OsMGD1-Mediated Membrane Lipid Remodeling Improves Salt Tolerance in Rice
Abstract
1. Introduction
2. Results
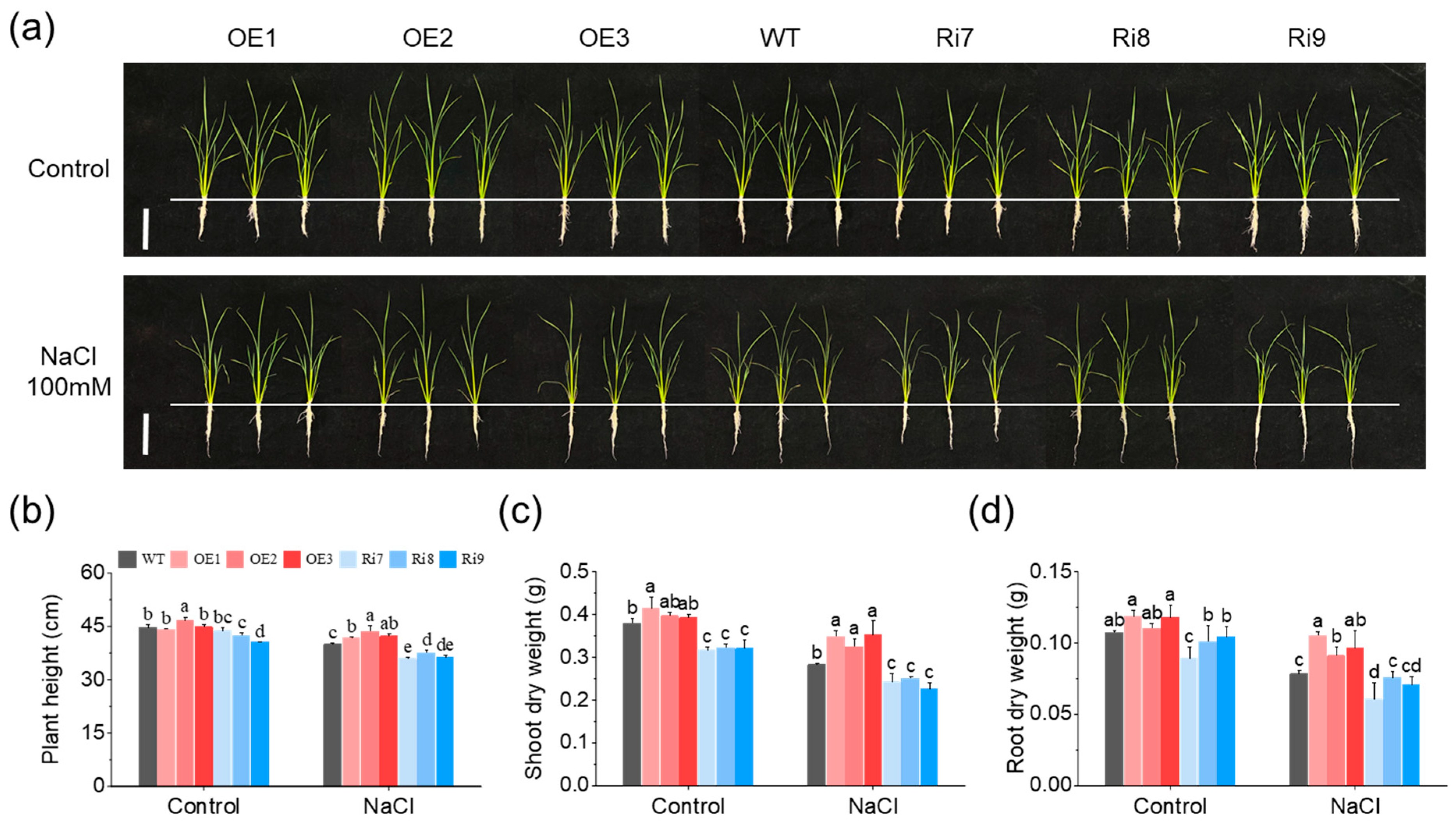
2.1. OsMGD1 Enhanced Growth of Salt-Stressed Rice Seedlings
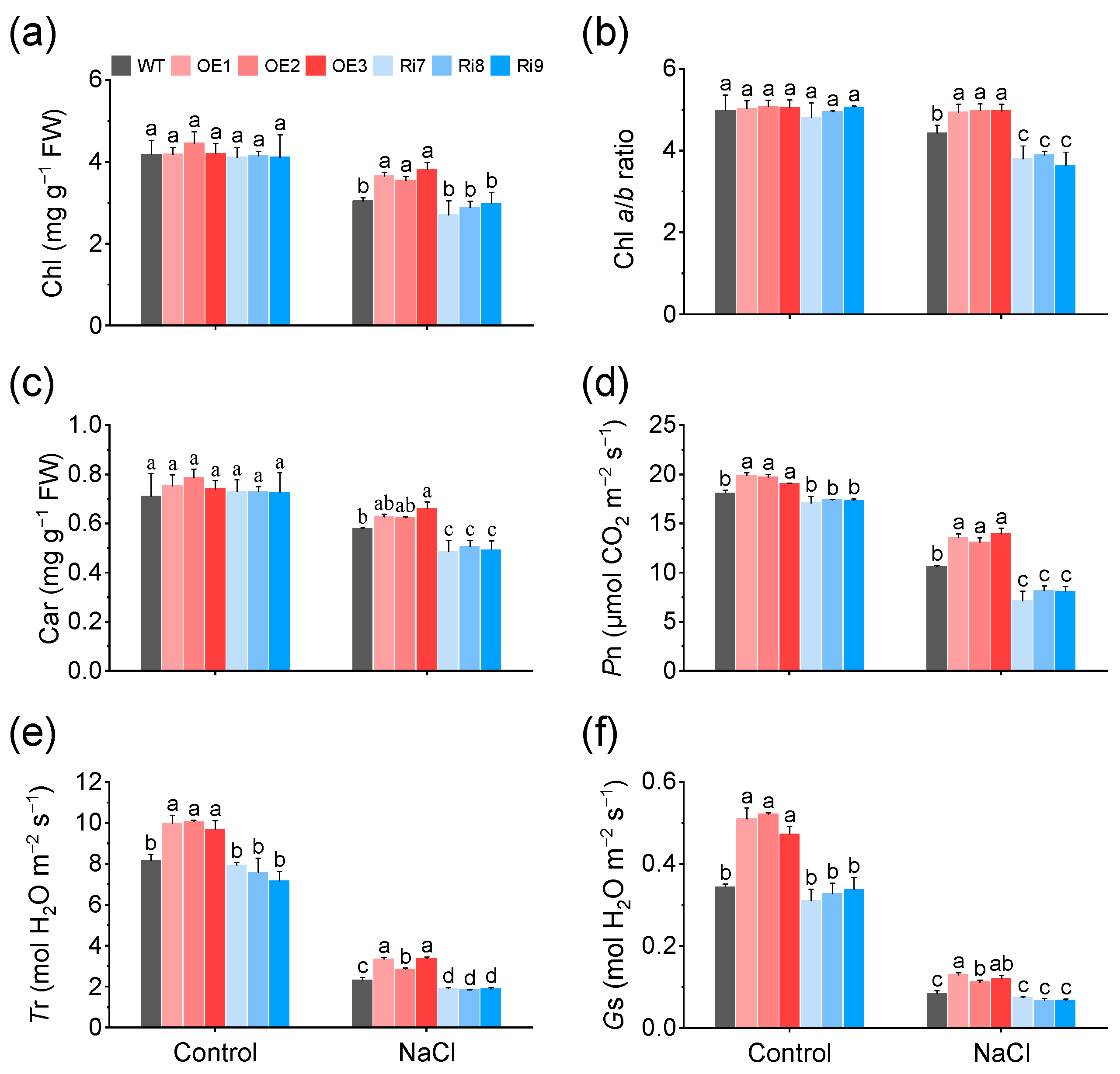
2.2. Overexpression of OsMGD1 Increased Photosynthetic Capacity under Salt Stress
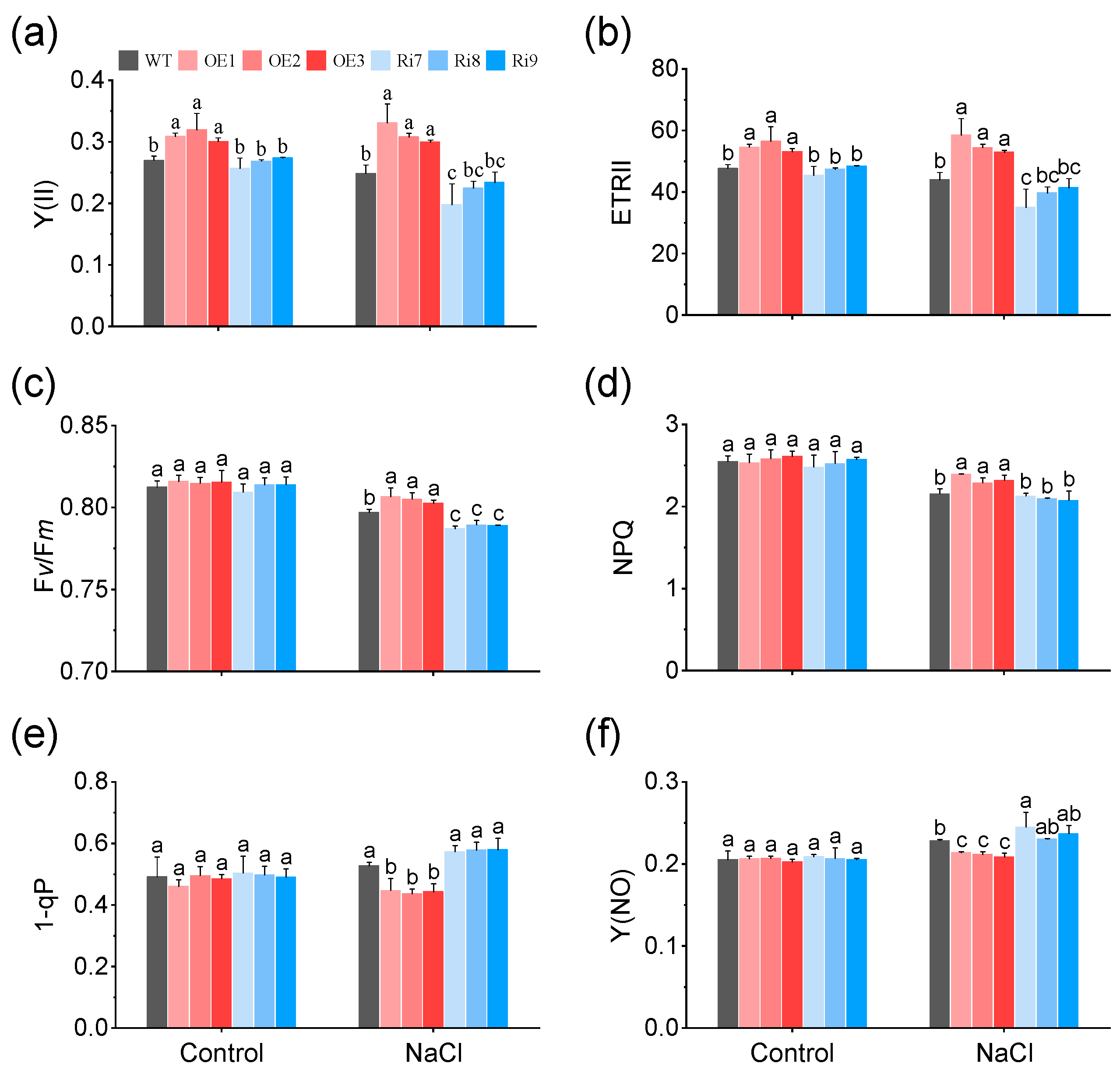
2.3. Effects of OsMGD1 on Chlorophyll Fluorescence under Salt Stress
2.4. Regulation of Na+/K+ Balance by Overexpression of OsMGD1 Conferred Salt Tolerance
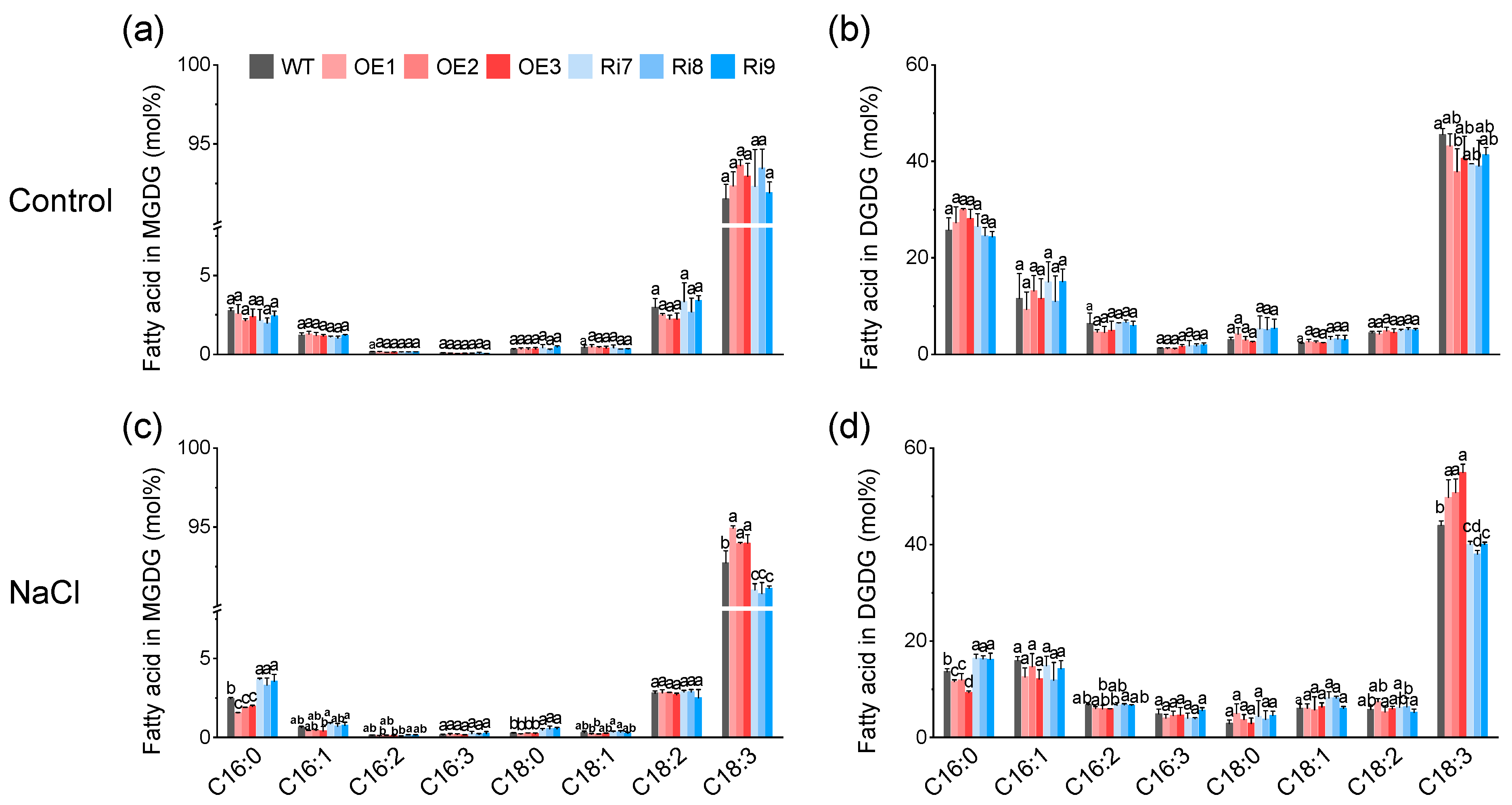
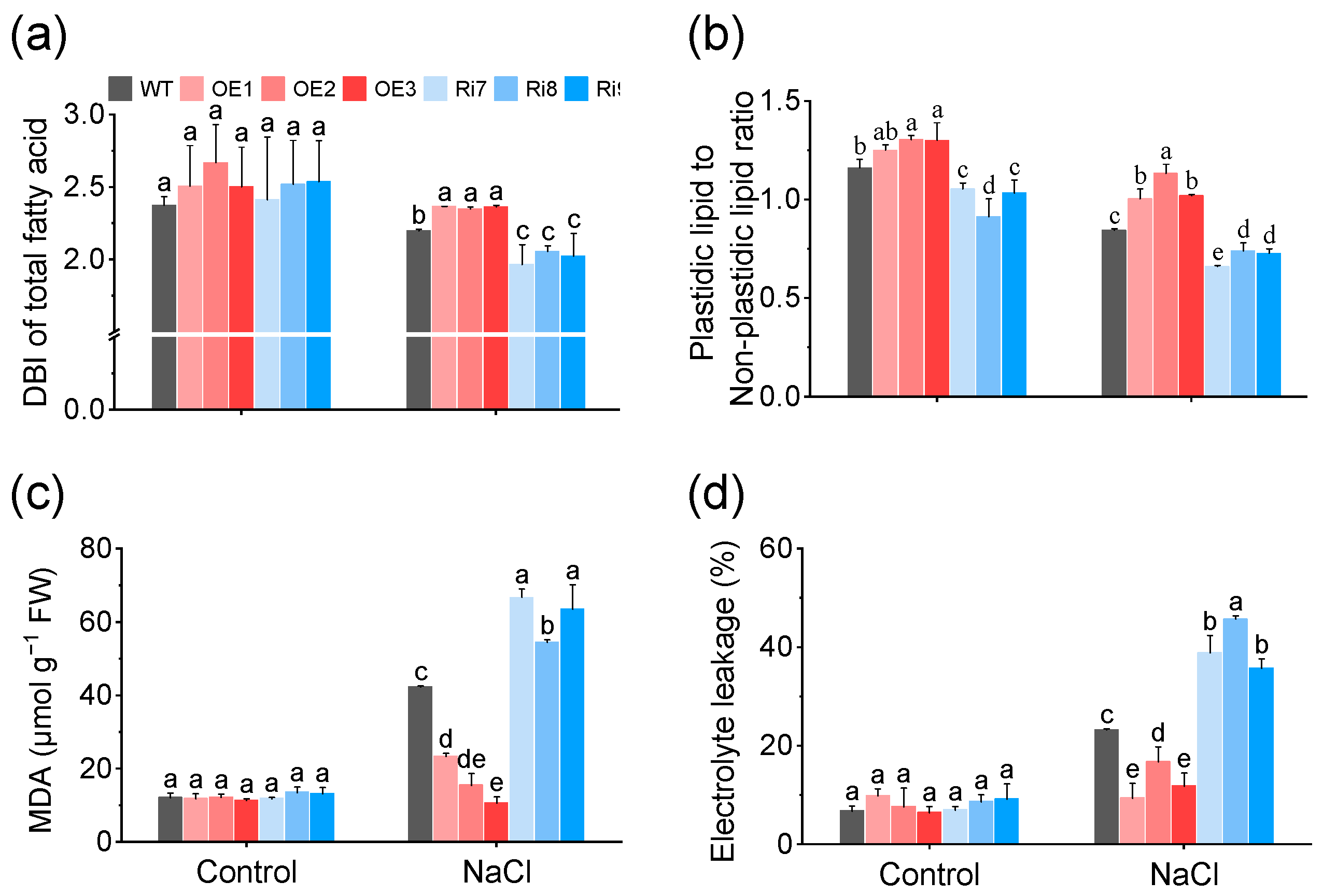
2.5. OsMGD1-Mediated Membrane Lipid Remodeling Positively Regulates Rice Salt Tolerance
2.6. OsMGD1 Increased Membrane Fluidity and Reduced Membrane Damage
3. Discussion
3.1. OsMGD1 Enhanced Salt Tolerance in Rice by Remodeling Membrane Lipids
3.2. Photosynthetic Capacity of Rice under Salt Stress Was Maintained by OsMGD1
3.3. OsMGD1 Improved Antioxidant Capacity and Maintained Shoot Na+/K+ Homeostasis under Salt Stress
4. Materials and Methods
4.1. Plant Materials and Salt Stress Treatment
4.2. Pigment Content Determination
4.3. Photosynthetic Parameters
4.4. Chlorophyll Fluorescence Measurement
4.5. Ion Content Measurement
4.6. Lipid Analysis
4.7. Determination of Lipid Peroxidation and Membrane Integrity
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gong, J.; Liu, Q.; Cai, L.; Yang, Q.; Tong, Y.; Chen, X.; Kotha, S.; Mao, X.; He, W. Multimechanism collaborative superior antioxidant CDzymes to alleviate salt stress-induced oxidative damage in plant growth. ACS Sustain. Chem. Eng. 2023, 11, 4237–4247. [Google Scholar] [CrossRef]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef] [PubMed]
- OECD; Food and Agriculture Organization of the United Nations. OECD-FAO Agricultural Outlook 2023–2032; OECD: Paris, France; FAO: Rome, Italy, 2023. [Google Scholar]
- Pan, T.; Liu, M.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Nie, C.; Yu, M.; Kuznetsov, V.V.; Allakhverdiev, S.I.; Shabala, S. Non-stomatal limitation of photosynthesis by soil salinity. Crit. Rev. Environ. Sci. Technol. 2021, 51, 791–825. [Google Scholar] [CrossRef]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Heß, D.; Heise, C.M.; Schubert, H.; Hess, W.R.; Hagemann, M. The impact of salt stress on the physiology and the transcriptome of the model streptophyte green alga Chara braunii. Physiol. Plant. 2023, 175, e14123. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Agarwal, R.; Bajada, C.; Arshinder, K. Redesigning a food supply chain for environmental sustainability—An analysis of resource use and recovery. J. Clean Prod. 2020, 242, 118374. [Google Scholar] [CrossRef]
- Yu, H.; Du, Q.; Campbell, M.; Yu, B.; Walia, H.; Zhang, C. Genome-wide discovery of natural variation in pre-mRNA splicing and prioritising causal alternative splicing to salt stress response in rice. New Phytol. 2021, 230, 1273–1287. [Google Scholar] [CrossRef]
- Li, G.; Xu, B.; Zhang, Y.; Xu, Y.; Khan, N.U.; Xie, J.; Sun, X.; Guo, H.; Wu, Z.; Wang, X.; et al. RGN1 controls grain number and shapes panicle architecture in rice. Plant Biotechnol. J. 2022, 20, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mukherjee, A.; Das, P.; Bandyopadhyay, S.; Chattopadhyay, D.; Chatterjee, J.; Majumder, A.L. A salt-tolerant chloroplastic FBPase from Oryza coarctata confers improved photosynthesis with higher yield and multi-stress tolerance to indica rice. Plant Cell Tiss. Org. 2021, 145, 561–578. [Google Scholar] [CrossRef]
- Chen, L.; Yang, W.; Liu, S.; Meng, Y.; Zhu, Z.; Liang, R.; Cao, K.; Xie, Y.; Li, X. Genome-wide analysis and identification of light-harvesting chlorophyll a/b binding (LHC) gene family and BSMV-VIGS silencing TaLHC86 reduced salt tolerance in wheat. Int. J. Biol. Macromol. 2023, 242, 124930. [Google Scholar] [CrossRef]
- Tamizhselvan, P.; Madhavan, S.; Constan-Aguilar, C.; Elrefaay, E.R.; Liu, J.; Pěnčík, A.; Novák, O.; Cairó, A.; Hrtyan, M.; Geisler, M.; et al. Chloroplast auxin efflux mediated by ABCB28 and ABCB29 fine-tunes salt and drought stress responses in Arabidopsis. Plants 2023, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, H. Structure-function relationships in photosynthetic membranes: Challenges and emerging fields. Plant Sci. 2018, 266, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhou, C.; Fan, J.; Shanklin, J.; Xu, C. Mechanisms and functions of membrane lipid remodeling in plants. Plant J. 2021, 107, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.M.; James, M.G.; Lin, Q.; Yi, G.; Stinard, P.S.; Hennen-Bierwagen, T.A.; Becraft, P.W. Maize opaque5 encodes monogalactosyldiacylglycerol synthase and specifically affects galactolipids necessary for amyloplast and chloroplast function. Plant Cell 2011, 23, 2331–2347. [Google Scholar] [CrossRef] [PubMed]
- Loll, B.; Kern, J.; Saenger, W.; Zouni, A.; Biesiadka, J. Lipids in photosystem II: Interactions with protein and cofactors. BBA-Bioenergetics 2007, 1767, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Mizusawa, N.; Wada, H. The role of lipids in photosystem II. BBA-Bioenergetics 2012, 1817, 194–208. [Google Scholar] [CrossRef]
- Lee, A.G. Membrane lipids: It’s only a phase. Curr. Biol. 2000, 10, R377–R380. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Chen, L.J.; Herrfurth, C.; Feussner, I.; Li, H.M. Reduced biosynthesis of digalactosyldiacylglycerol, a major chloroplast membrane lipid, leads to oxylipin overproduction and phloem cap lignification in Arabidopsis. Plant Cell 2016, 28, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Iwai, M.; Yamada-Oshima, Y.; Asami, K.; Kanamori, T.; Yuasa, H.; Shimojima, M.; Ohta, H. Recycling of the major thylakoid lipid MGDG and its role in lipid homeostasis in Chlamydomonas reinhardtii. Plant Physiol. 2021, 187, 1341–1356. [Google Scholar] [CrossRef]
- Awai, K.; Maréchal, E.; Block, M.A.; Brun, D.; Masuda, T.; Shimada, H.; Takamiya, K.; Ohta, H.; Joyard, J. Two types of MGDG synthase genes, found widely in both 16:3 and 18:3 plants, differentially mediate galactolipid syntheses in photosynthetic and nonphotosynthetic tissues in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2001, 98, 10960–10965. [Google Scholar] [CrossRef]
- Basnet, R.; Zhang, J.; Hussain, N.; Shu, Q. Characterization and mutational analysis of a monogalactosyldiacylglycerol synthase gene OsMGD2 in rice. Front. Plant Sci. 2019, 10, 992. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Awai, K.; Takamiya, K.; Ohta, H. Arabidopsis type B monogalactosyldiacylglycerol synthase genes are expressed during pollen tube growth and induced by phosphate starvation. Plant Physiol. 2004, 134, 640–648. [Google Scholar] [CrossRef]
- Aronsson, H.; Schottler, M.A.; Kelly, A.A.; Sundqvist, C.; Dormann, P.; Karim, S.; Jarvis, P. Monogalactosyldiacylglycerol deficiency in Arabidopsis affects pigment composition in the prolamellar body and impairs thylakoid membrane energization and photoprotection in leaves. Plant Physiol. 2008, 148, 580–592. [Google Scholar] [CrossRef]
- Fujii, S.; Kobayashi, K.; Nakamura, Y.; Wada, H. Inducible knockdown of monogalactosyldiacylglycerol synthase1 reveals roles of galactolipids in organelle differentiation in Arabidopsis cotyledons. Plant Physiol. 2014, 166, 1436–1449. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kondo, M.; Fukuda, H.; Nishimura, M.; Ohta, H. Galactolipid synthesis in chloroplast inner envelope is essential for proper thylakoid biogenesis, photosynthesis, and embryogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 17216–17221. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, P.; Dörmann, P.; Peto, C.A.; Lutes, J.; Benning, C.; Chory, J. Galactolipid deficiency and abnormal chloroplast development in the Arabidopsis MGD synthase 1 mutant. Proc. Natl. Acad. Sci. USA 2000, 97, 8175–8179. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Wang, S.; Ke, Q.; Yin, L.; Deng, X.; Feng, B. Arabidopsis mgd mutants with reduced monogalactosyldiacylglycerol contents are hypersensitive to aluminium stress. Ecotox. Environ. Safe 2020, 203, 110999. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Liu, D.; Wang, Y.; Li, S.; Yin, L.; Wang, S. Overexpression of rice monogalactosyldiacylglycerol synthase OsMGD leads to enhanced salt tolerance in rice. Agronomy 2022, 12, 568. [Google Scholar] [CrossRef]
- Wang, S.; Uddin, M.I.; Tanaka, K.; Yin, L.; Shi, Z.; Qi, Y.; Mano, J.; Matsui, K.; Shimomura, N.; Sakaki, T.; et al. Maintenance of chloroplast structure and function by overexpression of the rice monogalactosyldiacylglycerol synthase gene leads to enhanced salt tolerance in Tobacco. Plant Physiol. 2014, 165, 1144–1155. [Google Scholar] [CrossRef]
- Kobayashi, K.; Nakamura, Y.; Ohta, H. Type A and type B monogalactosyldiacylglycerol synthases are spatially and functionally separated in the plastids of higher plants. Plant Physiol. Biochem. 2009, 47, 518–525. [Google Scholar] [CrossRef]
- Zhan, X.; Qian, Y.; Mao, B. Identification and expression profiling of nonphosphorus glycerolipid synthase genes in response to abiotic stresses in Dendrobium catenatum. Plants 2021, 10, 1204. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Saito, K. Lipidomic studies of membrane glycerolipids in plant leaves under heat stress. Prog. Lipid Res. 2019, 75, 100990. [Google Scholar] [CrossRef] [PubMed]
- Floris, D.; Kühlbrandt, W. Molecular landscape of etioplast inner membranes in higher plants. Nat. Plants 2021, 7, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xin, W.; Wang, Y.; Zhang, D.; Wang, J.; Zheng, H.; Yang, L.; Nie, S.; Zou, D. An integrated analysis of the rice transcriptome and lipidome reveals lipid metabolism plays a central role in rice cold tolerance. BMC Plant Biol. 2022, 22, 91. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Liu, D.; Chu, M.; Liu, X.; Wei, Y.; Che, X.; Zhu, L.; He, L.; Xu, J. Dynamic and adaptive membrane lipid remodeling in leaves of sorghum under salt stress. Crop J. 2022, 10, 1557–1569. [Google Scholar] [CrossRef]
- Joynson, R.; Molero, G.; Coombes, B.; Gardiner, L.J.; Rivera-Amado, C.; Piñera-Chávez, F.J.; Evans, J.R.; Furbank, R.T.; Reynolds, M.P.; Hall, A. Uncovering candidate genes involved in photosynthetic capacity using unexplored genetic variation in Spring Wheat. Plant Biotechnol. J. 2021, 19, 1537–1552. [Google Scholar] [CrossRef] [PubMed]
- Tzfadia, O.; Amar, D.; Bradbury, L.M.; Wurtzel, E.T.; Shamir, R. The MORPH algorithm: Ranking candidate genes for membership in Arabidopsis and tomato pathways. Plant Cell 2012, 24, 4389–4406. [Google Scholar] [CrossRef] [PubMed]
- Szilágyi, A.; Sommarin, M.; Åkerlund, H.-E. Membrane curvature stress controls the maximal conversion of violaxanthin to zeaxanthin in the violaxanthin cycle—Influence of α-tocopherol, cetylethers, linolenic acid, and temperature. BBA-Bioenergetics 2007, 1768, 2310–2318. [Google Scholar] [CrossRef] [PubMed]
- Callahan, G.; Fillier, T.; Pham, T.H.; Zhu, X.; Thomas, R. The effects of clearcut harvesting on moss chloroplast lipidome and adaptation to light stress during boreal forest regeneration. J. Environ. Manag. 2022, 315, 115126. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Emam, M.M.; Salama, K.H.A.; Morsy, A.A. Sorghum under saline conditions: Responses, tolerance mechanisms, and management strategies. Planta 2021, 254, 24. [Google Scholar] [CrossRef]
- Zahra, N.; Al Hinai, M.S.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.M.; Farooq, M. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiol. Biochem. 2022, 178, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Mieulet, D.; Hubberten, H.M.; Obata, T.; Hoefgen, R.; Fernie, A.R.; Fisahn, J.; San Segundo, B.; Guiderdoni, E.; Schippers, J.H.; et al. Salt-responsive ERF1 regulates reactive oxygen species-dependent signaling during the initial response to salt stress in rice. Plant Cell 2013, 25, 2115–2131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Z.; Zhao, X.; Liu, L.; Tang, Q.; Fu, J.; Tang, X.; Yang, R.; Lin, J.; Liu, X.; et al. Receptor-Like Cytoplasmic Kinase STK Confers Salt Tolerance in Rice. Rice 2023, 16, 21. [Google Scholar] [CrossRef]
- Song, T.; Shi, Y.; Shen, L.; Cao, C.; Shen, Y.; Jing, W.; Tian, Q.; Lin, F.; Li, W.; Zhang, W. An endoplasmic reticulum-localized cytochrome b5 regulates high-affinity K+ transport in response to salt stress in rice. Proc. Natl. Acad. Sci. USA 2021, 118, e2114347118. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lim, S.D.; Jung, K.-H.; Jang, C.S. Overexpression of a C3HC4-type RING E3 ligase gene, OsRFPHC-13, improves salinity resistance in rice, Oryza sativa, by altering the expression of Na+/K+ transporter genes. Environ. Exp. Bot. 2023, 207, 105224. [Google Scholar] [CrossRef]
- Sun, J.; Cao, H.; Cheng, J.; He, X.; Sohail, H.; Niu, M.; Huang, Y.; Bie, Z. Pumpkin CmHKT1;1 controls shoot Na+ accumulation via limiting Na+ transport from rootstock to scion in Grafted Cucumber. Int. J. Mol. Sci. 2018, 19, 2648. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Forno, D.A.; Cock, J. Laboratory manual for physiological studies of rice. Res. Inst. 1976, 17, 61–66. [Google Scholar]
- Breeze, E.; Harrison, E.; McHattie, S.; Hughes, L.; Hickman, R.; Hill, C.; Kiddle, S.; Kim, Y.S.; Penfold, C.A.; Jenkins, D.; et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 2011, 23, 873–894. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Benning, C. Arabidopsis thaliana polar glycerolipid profiling by thin layer chromatography (TLC) coupled with gas-liquid chromatography (GLC). J. Vis. Exp. 2011, 49, 2519. [Google Scholar]
- Chen, D.; Wang, S.; Qi, L.; Yin, L.; Deng, X. Galactolipid remodeling is involved in drought-induced leaf senescence in maize. Environ. Exp. Bot. 2018, 150, 57–68. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Cao, B.; Cao, D.; Leng, G.; Li, H.; Yin, L.; Shan, L.; Deng, X. Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front. Plant Sci. 2016, 6, 1241. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, S.; Deng, X.; Zhang, Z.; Yin, L. Comprehensive evaluation of physiological traits under nitrogen stress and participation of linolenic acid in nitrogen-deficiency response in wheat seedlings. BMC Plant Biol. 2020, 20, 501. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Hui, L.; Li, J.; Xi, Y.; Xu, J.; Wang, L.; Yin, L. OsMGD1-Mediated Membrane Lipid Remodeling Improves Salt Tolerance in Rice. Plants 2024, 13, 1474. https://doi.org/10.3390/plants13111474
Li S, Hui L, Li J, Xi Y, Xu J, Wang L, Yin L. OsMGD1-Mediated Membrane Lipid Remodeling Improves Salt Tolerance in Rice. Plants. 2024; 13(11):1474. https://doi.org/10.3390/plants13111474
Chicago/Turabian StyleLi, Shasha, Lei Hui, Jingchong Li, Yuan Xi, Jili Xu, Linglong Wang, and Lina Yin. 2024. "OsMGD1-Mediated Membrane Lipid Remodeling Improves Salt Tolerance in Rice" Plants 13, no. 11: 1474. https://doi.org/10.3390/plants13111474
APA StyleLi, S., Hui, L., Li, J., Xi, Y., Xu, J., Wang, L., & Yin, L. (2024). OsMGD1-Mediated Membrane Lipid Remodeling Improves Salt Tolerance in Rice. Plants, 13(11), 1474. https://doi.org/10.3390/plants13111474






