Investigating the Potential of Essential Oils from Citrus reticulata Leaves in Mitigating Memory Decline and Oxidative Stress in the Scopolamine-Treated Zebrafish Model
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of PGEO
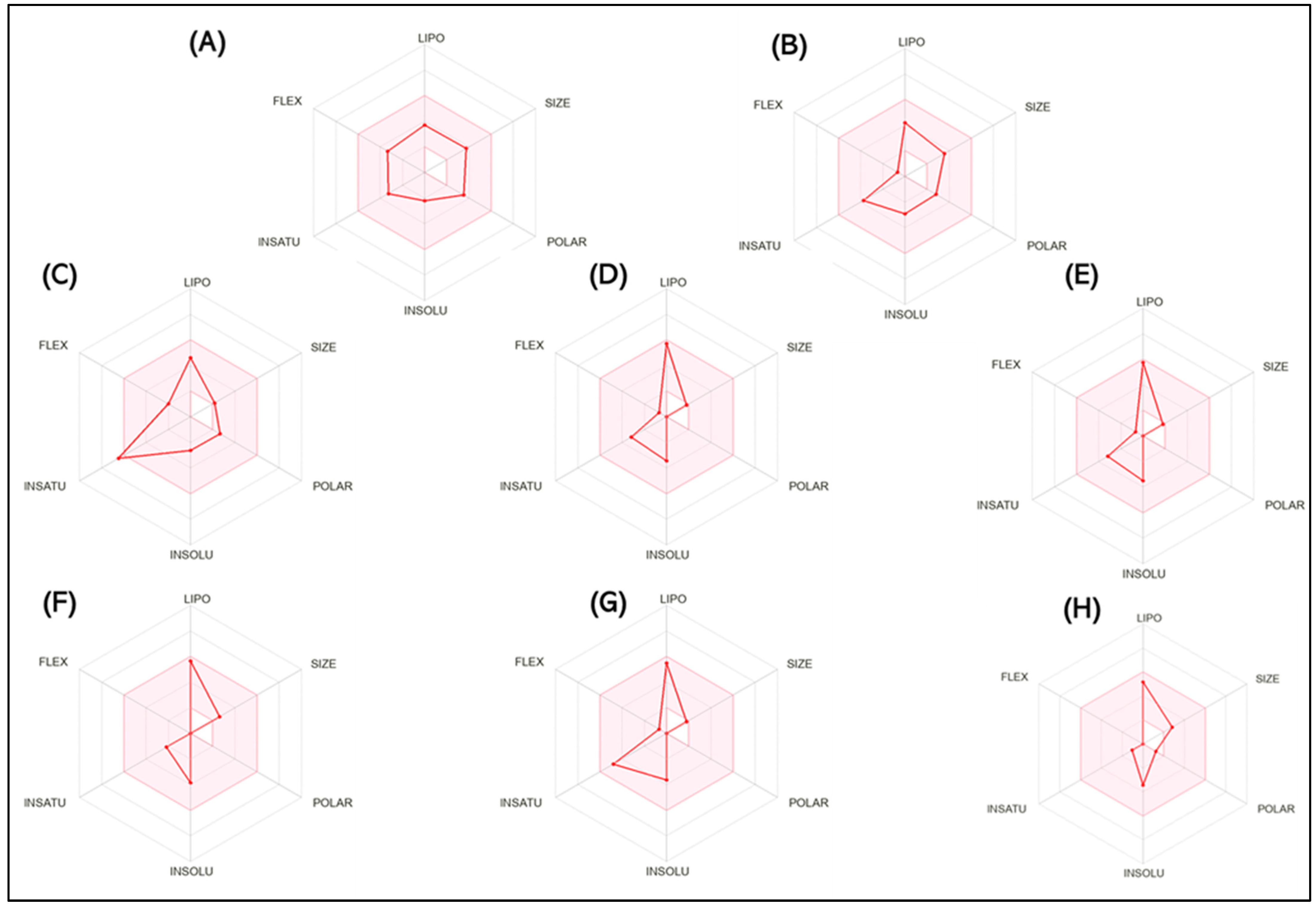
2.2. Highlighting the Biomedical Alert Structures of PGEO Main Biocompounds
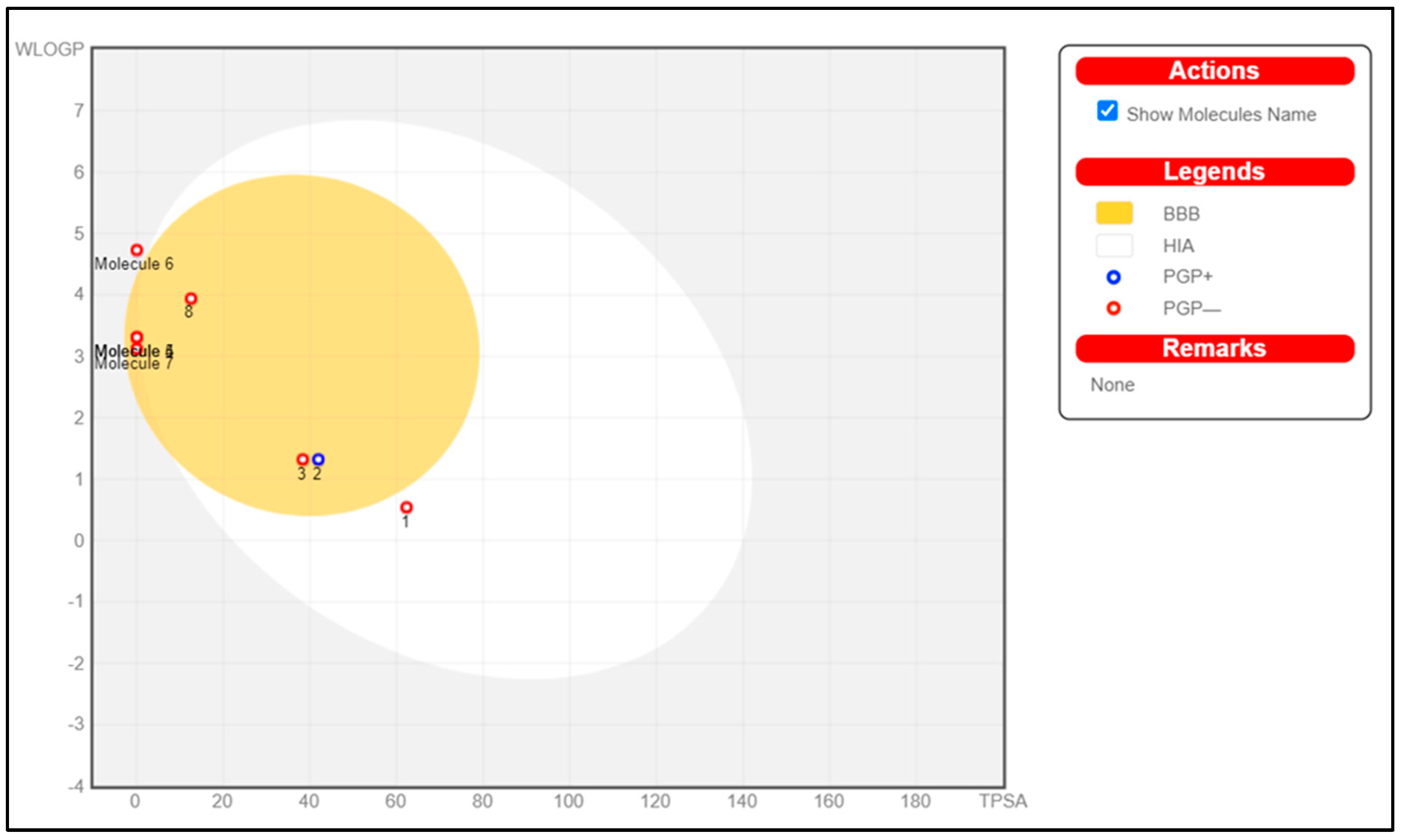
2.3. The Similarities between the Pharmacokinetic Properties of the Compounds
2.4. Evaluation of the Pharmacological Properties of PGEO
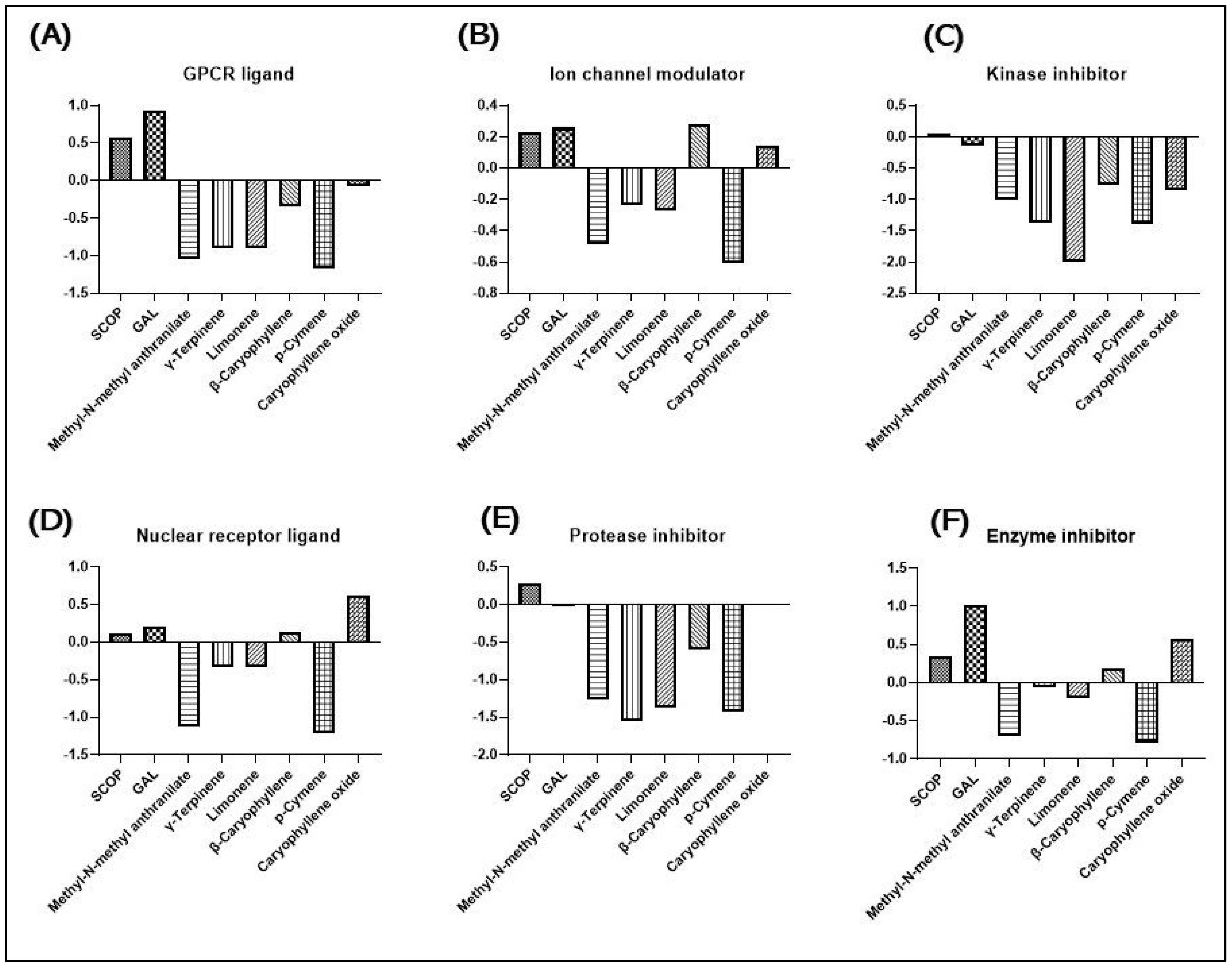
2.5. In Silico Protein Target Prediction of PGEO Biocompounds
2.6. Evaluation of PGEO Activity on the Anxiety Response
2.7. Evaluation of PGEO Impact on Spatial Memory and Response to Novelty
2.8. Evaluation of the PGEO Impact on Recognition Memory
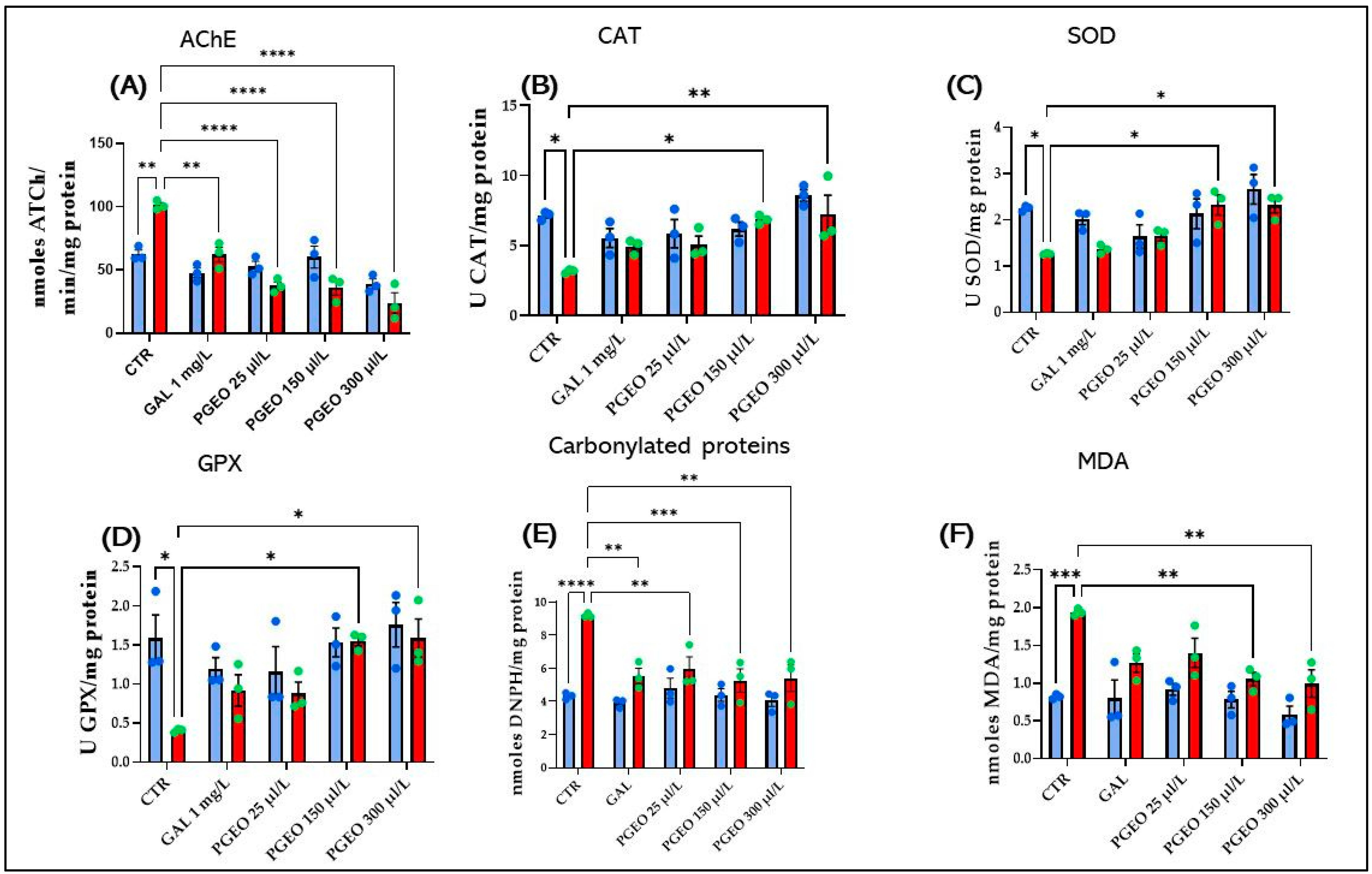
2.9. Effects of PGEO on AChE Activity
2.10. Effects of PGEO on Brain Oxidative Stress
2.11. Correlation Analyses between Behavioral and Biochemical Parameters
3. Materials and Methods
3.1. Plant Material and Essential Oil Preparation
3.2. Gas Chromatography/Mass Spectrometry (GC/MS) Analysis
3.3. Biomedical Alert and Structural Prediction of Biocompounds from PGEO, Drug Analogy
3.4. In Silico Estimation of Pharmacokinetic Profile of Biocompounds from PGEO
3.5. In Silico Protein Target Prediction for PGEO Compounds
3.6. Experimental Animals
3.7. Animal Care and Housing
3.8. Ethical Considerations
3.9. Experimental Animals and Method of Work
3.10. Animal Treatment
3.11. Behavioral Analysis
3.11.1. An Assessment of Anxiety State Using the Novel Tank Diving Test (NTT)
3.11.2. An Assessment of Spatial Memory and Response to Novelty Using the Y-Maze
3.11.3. The Novel Object Recognition Test (NOR): Assessing Reinstatement Memory in Zebrafish
3.11.4. Biochemical Parameters
3.12. Data Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Korczyn, A.D.; Grinberg, L.T. Is Alzheimer Disease a Disease? Nat. Rev. Neurol. 2024, 20, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer Disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Hoover, B.R.; Reed, M.N.; Su, J.; Penrod, R.D.; Kotilinek, L.A.; Grant, M.K.; Pitstick, R.; Carlson, G.A.; Lanier, L.M.; Yuan, L.L.; et al. Tau Mislocalization to Dendritic Spines Mediates Synaptic Dysfunction Independently of Neurodegeneration. Neuron 2010, 68, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Madnani, R.S. Alzheimer’s Disease: A Mini-Review for the Clinician. Front. Neurol. 2023, 14, 1178588. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Slowikowski, S.P.; Westaway, D.; Mount, T.J. Interactions between β-Amyloid and Central Cholinergic Neurons: Implications for Alzheimer’s Disease. CA J. Psychiatry Neurosci. 2004, 29, 427–468. [Google Scholar] [PubMed]
- Campanari, M.L.; García-Ayllón, M.S.; Belbin, O.; Galcerán, J.; Lleó, A.; Sáez-Valero, J. Acetylcholinesterase Modulates Presenilin-1 Levels and γ-Secretase Activity. J. Alzheimer’s Dis. 2014, 41, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.P.; de Castro, A.A.; Soares, F.V.; da Cunha, E.F.F.; Ramalho, T.C. Future Therapeutic Perspectives into the Alzheimer’s Disease Targeting the Oxidative Stress Hypothesis. Molecules 2019, 24, 4410. [Google Scholar] [CrossRef]
- Golde, T.E. Alzheimer’s Disease—The Journey of a Healthy Brain into Organ Failure. Mol. Neurodegener. 2022, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.; Nazar, F.H.; Makpol, S.; Teoh, S.L. Zebrafish: A Pharmacological Model for Learning and Memory Research. Molecules 2022, 27, 7374. [Google Scholar] [CrossRef]
- Shenoy, A.; Banerjee, M.; Upadhya, A.; Bagwe-Parab, S.; Kaur, G. The Brilliance of the Zebrafish Model: Perception on Behavior and Alzheimer’s Disease. Front. Behav. Neurosci. 2022, 16, 861155. [Google Scholar] [CrossRef]
- Elhawary, E.A.; Nilofar, N.; Zengin, G.; Eldahshan, O.A. Variation of the Essential Oil Components of Citrus aurantium Leaves upon Using Different Distillation Techniques and Evaluation of Their Antioxidant, Antidiabetic, and Neuroprotective Effect against Alzheimer’s Disease. BMC Complement. Med. Ther. 2024, 24, 73. [Google Scholar] [CrossRef]
- Abdelghffar, E.A.; El-Nashar, H.A.S.; Al-Mohammadi, A.G.A.; Eldahshan, O.A. Orange Fruit (Citrus sinensis) Peel Extract Attenuates Chemotherapy-Induced Toxicity in Male Rats. Food Funct. 2021, 12, 9443–9455. [Google Scholar] [CrossRef] [PubMed]
- Ashmawy, A.; Mostafa, N.; Eldahshan, O. GC/MS Analysis and Molecular Profiling of Lemon Volatile Oil against Breast Cancer. J. Essent. Oil Bear. Plants 2019, 22, 903–916. [Google Scholar] [CrossRef]
- Eldahshan, O.A.; Halim, A.F. Comparison of the Composition and Antimicrobial Activities of the Essential Oils of Green Branches and Leaves of Egyptian Navel Orange (Citrus sinensis (L.) Osbeck Var. Malesy). Chem. Biodivers. 2016, 13, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, Z.; Fleisher, A. Mandarin Leaf Oil (Citrus reticulata Blanco). J. Essent. Oil Res. 1990, 2, 331–334. [Google Scholar] [CrossRef]
- Yi, F.; Jin, R.; Sun, J.; Ma, B.; Bao, X. Evaluation of Mechanical-Pressed Essential Oil from Nanfeng Mandarin (Citrus reticulata Blanco Cv. Kinokuni) as a Food Preservative Based on Antimicrobial and Antioxidant Activities. LWT 2018, 95, 346–353. [Google Scholar] [CrossRef]
- Tao, N.; Jia, L.; Zhou, H. Anti-Fungal Activity of Citrus reticulata Blanco Essential Oil against Penicillium italicum and Penicillium digitatum. Food Chem. 2014, 153, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Eldahshan, O.A. Comparison of Chemical and Antimicrobial Studies of Egyptian Mandarin Leaves and Green Branches Volatile Oil. Eur. J. Med. Plants 2015, 5, 248–254. [Google Scholar] [CrossRef]
- Elkousy, R.H.; Mostafa, N.M.; Abd-alkhalek, A.M.; El Hassab, M.A.; Al-Rashood, S.T.; Eldehna, W.M.; Eldahshan, O.A. GC/MS Analysis and Potential Synergistic Effect of Mandarin and Marjoram Oils on Helicobacter Pylori. J. Enzyme Inhib. Med. Chem. 2022, 37, 1610–1619. [Google Scholar] [CrossRef]
- Fayed, S.A. Antioxidant and Anticancer Activities of Citrus Reticulate (Petitgrain Mandarin) and Pelargonium Graveolens (Geranium) Essential Oils. Res. J. Agric. Biol. Sci. 2009, 5, 740–747. [Google Scholar]
- Petretto, G.L.; Sarais, G.; Maldini, M.T.; Foddai, M.; Tirillini, B.; Rourke, J.P.; Chessa, M.; Pintore, G. Citrus Monstruosa Discrimination among Several Citrus Species by Multivariate Analysis of Volatiles: A Metabolomic Approach. J. Food Process. Preserv. 2016, 40, 950–957. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Amparo Blázquez, M.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 433929. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An Integrated Online Platform for Accurate and Comprehensive Predictions of ADMET Properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Bickerton, G.R.; Paolini, G.V.; Besnard, J.; Muresan, S.; Hopkins, A.L. Quantifying the Chemical Beauty of Drugs. Nat. Chem. 2012, 4, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Azman, M.; Sabri, A.H.; Anjani, Q.K.; Mustaffa, M.F.; Hamid, K.A. Intestinal Absorption Study: Challenges and Absorption Enhancement Strategies in Improving Oral Drug Delivery. Pharmaceuticals 2022, 15, 975. [Google Scholar] [CrossRef] [PubMed]
- Waldeck, F. The Intestinal Absorption of Scopolamine-n-Butylbromide (Buscopan®) from Isolated Loops in Conscious Rats. Eur. J. Pharmacol. 1969, 8, 108–113. [Google Scholar] [CrossRef]
- Rodríguez-Mejía, U.U.; Viveros-Paredes, J.M.; Zepeda-Morales, A.S.M.; Carrera-Quintanar, L.; Zepeda-Nuño, J.S.; Velázquez-Juárez, G.; Delgado-Rizo, V.; García-Iglesias, T.; Camacho-Padilla, L.G.; Varela-Navarro, E.; et al. β-Caryophyllene: A Therapeutic Alternative for Intestinal Barrier Dysfunction Caused by Obesity. Molecules 2022, 27, 6156. [Google Scholar] [CrossRef]
- Lombardo, F.; Bentzien, J.; Berellini, G.; Muegge, I. In Silico Models of Human PK Parameters. Prediction of Volume of Distribution Using an Extensive Data Set and a Reduced Number of Parameters. J. Pharm. Sci. 2021, 110, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Bhukya, R.; Kumari, A.; Amilpur, S.; Dasari, C.M. PPred-PCKSM: A Multi-Layer Predictor for Identifying Promoter and Its Variants Using Position Based Features. Comput. Biol. Chem. 2022, 97, 107623. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, E.A.; Noorani, B.; Alqahtani, F.; Bhalerao, A.; Raut, S.; Sivandzade, F.; Cucullo, L. Understanding the Brain Uptake and Permeability of Small Molecules through the BBB: A Technical Overview. J. Cereb. Blood Flow Metab. 2021, 41, 1797–1820. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel Integral Membrane Proteins Localizing at Tight Junctions with No Sequence Similarity to Occludin. J. Cell Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Klinkenberg, I.; Blokland, A. The Validity of Scopolamine as a Pharmacological Model for Cognitive Impairment: A Review of Animal Behavioral Studies. Neurosci. Biobehav. Rev. 2010, 34, 1307–1350. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.A. Anticholinergic Drugs, Blood-Brain-Barrier and Tonic Immobility in Chickens. Physiol. Behav. 1982, 29, 67–71. [Google Scholar] [CrossRef]
- Basagni, F.; Ortega, J.A.; Bertozzi, S.M.; Armirotti, A.; Summa, M.; Bertorelli, R.; Bartolini, M.; Mellor, I.R.; Bedeschi, M.; Bottegoni, G.; et al. Galantamine-Memantine Hybrids for Alzheimer’s Disease: The Influence of Linker Rigidity in Biological Activity and Pharmacokinetic Properties. Eur. J. Med. Chem. 2023, 261, 115803. [Google Scholar] [CrossRef]
- Ravikumar, C.; Ranjith, D. SwissADME Predictions of Pharmacokinetics and Drug-Likeness Properties of Small Molecules Present in Ipomoea mauritiana Jacq. J. Pharmacogn. Phytochem. 2019, 8, 2063–2073. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Martinez, J.D.; Bueno, M.; Alvarez-Rivera, G.; Tudela, J.; Ibanez, E.; Cifuentes, A. In Vitro Neuroprotective Potential of Terpenes from Industrial Orange Juice By-Products. Food Funct. 2021, 12, 302–314. [Google Scholar] [CrossRef]
- Satou, T.; Hayakawa, M.; Kasuya, H.; Masuo, Y.; Koike, K. Mouse Brain Concentrations of α-Pinene, Limonene, Linalool, and 1,8-Cineole Following Inhalation. Flavour. Fragr. J. 2017, 32, 36–39. [Google Scholar] [CrossRef]
- Van Beijsterveldt, L.; Geerts, R.; Verhaeghe, T.; Willems, B.; Bode, W.; Lavrijsen, K.; Meuldermans, W. Pharmacokinetics and Tissue Distribution of Galantamine and Galantamine-Related Radioactivity after Single Intravenous and Oral Administration in the Rat. Arzneim.-Forsch./Drug Res. 2004, 54, 85–94. [Google Scholar] [CrossRef]
- Noetzli, M.; Eap, C.B. Pharmacodynamic, Pharmacokinetic and Pharmacogenetic Aspects of Drugs Used in the Treatment of Alzheimer’s Disease. Clin. Pharmacokinet. 2013, 52, 225–241. [Google Scholar] [CrossRef]
- Putcha, L.; Cintrón, N.M.; Tsui, J.; Vanderploeg, J.M.; Kramer, W.G. Pharmacokinetics and Oral Bioavailability of Scopolamine in Normal Subjects. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1989, 6, 481–485. [Google Scholar] [CrossRef]
- Oboh, G.; Olasehinde, T.A.; Ademosun, A.O. Essential Oil from Lemon Peels Inhibit Key Enzymes Linked to Neurodegenerative Conditions and Pro-Oxidant Induced Lipid Peroxidation. J. Oleo Sci. 2014, 63, 373–381. [Google Scholar] [CrossRef]
- Kim, K.N.; Ko, Y.J.; Yang, H.M.; Ham, Y.M.; Roh, S.W.; Jeon, Y.J.; Ahn, G.; Kang, M.C.; Yoon, W.J.; Kim, D.; et al. Anti-Inflammatory Effect of Essential Oil and Its Constituents from Fingered Citron (Citrus medica L. Var. Sarcodactylis) through Blocking JNK, ERK and NF-ΚB Signaling Pathways in LPS-Activated RAW 264.7 Cells. Food Chem. Toxicol. 2013, 57, 126–131. [Google Scholar] [CrossRef]
- Laczó, J.; Markova, H.; Lobellova, V.; Gazova, I.; Parizkova, M.; Cerman, J.; Nekovarova, T.; Vales, K.; Klovrzova, S.; Harrison, J.; et al. Scopolamine Disrupts Place Navigation in Rats and Humans: A Translational Validation of the Hidden Goal Task in the Morris Water Maze and a Real Maze for Humans. Psychopharmacology 2017, 234, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Volgin, A.D.; Yakovlev, O.A.; Demin, K.A.; Alekseeva, P.A.; Kalueff, A.V. Acute Behavioral Effects of Deliriant Hallucinogens Atropine and Scopolamine in Adult Zebrafish. Behav. Brain Res. 2019, 359, 274–280. [Google Scholar] [CrossRef] [PubMed]
- De Moraes Pultrini, A.; Almeida Galindo, L.; Costa, M. Anxiolytic and Sedative Effects of Extracts and Essential Oil from Citrus aurantium L. Biol. Pharm. Bull. 2002, 25, 1629–1633. [Google Scholar] [CrossRef]
- De Moraes Pultrini, A.; Almeida Galindo, L.; Costa, M. Effects of the Essential Oil from Citrus aurantium L. in Experimental Anxiety Models in Mice. Life Sci. 2006, 78, 1720–1725. [Google Scholar] [CrossRef]
- Agarwal, P.; Sebghatollahi, Z.; Kamal, M.; Dhyani, A.; Shrivastava, A.; Singh, K.K.; Sinha, M.; Mahato, N.; Mishra, A.K.; Baek, K.H. Citrus Essential Oils in Aromatherapy: Therapeutic Effects and Mechanisms. Antioxidants 2022, 11, 2374. [Google Scholar] [CrossRef] [PubMed]
- Brinza, I.; Boiangiu, R.S.; Hancianu, M.; Cioanca, O.; Orhan, I.E.; Hritcu, L. Bay Leaf (Laurus nobilis L.) Incense Improved Scopolamine-Induced Amnesic Rats by Restoring Cholinergic Dysfunction and Brain Antioxidant Status. Antioxidants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Ocampo, L.M.; López-Camacho, P.Y.; Aguirre-Hernández, E.; Cárdenas-Vázquez, R.; Bonilla-Jaime, H.; Basurto-Islas, G. Neuroprotective Effects of Petiveria Alliacea on Scopolamine-Induced Learning and Memory Impairment Mouse Model. J. Ethnopharmacol. 2024, 318, 116881. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, I.A.; Oregbesan, P.O.; Adesanya, A.; Olubori, M.A.; Olayinka, G.S.; Ajayi, A.M.; Onasanwo, S.A. Olax Subscorpioidea Prevented Scopolamine-Induced Memory Impairment through the Prevention of Oxido-Inflammatory Damage and Modulation of Cholinergic Transmission. J. Ethnopharmacol. 2024, 318, 116995. [Google Scholar] [CrossRef]
- Singsai, K.; Saksit, N.; Chaikhumwang, P. Brain Acetylcholinesterase Activity and the Protective Effect of Gac Fruit on Scopolamine-Induced Memory Impairment in Adult Zebrafish. IBRO Neurosci. Rep. 2024, 16, 368–372. [Google Scholar] [CrossRef]
- Ueda, K.; Horita, T.; Suzuki, T. Effects of Inhaling Essential Oils of Citrus limonum L., Santalum album, and Cinnamomum camphora on Human Brain Activity. Brain Behav. 2023, 13, e2889. [Google Scholar] [CrossRef]
- Liu, B.; Kou, J.; Li, F.; Huo, D.; Xu, J.; Zhou, X.; Meng, D.; Ghulam, M.; Artyom, B.; Gao, X.; et al. Lemon Essential Oil Ameliorates Age-Associated Cognitive Dysfunction via Modulating Hippocampal Synaptic Density and Inhibiting Acetylcholinesterase. Aging 2020, 12, 8622–8639. [Google Scholar] [CrossRef]
- Braidy, N.; Behzad, S.; Habtemariam, S.; Ahmed, T.; Daglia, M.; Mohammad Nabavi, S.; Sobarzo-Sanchez, E.; Fazel Nabavi, S. Neuroprotective Effects of Citrus Fruit-Derived Flavonoids, Nobiletin and Tangeretin in Alzheimer’s and Parkinson’s Disease. CNS Neurol. Disord. Drug Targets 2017, 16, 387–397. [Google Scholar] [CrossRef]
- Sun, K.; Bai, Y.; Zhao, R.; Guo, Z.; Su, X.; Li, P.; Yang, P. Neuroprotective Effects of Matrine on Scopolamine-Induced Amnesia via Inhibition of AChE/BuChE and Oxidative Stress. Metab. Brain Dis. 2019, 34, 173–181. [Google Scholar] [CrossRef]
- Baek, S.Y.; Li, F.Y.; Kim, D.H.; Kim, S.J.; Kim, M.R. Enteromorpha Prolifera Extract Improves Memory in Scopolamine-Treated Mice via Downregulating Amyloid-β Expression and Upregulating BDNF/TrkB Pathway. Antioxidants 2020, 9, 620. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, Y.; Kim, D.; Jung, M.W.; Lee, C.J. Scopolamine-Induced Learning Impairment Reversed by Physostigmine in Zebrafish. Neurosci. Res. 2010, 67, 156–161. [Google Scholar] [CrossRef]
- Smeriglio, A.; Alloisio, S.; Raimondo, F.M.; Denaro, M.; Xiao, J.; Cornara, L.; Trombetta, D. Essential Oil of Citrus Lumia Risso: Phytochemical Profile, Antioxidant Properties and Activity on the Central Nervous System. Food Chem. Toxicol. 2018, 119, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Zarrad, K.; Hamouda, A.B.; Chaieb, I.; Laarif, A.; Jemâa, J.M.B. Chemical Composition, Fumigant and Anti-Acetylcholinesterase Activity of the Tunisian Citrus aurantium L. Essential Oils. Ind. Crops Prod. 2015, 76, 121–127. [Google Scholar] [CrossRef]
- Oyeniran, O.H.; Omotosho, O.-P.I.; Ademola, I.I.; Ibraheem, O.; Nwagwe, O.R.; Onodugo, C.A. Lemon (Citrus Limon) Leaf Alkaloid-Rich Extracts Ameliorate Cognitive and Memory Deficits in Scopolamine-Induced Amnesic Rats. Pharmacol. Res. Mod. Chin. Med. 2024, 10, 100395. [Google Scholar] [CrossRef]
- Aumeeruddy-Elalfi, Z.; Lall, N.; Fibrich, B.; Blom van Staden, A.; Hosenally, M.; Mahomoodally, M.F. Selected Essential Oils Inhibit Key Physiological Enzymes and Possess Intracellular and Extracellular Antimelanogenic Properties in Vitro. J. Food Drug Anal. 2018, 26, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [PubMed]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, And Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Mani, V.M.; Sadiq, A.M.M. Naringin Modulates the Impairment of Memory, Anxiety, Locomotor, and Emotionality Behaviors in Rats Exposed to Deltamethrin; a Possible Mechanism Association with Oxidative Stress, Acetylcholinesterase and ATPase. Biomed. Prev. Nutr. 2014, 4, 527–533. [Google Scholar] [CrossRef]
- Pruthi, S.; Kaur, K.; Singh, V.; Shri, R. Improvement of Cognitive Function in Mice by Citrus reticulata Var. Kinnow via Modulation of Central Cholinergic System and Oxidative Stress. Metab. Brain Dis. 2021, 36, 901–910. [Google Scholar] [CrossRef]
- Rahnama, S.; Rabiei, Z.; Alibabaei, Z.; Mokhtari, S.; Rafieian-kopaei, M.; Deris, F. Anti-Amnesic Activity of Citrus aurantium Flowers Extract against Scopolamine-Induced Memory Impairments in Rats. Neurol. Sci. 2015, 36, 553–560. [Google Scholar] [CrossRef] [PubMed]
- El-Khadragy, M.F.; Al-Olayan, E.M.; Abdel Moneim, A.E. Neuroprotective Effects of Citrus reticulata in Scopolamine-Induced Dementia Oxidative Stress in Rats. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2014, 13, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Falls, N.; Singh, D.; Anwar, F.; Verma, A.; Kumar, V. Amelioration of Neurodegeneration and Cognitive Impairment by Lemon Oil in Experimental Model of Stressed Mice. Biomed. Pharmacother. 2018, 106, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Lambe, A.T.; Krechmer, J.E.; Peng, Z.; Casar, J.R.; Carrasquillo, A.J.; Raff, J.D.; Jimenez, J.L.; Worsnop, D.R. HOx and NOx Production in Oxidation Flow Reactors via Photolysis of Isopropyl Nitrite, Isopropyl Nitrite-d7, and 1,3-Propyl Dinitrite at λ = 254, 350, and 369 nm. Atmos. Meas. Tech. 2019, 12, 299–311. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Baranowska-Wójcik, E.; Kwiecień, M.; Grela, E.R.; Szwajgier, D.; Kwiatkowska, K.; Kiczorowska, B. The Role of Dietary Antioxidants in the Pathogenesis of Neurodegenerative Diseases and Their Impact on Cerebral Oxidoreductive Balance. Nutrients 2020, 12, 435. [Google Scholar] [CrossRef]
- Casado, Á.; Encarnación López-Fernández, M.; Concepción Casado, M.; De La Torre, R. Lipid Peroxidation and Antioxidant Enzyme Activities in Vascular and Alzheimer Dementias. Neurochem. Res. 2008, 33, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, G.; Flaherty, J.T.; Duilio, C.; Tritto, I.; Santoro, G.; Elia, P.P.; Condorelli, M.; Chiariello, M. Oxygen Radicals Generated at Reflow Induce Peroxidation of Membrane Lipids in Reperfused Hearts. J. Clin. Investig. 1991, 87, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Dhapola, R.; Beura, S.K.; Sharma, P.; Singh, S.K.; HariKrishnaReddy, D. Oxidative Stress in Alzheimer’s Disease: Current Knowledge of Signaling Pathways and Therapeutics. Mol. Biol. Rep. 2024, 51, 48. [Google Scholar] [CrossRef] [PubMed]
- Gambetta, C.; Natera, J.; Massad, W.A.; García, N.A. Methyl Anthranilate as Generator and Quencher of Reactive Oxygen Species: A Photochemical Study. J. Photochem. Photobiol. A Chem. 2013, 269, 27–33. [Google Scholar] [CrossRef]
- Foti, M.C.; Ingold, K.U. Mechanism of Inhibition of Lipid Peroxidation by γ-Terpinene, an Unusual and Potentially Useful Hydrocarbon Antioxidant. J. Agric. Food Chem. 2003, 51, 2758–2765. [Google Scholar] [CrossRef]
- Asikin, Y.; Shimizu, K.; Iwasaki, H.; Oku, H.; Wada, K. Stress Amelioration and Anti-Inflammatory Potential of Shiikuwasha (Citrus Depressa Hayata) Essential Oil, Limonene, and γ-Terpinene. J. Food Drug Anal. 2022, 30, 454. [Google Scholar] [CrossRef]
- Ojha, S.; Javed, H.; Azimullah, S.; Haque, M.E. β-Caryophyllene, a Phytocannabinoid Attenuates Oxidative Stress, Neuroinflammation, Glial Activation, and Salvages Dopaminergic Neurons in a Rat Model of Parkinson Disease. Mol. Cell Biochem. 2016, 418, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Mazhar, Z.; Alsayrafi, D.; Garelnabi, M. P-Cymene Modulate Oxidative Stress and Inflammation in Murine Macrophages: Potential Implication in Atherosclerosis. Cardiovasc. Hematol. Agents Med. Chem. 2020, 18, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corp: Carol Stream, IL, USA, 2007; ISBN 1932633219. [Google Scholar]
- Lim, S.; Lee, Y.O. Predicting Chemical Properties Using Self-Attention Multi-Task Learning Based on SMILES Representation. In Proceedings of the 2020 25th International Conference on Pattern Recognition (ICPR), Milan, Italy, 10–15 January 2021; pp. 3146–3153. [Google Scholar] [CrossRef]
- Sha, C.; Cuperlovic-Culf, M.; Hu, T. SMILE: Systems Metabolomics Using Interpretable Learning and Evolution. BMC Bioinform. 2021, 22, 284. [Google Scholar] [CrossRef]
- Jorissen, E.; Prox, J.; Bernreuther, C.; Weber, S.; Schwanbeck, R.; Serneels, L.; Snellinx, A.; Craessaerts, K.; Thathiah, A.; Tesseur, I.; et al. The Disintegrin/Metalloproteinase ADAM10 Is Essential for the Establishment of the Brain Cortex. J. Neurosci. 2010, 30, 4833–4844. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 6 November 2023).
- SwissADME. Available online: http://www.swissadme.ch/ (accessed on 13 October 2023).
- PkCSM. Available online: https://biosig.lab.uq.edu.au/pkcsm/ (accessed on 13 October 2023).
- Tripathy, A.; Raichur, A.M.; Chandrasekaran, N.; Prathna, T.C.; Mukherjee, A. Process Variables in Biomimetic Synthesis of Silver Nanoparticles by Aqueous Extract of Azadirachta Indica (Neem) Leaves. J. Nanoparticle Res. 2010, 12, 237–246. [Google Scholar] [CrossRef]
- Rusu, E.; Fizesan, I.; Brinza, I.; Boiangiu, R.S.; Cioanca, O.; Hancianu, M.; Dumitru, G.; Hritcu, L.; Birsan, G.-C.; Todirascu-Ciornea, E. Direct Evidence for Using Coriandrum Sativum Var. Microcarpum Essential Oil to Ameliorate Scopolamine-Induced Memory Impairment and Brain Oxidative Stress in the Zebrafish Model. Antioxidants 2023, 12, 1534. [Google Scholar] [CrossRef] [PubMed]
- Brinza, I.; Ayoub, I.M.; Eldahshan, O.A.; Hritcu, L. Baicalein 5,6-Dimethyl Ether Prevents Memory Deficits in the Scopolamine Zebrafish Model by Regulating Cholinergic and Antioxidant Systems. Plants 2021, 10, 1245. [Google Scholar] [CrossRef] [PubMed]
- Blaser, R.E.; Rosemberg, D.B. Measures of Anxiety in Zebrafish (Danio Rerio): Dissociation of Black/White Preference and Novel Tank Test. PLoS ONE 2012, 7, e36931. [Google Scholar] [CrossRef]
- Levin, E.D.; Bencan, Z.; Cerutti, D.T. Anxiolytic Effects of Nicotine in Zebrafish. Physiol. Behav. 2007, 90, 54–58. [Google Scholar] [CrossRef]
- Fontana, B.D.; Alnassar, N.; Parker, M.O. The Zebrafish (Danio Rerio) Anxiety Test Battery: Comparison of Behavioral Responses in the Novel Tank Diving and Light–Dark Tasks Following Exposure to Anxiogenic and Anxiolytic Compounds. Psychopharmacology 2022, 239, 287–296. [Google Scholar] [CrossRef]
- Cachat, J.M.; Canavello, P.R.; Elkhayat, S.I.; Bartels, B.K.; Hart, P.C.; Elegante, M.F.; Beeson, E.C.; Laffoon, A.L.; Haymore, W.A.M.; Tien, D.H.; et al. Video-Aided Analysis of Zebrafish Locomotion and Anxiety-Related Behavioral Responses. In Zebrafish Neurobehavioral Protocols; Neuromethods series; Humana Press: Totowa, NJ, USA, 2011; Volume 51, pp. 1–14. [Google Scholar]
- Fontana, B.D.; Gibbon, A.J.; Cleal, M.; Sudwarts, A.; Pritchett, D.; Petrazzini, M.E.M.; Brennan, C.H.; Parker, M.O. Moderate Early-Life Stress Improves Adult Zebrafish (Danio Rerio) Spatial Short-Term Memory but Does Not Affect Social and Anxiety-like Responses. bioRxiv 2020. [Google Scholar] [CrossRef]
- Gaikwad, S.; Stewart, A.; Hart, P.; Wong, K.; Piet, V.; Cachat, J.; Kalueff, A.V. Acute Stress Disrupts Performance of Zebrafish in the Cued and Spatial Memory Tests: The Utility of Fish Models to Study Stress-Memory Interplay. Behav. Process. 2011, 87, 224–230. [Google Scholar] [CrossRef]
- Cognato, G.d.P.; Bortolotto, J.W.; Blazina, A.R.; Christoff, R.R.; Lara, D.R.; Vianna, M.R.; Bonan, C.D. Y-Maze Memory Task in Zebrafish (Danio Rerio): The Role of Glutamatergic and Cholinergic Systems on the Acquisition and Consolidation Periods. Neurobiol. Learn. Mem. 2012, 98, 321–328. [Google Scholar] [CrossRef]
- Barbosa, F.F.; Silva, R.H. Immediate-Early Gene Expression in Neural Circuits Related to Object Recognition Memory. Handb. Behav. Neurosci. 2018, 27, 261–271. [Google Scholar] [CrossRef]
- Stefanello, F.V.; Fontana, B.D.; Ziani, P.R.; Müller, T.E.; Mezzomo, N.J.; Rosemberg, D.B. Exploring Object Discrimination in Zebrafish: Behavioral Performance and Scopolamine-Induced Cognitive Deficits at Different Retention Intervals. Zebrafish 2019, 16, 370–378. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-Stone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Sinha, A.K. Calorimetric Assay of Catalase. Anal. Biocijemistry 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Hawkins, R.E.; Brian, M.; Carrell, R.W. The Estimation of Red Cell Superoxide Dismutase Activity. J. Lab. Clin. Med. 1975, 85, 337–341. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Tokumura, A. Glutathione Peroxidase Activity in Tissues of Vitamin E-Deficient Mice. J. Nutr. Sci. Vitaminol. 1976, 22, 405–407. [Google Scholar] [CrossRef]
- Oliver, C.N.; Ahn, B.W.; Moerman, E.J.; Goldstein, S.; Stadtman, E.R. Age-Related Changes in Oxidized Proteins. J. Biol. Chem. 1987, 262, 5488–5491. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein Carbonyl Groups as Biomarkers of Oxidative Stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]

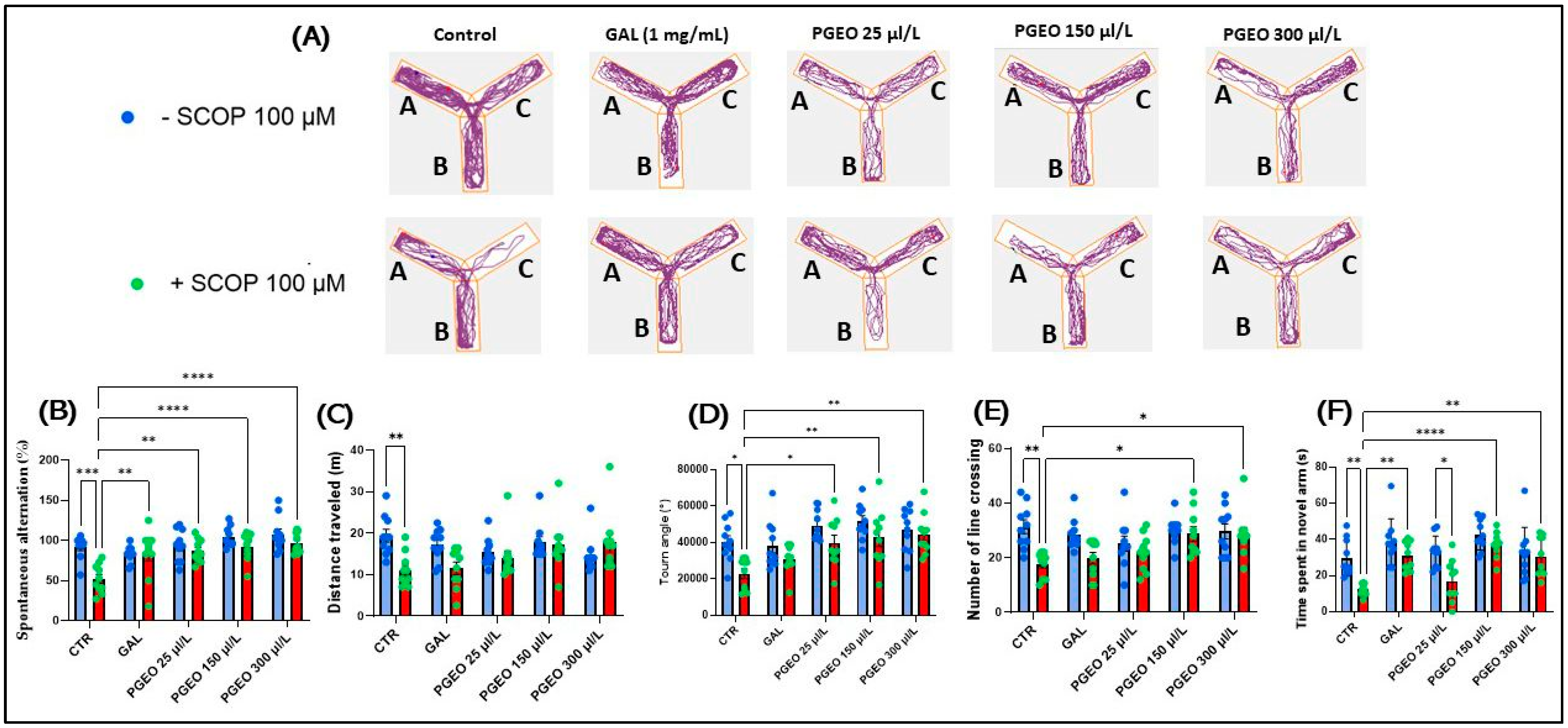
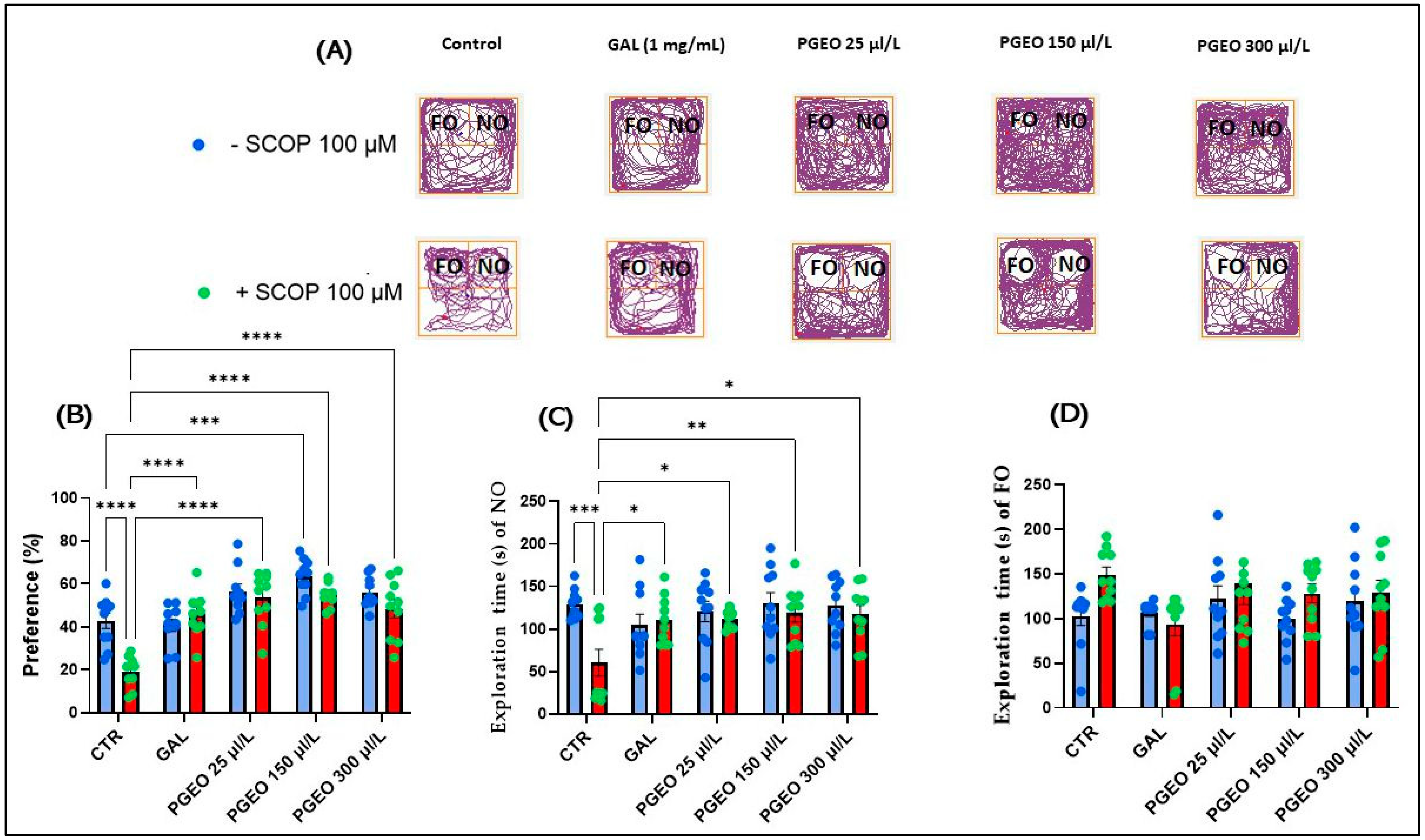
 ) control, (
) control, ( ) Galantamine (GAL, 1 mg/mL), (
) Galantamine (GAL, 1 mg/mL), ( ) petitgrain essential oil (PGEO, 1 μL/L), (
) petitgrain essential oil (PGEO, 1 μL/L), ( ) PGEO 3 μL/L, (
) PGEO 3 μL/L, ( ) PGEO 6 μL/L, (
) PGEO 6 μL/L, ( ) Scopolamine (SCOP, 100 μM), (
) Scopolamine (SCOP, 100 μM), ( ) SCOP (100 μM) + GAL 1 mg/mL, (
) SCOP (100 μM) + GAL 1 mg/mL, ( ) SCOP (100 μM) + PGEO 1 μL/L, (
) SCOP (100 μM) + PGEO 1 μL/L, ( ) SCOP (100 μM) + PGEO 3 μL/L, and (
) SCOP (100 μM) + PGEO 3 μL/L, and ( ) SCOP (100 μM) + PGEO 6 μL/L.
) SCOP (100 μM) + PGEO 6 μL/L.
 ) control, (
) control, ( ) Galantamine (GAL, 1 mg/mL), (
) Galantamine (GAL, 1 mg/mL), ( ) petitgrain essential oil (PGEO, 1 μL/L), (
) petitgrain essential oil (PGEO, 1 μL/L), ( ) PGEO 3 μL/L, (
) PGEO 3 μL/L, ( ) PGEO 6 μL/L, (
) PGEO 6 μL/L, ( ) Scopolamine (SCOP, 100 μM), (
) Scopolamine (SCOP, 100 μM), ( ) SCOP (100 μM) + GAL 1 mg/mL, (
) SCOP (100 μM) + GAL 1 mg/mL, ( ) SCOP (100 μM) + PGEO 1 μL/L, (
) SCOP (100 μM) + PGEO 1 μL/L, ( ) SCOP (100 μM) + PGEO 3 μL/L, and (
) SCOP (100 μM) + PGEO 3 μL/L, and ( ) SCOP (100 μM) + PGEO 6 μL/L.
) SCOP (100 μM) + PGEO 6 μL/L.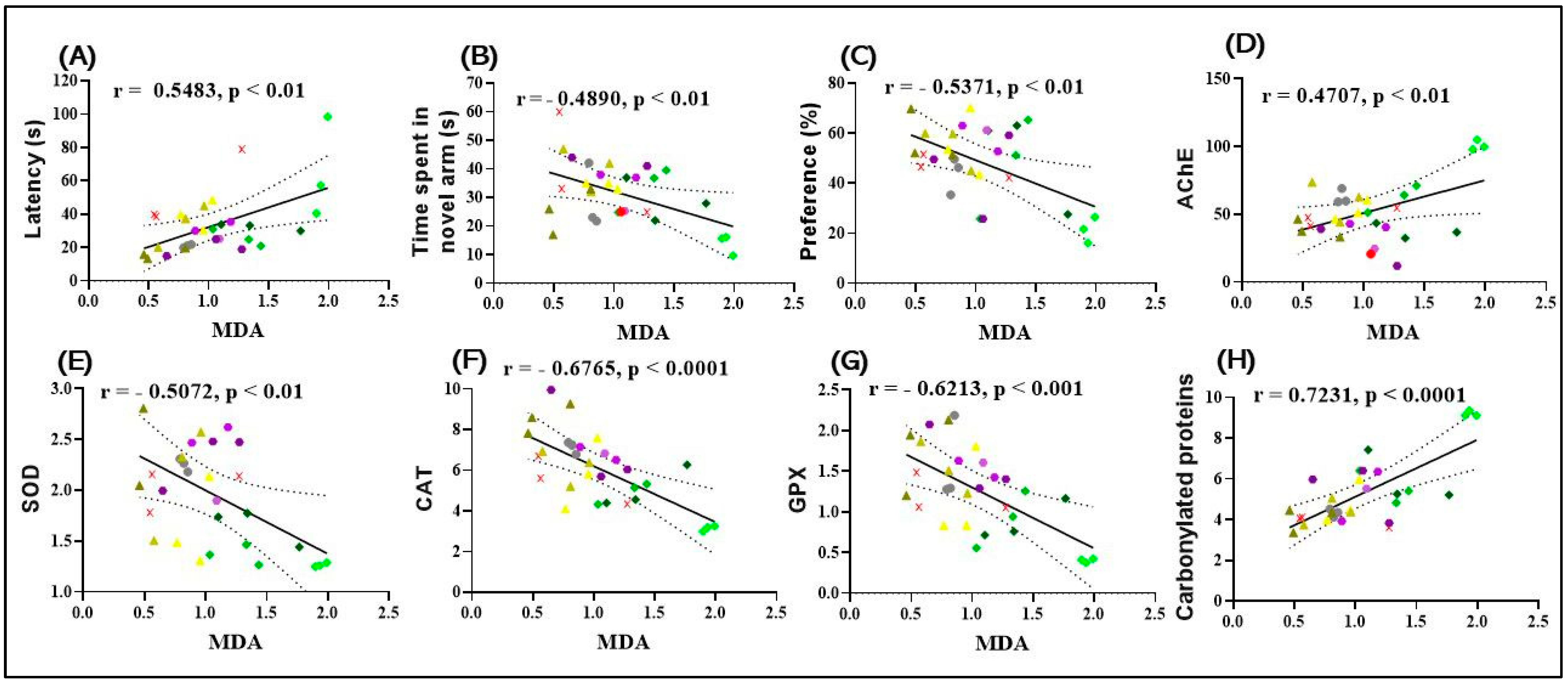

| Identified Compounds | RIexp | RIlit | Content % |
|---|---|---|---|
| p-cymene | 1015 | 1018 | 0.72 (±0.01) |
| Limonene | 1019 | 1022 | 1.35 (±0.03) |
| γ-Terpinene | 1050 | 1055 | 6.25 (±0.01) |
| Methyl-N-methyl anthranilate | 1406 | 1402 | 89.93 (±0.08) |
| β-Caryophyllene | 1411 | 1418 | 1.11 (±0.01) |
| Caryophyllene oxide | 1576 | 1572 | 0.63 (±0.01) |
| Total identified | 100 |
| Descriptor | SCOP | GAL | Methyl-N-Methyl Anthranilate | γ-Terpinene | Limonene | β-Caryophyllene | p-Cymene | Caryophyllene Oxide |
|---|---|---|---|---|---|---|---|---|
| Formula | C16H22O9 | C17H21NO3 | C9H11NO2 | C10H16 | C10H16 | C15H24 | C10H14 | C15H24O |
| Molecular weight (g/mol) | 303.35 | 287.359 | 165.19 | 136.23 | 136.23 | 204.35 | 134.22 | 220.35 |
| Num. heavy atoms | 22 | 21 | 12 | 10 | 10 | 15 | 10 | 16 |
| Num. arom. heavy atoms | 6 | 6 | 6 | 0 | 0 | 0 | 6 | 0 |
| Fraction Csp3 | 0.59 | 0.53 | 0.22 | 0.60 | 0.60 | 0.73 | 0.40 | 0.87 |
| Num. rotatable bonds | 5 | 1 | 3 | 1 | 1 | 0 | 1 | 0 |
| Num. H-bond acceptors | 5 | 4 | 2 | 0 | 0 | 0 | 0 | 1 |
| Num. H-bond donors | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Molar Refractivity | 83.48 | 84.05 | 47.03 | 47.12 | 47.12 | 68.78 | 45.99 | 68.27 |
| TPSA | 62.30 Å2 | 41.93 Å2 | 38.33 Å2 | 0.00 Å2 | 0.00 Å2 | 0.00 Å | 0.00 Å | 12.53 Å2 |
| Druglikeness | SCOP | GAL | Methyl-N-Methyl Anthranilate | γ-Terpinene | Limonene | β-Caryophyllene | p-Cymene | Caryophyllene Oxide |
|---|---|---|---|---|---|---|---|---|
| Lipinski | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation | Yes; 1 violation: MLOGP > 4.15 | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation |
| Ghose | Yes | Yes | Yes | No; 1 violation: MW < 160 | No; 1 violation: MW < 160 | Yes | No; 1 violation: MW < 160 | No; 1 violation: MW < 160 |
| Veber | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Egan | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Muegge | Yes | Yes | Yes/No; 1 violation: MW < 200 | No; 2 violations: MW < 200, Heteroatoms < 2 | No; 1 violation: Heteroatoms < 2 | No; 1 violation: MW < 200 | No; 2 violations: MW < 200, Heteroatoms < 2 | No; 1 violation: MW < 200 |
| Bioavailability Score | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| Property | Compound Model Name | SCOP | GAL | Methyl-N-Methyl Anthranilate | γ-Terpinene | Limonene | β-Caryophyllene | p-Cymene | Caryophyllene Oxide | Unit |
|---|---|---|---|---|---|---|---|---|---|---|
| Absorption | Intestinal absorption (human) (low < 30%, high > 30%) | 72.626 | 94.994 | 93.334 | 96.219 | 95.301 | 95.898 | 95.561 | 94.547 | Numeric (% Absorbed) |
| Skin permeability (low logKp > −2.5, high logKp < −2.5) | −4.097 | −3.75 | −2.165 | −1.489 | −1.438 | −1.721 | −1.575 | −1.017 | Numeric (log Kp) | |
| Distribution | VDss (human) (low log VDss < −0.15, high VDss > 0.45) | 0.583 | 0.89 | −0.15 | 0.412 | 0.412 | 0.396 | −0.663 | 0.686 | Numeric (log L/kg) |
| Fraction unbound (human) | 0.414 | 0.36 | 0.413 | 0.42 | 0.424 | 0.396 | 0.258 | 0.213 | Numeric (Fu) | |
| BBB permeability (log BB > 0.3 cross BB, log BB < 0.1 do not cross BB) | −0.043 | −0.081 | −0.087 | 0.754 | 0.741 | 0.484 | 0.73 | 0.531 | Numeric (log BB) | |
| CNS permeability (log PS > −2, penetrate CNS, log PS < −3 do not penetrate) | −3.031 | −2.511 | −1.785 | −2.049 | −2.029 | −2.172 | −2.172 | −1.398 | Numeric (log PS) | |
| Metabolism | CYP3A4 substrate | Yes | Yes | No | No | No | No | No | No | Categorical (Yes/No) |
| CYP1A2 inhibitor | No | No | No | No | No | No | No | Yes | Categorical (Yes/No) | |
| Excretion | Total Clearance | 1.096 | 0.991 | 0.75 | 0.217 | 0.217 | 0.213 | 1.088 | 0.239 | Numeric (log ml/min/kg) |
| Renal OCT2 substrate | No | Yes | No | No | No | No | No | No | Categorical (Yes/No) | |
| Toxicity | Max. tolerated dose (human) (low < 0.447, high > 0.477) | −0.319 | −0.423 | 0.769 | 0.756 | 0.63 | 0.77 | 0.271 | 0.858 | Numeric (log mg/kg/day) |
| Hepatotoxicity | No | Yes | No | No | No | No | No | No | Categorical (Yes/No) |
| Name | Smile | Chemical Structure |
|---|---|---|
| SCOP | CN1C2CC(CC1C3C2O3)OC(=O)C(CO)C4=CC=CC=C4 | 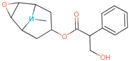 |
| GAL | CN1CCC23C=CC(CC2OC4=C(C=CC(=C34)C1)OC)O |  |
| Methyl-N-methyl anthranilate | CNc1ccccc1C(=O)OC |  |
| γ-Terpinene | CC1=CCC(C(C)C)=CC1 | 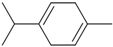 |
| Limonene | C=C(C)C1CC=C(C)CC1 | 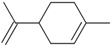 |
| β-Caryophyllene | C=C1CCC=C(C)CCC2C1CC2(C)C | 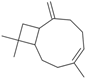 |
| p-Cymene | Cc1ccc(C(C)C)cc1 | 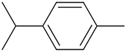 |
| Caryophyllene oxide | C=C1CCC2OC2(C)CCC2C1CC2(C)C | 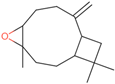 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brinza, I.; Boiangiu, R.S.; Honceriu, I.; Abd-Alkhalek, A.M.; Eldahshan, O.A.; Dumitru, G.; Hritcu, L.; Todirascu-Ciornea, E. Investigating the Potential of Essential Oils from Citrus reticulata Leaves in Mitigating Memory Decline and Oxidative Stress in the Scopolamine-Treated Zebrafish Model. Plants 2024, 13, 1648. https://doi.org/10.3390/plants13121648
Brinza I, Boiangiu RS, Honceriu I, Abd-Alkhalek AM, Eldahshan OA, Dumitru G, Hritcu L, Todirascu-Ciornea E. Investigating the Potential of Essential Oils from Citrus reticulata Leaves in Mitigating Memory Decline and Oxidative Stress in the Scopolamine-Treated Zebrafish Model. Plants. 2024; 13(12):1648. https://doi.org/10.3390/plants13121648
Chicago/Turabian StyleBrinza, Ion, Razvan Stefan Boiangiu, Iasmina Honceriu, Ahmed M. Abd-Alkhalek, Omayma A. Eldahshan, Gabriela Dumitru, Lucian Hritcu, and Elena Todirascu-Ciornea. 2024. "Investigating the Potential of Essential Oils from Citrus reticulata Leaves in Mitigating Memory Decline and Oxidative Stress in the Scopolamine-Treated Zebrafish Model" Plants 13, no. 12: 1648. https://doi.org/10.3390/plants13121648










