Primary Metabolic Response of Aristolochia contorta to Simulated Specialist Herbivory under Elevated CO2 Conditions
Abstract
:1. Introduction
2. Results
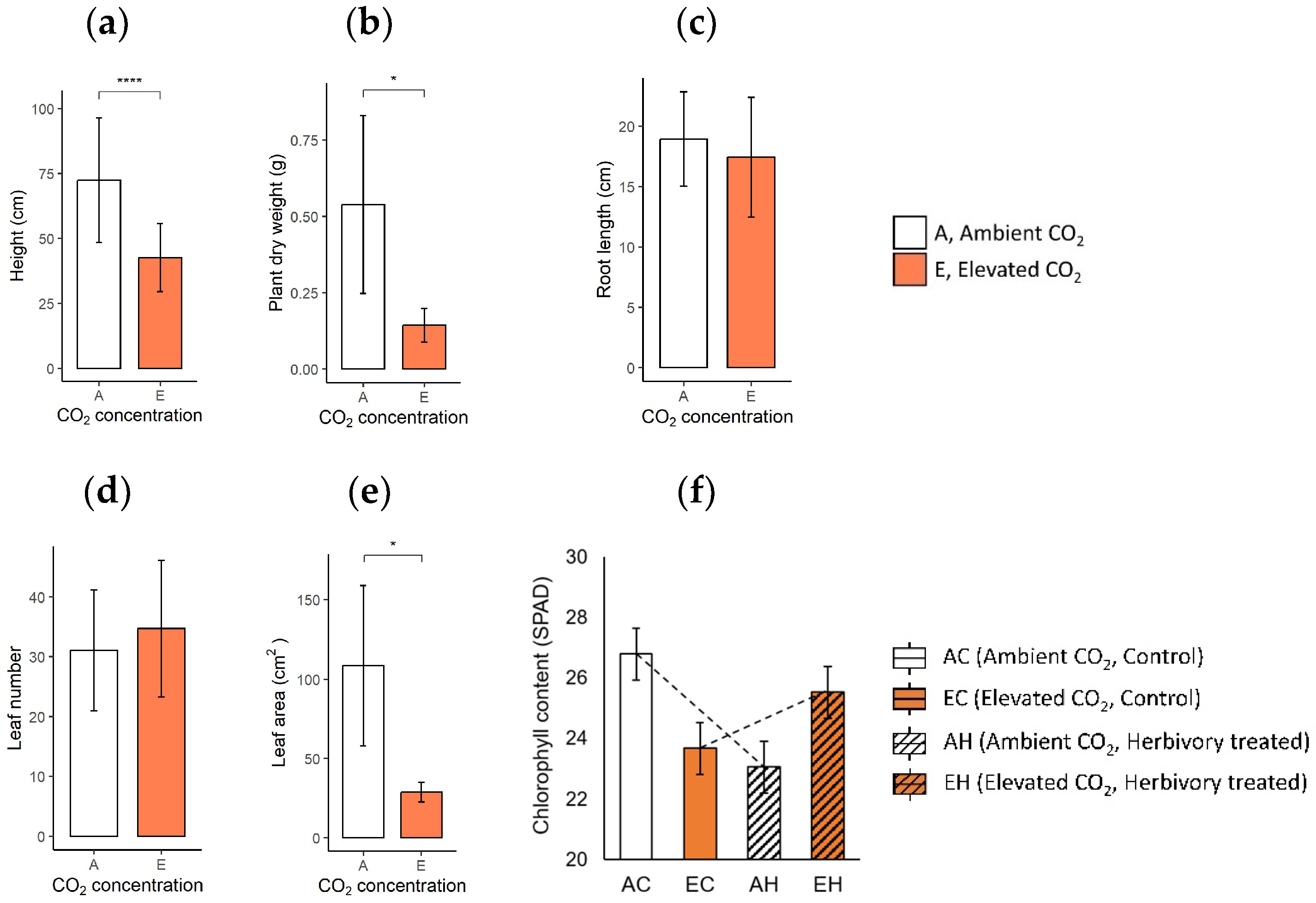
2.1. Growth Characteristics and Chlorophyll Content of the Plants
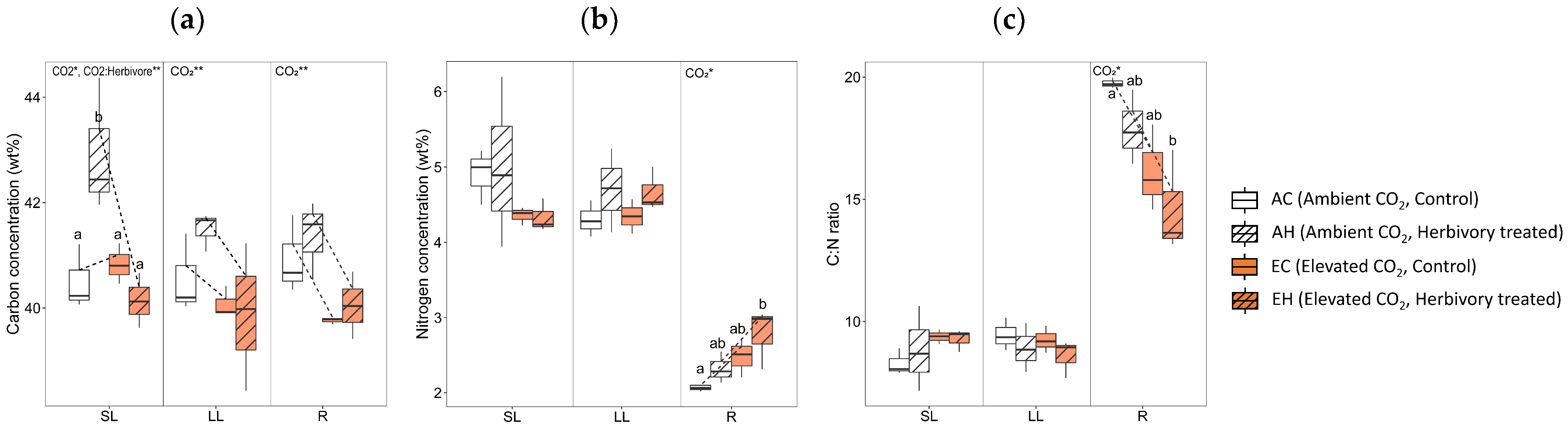
2.2. Carbon and Nitrogen Concentrations
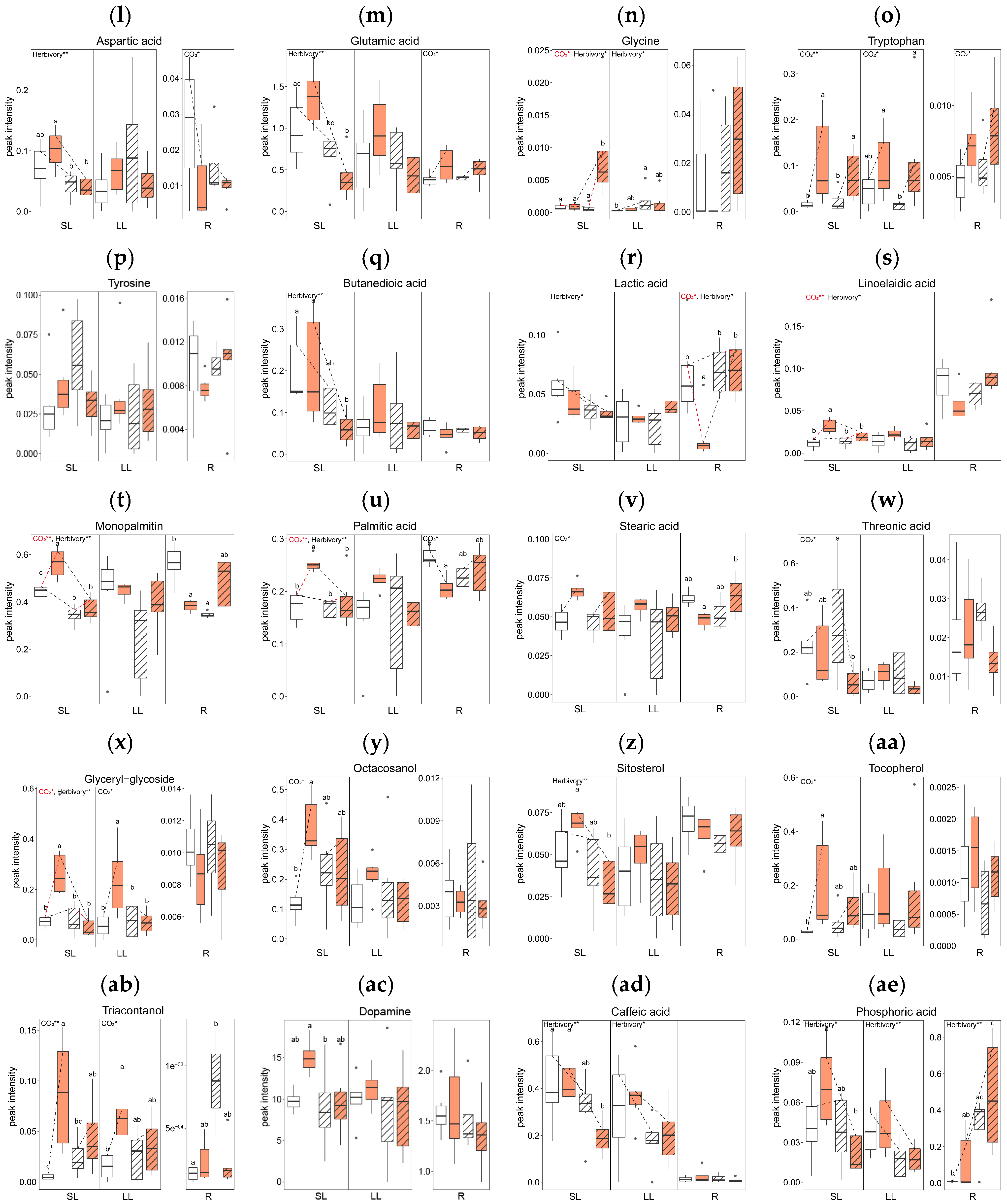
2.3. Primary Metabolite Concentrations
2.3.1. Effects of Elevated CO2 on Primary Metabolites
2.3.2. Effects of Herbivory on Primary Metabolites under Ambient CO2 Conditions
2.3.3. Combined Effects of Elevated CO2 and Herbivory on Primary Metabolites
3. Discussion
3.1. Effects of Elevated CO2: Growth Inhibition and Changes of Stress-Related Primary Metabolites
3.2. Effects of Herbivory: Local and Systemic Responses
3.3. Combined Effects of Elevated CO2 and Herbivory: Shifts in Defense Response
4. Materials and Methods
4.1. Plant Material and Growth Condition
4.2. CO2 and Herbivory Treatment
4.3. Measuring Chlorophyll Content and Biomass
4.4. Carbon and Nitrogen Concentrations
4.5. Metabolic Profiling of Primary Metabolite
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassia, R.; Nocioni, M.; Correa-Aragunde, N.; Lamattina, L. Climate Change and the Impact of Greenhouse Gasses: CO2 and NO, Friends and Foes of Plant Oxidative Stress. Front. Plant Sci. 2018, 9, 273. [Google Scholar] [CrossRef]
- Körner, C. Significance of temperature in plant life. In Plant Growth and Climate Change; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 48–69. [Google Scholar]
- Ziska, L.H.; Bunce, J.A. Plant responses to rising atmospheric carbon dioxide. In Plant Growth and Climate Change; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 17–47. [Google Scholar]
- DeLucia, E.H.; Nabity, P.D.; Zavala, J.A.; Berenbaum, M.R. Climate change: Resetting plant-insect interactions. Plant Physiol. 2012, 160, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A. Interplay between insects and plants: Dynamic and complex interactions that have coevolved over millions of years but act in milliseconds. J. Exp. Bot. 2015, 66, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, M.A.; Trowbridge, A.M.; Raffa, K.F.; Lindroth, R.L. Consequences of climate warming and altered precipitation patterns for plant-insect and multitrophic interactions. Plant Physiol. 2012, 160, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- De Graaff, M.-A.; Van Groenigen, K.-J.; Six, J.; Hungate, B.; Van Kessel, C. Interactions between plant growth and soil nutrient cycling under elevated CO2: A meta-analysis. Glob. Chang. Biol. 2006, 12, 2077–2091. [Google Scholar] [CrossRef]
- Eamus, D. The interaction of rising CO2 and temperatures with water use efficiency. Plant Cell Environ. 1991, 14, 843–852. [Google Scholar] [CrossRef]
- Hellmers, H.; Sionit, N.; Strain, B.R. Effects of different concentrations of atmospheric CO2 on growth and yield components of wheat. J. Agric. Sci. 1981, 97, 335–339. [Google Scholar] [CrossRef]
- Nilsen, S.; Hovland, K.; Dons, C.; Sletten, S.P. Effect of CO2 enrichment on photosynthesis, growth and yield of tomato. Sci. Hortic. 1983, 20, 1–14. [Google Scholar] [CrossRef]
- Rogers, H.H.; Cure, J.D.; Thomas, J.F.; Smith, J.M. Influence of elevated CO2 on growth of Soybean plants. Crop Sci. 1984, 24, 361–366. [Google Scholar] [CrossRef]
- Toreti, A.; Deryng, D.; Tubiello, F.N.; Müller, C.; Kimball, B.A.; Moser, G.; Boote, K.; Asseng, S.; Pugh, T.A.M.; Vanuytrecht, E.; et al. Narrowing uncertainties in the effects of elevated CO2 on crops. Nat. Food 2020, 1, 775–782. [Google Scholar] [CrossRef]
- Wang, D.; Heckathorn, S.A.; Wang, X.; Philpott, S.M. A meta-analysis of plant physiological and growth responses to temperature and elevated CO2. Oecologia 2012, 169, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Nam, B.E.; Lee, G.; Kim, S.-G.; Joo, Y.; Kim, J.G. Ontogeny-dependent effects of elevated CO2 and watering frequency on interaction between Aristolochia contorta and its herbivores. Sci. Total Environ. 2022, 838, 156065. [Google Scholar] [CrossRef] [PubMed]
- Nakonechnaya, O.V.; Kholina, A.B.; Koren, O.G.; Zhuravlev, Y.N. Genetic diversity of a rare species Aristolochia contorta Bunge (Aristolochiaceae) in Primorsky Krai. Russ. J. Genet. 2012, 48, 152–162. [Google Scholar] [CrossRef]
- Rücker, G.; Chung, B. Aristolochiasäuren aus Aristolochia manshuriensis. Planta Med. 1975, 27, 68–71. [Google Scholar] [CrossRef]
- Tada, A.; Sase, K.; Ohmura, I.; Shoji, J.; Tanaka, O. Studies on the Aristolochiaceous plants. I. The isolation of aristolochic acid from Aristolochia griffithii Hook f. et Thoms. Syoyakugaku Zasshi 1969, 23, 99–101. [Google Scholar]
- Li, X.; Luo, Y.; Yang, H.; Yang, Q.; Settele, J.; Schweiger, O. On the ecology and conservation of Sericinus montelus (Lepidoptera: Papilionidae)–Its threats in Xiaolongshan forests area (China). PLoS ONE 2016, 11, e0150833. [Google Scholar] [CrossRef] [PubMed]
- Nishida, R. Sequestration of plant secondary compounds by butterflies and moths. Chemoecology 1994, 5, 127–138. [Google Scholar] [CrossRef]
- Nishida, R.; Fukami, H. Ecological adaptation of an Aristolochiaceae-feeding swallowtail butterfly, Atrophaneura alcinous, to aristolochic acids. J. Chem. Ecol. 1989, 15, 2549–2563. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Biological Resources. Red Data Book of Endangered Insects in Korea I; National Institute of Biological Resources: Incheon, Republic of Korea, 2012; Available online: https://archive.nationalredlist.org/files/2016/04/7.-Red-Data-Book-of-Endangered-Insects-in-Korea-I-Korean.pdf (accessed on 20 May 2024). (In Korean)
- Wang, M.; Gu, Z.; Wang, R.; Guo, J.; Ling, N.; Firbank, L.G.; Guo, S. Plant primary metabolism regulated by nitrogen contributes to plant–pathogen interactions. Plant Cell Physiol. 2019, 60, 329–342. [Google Scholar] [CrossRef]
- Salam, U.; Ullah, S.; Tang, Z.-H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant metabolomics: An overview of the role of primary and secondary metabolites against different environmental stress factors. Life 2023, 13, 706. [Google Scholar] [CrossRef]
- Watts, S.M.; Dodson, C.D.; Reichman, O.J. The roots of defense: Plant resistance and tolerance to belowground herbivory. PLoS ONE 2011, 6, e18463. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Brennan, R.M.; Graham, J.; Karley, A.J. Plant defense against herbivorous pests: Exploiting resistance and tolerance traits for sustainable crop protection. Front. Plant Sci. 2016, 7, 1132. [Google Scholar] [CrossRef] [PubMed]
- Janzen, D.H. When is it coevolution. Evolution 1980, 34, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Stowe, K.A.; Marquis, R.J.; Hochwender, C.G.; Simms, E.L. The evolutionary ecology of tolerance to consumer damage. Annu. Rev. Ecol. Evol. Syst. 2000, 31, 565–595. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Agrawal, A.A. The ecology and evolution of plant tolerance to herbivory. Trends Ecol. Evol. 1999, 14, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Farfán, J.; Fornoni, J.; Valverde, P.L. The evolution of resistance and tolerance to herbivores. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 541–566. [Google Scholar] [CrossRef]
- War, A.R.; Taggar, G.K.; Hussain, B.; Taggar, M.S.; Nair, R.M.; Sharma, H.C. Plant defence against herbivory and insect adaptations. AoB Plants 2018, 10, ply037. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, J.H.; Kim, J.G. Effects of human activities on Sericinus montela and its host plant Aristolochia contorta. Sci. Rep. 2023, 13, 8289. [Google Scholar] [CrossRef]
- El Tahir, K.E.H. Pharmacological Actions of Magnoflorine and Aristolochic Acid-1 Isolated from the Seeds of Aristolochia bracteata. Int. J. Pharm. 1991, 29, 101–110. [Google Scholar] [CrossRef]
- McKenzie, A.; Price, J. The alkaloids of Gyrocarpus americanus Jacq. Aust. J. Chem. 1953, 6, 180–185. [Google Scholar] [CrossRef]
- Park, H.J. Effects of Environmental Factors on Host Plant and Its Specialist Herbivore, Aristolochia contorta and Sericinus montela. Ph.D. Thesis, Seoul National University Graduate School, Seoul, Republic of Korea, 2021. [Google Scholar]
- Dewar, R.C. The correlation between plant growth and intercepted radiation: An interpretation in terms of optimal plant nitrogen content. Ann. Bot. 1996, 78, 125–136. [Google Scholar] [CrossRef]
- Smith, N.G.; Keenan, T.F.; Colin Prentice, I.; Wang, H.; Wright, I.J.; Niinemets, Ü.; Crous, K.Y.; Domingues, T.F.; Guerrieri, R.; Yoko Ishida, F.; et al. Global photosynthetic capacity is optimized to the environment. Ecol. Lett. 2019, 22, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Valluru, R.; Van den Ende, W. Myo-inositol and beyond–Emerging networks under stress. Plant Sci. 2011, 181, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.; Chibnall, A.C.; Piper, S.H. The isolation of n-octacosanol from wheat wax. Biochem. J. 1933, 27, 1889–1893. [Google Scholar] [CrossRef] [PubMed]
- Simões, R.; Rodrigues, A.; Ferreira-Dias, S.; Miranda, I.; Pereira, H. Chemical composition of cuticular waxes and pigments and morphology of leaves of Quercus suber trees of different provenance. Plants 2020, 9, 1165. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Mukherjee, S.; Basak, P.; Majumder, A.L. Significance of galactinol and raffinose family oligosaccharide synthesis in plants. Front. Plant Sci. 2015, 6, 656. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S. The role of alpha-tocopherol in plant stress tolerance. J. Plant Physiol. 2005, 162, 743–748. [Google Scholar] [CrossRef]
- Islam, S.; Mohammad, F. Triacontanol as a dynamic growth regulator for plants under diverse environmental conditions. Physiol. Mol. Biol. Plants 2020, 26, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J. Plant defense compounds: Systems approaches to metabolic analysis. Annu. Rev. Phytopathol. 2012, 50, 155–173. [Google Scholar] [CrossRef]
- Baldwin, I.T. Herbivory simulations in ecological research. Trends Eco. Evol. 1990, 5, 91–93. [Google Scholar] [CrossRef]
- Green, T.R.; Ryan, C.A. Wound-induced proteinase inhibitor in plant leaves: A possible defense mechanism against insects. Science 1972, 175, 776–777. [Google Scholar] [CrossRef] [PubMed]
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Jaremo, J.; Tuomi, J.; Nilsson, P. Adaptive status of localized and systemic defense responses in plants. In The Ecology and Evolution of Inducible Defenses; Princeton University Press: Princeton, NJ, USA, 1999; pp. 33–44. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Kano, A.; Gomi, K.; Yamasaki-Kokudo, Y.; Satoh, M.; Fukumoto, T.; Ohtani, K.; Tajima, S.; Izumori, K.; Tanaka, K.; Ishida, Y.; et al. A rare sugar, d-allose, confers resistance to rice bacterial blight with upregulation of defense-related genes in Oryza sativa. Phytopathology 2010, 100, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.J., Jr.; Fichman, Y.; Zandalinas, S.I.; Mittler, R. Jasmonic acid and salicylic acid modulate systemic reactive oxygen species signaling during stress responses. Plant Physiol. 2022, 191, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Stenzel, I.; Hause, B.; Hause, G.; Kutter, C.; Maucher, H.; Neumerkel, J.; Feussner, I.; Miersch, O. The wound response in tomato–Role of jasmonic acid. J. Plant Physiol. 2006, 163, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Lee, J.S. Sugar acts as a regulatory signal on the wound-inducible expression of SbHRGP3::GUS in transgenic plants. Plant Cell Rep. 2003, 22, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, O.; Béthencourt, L.; Quero, A.; Sangwan, R.S.; Clément, C. Trehalose and plant stress responses: Friend or foe? Trends Plant Sci. 2010, 15, 409–417. [Google Scholar] [CrossRef]
- Park, S.-H.; Scheffler, J.; Scheffler, B.; Cantrell, C.L.; Pauli, C.S. Chemical defense responses of upland cotton, Gossypium hirsutum L. to physical wounding. Plant Direct 2019, 3, e00141. [Google Scholar] [CrossRef]
- Sharma, V.; Jain, S.; Bhakuni, D.S.; Kapil, R.S. Biosynthesis of aristolochic acid. J. Chem. Soc. Perkin Trans. 1982, 1, 1153–1155. [Google Scholar] [CrossRef]
- Erb, M.; Lenk, C.; Degenhardt, J.; Turlings, T.C.J. The underestimated role of roots in defense against leaf attackers. Trends Plant Sci. 2009, 14, 653–659. [Google Scholar] [CrossRef]
- Bezemer, T.M.; van Dam, N.M. Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol. Evol. 2005, 20, 617–624. [Google Scholar] [CrossRef]
- Kaplan, I.; Halitschke, R.; Kessler, A.; Rehill, B.J.; Sardanelli, S.; Denno, R.F. Physiological integration of roots and shoots in plant defense strategies links above- and belowground herbivory. Ecol. Lett. 2008, 11, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Balmer, D.; Mauch-Mani, B. More beneath the surface? Root versus shoot antifungal plant defenses. Front. Plant Sci. 2013, 4, 54921. [Google Scholar] [CrossRef]
- Comer, F.; Tiwari, H.P.; Spenser, I.D. Biosynthesis of aristolochic acid. Can. J. Chem. 1969, 47, 481–487. [Google Scholar] [CrossRef]
- Aldea, M.; Hamilton, J.G.; Resti, J.P.; Zangerl, A.R.; Berenbaum, M.R.; DeLucia, E.H. Indirect effects of insect herbivory on leaf gas exchange in soybean. Plant Cell Environ. 2005, 28, 402–411. [Google Scholar] [CrossRef]
- Bueno, A.D.F.; Bueno, R.C.O.D.F.; Nabity, P.D.; Higley, L.G.; Fernandes, O.A. Photosynthetic response of soybean to twospotted spider mite (Acari: Tetranychydae) injury. Braz. Arch. Biol. Technol. 2009, 52, 825–834. [Google Scholar] [CrossRef]
- Hermsmeier, D.; Schittko, U.; Baldwin, I.T. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. I. Large-scale changes in the accumulation of growth- and defense-related plant mRNAs1. Plant Physiol. 2001, 125, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.D.; Novak, N.G.; Ju, C.J.T.; Cooke, J.E.K. Potato, Solanum Tuberosum, defense against colorado potato beetle, Leptinotarsa Decemlineata (Say): Microarray gene expression profiling of potato by Colorado potato beetle regurgitant treatment of wounded leaves. J. Chem. Ecol. 2008, 34, 1013–1025. [Google Scholar] [CrossRef]
- Ralph, S.G.; Yueh, H.; Friedmann, M.; Aeschliman, D.; Zeznik, J.A.; Nelson, C.C.; Butterfield, Y.S.N.; Kirkpatrick, R.; Liu, J.; Jones, S.J.M.; et al. Conifer defence against insects: Microarray gene expression profiling of Sitka spruce (Picea sitchensis) induced by mechanical wounding or feeding by spruce budworms (Choristoneura occidentalis) or white pine weevils (Pissodes strobi) reveals large-scale changes of the host transcriptome. Plant Cell Environ. 2006, 29, 1545–1570. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Kerchev, P.I.; Fenton, B.; Foyer, C.H.; Hancock, R.D. Plant responses to insect herbivory: Interactions between photosynthesis, reactive oxygen species and hormonal signalling pathways. Plant Cell Environ. 2012, 35, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, A.R.; Heng-Moss, T.M.; Higley, L.G.; Sarath, G.; Mornhinweg, D.W. Physiological responses of resistant and susceptible barley, Hordeum vulgare to the Russian wheat aphid, Diurpahis noxia (Mordvilko). Arthropod-Plant Interact. 2009, 3, 233–240. [Google Scholar] [CrossRef]
- Haile, F.J.; Higley, L.G.; Ni, X.; Quisenberry, S.S. Physiological and growth tolerance in wheat to Russian wheat aphid (Homoptera: Aphididae) injury. Environ. Entomol. 1999, 28, 787–794. [Google Scholar] [CrossRef]
- Jeong, S.J.; Nam, B.E.; Jeong, H.J.; Jang, J.Y.; Joo, Y.; Kim, J.G. Age-dependent resistance of a perennial herb, Aristolochia contorta against specialist and generalist leaf-chewing herbivores. Front. Plant Sci. 2023, 14, 1145363. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, H.; Son, G.Y.; Nam, B.E.; Kim, J.G. Biotic and abiotic effects on the growth and reproduction of Aristolochia contorta. J. Wet. Res. 2020, 22, 113–120. [Google Scholar]
- Park, S.-H.; Nam, B.E.; Kim, J.G. Shade and physical support are necessary for conserving the Aristolochia contorta population. Ecol. Eng. 2019, 135, 108–115. [Google Scholar] [CrossRef]
- Meinshausen, M.; Smith, S.J.; Calvin, K.; Daniel, J.S.; Kainuma, M.L.T.; Lamarque, J.F.; Matsumoto, K.; Montzka, S.A.; Raper, S.C.B.; Riahi, K.; et al. The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim. Chang. 2011, 109, 213. [Google Scholar] [CrossRef]
- Halitschke, R.; Schittko, U.; Pohnert, G.; Boland, W.; Baldwin, I.T. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 2001, 125, 711–717. [Google Scholar] [CrossRef]
- McGale, E.; Diezel, C.; Schuman, M.C.; Baldwin, I.T. Cry1Ac production is costly for native plants attacked by non-Cry1Ac-targeted herbivores in the field. New Phytol. 2018, 219, 714–727. [Google Scholar] [CrossRef]
- Ohnmeiss, T.E.; Baldwin, I.T. The allometry of nitrogen to growth and an inducible defense under nitrogen-limited growth. Ecology 1994, 75, 995–1002. [Google Scholar] [CrossRef]
- Munné-Bosch, S. Perennial roots to immortality. Plant Physiol. 2014, 166, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Palta, J.P. Leaf chlorophyll content. Remote Sens. Rev. 1990, 5, 207–213. [Google Scholar] [CrossRef]
- Süß, A.; Danner, M.; Obster, C.; Locherer, M.; Hank, T.; Richter, K. Measuring Leaf Chlorophyll Content with the Konica Minolta SPAD-502Plus–Theory, Measurement, Problems, Interpretation; EnMAP Field Guides Technical Report; GFZ Data Services: Potsdam, Germany, 2015. [Google Scholar]
- Royer, M.; Larbat, R.; Le Bot, J.; Adamowicz, S.; Robin, C. Is the C:N ratio a reliable indicator of C allocation to primary and defence-related metabolisms in tomato? Phytochemistry 2013, 88, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C:N:P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. 2023. Available online: https://rpkgs.datanovia.com/ggpubr/ (accessed on 20 May 2024).
- Vincent Bonhomme, S.P.; Gaucherel, C.; Claude, J. Momocs: Outline analysis using R. J. Stat. Softw. 2014, 56, 1–24. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 20 May 2024).
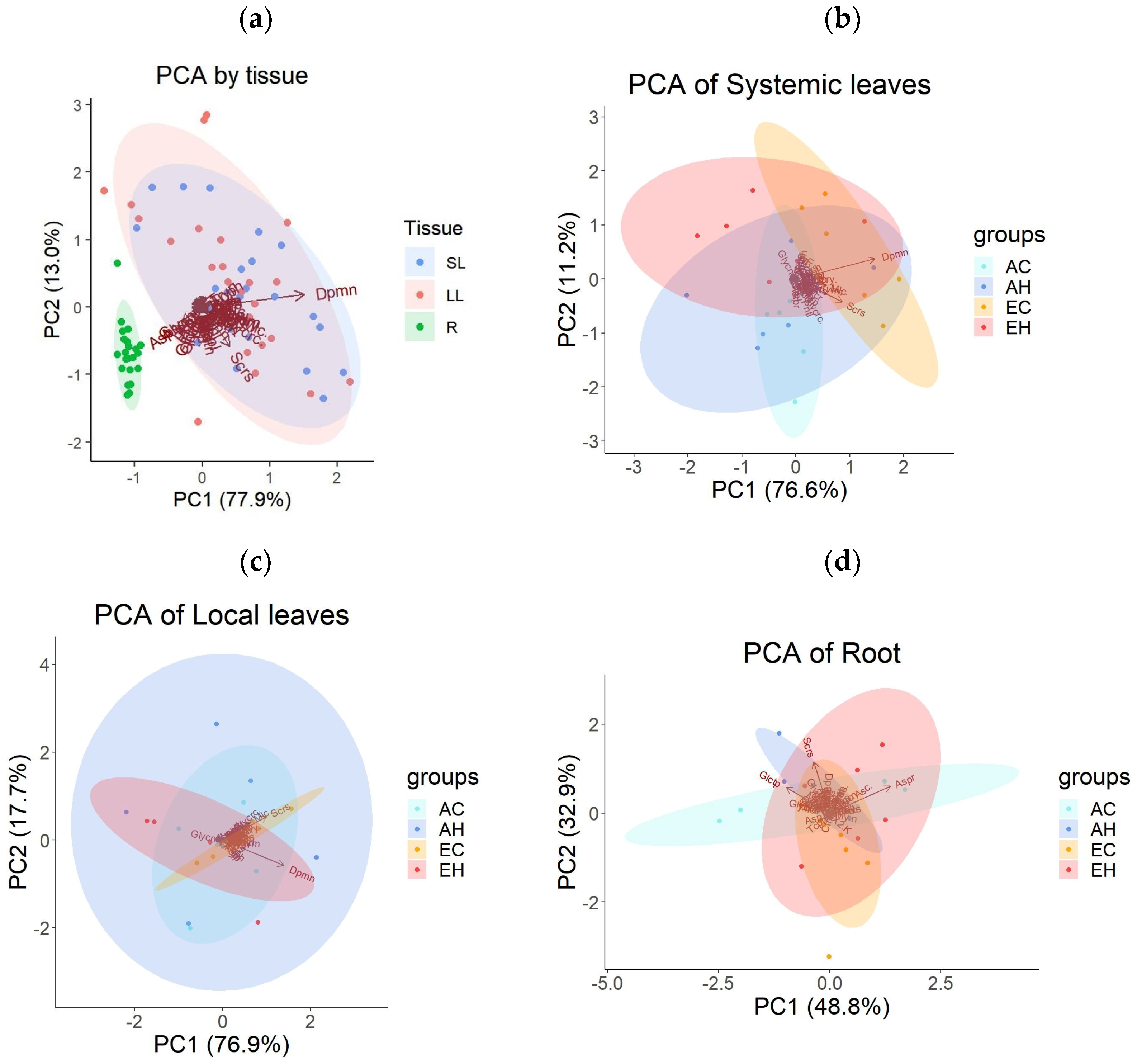


| Df | Hotelling-Lawley | approx F | num Df | den Df | Pr (>F) | |
|---|---|---|---|---|---|---|
| Tissue | 2 | 10.74 | 99.726 | 14 | 260 | <0.001 |
| Residuals | 137 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.J.; Nam, B.E.; Jeong, S.J.; Lee, G.; Kim, S.-G.; Kim, J.G. Primary Metabolic Response of Aristolochia contorta to Simulated Specialist Herbivory under Elevated CO2 Conditions. Plants 2024, 13, 1456. https://doi.org/10.3390/plants13111456
Jeong HJ, Nam BE, Jeong SJ, Lee G, Kim S-G, Kim JG. Primary Metabolic Response of Aristolochia contorta to Simulated Specialist Herbivory under Elevated CO2 Conditions. Plants. 2024; 13(11):1456. https://doi.org/10.3390/plants13111456
Chicago/Turabian StyleJeong, Hyeon Jin, Bo Eun Nam, Se Jong Jeong, Gisuk Lee, Sang-Gyu Kim, and Jae Geun Kim. 2024. "Primary Metabolic Response of Aristolochia contorta to Simulated Specialist Herbivory under Elevated CO2 Conditions" Plants 13, no. 11: 1456. https://doi.org/10.3390/plants13111456
APA StyleJeong, H. J., Nam, B. E., Jeong, S. J., Lee, G., Kim, S. -G., & Kim, J. G. (2024). Primary Metabolic Response of Aristolochia contorta to Simulated Specialist Herbivory under Elevated CO2 Conditions. Plants, 13(11), 1456. https://doi.org/10.3390/plants13111456









