High Concentrations of Se Inhibited the Growth of Rice Seedlings
Abstract
:1. Introduction
2. Results
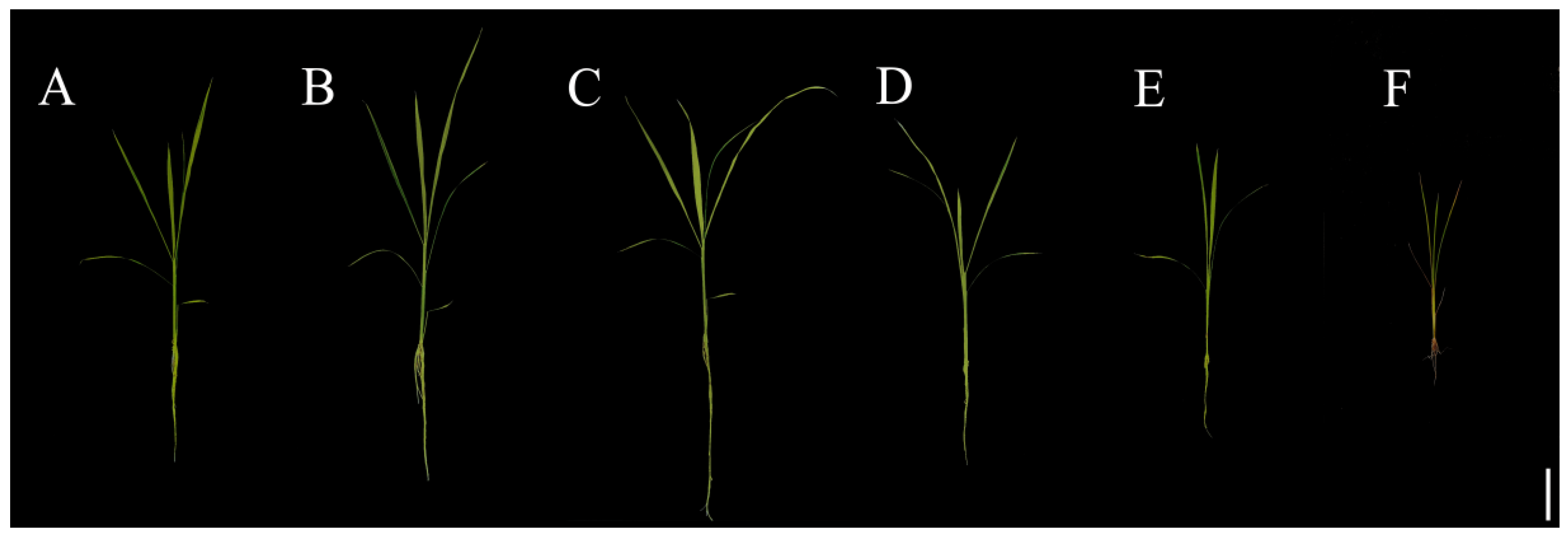
2.1. Influences of Various Concentrations of Se on Rice Development
2.2. Influences of Various Levels of Se on Root Development in Rice
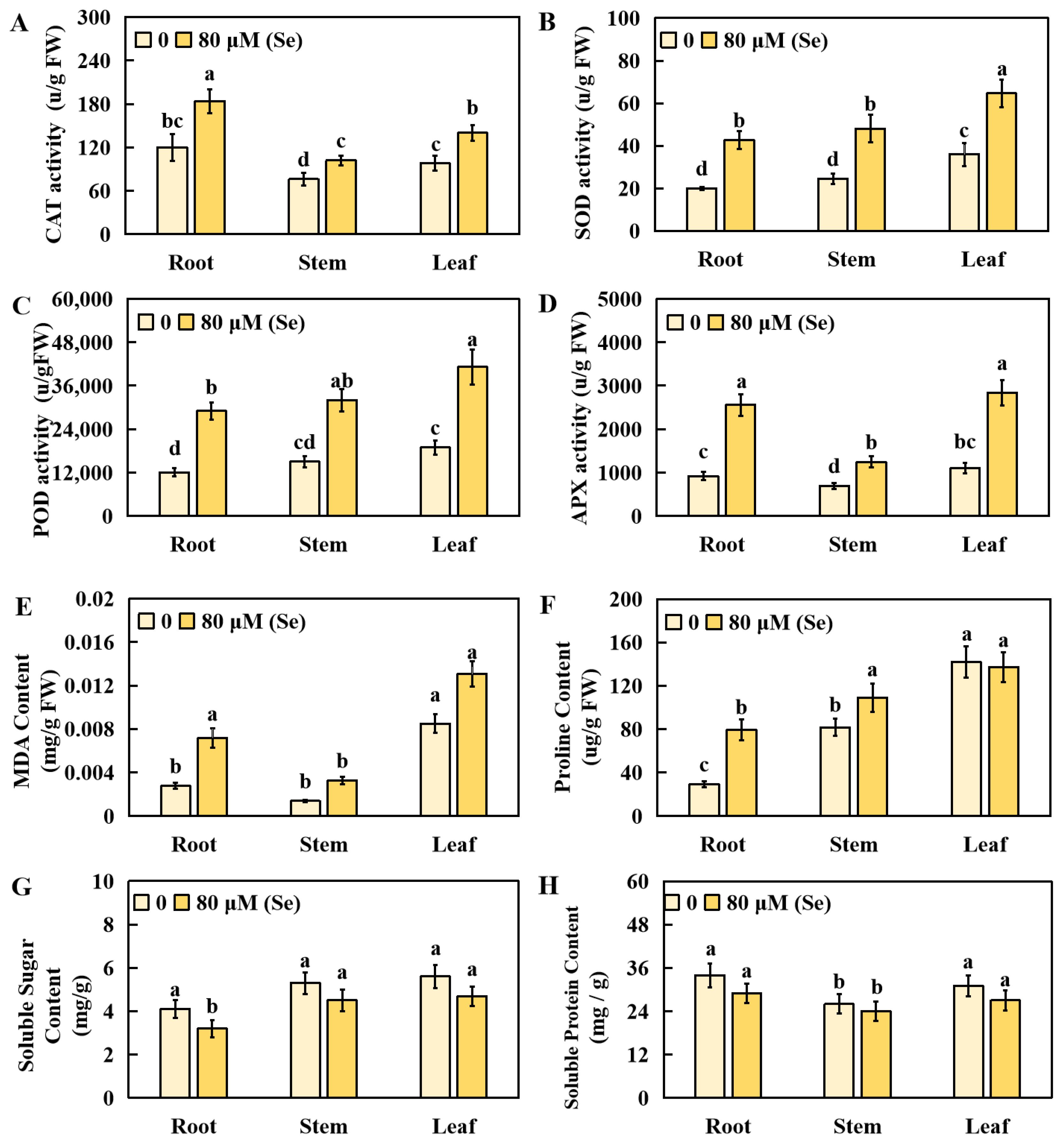
2.3. Effects of Se Stress on the Physiological Indicators of Rice
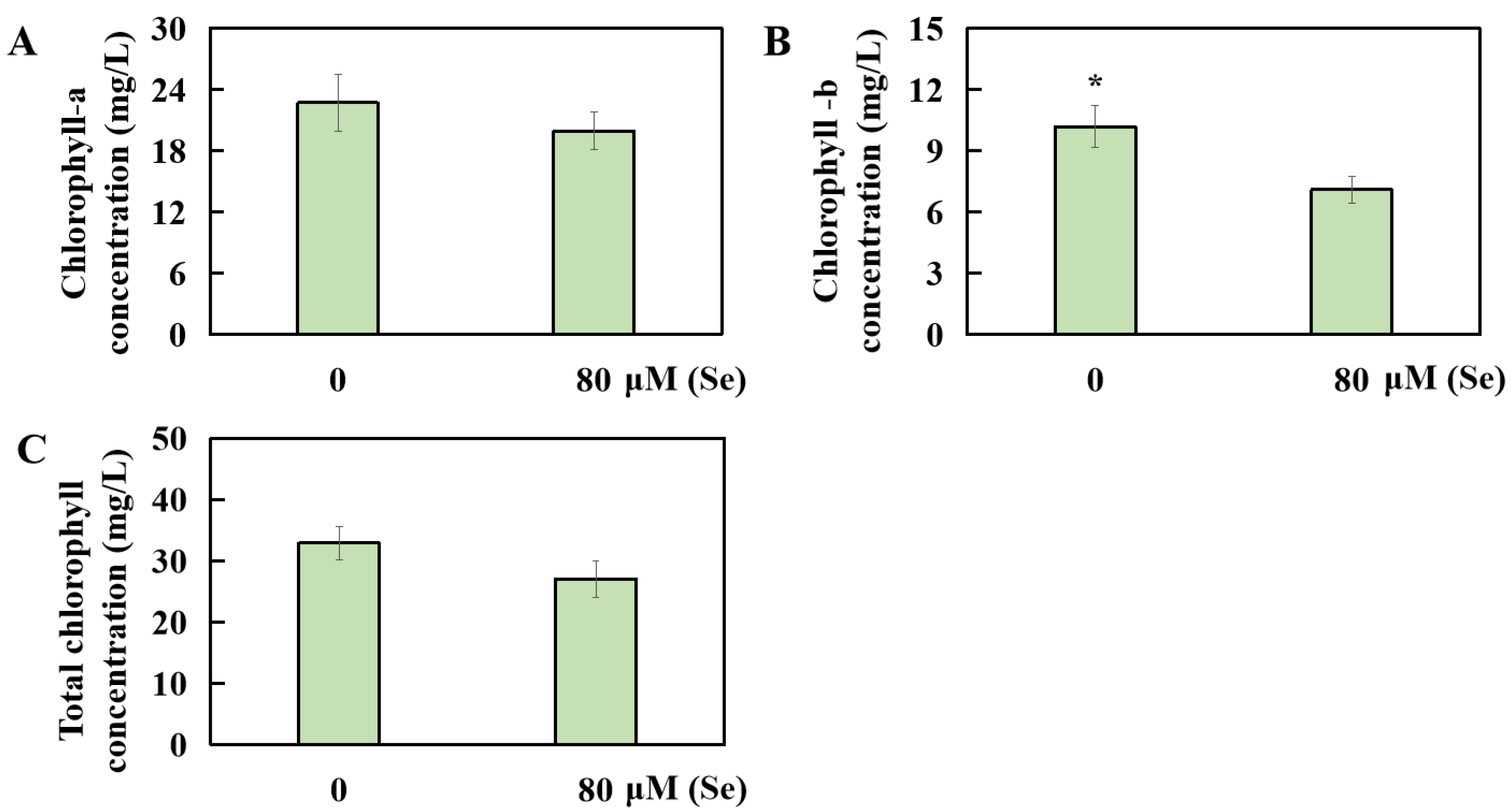
2.4. Influences of Se Poisoning Stress on Chlorophyll Levels in Rice
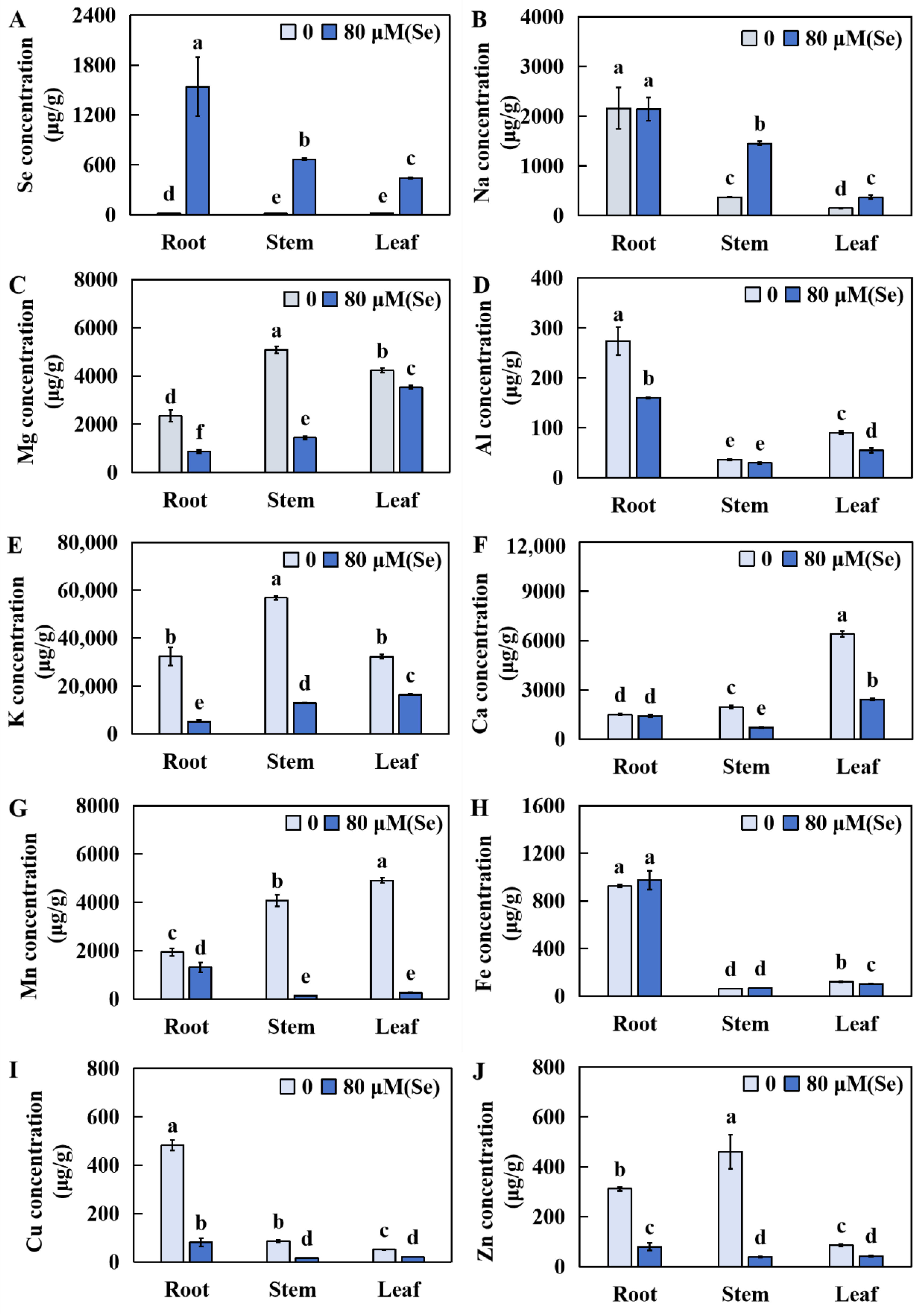
2.5. Influences of Se Stress on the Accumulation of Several Elements in Rice
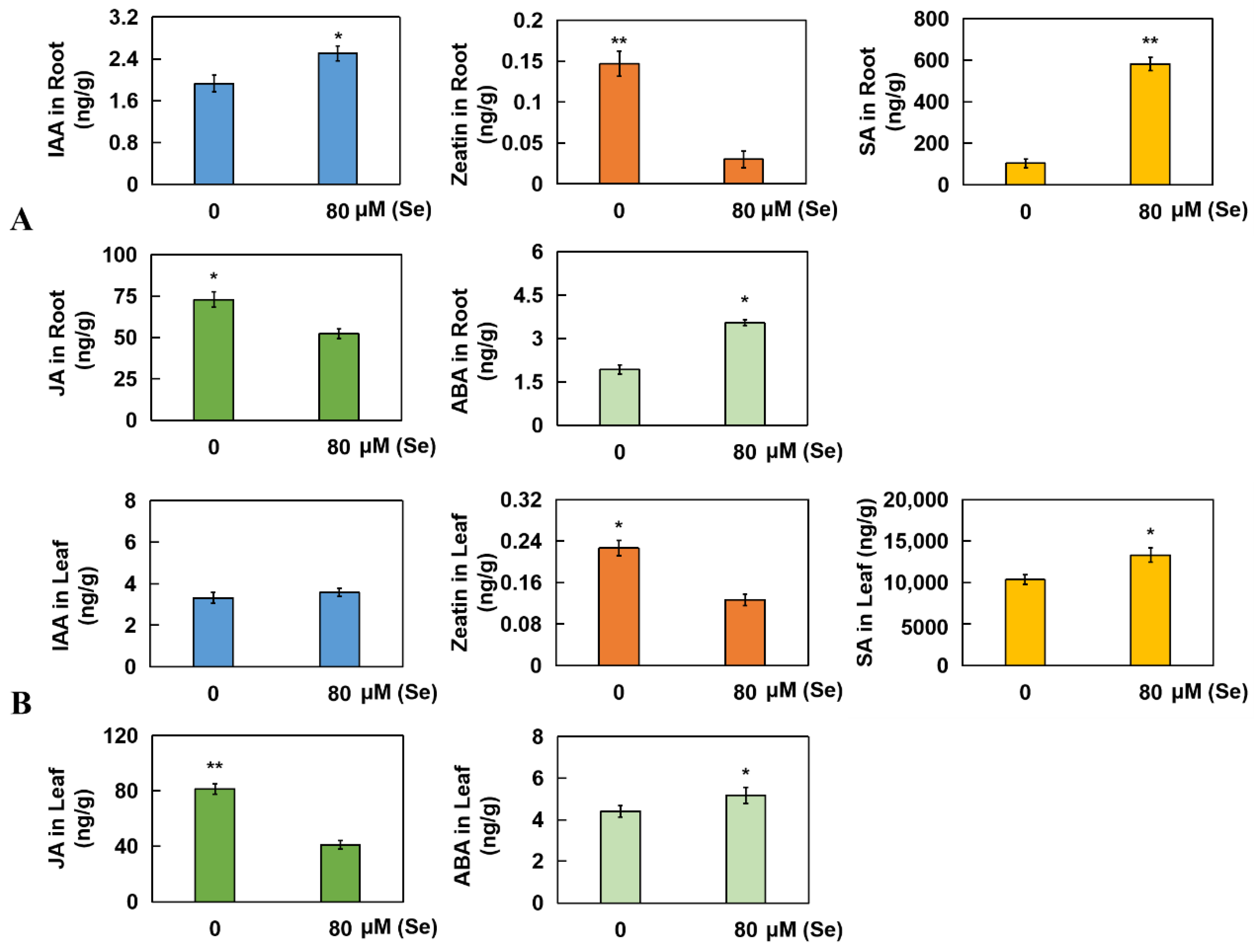
2.6. Determination of Five Kinds of Hormones from Roots and Leaves in Rice
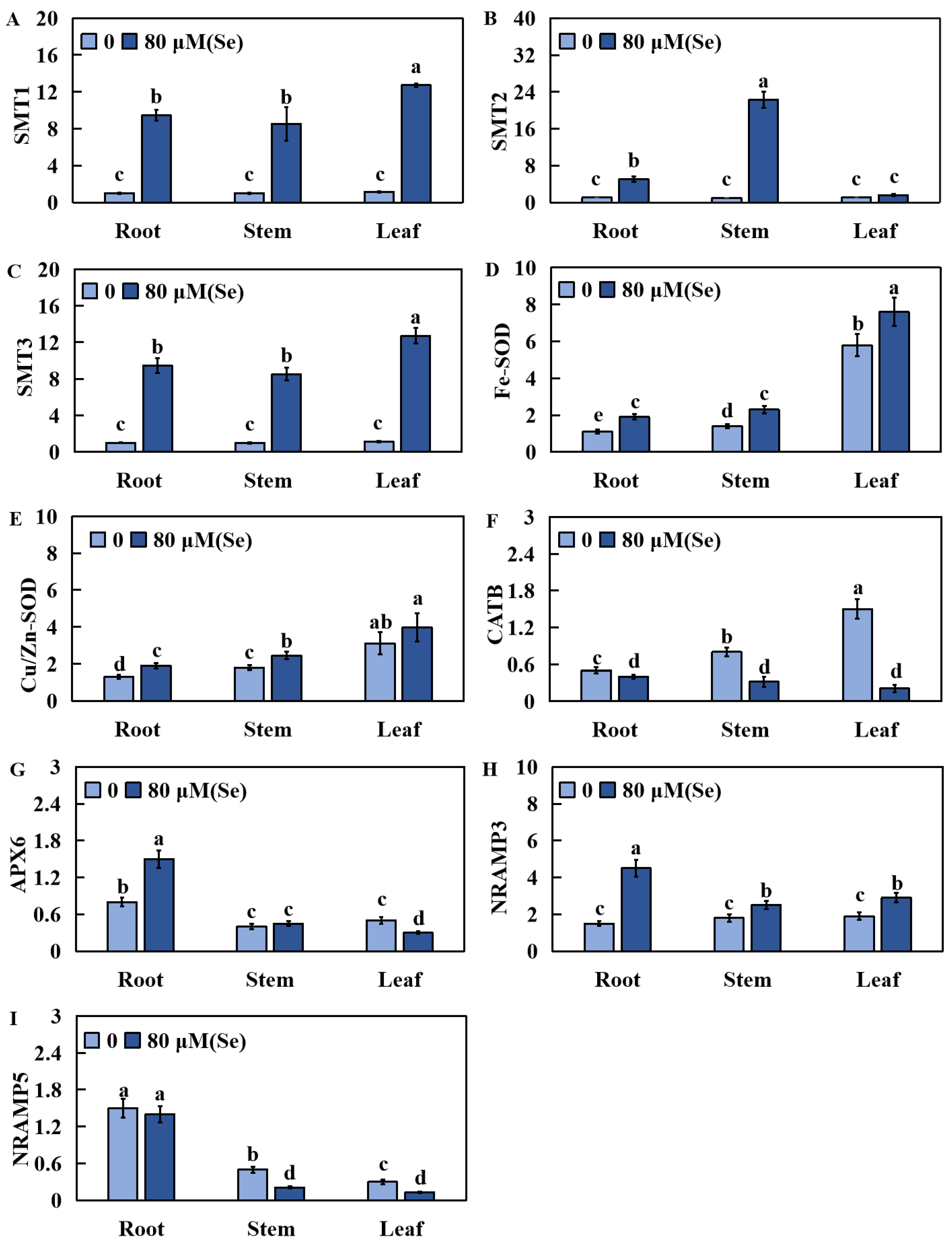
2.7. Effects of Se Poisoning Stress on the Relative Expression of Nine Related Genes in Rice
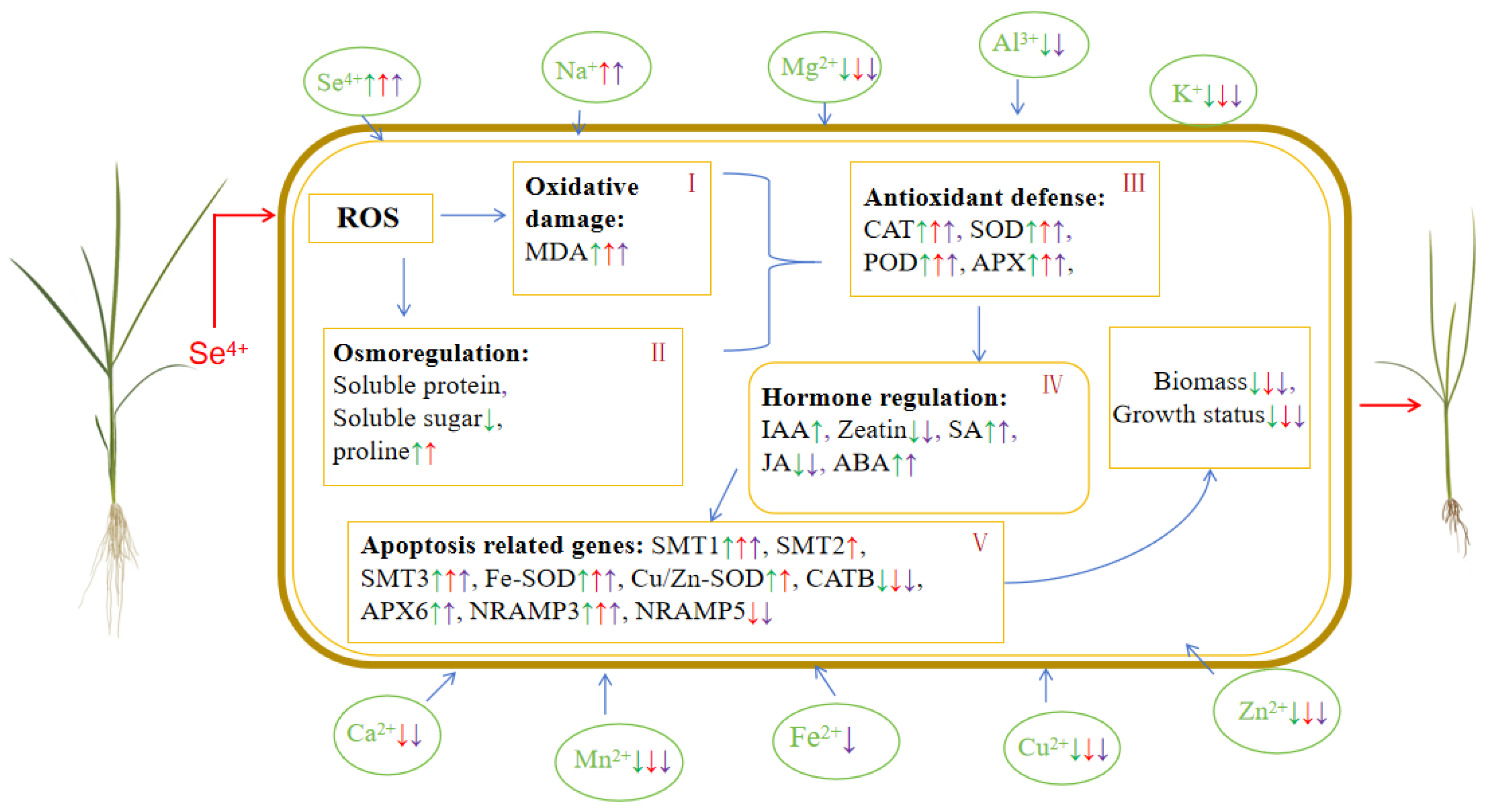
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Material Handling
4.3. Determination of Fresh and Dry Weight and Plant Height of Rice
4.4. Surveying of Root Phenotype Data
4.5. Surveying of Metal Ion Levels in Rice Stems, Leaves, and Roots
4.6. Determination of Physiological Response Indices and Chlorophyll Content in Rice
4.7. Determination of the Levels of Six Kinds of Hormones in Rice Leaves and Roots
4.8. cDNA Extraction and qRNA Analysis
4.9. Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richard, C.; Elizabeth, A.H. Selenium transport and metabolism in plants: Phytoremediation and biofortification implications. J. Hazard. Mater. 2021, 404, 124178. [Google Scholar]
- Schiavon, M.; Nardi, S.; Dalla, F.; Ertani, A. Selenium biofortification in the 21st century: Status and challenges for healthy human nutrition. Plant Soil. 2020, 453, 245–270. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Z.; Gong, P.; Yao, W.; Ba, Q.; Wang, H. Review on the health-promoting effect of adequate selenium status. Front. Nutr. 2023, 10, 1136458. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, X.; Liu, T.; Fu, H.; Yuan, X.; Cheng, Q.; Li, X. Strategies for fertilizer management to achieve higher yields and environmental and fertilizer benefits of rice production in China. Sci. Total Environ. 2023, 904, 166325. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhu, Y.; Zhang, S.; Xu, Q.; Guo, Y. Nitrate reductase involves in selenite reduction in Rahnella aquatilis HX2 and the characterization and anticancer activity of the biogenic selenium nanoparticles. J. Trace Elem. Med. Biol. 2024, 83, 127387. [Google Scholar] [CrossRef]
- Kuria, A.; Tian, H.; Li, M.; Wang, Y.; Aaseth, J.O.; Zang, J.; Cao, Y. Selenium status in the body and cardiovascular disease: A systematic review and meta-analysis. Cirt. Rev. Food Sci. 2021, 61, 3616–3625. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Deng, Y.; Zhao, Z.; Yang, D.; Wan, G.; Xu, X. Selenium nanoparticles based on Morinda officinalis polysaccharides: Characterization, anti-cancer activities, and immune-enhancing activities evaluation in vitro. Molecules 2023, 28, 2426. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Liu, X.; Ma, L.; Yang, Z.; Ma, C. Association between dietary selenium intake and severe abdominal aortic calcification in the United States: A cross-sectional study. Food Funct. 2024, 15, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Padariya, C.; Rutkowska, M.; Konieczka, P. The accessibility, necessity, and significance of certified reference materials for total selenium content and its species to improve food laboratories’ performance. Food Chem. 2023, 425, 136460. [Google Scholar] [CrossRef]
- Jabbari, P.; Yazdanpanah, O.; Benjamin, D.J.; Rezazadeh, K.A. Supplement use and increased risks of cancer: Unveiling the other side of the coin. Cancers 2024, 16, 880. [Google Scholar] [CrossRef]
- Chang, C.Y.; Yin, R.S.; Wang, X.; Shao, S.X.; Chen, C.Y.; Zhang, H. Selenium translocation in the soil-rice system in the Enshi seleniferous area, Central China. Sci. Total Environ. 2019, 669, 83–90. [Google Scholar] [CrossRef]
- Schweizer, U.; Fradejas, V.N. The significance of selenoproteins for human health revealed by inborn errors of metabolism. FASEB J. 2016, 30, 3669–3681. [Google Scholar] [CrossRef]
- Yang, H.; Yang, X.; Ning, Z.; Kwon, S.Y.; Li, M.L.; Tack, F.M.; Yin, R. The beneficial and hazardous effects of selenium on the health of the soil-plant-human system: An overview. J. Hazard. Mater. 2022, 422, 126876. [Google Scholar] [CrossRef]
- Ikram, S.; Li, Y.; Lin, C.; Yi, D.; Heng, W.; Li, Q.; Tao, L.; Hong, J.Y.; Wei, J.J. Selenium in plants: A nexus of growth, antioxidants, and phytohormones. J. Plant Physiol. 2024, 21, 154237. [Google Scholar] [CrossRef]
- Zou, J.; Su, S.; Chen, Z.; Liang, F.; Zeng, Y.; Cen, W.; Zhang, X.; Huang, D. Hyaluronic acid-modified selenium nanoparticles for enhancing the therapeutic efficacy of paclitaxel in lung cancer therapy. Artif. Cells Nanomed. B 2019, 47, 3456–3464. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Braat, S.; Graham, R.M. Selenium status is associated with insulin resistance markers in adults: Findings from the 2013 to 2018 National Health and Nutrition Examination Survey (NHANES). Front. Nutr. 2021, 8, 696024. [Google Scholar] [CrossRef]
- Casanova, P.; Monleon, D. Role of selenium in type 2 diabetes, insulin resistance and insulin secretion. World J. Diabetes 2023, 14, 147–158. [Google Scholar] [CrossRef]
- Tortelly, V.C.; Melo, D.F.; Matsunaga, A.M. The relevance of selenium to alopecias. Inter. J. Trichol. 2018, 10, 92–93. [Google Scholar] [CrossRef]
- Qu, L.; Xu, J.; Dai, Z.; Elyamine, A.M.; Huang, W.; Han, D.; Jia, W. Selenium in soil-plant system: Transport, detoxification and bioremediation. J. Hazard. Mater. 2023, 452, 131272. [Google Scholar] [CrossRef]
- Tao, J.; Leng, J.; Lei, X.; Wan, C.; Li, D.; Wu, Y.; Gao, J. Effects of selenium (Se) uptake on plant growth and yield in common buckwheat (Fagopyrum esculentum Moench). Field Crops Res. 2023, 302, 109070. [Google Scholar] [CrossRef]
- Jia, H.; Song, Z.; Wu, F.; Ma, M.; Li, Y.; Han, D.; Cui, H. Low selenium increases the auxin concentration and enhances tolerance to low phosphorous stress in tobacco. Environ. Exp. Bot. 2018, 153, 127–134. [Google Scholar] [CrossRef]
- Regni, L.; Palmerini, C.A.; Businelli, D.D.; Amato, R.; Mairech, H.; Proietti, P. Effects of selenium supplementation on olive under salt stress conditions. Sci. Hortic. 2021, 278, 109866. [Google Scholar] [CrossRef]
- Rady, M.M.; Belal, H.E.; Gadallah, F.M.; Semida, W.M. Selenium application in two methods promotes drought tolerance in Solanum lycopersicum plant by inducing the antioxidant defense system. Sci. Hortic. 2020, 266, 109290. [Google Scholar] [CrossRef]
- Shinmachi, F.; Buchner, P.; Stroud, J.L.; Parmar, S.; Zhao, F.J.; McGrath, S.P.; Hawkesford, M.J. Influence of sulfur deficiency on the expression of specific sulfate transporters and the distribution of sulfur, selenium, and molybdenum in wheat. Plant Physiol. 2010, 153, 327–336. [Google Scholar] [CrossRef]
- Terry, N.; Zayed, A.M.; De, S.M.; Tarun, A.S. Selenium in higher plants. Annu. Rev. Plant Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, B.; Li, W.; Che, R.; Deng, K.; Li, H.; Yu, F.; Ling, H.; Li, Y.; Chu, C. OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytol. 2014, 201, 1183–1191. [Google Scholar] [CrossRef]
- Cappa, J.J.; Cappa, P.J.; Mehdawi, A.F.; McAleer, J.M.; Simmons, M.; Pilon-Smits, E.A. Characterization of selenium and sulfur accumulation across the genus Stanleya (Brassicaceae): A field survey and common-garden experiment. Am. J. Bot. 2014, 101, 830–839. [Google Scholar] [CrossRef]
- Harris, J.; Schneberg, K.A.; Pilon-Smits, E.A. Sulfur–selenium–molybdenum interactions distinguish selenium hyperaccumulator Stanleya pinnata from non-hyperaccumulator Brassica juncea (Brassicaceae). Planta 2014, 239, 479–491. [Google Scholar] [CrossRef]
- Mazej, D.; Osvald, J.; Stibilj, V. Selenium species in leaves of chicory, dandelion, lamb’s lettuce and parsley. Food Chem. 2008, 107, 175–183. [Google Scholar] [CrossRef]
- Gigolashvili, T.; Kopriva, S. Transporters in plant sulfur metabolism. Front. Plant Sci. 2014, 5, 107212. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali, A.; Kour, N.; Bornhorst, J.; AlHarbi, K.; Rinklebe, J.; Chung, Y.S. Heavy metal induced oxidative stress mitigation and ROS scavenging in plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Najafi-Kakavand, S.; Abbas, S.; Shoaib, Y.; Anwar, S.; Sharifi, S.; Lu, G.; Siddique, K.H. Role of phytohormones in regulating cold stress tolerance: Physiological and molecular approaches for developing cold-smart crop plants. Plant Stress 2023, 8, 100152. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Mahmud, J.; Nahar, K.; Fujita, M. Selenium in plants: Boon or bane? Environ. Exp. Bot. 2020, 178, 104170. [Google Scholar] [CrossRef]
- Lanza, M.G.; Dos, R.A. Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiol. Biochem. 2020, 164, 27–43. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Role of selenium in plants against abiotic stresses: Phenological and molecular aspects. Mol. Plant Abiotic Stress Biol. Biotechnol. 2019, 12, 123–133. [Google Scholar]
- Chen, Z.; Lu, Y.; Dun, X.; Wang, X.; Wang, H. Research progress of selenium-enriched foods. Nutrients 2023, 15, 41–89. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.H.; Chen, L.C.; Xu, J.; Zhang, Y.L.; Pan, G.X. Determination of selenium concentration in rice and the effect of foliar application of Se-enriched fertiliser or sodium selenite on the selenium content of rice. J. Sci. Food Agric. 2002, 82, 869–872. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, W.; Wang, X. Selenium speciation and distribution characteristics in the rhizosphere soil of rice (Oryza sativa L.) Seedlings. Commun. Soil Sci. Plant Anal. 2010, 41, 1411–1425. [Google Scholar] [CrossRef]
- Sun, G.; Liu, X.; Williams, P.; Zhu, Y.G. Distribution and translocation of selenium from soil to grain and its speciation in paddy rice (Oryza sativa L.). Environ. Sci. Technol. 2010, 44, 6706–6711. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, X.; Yu, T.; Lin, K.; Ma, X.; Li, C.; Wang, Y. Environmental selenium and human longevity: An ecogeochemical perspective. Chemosphere 2024, 347, 140691. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, Y.; Yan, S.; Shi, H.; Nie, Y.; Zou, G.; Zhang, X.; Luo, L. Characterization of selenium accumulation of different rice genotypes in Chinese natural seleniferous soil. Plant Soil Environ. 2019, 65, 15–20. [Google Scholar] [CrossRef]
- Chen, L.; Yang, F.; Xu, J.; Hu, Y.; Hu, Q.; Zhang, Y.; Pan, G. Determination of selenium concentration of rice in China and effect of fertilization of selenite and selenate on selenium content of rice. J. Agric. Food. Chem. 2002, 50, 5128–5130. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, W.; Wang, C. Difference in selenium accumulation in shoots of two rice cultivars. Pedosphere 2006, 16, 646–653. [Google Scholar] [CrossRef]
- Song, T.; Su, X.; He, J.; Liang, Y.; Zhou, T.; Liu, C. Selenium (Se) uptake and dynamic changes of Se content in soil-plant systems. Environ. Sci. Pollut. R. 2018, 25, 34343–34350. [Google Scholar] [CrossRef]
- Kong, L.; Wang, M.; Bi, D. Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the growth of sorrel seedlings under salt stress. Plant Growth Regul. 2005, 45, 155–163. [Google Scholar] [CrossRef]
- Shen, J.; Jiang, C.; Yan, Y.; Zu, C. Selenium distribution and translocation in rice (Oryza sativa L.) under different naturally seleniferous soils. Sustainability 2019, 11, 520. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, X.; Yao, D.; Zhang, X.; Wong, M. Selenium geochemistry of paddy soils in Yangtze River Delta. Environ. Int. 2001, 26, 335–339. [Google Scholar] [CrossRef]
- Lima-Lessa, J.H.; Araujo, A.M.; Ferreira, L.A.; Silva-Júnior, E.C.; Oliveira, C.; Corguinha, A.P.; Martins, F.A.; Carvalho, H.W.; Guilherme, L.R.; Lopes, G. Agronomic biofortification of rice (Oryza sativa L.) with selenium and its effect on element distributions in biofortified grains. Plant Soil. 2019, 444, 331–342. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A. The fascinating facets of plant selenium accumulation–biochemistry, physiology, evolution and ecology. New Phytol. 2017, 213, 1582–1596. [Google Scholar] [CrossRef]
- Liang, Y.; Su, Y.; Li, L.; Huang, X.; Panhwar, F.H.; Zheng, T.; Tang, Z.; Ei, H.H.; Farooq, M.U.; Zeng, R.; et al. Quick selenium accumulation in the selenium-rich rice and its physiological responses in changing selenium environments. BMC Plant Biol. 2019, 19, 559. [Google Scholar] [CrossRef]
- Yin, H.; Qi, Z.; Li, M.; Ahammed, G.; Chu, X.; Zhou, J. Selenium forms and methods of application differentially modulate plant growth, photosynthesis, stress tolerance, selenium content and speciation in Oryza sativa L. Ecotoxicol. Environ. Saf. 2019, 169, 911–917. [Google Scholar]
- Hamilton, S.; Teven, J. Review of selenium toxicity in the aquatic food chain. Sci. Total Environ. 2004, 326, 1–31. [Google Scholar] [CrossRef]
- Wu, H.; Fan, S.; Gong, H.; Guo, J. Roles of salicylic acid in selenium-enhanced salt tolerance in tomato plants. Plant Soil. 2023, 484, 569–588. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, G.; Qin, H.; Guan, Y.; Wang, T.; Ni, W.; Ge, S. Metabolomics combined with physiology and transcriptomics reveal key metabolic pathway responses in apple plants exposure to different selenium concentrations. J. Hazard. Mater. 2024, 464, 132953. [Google Scholar] [CrossRef]
- Dos-Reis, A.R.; Boleta, E.H.; Alves, C.Z.; Cotrim, M.F.; Barbosa, J.Z.; Silva, V.M.; Porto, R.L.; Lanza, M.G.; Lavres, J.; Gomes, M.H.; et al. Selenium toxicity in upland field-grown rice: Seed physiology responses and nutrient distribution using the μ-XFR technique. Ecotoxicol. Environ. Saf. 2020, 190, 110147. [Google Scholar] [CrossRef]
- Du, B.; Luo, H.; He, L.; Zhang, L.; Liu, Y.; Mo, Z.; Tang, X. Rice seed priming with sodium selenate: Effects on germination, seedling growth, and biochemical attributes. Sci. Rep. 2019, 9, 4311. [Google Scholar] [CrossRef]
- Nedjimi, B. Selenium as a powerful trace element for mitigation of plant salt stress: A review. J. Trace Elem. Miner. 2024, 8, 100123. [Google Scholar] [CrossRef]
- Labanowska, M.; Filek, M.; Koscielniak, J.; Kurdziel, M.; Kulis, E.; Hartikainen, H. The effects of short-term selenium stress on Polish and Finnish wheat seedlings-EPR, enzymatic and fluorescence studies. Plant Physiol. 2012, 169, 275–284. [Google Scholar] [CrossRef]
- Jan, S.; Bhardwaj, R.; Sharma, N.R.; Singh, R. Unraveling the role of plant growth regulators and plant growth promoting rhizobacteria in phytoremediation. J. Plant Growth Regul. 2024, 43, 1–17. [Google Scholar] [CrossRef]
- De-Lima, J.H.; Raymundo, J.F.; Corguinha, A.P.; Martins, F.A.; Araujo, A.M.; Santiago, F.E.; Lopes, G. Strategies for applying selenium for biofortification of rice in tropical soils and their effect on element accumulation and distribution in grains. J. Cereal Sci. 2020, 96, 103125. [Google Scholar] [CrossRef]
- Andrade, F.R.; Silva, G.N.; Guimarães, K.C.; Barreto, H.B.F.; Souza, K.R.D.; Guilherme, L.R.G.; Dos-Reis, A.R. Selenium protects rice plants from water deficit stress. Ecotoxicol. Environ. Saf. 2018, 164, 562–570. [Google Scholar] [CrossRef]
- Dreyer, B.H.; Schippers, J.H.M. Copper-Zinc superoxide dismutases in plants: Evolution, enzymatic properties, and beyond. Annu. Plant Rev. 2018, 2, 933–968. [Google Scholar]
- Su, W.; Raza, A.; Gao, A.; Jia, Z.; Zhang, Y.; Hussain, M.A.; Mehmood, S.S.; Cheng, Y.; Lv, Y.; Zou, X. Genome-wide analysis and expression profile of superoxide dismutase (SOD) gene family in rapeseed (Brassica napus L.) under different hormones and abiotic stress conditions. Antioxidants 2021, 10, 1182. [Google Scholar] [CrossRef]
- Xiao, H.L.; Wang, C.P.; Khan, N.; Chen, M.; Fu, W.; Guan, L.; Leng, X. Genome-wide identification of the class III POD gene family and their expression profiling in grapevine (Vitis vinifera L.). BMC Genom. 2020, 21, 444. [Google Scholar] [CrossRef]
- Hiraga, S.; Sasaki, K.; Ito, H.; Ohashi, Y.; Matsui, H. A large family of class III plant peroxidases. Plant Cell Physiol. 2001, 42, 462. [Google Scholar] [CrossRef]
- Daudi, A.; Cheng, Z.; O’Brien, J.A.; Mammarella, N.; Khan, S.; Ausubel, F.M.; Bolwell, G.P. The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 2012, 24, 275–287. [Google Scholar] [CrossRef]
- Lin, C.Y.; Li, Q.; Tunlaya-Anukit, S.; Shi, R.; Sun, Y.H.; Wang, J.P.; Chiang, V.L. A cell wall-bound anionic peroxidase, PtrPO21, is involved in lignin polymerization in Populus trichocarpa. Tree Genet. Genomes 2016, 12, 22. [Google Scholar] [CrossRef]
- Kim, B.H.; Kim, S.Y.; Nam, K.H. Genes encoding plant-specific class III peroxidases are responsible for increased cold tolerance of the brassinosteroid-insensitive 1 mutant. Mol. Cells 2012, 34, 539–548. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.J.; Yun, L.; Ji, L.; Li, G.H.; Ji, M.C.; Shi, Y.; Zheng, X. Catalase (CAT) gene family in wheat (Triticum aestivum L.): Evolution, expression pattern and function analysis. Int. J. Mol. Sci. 2022, 23, 542. [Google Scholar] [CrossRef]
- Wu, B.; Binbin, W. Comparative analysis of ascorbate peroxidases (APXs) from selected plants with a special focus on Oryza sativa employing public databases. PLoS ONE 2019, 14, 0226543. [Google Scholar] [CrossRef]
- Raza, A.; Su, W.; Gao, A.; Mehmood, S.S.; Hussain, M.A.; Nie, W.L.; Lv, Y.; Zhang, X.L. Catalase (CAT) gene family in rapeseed (Brassica napus L.): Genome-wide analysis, identification, and expression pattern in response to multiple hormones and abiotic stress conditions. Int. J. Mol. Sci. 2021, 22, 4281. [Google Scholar] [CrossRef]
- Raza, A.; Sharif, Y.; Chen, K.; Wang, L.H.; Fu, H.W.; Zhuang, Y.H.; Chitikineni, A.; Chen, H.; Zhang, C.; Varshney, R.K. Genome-wide characterization of ascorbate peroxidase gene family in peanut (Arachis hypogea L.) revealed their crucial role in growth and multiple stress tolerance. Plant Sci. 2022, 13, 962182. [Google Scholar] [CrossRef]
- Ling, J.Y.; Tan, J.H.; Chen, H.; Yang, Z.Q.; Luo, Q.F.; Jia, J. Physiology, transcriptome and root exudates analysis of response to aluminum stress in Pinus massoniana. Forests 2023, 14, 1410. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, M.; Zhang, F.; Feng, D.; Yang, S.; Xue, Y.; Liu, Y. Physiological mechanism through which al toxicity inhibits peanut root growth. Plants 2024, 13, 325. [Google Scholar] [CrossRef]
- Pirzadah, T.B.; Malik, B.; Tahir, I.; Rehman, R.U.; Hakeem, K.R.; Alharby, H.F. Aluminium stress modulates the osmolytes and enzyme defense system in Fagopyrum species. Plant Physiol. Biochem. 2019, 144, 178–186. [Google Scholar] [CrossRef]
- Li, F.; Wu, X.J.; Wu, W. Effects of malondialdehyde-induced protein oxidation on the structural characteristics of rice protein. Int. J. Food Sci. Technol. 2020, 55, 760–768. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, W.; Dai, H.; Cao, F.; Zhang, G.; Wu, F. Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J. Hazard. Mater. 2012, 235, 343–351. [Google Scholar] [CrossRef]
- Cakmak, I.; Wang, S.; Zhang, F.; Guo, S. Synergistic and antagonistic interactions between potassium and magnesium in higher plants. Crop J. 2021, 9, 249–256. [Google Scholar]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role of magnesium fertilisers in agriculture: Plant–soil continuum. Crop Pasture Sci. 2015, 66, 1219–1229. [Google Scholar] [CrossRef]
- Taylor, G.J.; Blarney, F.P.; Edwards, D. Antagonistic and synergistic interactions between aluminum and manganese on growth of Vigna unguiculata at low ionic strength. Physiol. Plant. 1998, 104, 183–194. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Sors, T.G.; Ellis, D.R.; Salt, D.E. Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth. Res. 2005, 86, 373–389. [Google Scholar] [CrossRef]
- LeDuc, D.L.; Tarun, A.S.; Montes-Bayon, M.; Meija, J.; Malit, M.F.; Wu, C.P.; AbdelSamie, M.; Chiang, C.Y.; Tagmount, A.; DeSouza, M.; et al. Overexpression of selenocysteine methyltransferase in Arabidopsis and Indian mustard increases selenium tolerance and accumulation. Plant Physiol. 2004, 135, 377–383. [Google Scholar] [CrossRef]
- Nikiforova, V.; Freitag, J.; Kempa, S. Transcriptome analysis of sulfur depletion in Arabidopsis thaliana: Interlacing of biosynthetic pathways provides response specificity. Plant J. 2003, 33, 633–650. [Google Scholar] [CrossRef]
- Tan, Z.J.; Li, J.X.; Guan, J.H.; Wang, C.H.; Zhang, Z.; Shi, G.R. Genome-wide identification and expression analysis reveals roles of the NRAMP gene family in iron/cadmium interactions in peanut. Int. J. Mol. Sci. 2023, 24, 1713. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Han, P.P.; Chen, L.Y.; Walk, T.C.; Li, Y.S.; Hu, X.J.; Xie, L.H.; Liao, H.; Liao, X. Genome-wide identification and expression analysis of NRAMP family genes in soybean (Glycine max L.). Plant Sci. 2017, 8, 1436. [Google Scholar] [CrossRef]
- Yamaji, N.; Sasaki, A.; Yokosho, K.; Ma, J.F. A node-based switch for preferential distribution of manganese in rice. Nat. Commun. 2013, 4, 2442. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Takahashi, R.; Bashir, K.; Shimo, H.; Senoura, T.; Sugimoto, K.; Nishizawa, N.K. Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci. Rep. 2012, 2, 1286. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, Z.; Li, X.; Liu, H.; Li, N.; Wei, S. Mitigation of rice cadmium (Cd) accumulation by joint application of organic amendments and selenium (Se) in high-Cd-contaminated soils. Chemosphere 2020, 241, 125106. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Chen, W.; Zhu, Y.; Yang, J.; Mazhar, S.H.; Zhao, P.; Feng, R. Efficiency and risks of selenite combined with different water conditions in reducing uptake of arsenic and cadmium in paddy rice. Environ. Pollut. 2020, 262, 114283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium biofortification and interaction with other elements in plants: A review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef]
- Tao, Q.; Jupa, R.; Liu, Y.K.; Luo, J.P.; Li, J.X.; Kováč, J.; Li, T.Q. Abscisic acid-mediated modifications of radial apoplastic transport pathway play a key role in cadmium uptake in hyperaccumulator Sedum alfredii. Plant Cell Environ. 2019, 42, 1425–1440. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Wang, X.; Lu, W.; Zhao, Z.; Hao, W.; Du, R.; Li, Z.; Lin, L. Abscisic acid promotes selenium absorption, metabolism and toxicity via stress-related phytohormones regulation in Cyphomandra betacea Sendt (Solanum betaceum Cav). J. Hazard. Mater. 2024, 461, 132642. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, Z.; Chu, Z. Emerging roles of salicylic acid in plant saline stress tolerance. Int. J. Mol. Sci. 2023, 24, 43388. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; La, S.K.; Bishi, S.K.; Singh, A.K. Phytohormone signalling and cross-talk to alleviate aluminium toxicity in plants. Plant Cell Rep. 2021, 40, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Rahman, M.M.; Siddiqui, M.N.; Fujita, M.; Tran, L.S. Salicylic acid antagonizes selenium phytotoxicity in rice: Selenium homeostasis, oxidative stress metabolism and methylglyoxal detoxification. J. Hazard. Mater. 2020, 394, 122572. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, H.; Hu, T.; Wang, Z.; Wu, W.; Liang, Y.; Guo, Y. Sunflower resistance against Sclerotinia sclerotiorum is potentiated by selenium through regulation of redox homeostasis and hormones signaling pathways. Environ. Sci. Pollut. R. 2022, 29, 38097–38109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.L.; Zhou, S.; Yang, D.Y.; Fan, Z.J. Revealing shared and distinct genes responding to JA and SA signaling in Arabidopsis by meta-analysis. Plant Sci. 2020, 11, 908. [Google Scholar]
- Dai, Z.; Yuan, Y.; Huang, H.; Hossain, M.M.; Xiong, S.; Cao, M.; Ma, L.Q.; Tu, S. Methyl jasmonate mitigates high selenium damage of rice via altering antioxidant capacity, selenium transportation and gene expression. Sci. Total Environ. 2021, 756, 143848. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.D.; Strader, L.C. Intrinsic and extrinsic regulators of Aux/IAA protein degradation dynamics. Trends Biochem. Sci. 2022, 47, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Sheen, J.; Müller, B. Cytokinin signaling networks. Annu. Rev. Plant Biol. 2012, 63, 353–380. [Google Scholar] [CrossRef] [PubMed]
- Yürekli, F.; Türkan, I.; Porgali, Z.B.; Topçuoglu, S.F. Indoleacetic acid, gibberellic acid, zeatin, and abscisic acid levels in NaCl-treated tomato species differing in salt tolerance. Isr. J. Plant Sci. 2001, 49, 269–278. [Google Scholar] [CrossRef]
- Kasahara, H. Current aspects of auxin biosynthesis in plants. Biosci. Biotechnol. Biochem. 2016, 80, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Petrášek, J. Why plants need more than one type of auxin. Plant Sci. 2011, 180, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Tripathi, D.K.; Baluška, F.; Mukherjee, S. Auxin-mediated molecular mechanisms of heavy metal and metalloid stress regulation in plants. Environ. Exp. Bot. 2022, 196, 104796. [Google Scholar] [CrossRef]
- Qi, T.; Yang, W.; Hassan, M.J.; Liu, J.; Yang, Y.; Zhou, Q.; Peng, Y. Genome-wide identification of Aux/IAA gene family in white clover (Trifolium repens L.) and functional verification of TrIAA18 under different abiotic stress. BMC Plant Biol. 2024, 24, 346. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Cheng, Y.; Suzuki, N.; Guo, X.; Guo, H.; Ogra, Y. Speciation of selenium in brown rice fertilized with selenite and effects of selenium fertilization on rice proteins. Int. J. Mol. Sci. 2018, 19, 3494. [Google Scholar] [CrossRef]
- He, L.X.; Zheng, A.X.; Du, B.; Luo, H.W.; Lu, R.H.; Chen, Y.L.; Zhang, T.T.; Lai, R.F.; Tang, X.R. Low-concentration sodium selenite applications improve oxidation resistance of filling-stage rice. Appl. Ecol. Environ. Res. 2019, 17, 989–998. [Google Scholar] [CrossRef]
- Zhang, W.; Guan, M.; Chen, M.; Lin, X.; Xu, P.; Cao, Z. Mutation of OsNRAMP5 reduces cadmium xylem and phloem transport in rice plants and its physiological mechanism. Environ. Pollut. 2024, 341, 122928. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.H.; Li, J.Y.; Chen, J.Y.; Yang, S.X.; Zhao, M.; Xue, Y.B. Transcriptome sequencing analysis of root in soybean responding to Mn poisoning. Int. J. Mol. Sci. 2023, 24, 12727. [Google Scholar] [CrossRef]
- Pornaro, C.; Macolino, S.; Menegon, A.; Richardson, M. WinRHIZO technology for measuring morphological traits of bermudagrass stolons. Agron. J. 2017, 109, 3007–3010. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Manousi, N.; Zachariadis, G.A. Determination of the toxic and nutrient element content of almonds, walnuts, hazelnuts and pistachios by ICP-AES. Separations 2021, 8, 28. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Zhou, J.; Shang, R.; Wang, Y. Comparative study on several determination methods of chlorophyll content in plants. IOP Conf. Ser. Mater. Sci. Eng. 2020, 730, 012066. [Google Scholar] [CrossRef]
- Cao, K.L.; Yin, Y.; Li, S.S.; Li, M.Q.; Tajdar, A.; Tan, S.Q.; Shi, W.P. Chemical composition and amino acids of Locusta migratoria (Orthoptera Acrididae) fed wheat seedlings (Triticum aestivum) or rape leaves (Brassica napus). J. Insects Food Feed 2024, 1, 1–10. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tadayon, M.R.; Nadeem, M.; Cheema, M.; Razmjoo, J. Proline-mediated changes in antioxidant enzymatic activities and the physiology of sugar beet under drought stress. Acta Physiol. Plant. 2019, 41, 23. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Escriva, C.; Dromant, M.; Borras, C.; Vina, J. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch. Biochem. Biophys. 2021, 709, 108641. [Google Scholar] [CrossRef]
- Yang, Z.; Qiu, D.; Jiang, K.; Du, M.; Li, H. Abatement effects of different soil amendments on continuous crop of Codonopsis pilosula. Plant Sci. 2024, 15, 1376362. [Google Scholar]
- Sadak, M.S.; El-Hameid, A.R.; Zaki, F.S.; Dawood, M.G.; El-Awadi, M.E. Physiological and biochemical responses of soybean (Glycine max L.) to cysteine application under sea salt stress. Bull. Nat. Res. Cent. 2020, 44, 1. [Google Scholar]
- Hadwan, M.H.; Hussein, M.J.; Mohammed, R.M.; Hadwan, A.M.; Saad Al-Kawaz, H.; Al-Obaidy, S.S.; Al-Talebi, Z.A. An improved method for measuring catalase activity in biological samples. Biol. Methods Protoc. 2024, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.; Mosaddeghi, M.R.; Dexter, A.R.; Sepehri, M. Maize water status and physiological traits as affected by root endophytic fungus Piriformospora indica under combined drought and mechanical stresses. Planta 2018, 247, 1229–1245. [Google Scholar] [CrossRef] [PubMed]
- Khew, C.Y.; Mori, I.C.; Matsuura, T.; Hirayama, T.; Harikrishna, J.A.; Lau, E.T.; Mercer, Z.J.A.; Hwang, S.S. Hormonal and transcriptional analyses of fruit development and ripening in different varieties of black pepper (Piper nigrum). J. Plant Res. 2020, 133, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.B.; Zhuang, Q.L.; Zhu, S.N.; Xiao, B.X.; Liang, C.Y.; Liao, H.; Tian, J. Genome wide transcriptome analysis reveals complex regulatory mechanisms underlying phosphate homeostasis in soybean nodules. Int. J. Mol. Sci. 2018, 19, 2924. [Google Scholar] [CrossRef]
- Zhuang, Q.L.; Xue, Y.B.; Yao, Z.F.; Zhu, S.N.; Liang, C.Y.; Liao, H.; Tian, J. Phosphate starvation responsive GmSPX5 mediates nodule growth through interaction with GmNF-YC4 in soybean (Glycine max). Plant J. 2021, 108, 1422–1438. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ma, J.; Li, F.; Zeng, X.; Wu, Z.; Huang, Y.; Xue, Y.; Wang, Y. High Concentrations of Se Inhibited the Growth of Rice Seedlings. Plants 2024, 13, 1580. https://doi.org/10.3390/plants13111580
Liu Y, Ma J, Li F, Zeng X, Wu Z, Huang Y, Xue Y, Wang Y. High Concentrations of Se Inhibited the Growth of Rice Seedlings. Plants. 2024; 13(11):1580. https://doi.org/10.3390/plants13111580
Chicago/Turabian StyleLiu, Ying, Jiayu Ma, Feng Li, Xiang Zeng, Zhengwei Wu, Yongxiang Huang, Yingbin Xue, and Yanyan Wang. 2024. "High Concentrations of Se Inhibited the Growth of Rice Seedlings" Plants 13, no. 11: 1580. https://doi.org/10.3390/plants13111580
APA StyleLiu, Y., Ma, J., Li, F., Zeng, X., Wu, Z., Huang, Y., Xue, Y., & Wang, Y. (2024). High Concentrations of Se Inhibited the Growth of Rice Seedlings. Plants, 13(11), 1580. https://doi.org/10.3390/plants13111580






