Silicon Dioxide Nanoparticles-Based Amelioration of Cd Toxicity by Regulating Antioxidant Activity and Photosynthetic Parameters in a Line Developed from Wild Rice
Abstract
1. Introduction
2. Results
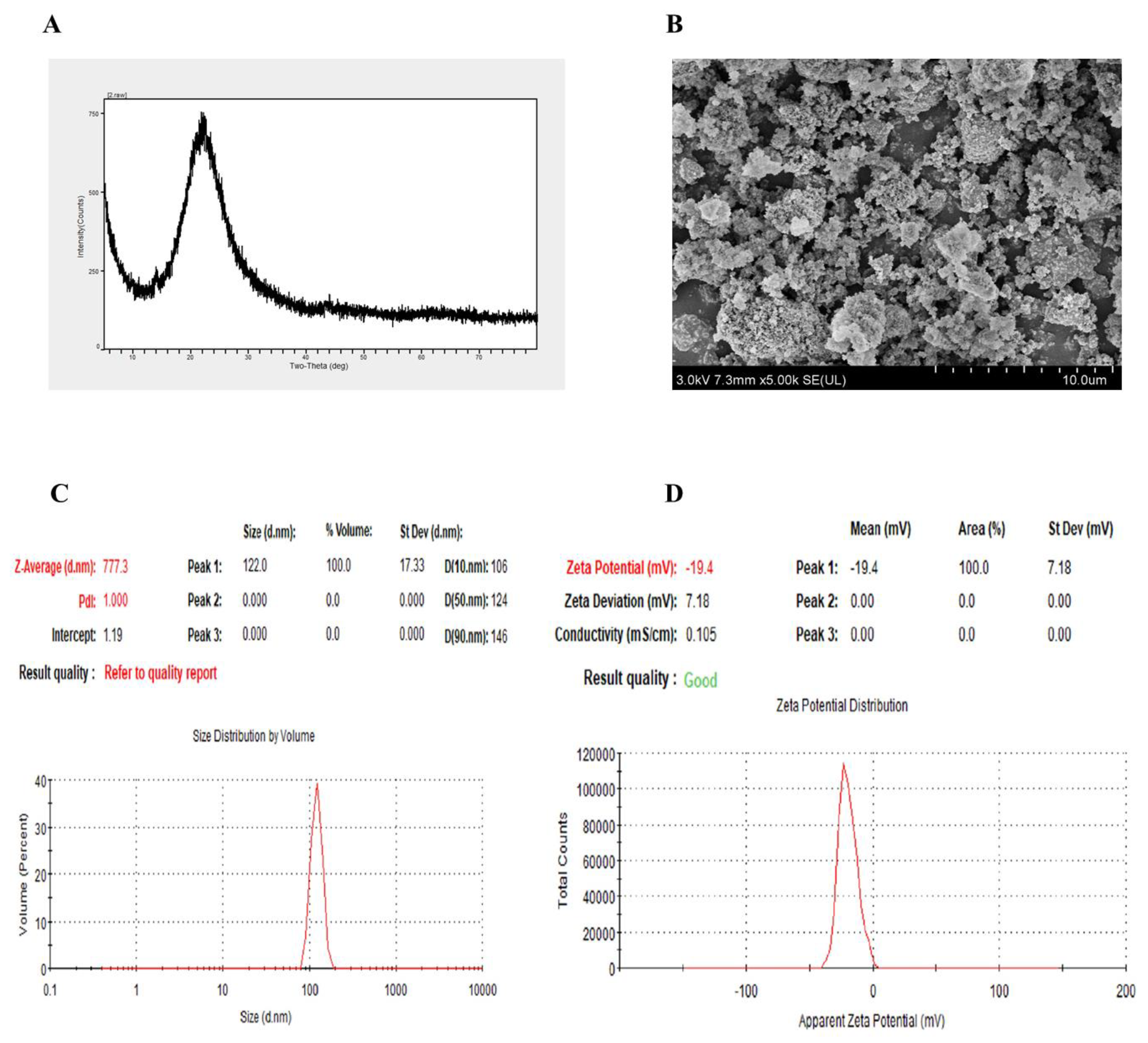
2.1. Characterization of SiO2NPs
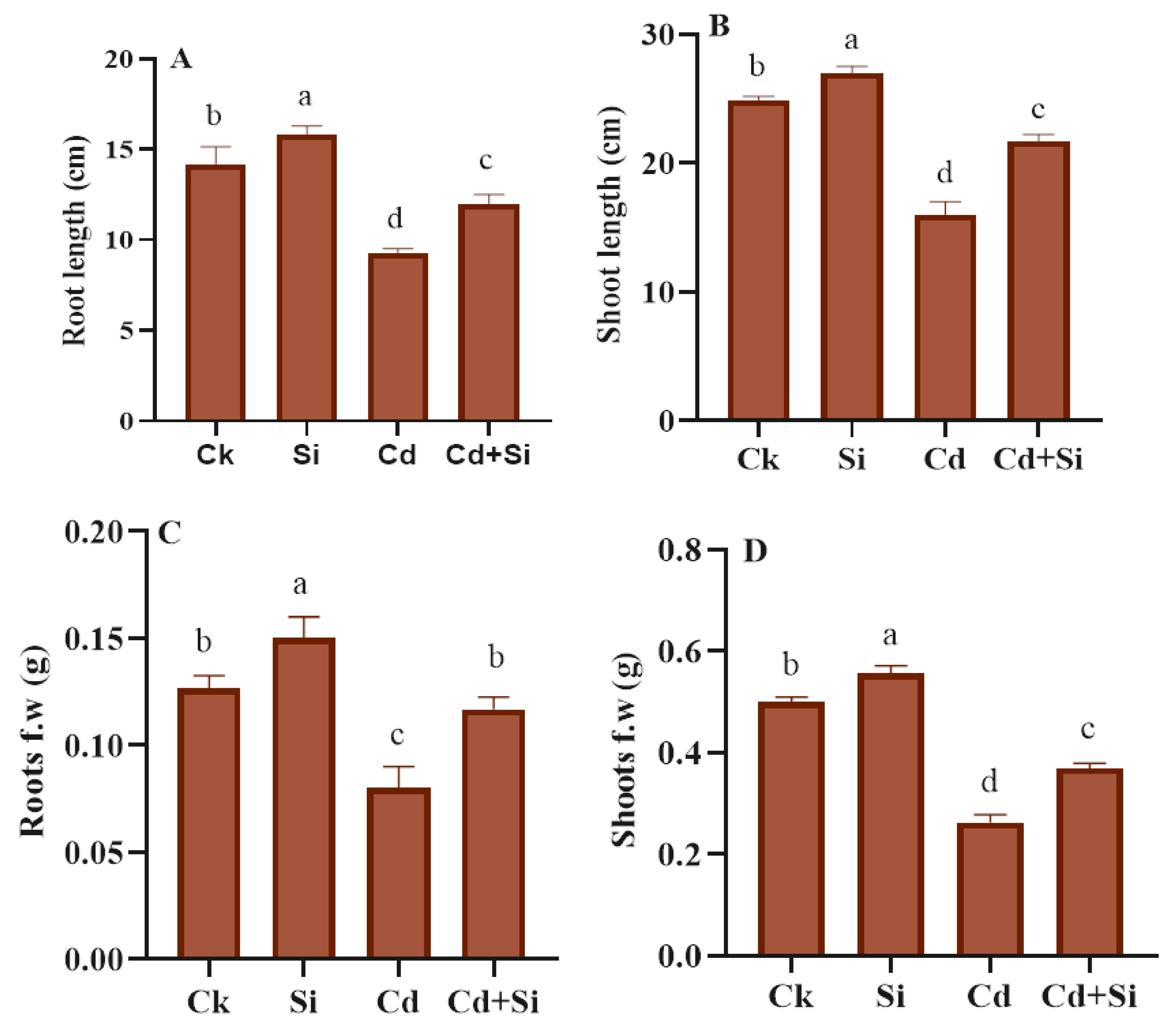
2.2. Treatment of SiO2NPs Enhanced Plant Growth under Cd Toxicity
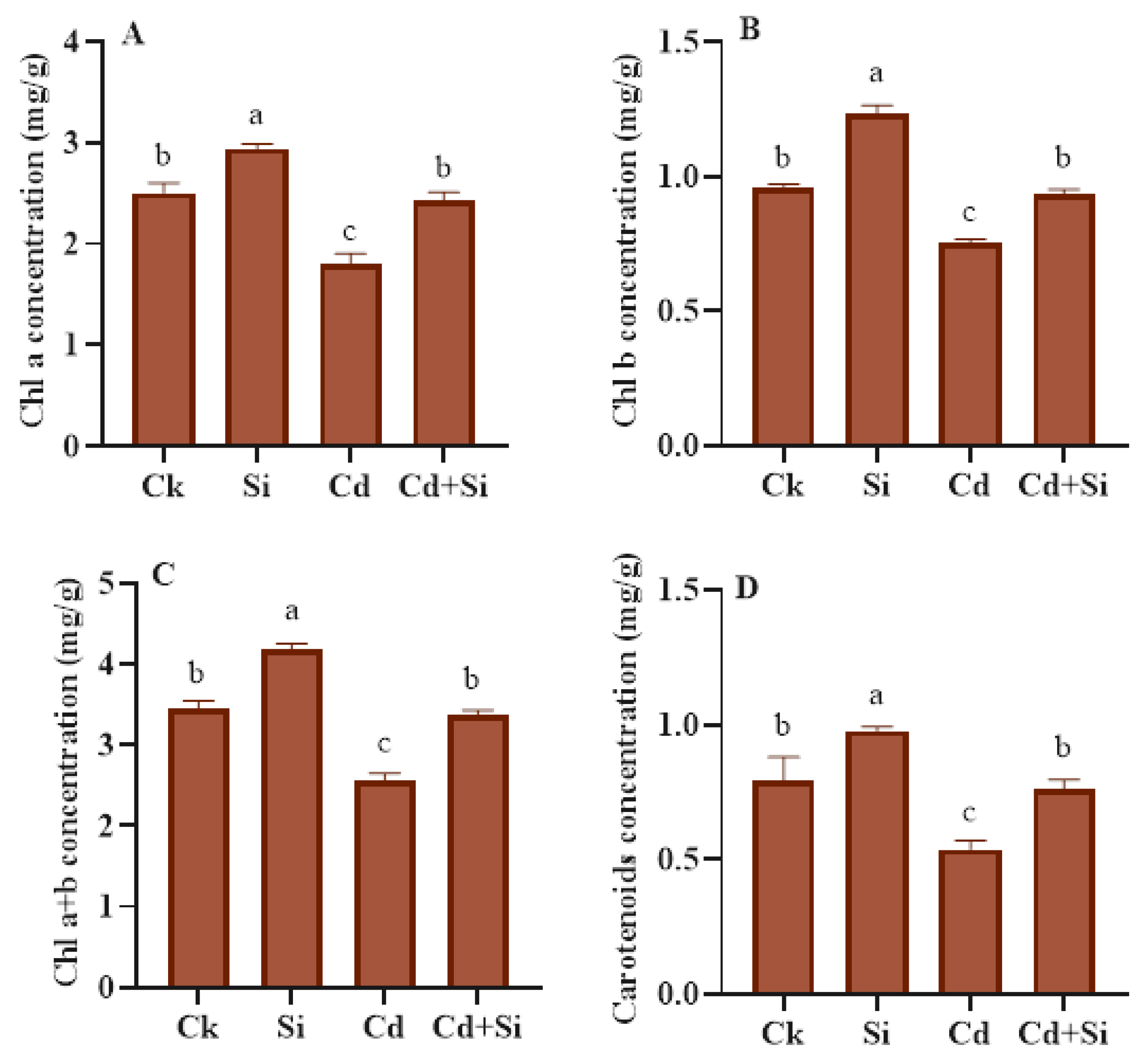
2.3. Effect of SiO2NPs on Photosynthetic Characteristics of Wild Rice under Cd Stress
2.4. Effect of SiO2NPs on MDA and H2O2 Levels in Wild Rice Subjected to Cd Stress
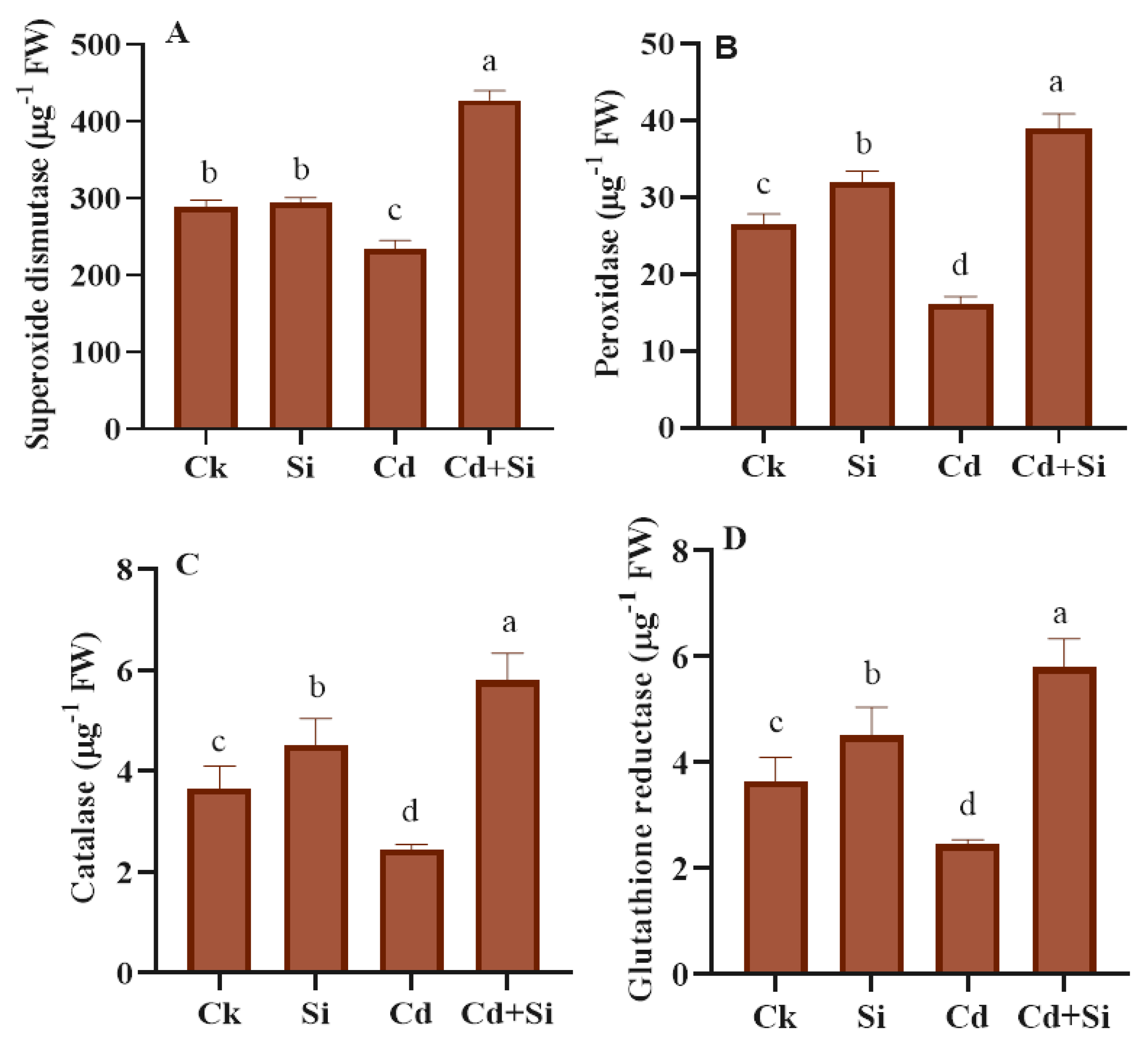
2.5. Effects of Antioxidant Enzymatic Defense in Wild Rice under Cadmium Toxicity
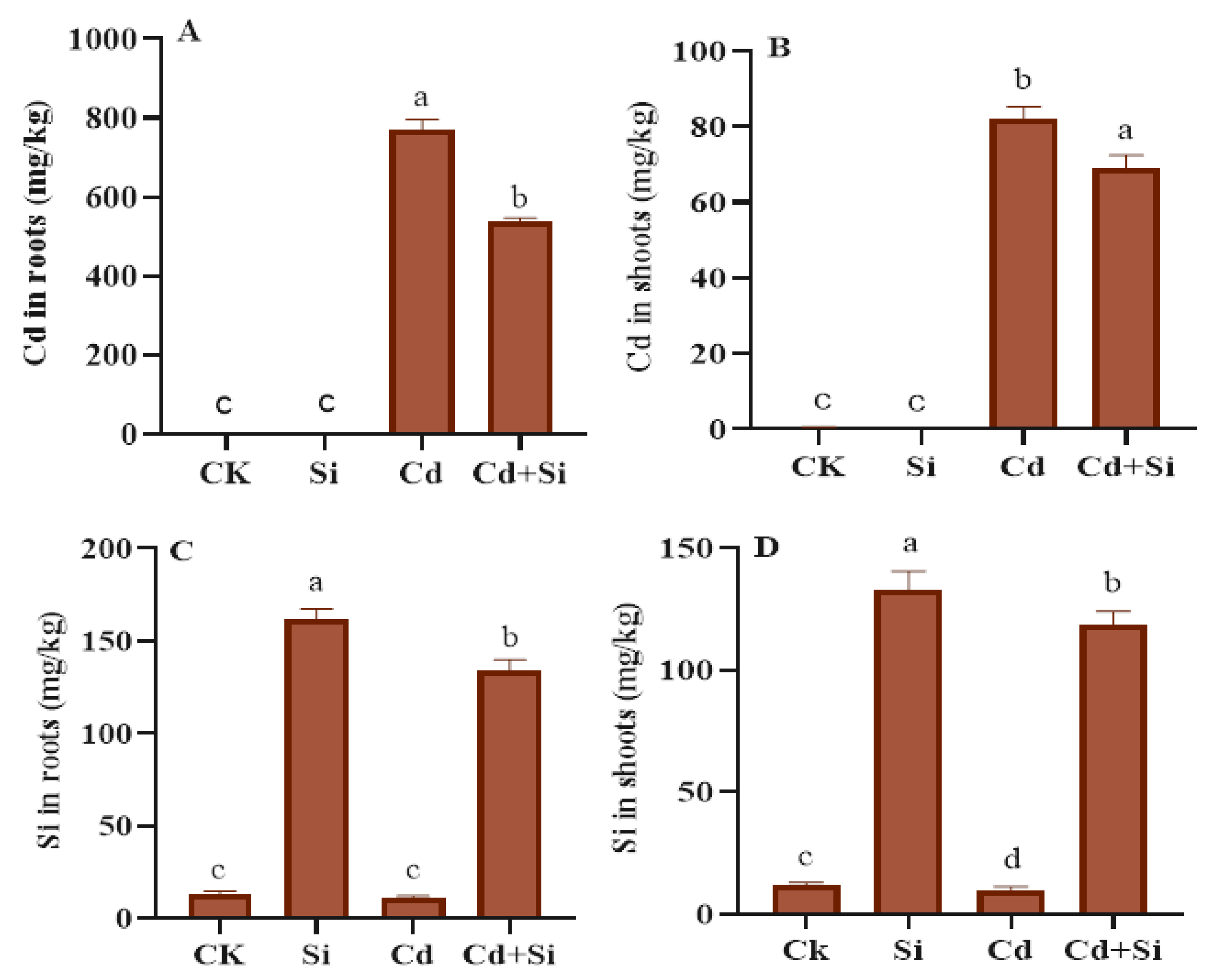
2.6. Effects of SiO2NPs on Cd and Si Concentration in Wild Rice
2.7. Expression Level of Transporters and Genes
3. Discussion
4. Materials and Methods
4.1. Experimental Strategy
4.2. Monitoring of Growth Metrics in Plants
4.3. Assessment of Chlorophyll Contents
4.4. Measurement of Oxidative Damage in Rice Plants
4.5. Antioxidant Compounds Evaluation
4.6. Characterization of SiO2NPs
4.7. Observation of Cd and SiO2NPs Contents in Wild Rice
4.8. Profound Insights into Transporters and Gene Expression Using qRT-PCR Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, S.; Lian, X.; Zhang, B.; Liu, X.; Yu, J.; Gao, Y.; Zhang, Q.; Sun, H. Nano silicon dioxide reduces cadmium uptake, regulates nutritional homeostasis and antioxidative enzyme system in barley seedlings (Hordeum vulgare L.) under cadmium stress. Environ. Sci. Pollut. Res. 2023, 30, 67552–67564. [Google Scholar] [CrossRef]
- Aqeel, M.; Khalid, N.; Tufail, A.; Ahmad, R.Z.; Akhter, M.S.; Luqman, M.; Javed, M.T.; Irshad, M.K.; Alamri, S.; Hashem, M. Elucidating the distinct interactive impact of cadmium and nickel on growth, photosynthesis, metal-homeostasis, and yield responses of mung bean (Vigna radiata L.) varieties. Environ. Sci. Pollut. Res. 2021, 28, 27376–27390. [Google Scholar] [CrossRef]
- Xiao, L.; Guo, H.; Wang, S.; Li, J.; Wang, Y.; Xing, B. Carbon dots alleviate the toxicity of cadmium ions (Cd2+) toward wheat seedlings. Environ. Sci. Nano 2019, 6, 1493–1506. [Google Scholar] [CrossRef]
- Panda, G.; Pobi, K.K.; Gangopadhyay, S.; Gope, M.; Rai, A.K.; Nayek, S. Contamination level, source identification and health risk evaluation of potentially toxic elements (PTEs) in groundwater of an industrial city in eastern India. Environ. Geochem. Health 2021, 44, 2685–2709. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, D.; Ren, F.; Huang, L. Spatiotemporal variation of soil heavy metals in China: The pollution status and risk assessment. Sci. Total Environ. 2023, 871, 161768. [Google Scholar] [CrossRef]
- Yan, S.; Wu, F.; Zhou, S.; Yang, J.; Tang, X.; Ye, W. Zinc oxide nanoparticles alleviate the arsenic toxicity and decrease the accumulation of arsenic in rice (Oryza sativa L.). BMC Plant Biol. 2021, 21, 150. [Google Scholar] [CrossRef]
- Luo, J.-S.; Zhang, Z. Mechanisms of cadmium phytoremediation and detoxification in plants. Crop J. 2021, 9, 521–529. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Sunoj, V.; Wen, Y.; Zhu, J.; Muralidharan, G.; Cao, K. Foliar application of nanoparticles mitigates the chilling effect on photosynthesis and photoprotection in sugarcane. Plant Physiol. Biochem. 2020, 149, 50–60. [Google Scholar] [CrossRef]
- Chen, F.; Aqeel, M.; Maqsood, M.F.; Khalid, N.; Irshad, M.K.; Ibrahim, M.; Akhter, N.; Afzaal, M.; Ma, J.; Hashem, M. Mitigation of lead toxicity in Vigna radiata genotypes by silver nanoparticles. Environ. Pollut. 2022, 308, 119606. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, C.; Zhao, Y.; Huang, Y.; Liu, Z. Foliar application with nano-silicon reduced cadmium accumulation in grains by inhibiting cadmium translocation in rice plants. Environ. Sci. Pollut. Res. 2018, 25, 2361–2368. [Google Scholar] [CrossRef]
- Ghouri, F.; Shahid, M.J.; Liu, J.; Sun, L.; Riaz, M.; Imran, M.; Ali, S.; Liu, X.; Shahid, M.Q. The protective role of tetraploidy and nanoparticles in arsenic-stressed rice: Evidence from RNA sequencing, ultrastructural and physiological studies. J. Hazard. Mater. 2023, 458, 132019. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, Q.; Ali, S. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut. Res. 2019, 26, 7579–7588. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Malik, S.; Adrees, M.; Qayyum, M.F.; Alamri, S.A.; Alyemeni, M.N.; Ahmad, P. Effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa). Acta Physiol. Plant. 2019, 41, 35. [Google Scholar] [CrossRef]
- Attia, T.S.; Elsheery, N. Nanomaterials: Scope, applications, and challenges in agriculture and soil reclamation. In Sustainable Agriculture Reviews 41: Nanotechnology for Plant Growth and Development; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–39. [Google Scholar]
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy 2020, 10, 558. [Google Scholar] [CrossRef]
- Ghouri, F.; Shahid, M.J.; Zhong, M.; Zia, M.A.; Alomrani, S.O.; Liu, J.; Sun, L.; Ali, S.; Liu, X.; Shahid, M.Q. Alleviated lead toxicity in rice plant by co-augmented action of genome doubling and TiO2 nanoparticles on gene expression, cytological and physiological changes. Sci. Total Environ. 2024, 911, 168709. [Google Scholar] [CrossRef]
- Manzoor, N.; Ahmed, T.; Noman, M.; Shahid, M.; Nazir, M.M.; Ali, L.; Alnusaire, T.S.; Li, B.; Schulin, R.; Wang, G. Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci. Total Environ. 2021, 769, 145221. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.Á.; Afzal, S.; Singh, T.; Hussain, I. Zinc oxide nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2018, 53, 185–201. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Rehman, M.Z.U.; Rizwan, M.; Ali, S. Foliar spray of silicon nanoparticles improved the growth and minimized cadmium (Cd) in wheat under combined Cd and water-limited stress. Environ. Sci. Pollut. Res. 2022, 29, 77321–77332. [Google Scholar] [CrossRef]
- Sun, H.; He, S.; Liu, T.; Zhang, Q.; Yu, J.; Gao, Y.; Wang, X. Alleviation of cadmium toxicity by nano-silicon dioxide in Momordica charantia L. seedlings. J. Soil Sci. Plant Nutr. 2023, 23, 1060–1069. [Google Scholar] [CrossRef]
- Cui, J.; Liu, T.; Li, F.; Yi, J.; Liu, C.; Yu, H. Silica nanoparticles alleviate cadmium toxicity in rice cells: Mechanisms and size effects. Environ. Pollut. 2017, 228, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Adrees, M.; Qayyum, M.F.; Khalid, S.; ur Rehman, M.Z.; Sarwar, M.A. Effects of silicon nanoparticles on growth and physiology of wheat in cadmium contaminated soil under different soil moisture levels. Environ. Sci. Pollut. Res. 2020, 27, 4958–4968. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, M.A.; Ahmad, Z.; Ashraf, M.Y.; Afzal, M.; Nawaz, F.; Nafees, M.; Jatoi, W.N.; Malghani, N.A.; Shah, A.N.; Manan, A. Silicon mitigates drought stress in wheat (Triticum aestivum L.) through improving photosynthetic pigments, biochemical and yield characters. Silicon 2021, 13, 4757–4772. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Hussain, A.; ur Rehman, M.Z.; Ali, B.; Yousaf, B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Yang, T.; Gu, H.; Yang, W.; Liu, B.; Liang, S.; Zhao, J. Artificially selected grain shape gene combinations in Guangdong Simiao varieties of rice (Oryza sativa L.). Rice 2023, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Barik, M.; Dash, G.K.; Pandit, E.; Dash, S.K.; Baig, M.J.; Mohanty, J.N.; Swain, P. Root attributes governing drought stress adaptation and the associated molecular markers in chromosome segment substitution lines in rice (Oryza sativa L.). J. Exp. Biol. Agric. Sci. 2023, 11, 947–963. [Google Scholar] [CrossRef]
- Zhang, G. Target chromosome-segment substitution: A way to breeding by design in rice. Crop J. 2021, 9, 658–668. [Google Scholar] [CrossRef]
- Ashraf, H.; Ghouri, F.; Baloch, F.S.; Nadeem, M.A.; Fu, X.; Shahid, M.Q. Hybrid Rice Production: A Worldwide Review of Floral Traits and Breeding Technology, with Special Emphasis on China. Plants 2024, 13, 578. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, L.; Xiong, T.; Wang, Z.; Liao, Y.; Zou, T.; Zheng, M.; Zhang, Z.; Pan, X.; He, N. Genetic characterization of the chromosome single-segment substitution lines of O. glumaepatula and O. barthii and identification of QTLs for yield-related traits. Mol. Breed. 2019, 39, 51. [Google Scholar] [CrossRef]
- Pramanik, P.; Krishnan, P.; Maity, A.; Mridha, N.; Mukherjee, A.; Rai, V. Application of nanotechnology in agriculture. Environ. Nanotechnol. 2020, 4, 317–348. [Google Scholar]
- Huang, Q.; Ayyaz, A.; Farooq, M.A.; Zhang, K.; Chen, W.; Hannan, F.; Sun, Y.; Shahzad, K.; Ali, B.; Zhou, W. Silicon dioxide nanoparticles enhance plant growth, photosynthetic performance, and antioxidants defence machinery through suppressing chromium uptake in Brassica napus L. Environ. Pollut. 2024, 342, 123013. [Google Scholar] [CrossRef]
- Ghouri, F.; Ali, Z.; Naeem, M.; Ul-Allah, S.; Babar, M.; Baloch, F.S.; Chattah, W.S.; Shahid, M.Q. Effects of silicon and selenium in alleviation of drought stress in rice. Silicon 2022, 14, 5453–5461. [Google Scholar] [CrossRef]
- Hong, J.; Wang, C.; Wagner, D.C.; Gardea-Torresdey, J.L.; He, F.; Rico, C.M. Foliar application of nanoparticles: Mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano 2021, 8, 1196–1210. [Google Scholar] [CrossRef]
- Thind, S.; Hussain, I.; Rasheed, R.; Ashraf, M.A.; Perveen, A.; Ditta, A.; Hussain, S.; Khalil, N.; Ullah, Z.; Mahmood, Q. Alleviation of cadmium stress by silicon nanoparticles during different phenological stages of Ujala wheat variety. Arab. J. Geosci. 2021, 14, 1028. [Google Scholar] [CrossRef]
- Wang, L.; Ning, C.; Pan, T.; Cai, K. Role of silica nanoparticles in abiotic and biotic stress tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 1947. [Google Scholar] [CrossRef] [PubMed]
- Jawad Hassan, M.; Ali Raza, M.; Ur Rehman, S.; Ansar, M.; Gitari, H.; Khan, I.; Wajid, M.; Ahmed, M.; Abbas Shah, G.; Peng, Y.; et al. Effect of cadmium toxicity on growth, oxidative damage, antioxidant defense system and cadmium accumulation in two sorghum cultivars. Plants 2020, 9, 1575. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Wu, L.; Wang, Q.; Wang, Y.; Luo, H.; Song, J.; Yang, M.; Yao, H.; Chen, S. Physiological and molecular mechanisms of medicinal plants in response to cadmium stress: Current status and future perspective. J. Hazard. Mater. 2023, 450, 131008. [Google Scholar] [CrossRef]
- El Rasafi, T.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Tack, F.M.; Sebastian, A.; Prasad, M.; Rinklebe, J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2022, 52, 675–726. [Google Scholar] [CrossRef]
- Koleva, L.; Umar, A.; Yasin, N.A.; Shah, A.A.; Siddiqui, M.H.; Alamri, S.; Riaz, L.; Raza, A.; Javed, T.; Shabbir, Z. Iron oxide and silicon nanoparticles modulate mineral nutrient homeostasis and metabolism in cadmium-stressed Phaseolus vulgaris. Front. Plant Sci. 2022, 13, 806781. [Google Scholar] [CrossRef]
- Aqeel, U.; Aftab, T.; Khan, M.M.A.; Naeem, M.; Khan, M.N. A comprehensive review of impacts of diverse nanoparticles on growth, development and physiological adjustments in plants under changing environment. Chemosphere 2022, 291, 132672. [Google Scholar] [CrossRef]
- Hayat, F.; Khanum, F.; Li, J.; Iqbal, S.; Khan, U.; Javed, H.U.; Razzaq, M.K.; Altaf, M.A.; Peng, Y.; Ma, X. Nanoparticles and their potential role in plant adaptation to abiotic stress in horticultural crops: A review. Sci. Hortic. 2023, 321, 112285. [Google Scholar] [CrossRef]
- Prakash, V.; Rai, P.; Sharma, N.C.; Singh, V.P.; Tripathi, D.K.; Sharma, S.; Sahi, S. Application of zinc oxide nanoparticles as fertilizer boosts growth in rice plant and alleviates chromium stress by regulating genes involved in oxidative stress. Chemosphere 2022, 303, 134554. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Adeel, M.; Shakoor, N.; Guo, M.; Hao, Y.; Azeem, I.; Li, M.; Liu, M.; Rui, Y. Application of nanoparticles alleviates heavy metals stress and promotes plant growth: An overview. Nanomaterials 2020, 11, 26. [Google Scholar] [CrossRef]
- Soni, A.T.; Rookes, J.E.; Arya, S.S. Chitosan Nanoparticles as Seed Priming Agents to Alleviate Salinity Stress in Rice (Oryza sativa L.) Seedlings. Polysaccharides 2023, 4, 129–141. [Google Scholar] [CrossRef]
- Rai-Kalal, P.; Jajoo, A. Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol. Biochem. 2021, 160, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.A.; Akther, M.S.; Reza, M.A.; Kabir, A.H. Cadmium tolerance is associated with the root-driven coordination of cadmium sequestration, iron regulation, and ROS scavenging in rice. Plant Physiol. Biochem. 2019, 136, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Helaly, M.N.; El-Hoseiny, H.M.; Elsheery, N.I.; Kalaji, H.M.; de Los Santos-Villalobos, S.; Wróbel, J.; Hassan, I.F.; Gaballah, M.S.; Abdelrhman, L.A.; Mira, A.M. 5-Aminolevulinic acid and 24-epibrassinolide improve the drought stress resilience and productivity of banana plants. Plants 2022, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Bhat, J.A.; Hessini, K.; Yu, F.; Ahmad, P. Zinc oxide nanoparticles alleviates the adverse effects of cadmium stress on Oryza sativa via modulation of the photosynthesis and antioxidant defense system. Ecotoxicol. Environ. Saf. 2021, 220, 112401. [Google Scholar] [CrossRef]
- Tan, J.; Li, Y.; Hou, D.-X.; Wu, S. The effects and mechanisms of cyanidin-3-glucoside and its phenolic metabolites in maintaining intestinal integrity. Antioxidants 2019, 8, 479. [Google Scholar] [CrossRef]
- Sardar, R.; Ahmed, S.; Yasin, N. Seed priming with karrikinolide improves growth and physiochemical features of coriandrum sativum under cadmium stress. Environ. Adv. 2021, 5, 100082. [Google Scholar] [CrossRef]
- Riaz, M.; Zhao, S.; Kamran, M.; Ur Rehman, N.; Mora-Poblete, F.; Maldonado, C.; Hamzah Saleem, M.; Parveen, A.; Ahmed Al-Ghamdi, A.; Al-Hemaid, F.M. Effect of nano-silicon on the regulation of ascorbate-glutathione contents, antioxidant defense system and growth of copper stressed wheat (Triticum aestivum L.) seedlings. Front. Plant Sci. 2022, 13, 986991. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y.; Zheng, X.; Wang, Y. Silicon dioxide nanoparticles improve plant growth by enhancing antioxidant enzyme capacity in bamboo (Pleioblastus pygmaeus) under lead toxicity. Trees 2020, 34, 469–481. [Google Scholar] [CrossRef]
- Elshayb, O.M.; Nada, A.M.; Ibrahim, H.M.; Amin, H.E.; Atta, A.M. Application of silica nanoparticles for improving growth, yield, and enzymatic antioxidant for the hybrid rice ehr1 growing under water regime conditions. Materials 2021, 14, 1150. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Ghouri, F.; Sarwar, S.; Alomrani, S.O.; Riaz, M.; Haider, F.U.; Liu, J.; Imran, M.; Ali, S.; Liu, X. Modulation of metal transporters, oxidative stress and cell abnormalities by synergistic application of silicon and titanium oxide nanoparticles: A strategy for cadmium tolerance in rice. Chemosphere 2023, 345, 140439. [Google Scholar] [CrossRef] [PubMed]
- Ghouri, F.; Sarwar, S.; Sun, L.; Riaz, M.; Haider, F.U.; Ashraf, H.; Lai, M.; Imran, M.; Liu, J.; Ali, S. Silicon and iron nanoparticles protect rice against lead (Pb) stress by improving oxidative tolerance and minimizing Pb uptake. Sci. Rep. 2024, 14, 5986. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Masood, H.A.; Noman, M.; Al-Huqail, A.A.; Alghanem, S.M.; Khan, M.M.; Muhammad, S.; Manzoor, N.; Rizwan, M.; Qi, X. Biogenic silicon nanoparticles mitigate cadmium (Cd) toxicity in rapeseed (Brassica napus L.) by modulating the cellular oxidative stress metabolism and reducing Cd translocation. J. Hazard. Mater. 2023, 459, 132070. [Google Scholar] [CrossRef] [PubMed]
- Memari-Tabrizi, E.F.; Yousefpour-Dokhanieh, A.; Babashpour-Asl, M. Foliar-applied silicon nanoparticles mitigate cadmium stress through physio-chemical changes to improve growth, antioxidant capacity, and essential oil profile of summer savory (Satureja hortensis L.). Plant Physiol. Biochem. 2021, 165, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Iqbal, M.; Ahmad, Z.; Iqbal, M.A.; Artyszak, A.; Sabagh, A.E.; Alharby, H.F.; Hossain, A. Foliar application of silicon-based nanoparticles improve the adaptability of maize (Zea mays L.) in cadmium contaminated soils. Environ. Sci. Pollut. Res. 2023, 30, 41002–41013. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhang, P.; He, M.; Cao, Y.; Adeel, M.; Shakoor, N.; Jiang, Y.; Zhao, W.; Li, Y.; Li, M. Iron-based nanomaterials reduce cadmium toxicity in rice (Oryza sativa L.) by modulating phytohormones, phytochelatin, cadmium transport genes and iron plaque formation. Environ. Pollut. 2023, 320, 121063. [Google Scholar] [CrossRef]
- Fang, C.; Li, L.; Zhang, P.; Wang, D.; Yang, L.; Reza, B.M.; Lin, W. Lsi1 modulates the antioxidant capacity of rice and protects against ultraviolet-B radiation. Plant Sci. 2019, 278, 96–106. [Google Scholar] [CrossRef]
- Ghouri, F.; Shahid, M.J.; Liu, J.; Lai, M.; Sun, L.; Wu, J.; Liu, X.; Ali, S.; Shahid, M.Q. Polyploidy and zinc oxide nanoparticles alleviated Cd toxicity in rice by modulating oxidative stress and expression levels of sucrose and metal-transporter genes. J. Hazard. Mater. 2023, 448, 130991. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Hussain, S.; He, L.; Ashraf, M.F.; Ihtisham, M.; Warraich, E.A.; Tang, X. Molybdenum-induced regulation of antioxidant defense-mitigated cadmium stress in aromatic rice and improved crop growth, yield, and quality traits. Antioxidants 2021, 10, 838. [Google Scholar] [CrossRef] [PubMed]
- Yong, Z.; Hao-Ru, T.; Ya, L. Variation in antioxidant enzyme activities of two strawberry cultivars with short-term low temperature stress. World J. Agric. Sci. 2008, 4, 458–462. [Google Scholar]
- Kamara, N.; Lu, Z.; Jiao, Y.; Zhu, L.; Wu, J.; Chen, Z.; Wang, L.; Liu, X.; Shahid, M.Q. An uncharacterized protein NY1 targets EAT1 to regulate anther tapetum development in polyploid rice. BMC Plant Biol. 2022, 22, 582. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashraf, H.; Ghouri, F.; Liang, J.; Xia, W.; Zheng, Z.; Shahid, M.Q.; Fu, X. Silicon Dioxide Nanoparticles-Based Amelioration of Cd Toxicity by Regulating Antioxidant Activity and Photosynthetic Parameters in a Line Developed from Wild Rice. Plants 2024, 13, 1715. https://doi.org/10.3390/plants13121715
Ashraf H, Ghouri F, Liang J, Xia W, Zheng Z, Shahid MQ, Fu X. Silicon Dioxide Nanoparticles-Based Amelioration of Cd Toxicity by Regulating Antioxidant Activity and Photosynthetic Parameters in a Line Developed from Wild Rice. Plants. 2024; 13(12):1715. https://doi.org/10.3390/plants13121715
Chicago/Turabian StyleAshraf, Humera, Fozia Ghouri, Jiabin Liang, Weiwei Xia, Zhiming Zheng, Muhammad Qasim Shahid, and Xuelin Fu. 2024. "Silicon Dioxide Nanoparticles-Based Amelioration of Cd Toxicity by Regulating Antioxidant Activity and Photosynthetic Parameters in a Line Developed from Wild Rice" Plants 13, no. 12: 1715. https://doi.org/10.3390/plants13121715
APA StyleAshraf, H., Ghouri, F., Liang, J., Xia, W., Zheng, Z., Shahid, M. Q., & Fu, X. (2024). Silicon Dioxide Nanoparticles-Based Amelioration of Cd Toxicity by Regulating Antioxidant Activity and Photosynthetic Parameters in a Line Developed from Wild Rice. Plants, 13(12), 1715. https://doi.org/10.3390/plants13121715





