04 c | 694.18 ± 7.24 b | 37.78 ± 0.41 cd | 45.67 ± 1.55 b | 682.57 ± 4.67 c | 2.37 ± 0.08 c |
| T6 (150 mM NaCl+ZnO-NPs 150 mg/L) | 2596 ± 0.11 c | 1271.37 ± 79.35 a | 121.83 ± 4.04 b | 552.14 ± 3.35 c | 38.89 ± 0.75 c | 41.38 ± 1.91 c | 558.76 ± 7.38 d | 3.55 ± 0.06 b |
| LSD (0.01) | 1408.995 | 152.394 | 9.852 | 22.406 | 3.206 | 4.661 | 32.934 | 0.448 |
| LSD (0.05) | 1028.595 | 111.251 | 7.192 | 16.357 | 2.341 | 3.403 | 24.043 | 0.327 |
| Coefficient of variation | 21.954 | 9.350 | 4.182 | 2.192 | 2.938 | 5.938 | 2.349 | 5.588 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.; Marrez, D.A.; Rizk, R.; Zedan, M.; Abdul-Hamid, D.; Decsi, K.; Kovács, G.P.; Tóth, Z. The Influence of Zinc Oxide Nanoparticles and Salt Stress on the Morphological and Some Biochemical Characteristics of Solanum lycopersicum L. Plants. Plants 2024, 13, 1418. https://doi.org/10.3390/plants13101418
Ahmed M, Marrez DA, Rizk R, Zedan M, Abdul-Hamid D, Decsi K, Kovács GP, Tóth Z. The Influence of Zinc Oxide Nanoparticles and Salt Stress on the Morphological and Some Biochemical Characteristics of Solanum lycopersicum L. Plants. Plants. 2024; 13(10):1418. https://doi.org/10.3390/plants13101418
Chicago/Turabian StyleAhmed, Mostafa, Diaa Attia Marrez, Roquia Rizk, Mostafa Zedan, Donia Abdul-Hamid, Kincső Decsi, Gergő Péter Kovács, and Zoltán Tóth. 2024. "The Influence of Zinc Oxide Nanoparticles and Salt Stress on the Morphological and Some Biochemical Characteristics of Solanum lycopersicum L. Plants" Plants 13, no. 10: 1418. https://doi.org/10.3390/plants13101418
APA StyleAhmed, M., Marrez, D. A., Rizk, R., Zedan, M., Abdul-Hamid, D., Decsi, K., Kovács, G. P., & Tóth, Z. (2024). The Influence of Zinc Oxide Nanoparticles and Salt Stress on the Morphological and Some Biochemical Characteristics of Solanum lycopersicum L. Plants. Plants, 13(10), 1418. https://doi.org/10.3390/plants13101418







