Trends and Directions in Oats Research under Drought and Salt Stresses: A Bibliometric Analysis (1993–2023)
Abstract
1. Introduction
2. Results
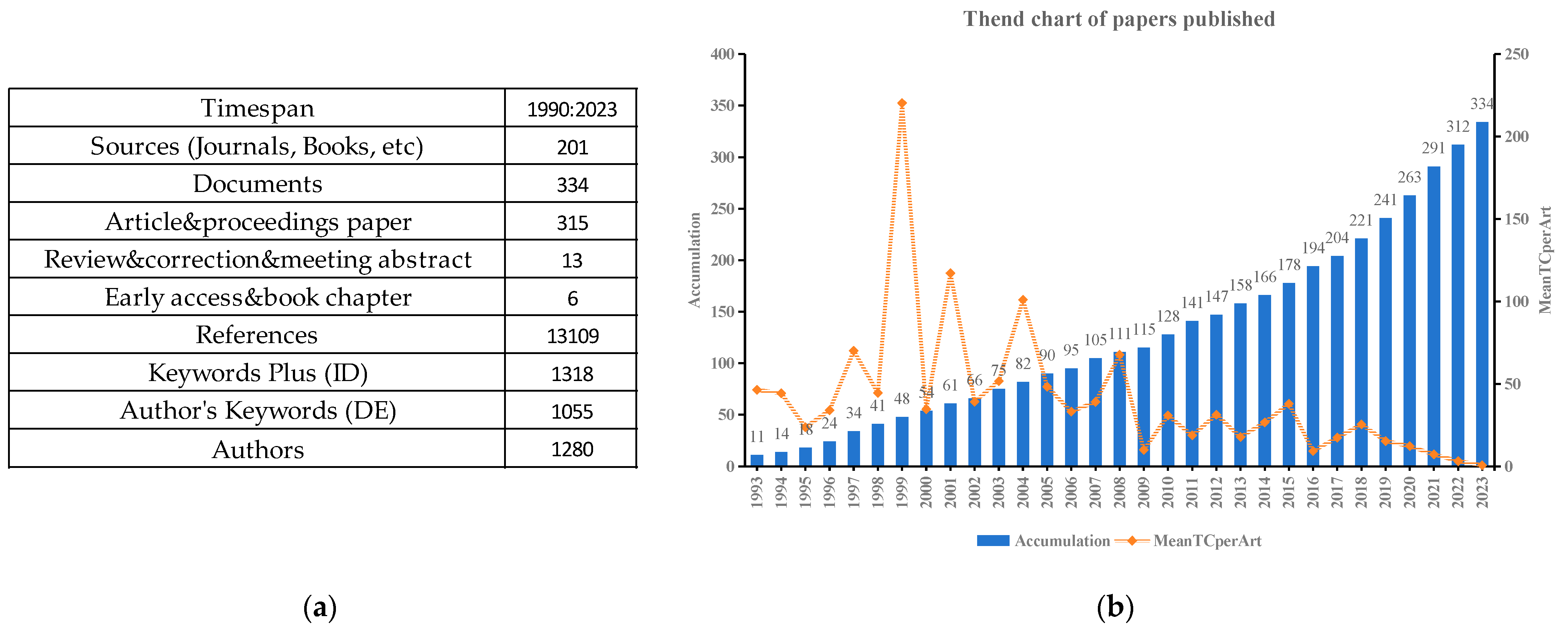
2.1. Descriptive Statistics of Publications and Main Information
2.2. Analysis of the Collaboration Network
2.2.1. Institutions
2.2.2. Countries
2.3. Publisher’s Citations and Sources of Analysis
2.4. Analysis of the Co-Citation Network
- (a)
- Physiological Changes and Responses: Notable contributions include those by Qi and Bai, who provided insights into the physiological and biochemical response mechanisms of oat under stress [15,35]. They explored the effects of drought stress on lipid peroxidation and antioxidant enzymes across various oat varieties. Zhao discussed the physiological responses of oat to key environmental stress factors at different stages [36]. Wang examined the role of stomatal closure and osmotic adjustment in drought adaptation, linking the higher yield of certain oat genotypes to drought tolerance strategies [37]. Additionally, Gao investigated the effect of melatonin on the antioxidant capacity of ruderalis under salt stress and analyzed the antioxidant mechanisms [38].
- (b)
- Yield and Quality: Pandey used oat as a model in field trials to report on scientific advancements regarding oat water use [39]. Sudras examined factors influencing oat yield under stresses [40], while Islam reviewed the impact of drought stress on oat productivity and growth [41]. The physiological mechanisms of drought stress on photosynthesis (Pn) and yield formation were also investigated by Zhao under two different genotypes, where drought stress decreased both oat yield and harvest index at the tassel stage [36]. Additionally, some researchers have analyzed that inoculation with IG3 strain can reduce the effect of salt stress on oat yield and growth under sodium chloride stress [42].
- (c)
- Technology Application: Researchers like Gao and Varghese explored the effects of exogenous melatonin (MT) on the antioxidant capacity of oat during drought and salt stresses, discussing how melatonin alters the plants’ light organs and antioxidant capacity to enhance stress resistance [42,43,44,45]. Sapre and Chang evaluated the inoculation effect of rhizosphere bacteria strain IG3, revealing its potential as a bio-fertilizer in saline-alkali land [21,42]. Song investigated how nitrogen application affects oat photosynthesis under salt stress, finding that increased nitrogen supply maintains photosynthesis and mitigates the adverse effects of stress [46].
- (d)
- Breeding: Studies by Willenborg and Gong focused on the types of oat that exhibit drought resistance and the assessment of oat variants’ biomass using molecular breeding, providing a theoretical foundation for developing high-yielding oats [47,48]. Additionally, Oraby studied third-generation transgenic oat and showed that transgenic R3 plants grew better and were more tolerant to salt stress [49].
- (e)
- Metabolism and Signal Transduction: Researchers like Sanchez, Zhao, and Xu compiled data on metabolic regulation under salt stress, emphasizing the timing, regulation, and integration of these stresses and their impact on stress resistance [50,51,52]. Xu revealed the adaptive response mechanisms of two oat cultivars under salt stress using their metabolomics and transcriptomics approaches. Heald and Wu elucidated the general pathway of stress signal transduction, emphasizing the significance of early induction of signal transduction-related secondary metabolic pathway enrichment in drought-stress management [53,54].
| Ranking | TC | Title | Author/Year | DOI |
|---|---|---|---|---|
| 1 | 94 | Soil Management and Supplemental Irrigation Effects on Potato: I. Soil Properties, Tuber Yield, and Quality | Zhang, M. (1999) [55] | 10.1016/S2095-3119(16)61515-0 |
| 2 | 64 | Effects of Melatonin on Antioxidant Capacity in Naked Oat Seedlings under Drought Stress | Gao, W. (2018) [43] | 10.21930/Rcta.Vol23_Num1_Art:2214 |
| 3 | 63 | Oat Germination Characteristics Differ Among Genotypes, Seed Sizes, and Osmotic Potentials | Willenborg (2005) [48] | 10.1002/Jsfa.12504 |
| 4 | 55 | A Metabolomic Study in Oats (Avena sativa) Highlights A Drought Tolerance Mechanism Based upon Salicylate Signaling Pathways and The Modulation of Carbon, Antioxidant and Photo-Oxidative Metabolism | Heald, J. (2015) [50] | 10.1590/S0100-83582012000100001 |
| 5 | 48 | Fatty Acid Profile Changes During Gradual Soil Water Depletion in Oats Suggests a Role for Jasmonates in Coping with Drought | Sanchez. (2018) [54] | 10.3390/Agriculture13020243 |
| 6 | 46 | Drought Stress Induced Changes in Lipid Peroxidation and Antioxidant System in Genus Avena | Pandey, H. (2010) [39] | 10.1016/0005-2736(91)90195-E |
| 7 | 38 | The Optimum Ridge-Furrow Ratio and Suitable Ridge-Covering Material in Rainwater Harvesting for Oats Production in Semiarid Regions of China | Qi, W. (2015) [56] | 10.1016/0005-2736(92)90253-I |
| 8 | 34 | Effects of water-saving superabsorbent polymer on antioxidant enzyme activities and lipid peroxidation in Oat (Avena sativa L.) under drought stress | Islam, M. (2011) [41] | 10.1002/jsfa.4234 |
| 9 | 30 | Early Activation of Plasma Membrane H+-Atpase and Its Relation to Drought Adaptation in Two Contrasting Oat (Avena sativa L.) Genotypes | Gong, D. (2010) [47] | 10.1016/j.envexpbot.2010.02.011 |
| 10 | 28 | Evaluation of Drought Tolerance Indices for Selection of Turkish Oat (Avena saliva L.) Landraces under Various Environmental Conditions | Akcura, M. (2011) [57] | 10.1016/j.tripleo.2009.03.011NA |
| 11 | 25 | Higher Rust Resistance and Similar Yield of Oat Landraces Versus Cultivars Under High Temperature And Drought | Dunn, M. (2017) [58] | 10.1007/Bf00267460 |
| 12 | 22 | Source-Sink Adjustment: A Mechanistic Understanding of the Timing and Severity of Drought Stress on Photosynthesis and Grain Yields of Two Contrasting Oat (Avena sativa L.) Genotypes | Zhao, B. (2021) [36] | 10.1007/s00344-020-10093-5 |
| 13 | 22 | Oat Phenotypes for Drought Adaptation and Yield Potential | Sadras, V. (2017) [40] | 10.1111/1365-2656.13189 |
| 14 | 20 | Recently-Released Genotypes of Naked Oat (Avena nuda L.) Out-Yield Early Releases under Water-Limited Conditions By Greater Reproductive Allocation and Desiccation Tolerance | Wang, T. (2017) [37] | 10.3390/Agriculture11040332 |
| 15 | 19 | Salicylic Acid Regulates Polyamine Biosynthesis during Drought Responses in Oat | Yule, K.(2015) [59] | 10.1007/S11829-010-9112-5 |
| 16 | 17 | Effect of Seed Size and Drought Stress on Germination and Seedling Growth of Naked Oat (Avena sativa L.) | Mut, Z. (2010) [60] | 10.1016/S1671-2927(09)60153-X |
| 17 | 16 | Influence of Water Stress on Absorption, Translocation and Phytotoxicity of Fenoxaprop-Ethyl and Imazamethabenz-Methyl in Avena Fatua | Xie, H. (1996) [61] | 10.1111/j.1365-3180. 1996.tb01802.x |
| 18 | 15 | Targeting Sources of Drought Tolerance Within an Avena spp. Collection through Multivariate Approaches | Lin, Y. (2012) [62] | 10.1007/S00572-020-00963-X |
| 19 | 15 | Water Use Efficiency and Physiological Responses of Oat under Alternate Partial Root-Zone Irrigation in The Semiarid Areas of Northeast China | Kavroulakis, N. (2012) [63] | 10.1016/J.Plaphy.2021.08.029 |
| 20 | 14 | Application of Photochemical Parameters and Several Indices Based on Phenotypical Traits to Assess Intraspecific Variation of Oat (Avena sativa L.) Tolerance to Drought | Arabia, S. (2017) [64] | 10.1007/S12298-021-01023-0 |
| Ranking | TC | Cited Document | Author/Year | DOI |
|---|---|---|---|---|
| 1 | 150 | Growth, Gas Exchange, Chlorophyll Fluorescence, and Ion Content of Naked Oat in Response to Salinity | Zhao, G. (2007) [23] | 10.2135/cropsci2006.06.0371 |
| 2 | 110 | Klebsiella Sp Confers Enhanced Tolerance to Salinity and Plant Growth Promotion in Oat Seedlings (Avena sativa) | Sapre, S. (2018) [42] | 10.1016/j.micres.2017.09.009 |
| 3 | 98 | Plant Growth-Promoting Bacteria Facilitate the Growth of Barley and Oats in Salt-Impacted Soil: Implications for Phytoremediation of Saline Soils | Chang, P. (2014) [21] | 10.1080/15226514.2013.821447 |
| 4 | 73 | Effects of Silicon Nanoparticles on Molecular, Chemical, Structural, and Ultrastructural Characteristics of Oat (Avena sativa L.) | Asgari, F. (2018) [65] | 10.1016/J.Plaphy.2018.03.021 |
| 5 | 59 | Melatonin-Mediated Regulation of Growth and Antioxidant Capacity in Salt-Tolerant Naked Oat under Salt Stress | Gao, W. (2019) [38] | 10.3390/ijms20051176 |
| 6 | 48 | Salicylic Acid, Hydrogen Peroxide and Calcium-Induced Saline Tolerance Associated with Endogenous Hydrogen Peroxide Homeostasis in Naked Oat Seedlings | Xu, Q. (2018) [66] | 10.1007/s10725-007-9247-2 |
| 7 | 40 | Transcriptome Analysis of Hexaploid Hulless Oat in Response to Salinity Stress | Wu, B. (2017) [53] | 10.1371/journal.pone.0171451 |
| 8 | 32 | Melatonin Positively Influences the Photosynthetic Machinery and Antioxidant System of Avena sativa during Salinity Stress | Varghese, N. (2019) [44] | 10.3390/plants8120610 |
| 9 | 27 | Integrative Analysis of Transcriptome and Metabolome Reveal Mechanism of Tolerance to Salt Stress in Oat (Avena sativa L.) | Xu, Z. (2021) [51] | 10.1016/j.plaphy.2021.01.027 |
| 10 | 26 | Barley Hva1 Gene Confers Salt Tolerance in R3 Transgenic Oat | Oraby, H. (2005) [49] | 10.2135/cropsci2004-0605 |
| 11 | 26 | Nitrogen Application Improved Photosynthetic Productivity, Chlorophyll Fluorescence, Yield and Yield Components of Two Oat Genotypes under Saline Conditions | Song, X. (2019) [46] | 10.3390/agronomy9030115 |
| 12 | 25 | Influence of Gibberellic Acid and Different Salt Concentrations on Germination Percentage and Physiological Parameters of Oat Cultivars | Chauhan, A. (2019) [67] | 10.1016/j.sjbs.2019.04.014 |
| 13 | 25 | Physiological and Tmt-Based Proteomic Analysis of Oat Early Seedlings in Response to Alkali Stress | Zhao, Z. (2019) [52] | 10.1016/j.jprot.2018.12.018 |
| 14 | 23 | Screening Oat Genotypes for Tolerance to Salinity and Alkalinity | Bai, J. (2018) [14] | 10.3389/fpls.2018.01302 |
| 15 | 23 | Physiological and Biochemical Changes of Cbf3 Transgenic Oat in Response to Salinity Stress | Oraby, H. (2012) [68] | 10.1016/j.plantsci.2012.01.003 |
| 16 | 23 | Potential Application of Oat for Phytoremediation of Salt Ions in Coastal Saline-Alkali Soil | Han, L. (2012) [16] | 10.1016/j.ecoleng.2013.09.034 |
| 17 | 23 | Proteomic Response of Oat Leaves to Long-Term Salinity Stress | Bai, J. (2016) [69] | 10.1007/s11356-016-8092-0 |
| 18 | 22 | Effect of Alkali Stress on Soluble Sugar, Antioxidant Enzymes and Yield of Oat | Bai, J. (2013) [70] | 10.1016/S2095-3119(13)60556-0 |
| 19 | 19 | Effects Of Saline and Alkaline Stresses on Growth and Physiological Changes in Oat (Avena sativa L.) Seedlings | Gao, Z. (2014) [71] | 10.15835/nbha4229441 |
| 20 | 19 | Use of Mixed Solid Waste as A Soil Amendment for Saline-Sodic Soil Remediation and Oat Seedling Growth Improvement | Fan, Y. (2019) [72] | 10.1007/s11356-016-7360-3 |
2.5. Analysis of the Co-Occurrence Network
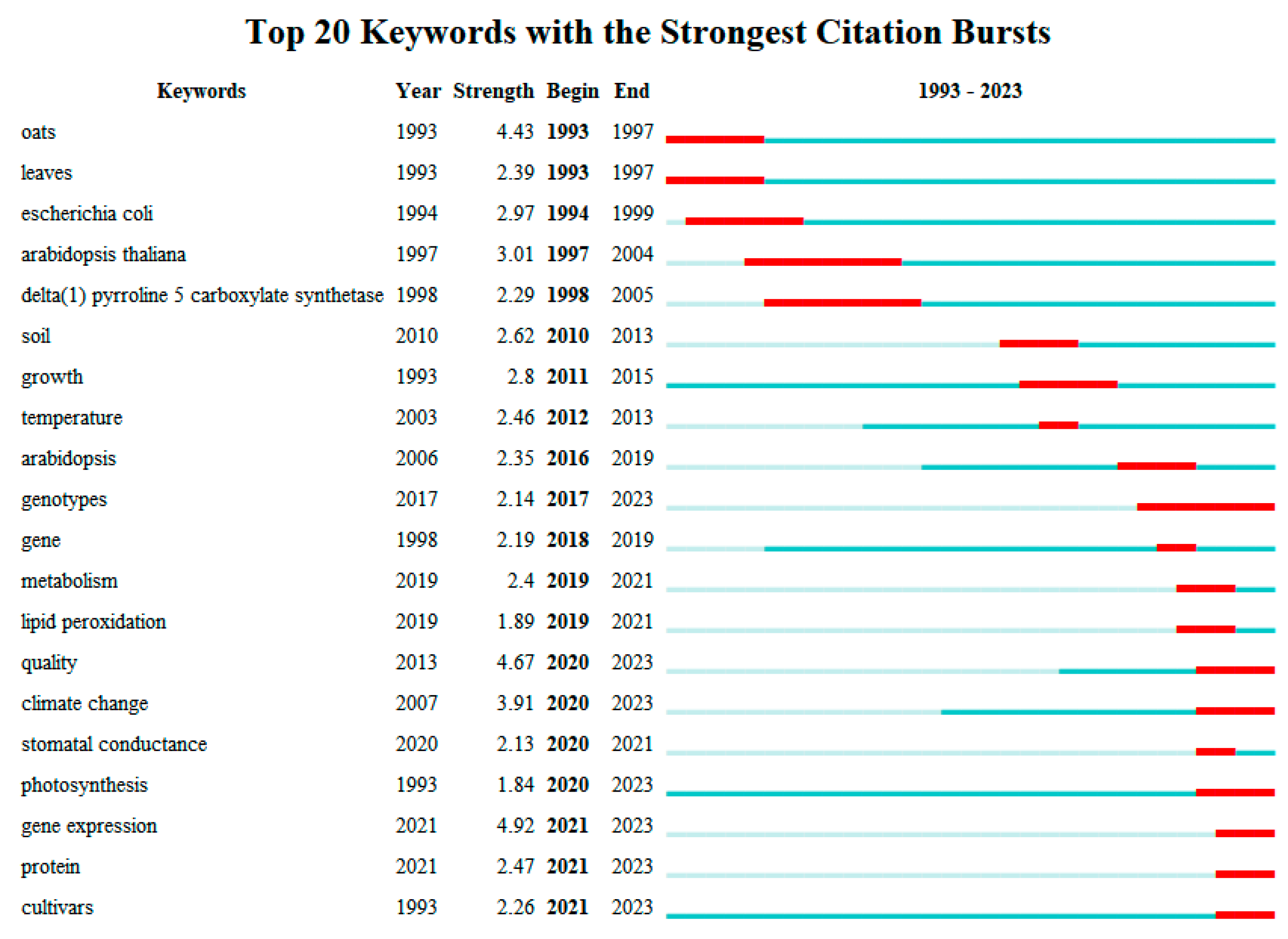
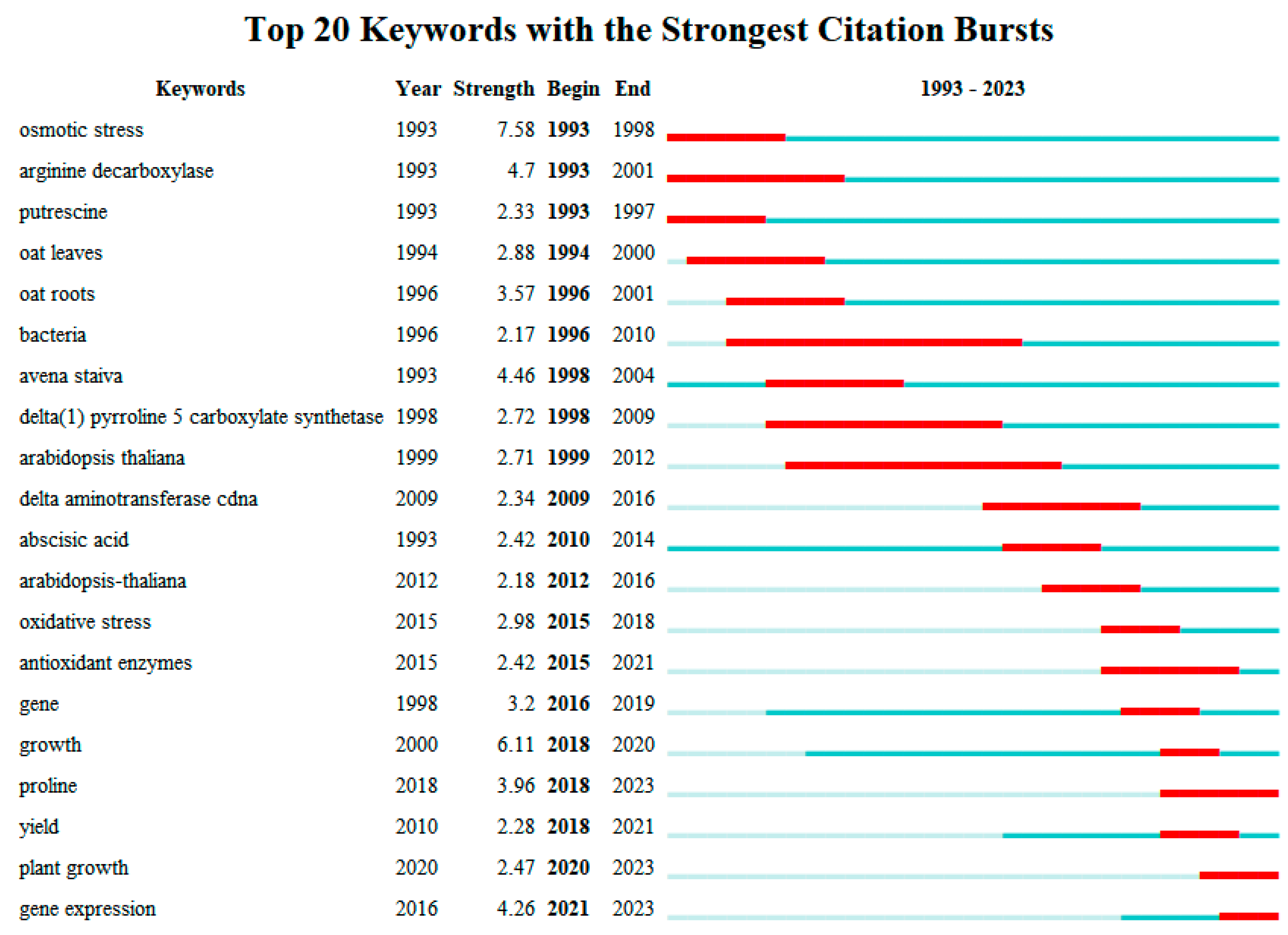
2.6. Keywords Centrality and Outbreak Analysis
3. Discussion
3.1. Yield and Quality
3.2. Molecular Response
3.2.1. Metabolomics and Transcriptomics
3.2.2. Proteomics
3.2.3. Genomics
3.3. Physiological and Biochemical Response
3.3.1. Antioxidant and Lipid Peroxidation
3.3.2. Photosynthesis
3.3.3. Plant Hormones
3.4. Cultivation and Breeding
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Allen, S.; Barros, V.; Burton, I.; Campbell-Lendrum, D.; Cardona, O.D.; Cutter, S.L.; Dube, O.P.; Ebi, K.L.; Field, C.B.; Handmer, J.; et al. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation: Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; pp. 3–21. [Google Scholar]
- Tramblay, Y.; Koutroulis, A.; Samaniego, L.; VicenteSerrano, S.M.; Volaire, F.; Boone, A.; Le Page, M.; Llasat, M.C.; Albergel, C.; Burak, S.; et al. Challenges for drought assessment in the Mediterranean region under future climate scenarios. Earth-Sci. Rev. 2020, 210, 103348. [Google Scholar] [CrossRef]
- ColladosLara, A.; PulidoVelazquez, D.; PardoIgúzquiza, E. An integrated statistical method to generate potential future climate scenarios to analyse droughts. Water 2018, 10, 1224. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alam, P.; Alyemeni, M.N.; Wijaya, L.; Ali, S.; Ashraf, M. Silicon (Si) supplementation alleviates nacl toxicity in mung bean [Vigna radiata (L.) Wilczek] through the modifications of physio-biochemical attributes and key antioxidant enzymes. J. Plant Growth Requl. 2019, 38, 70–82. [Google Scholar] [CrossRef]
- Willenborg, C.; Gulden, R.; Johnson, E.; Shirtliffe, S. Germination characteristics of polymer-coated canola (Brassica napus L.) seeds subjected to moisture stress at different temperatures. Agron. J. 2004, 96, 786–791. [Google Scholar] [CrossRef]
- Teklic, T.; Paradikovic, N.; Spoljarevic, M.; Zeljkovic, S.; Loncaric, Z.; Lisjak, M. Effects of ascorbic acid and/or α-tocopherol on agronomic and physio-biochemical traits of oat (Avena sativa L.) under drought condition. Agronomy 2021, 78, 169–191. [Google Scholar]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum. 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Boyer, J.S. Plant productivity and environment (crop genetic improvement). Field Crop. Res. 1982, 204, 169–179. [Google Scholar]
- Epstein, E.; Norlyn, J.D.; Rush, D.W.; Kingsbury, R.W.; Kelley, D.B.; Cunningham, G.A.; Wrona, A.F. Saline culture of crops: A genetic approach. Science 1980, 210, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S.; Iqbal, M.; Saeed, M.; Iqbal, N.; Zafar, S.; Mumtaz, T. Cysteine-induced alterations in physicochemical parameters of oat (Avena sativa L. var. Scott and F-411) under drought stress. Biol. Futura 2019, 70, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Zhang, C.; Zhao, N.N.; Sun, X.R.; Hou, S.; Wang, P.C. Physiological nitrogen uptake and utilisation responses in two native plants from the Qinghai-Tibet plateau under different water and fertiliser conditions. Agronomy 2024, 14, 440. [Google Scholar] [CrossRef]
- Bai, J.; Yan, W.; Wang, Y.; Yin, Q.; Liu, J.; Wight, C.; Ma, B. Screening oat genotypes for tolerance to salinity and alkalinity. Front. Plant Sci. 2018, 9, 01302. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Yang, J.; Gao, Y.; Li, J.Q.; Yang, Y.T.; Wang, P.C. Allocation, morphology, physiology: Multiple aspects of above-and below-ground responses to water table stress, duration of drainage in alpine wetland plants (Carex muliensis). Plant Soil 2024. [Google Scholar] [CrossRef]
- Han, L.; Liu, H.; Yu, S.; Wang, W.; Liu, J. Potential application of oat for phytoremediation of salt ions in coastal saline-alkali soil. Ecol. Eng. 2013, 61, 274–281. [Google Scholar] [CrossRef]
- Jan, S.; Abbas, N.; Ashraf, M.; Ahmad, P. Roles of potential plant hormones and transcription factors in controlling leaf senescence and drought tolerance. Protoplasma 2019, 256, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Ibrahim, M.; Riaz, M.; Arif, M.S.; Hafeez, F.; Al-Wabel, M.I.; Shahzad, A.N. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. Res. 2017, 24, 12700–12712. [Google Scholar] [CrossRef] [PubMed]
- Bijanzadeh, E.; Naderi, R.; Egan, T.P. Exogenous application of humic acid and salicylic acid to alleviate seedling drought stress in two corn (Zea mays L.) hybrids. J. Plant Nutr. 2019, 42, 1483–1495. [Google Scholar] [CrossRef]
- Neumann, P. Salinity resistance and plant growth revisited. Plant Cell Environ. 1997, 20, 1193–1198. [Google Scholar] [CrossRef]
- Chang, P.; Gerhardt, K.E.; Huang, X.D.; Yu, X.M.; Glick, B.R.; Gerwing, P.D.; Greenberg, B.M. Plant growth-promoting bacteria facilitate the growth of barley, and oats in salt-impacted soil: Implications for phytoremediation of saline soils. Int. J. Phytoremediat. 2014, 16, 1133–1147. [Google Scholar] [CrossRef] [PubMed]
- Canales, F.J.; Nagel, K.A.; Müller, C.; Rispail, N.; Prats, E. Deciphering root architectural traits involved to cope with water deficit in oat. Front. Plant Sci. 2019, 10, 1558. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Q.; Ma, B.L.; Ren, C.Z. Growth, Gas exchange, chlorophyll fluorescence, and ion content of naked oat in response to salinity. Crop Sci. 2007, 47, 123–131. [Google Scholar] [CrossRef]
- Ramegowda, V.; Senthil-Kumar, M. The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 2015, 176, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Comino, I.; de Lourdes Moreno, M.; Sousa, C. Role of oats in celiac disease. Microbiol. Res. 2015, 21, 11825–11831. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.L.; Zhang, X.S.; Lu, X.H.; Qin, R.; Bi, Y.M.; Gao, W.W. Effects of maize rotation on the physicochemical properties and microbial communities of American ginseng cultivated soil. Sci. Rep. 2019, 9, 8615. [Google Scholar] [CrossRef] [PubMed]
- Sadras, V.; Dreccer, M.F. Adaptation of wheat, barley, canola, field pea and chickpea to the thermal environments of Australia. Crop Pasture Sci. 2015, 66, 1137–1150. [Google Scholar] [CrossRef]
- Peterson, D.M. Oat Antioxidants. J. Cereal Sci. 2001, 33, 115–129. [Google Scholar] [CrossRef]
- Tang, Y.; Li, S.; Yan, J.; Peng, Y.; Weng, W.; Yao, X.; Gao, A.; Cheng, J.; Ruan, J.; Xu, B. Bioactive components and health functions of oat. Food Rev. Int. 2023, 39, 4545–4564. [Google Scholar] [CrossRef]
- Li, X.; Zhou, L.; Yu, Y.; Zhang, J.; Wang, J.; Sun, B. The potential functions and mechanisms of oat on cancer prevention: A review. J. Agric. Food Chem. 2022, 70, 14588–14599. [Google Scholar] [CrossRef] [PubMed]
- Choudhri, A.F.; Siddiqui, A.; Khan, N.R.; Cohen, H.L. Understanding bibliometric parameters and analysis. Radiographics 2015, 35, 736–746. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Li, J.Q.; Gao, Y.; Wang, R.; Hang, H.Y.; Zhang, Y.; Zhao, L.L.; Wang, P.C. Research on drought stress in Medicago sativa L. from 1998 to 2023: A Bibliometric Analysis. Front. Plant Sci. 2024, 15, 1406256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.Q.; Wang, X.T.; Yang, Y.T.; Zhou, Z.J.; Deng, X.L.; Gao, Y.; Wang, P.C. A bibliometric analysis review of the Pennisetum (1970–2023). Front. Sustain. Food Syst. 2014, 8, 1405684. [Google Scholar] [CrossRef]
- Durieux, V.; Gevenois, P.A. Bibliometric indicators: Quality measurements of scientific publication. Radiology 2010, 255, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, J.; Jiao, W.; Sa, R.; Zhang, N.; Jia, R. Proteomic analysis of salt-responsive proteins in oat roots (Avena sativa L.). J. Sci. Food Agric. 2016, 96, 3867–3875. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ma, B.L.; Hu, Y.; Liu, J. Source-Sink Adjustment: A mechanistic understanding of the timing and severity of drought stress on photosynthesis and grain yields of two contrasting oat (Avena sativa L.) genotypes. J. Plant Growth Regul. 2021, 40, 263–276. [Google Scholar] [CrossRef]
- Wang, T.; Du, Y.; He, J.; Turner, N.; Wang, B.; Zhang, C.; Cui, T.; Li, F. Recently released genotypes of naked oat (Avena nuda L.) out-yield early releases under water-limited conditions by greater reproductive allocation and desiccation tolerance. Field Crop. Res. 2017, 204, 169–179. [Google Scholar] [CrossRef]
- Gao, W.; Feng, Z.; Bai, Q.; He, J.; Wang, Y. Melatonin-mediated regulation of growth and antioxidant capacity in salt-tolerant naked oat under salt stress. Int. J. Mol. Sci. 2019, 20, 1176. [Google Scholar] [CrossRef] [PubMed]
- Pandey, H.; Baig, M.; Chandra, A.; Bhatt, R. Drought stress induced changes in lipid peroxidation and antioxidant system in genus avena. J. Environ. Biol. 2010, 31, 435–440. [Google Scholar] [PubMed]
- Sadras, V.O.; Mahadevan, M.; Zwer, P.K. Oat phenotypes for drought adaptation and yield potential. Field Crop. Res. 2017, 212, 135–144. [Google Scholar] [CrossRef]
- Islam, M.R.; Xue, X.; Mao, S.; Ren, C.; Eneji, A.E.; Hu, Y. Effects of water-saving superabsorbent polymer on antioxidant enzyme activities and lipid peroxidation in oat (Avena sativa L.) under drought stress. J. Sci. Food Agric. 2010, 91, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Sapre, S.; Gontia-Mishra, I.; Tiwari, S. Klebsiella sp confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa). Microbiolo. Res. 2018, 206, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, Y.; Feng, Z.; Bai, Q.; He, J.; Wang, Y. Effects of melatonin on antioxidant capacity in naked oat seedlings under drought stress. Molecules 2018, 23, 1580. [Google Scholar] [CrossRef] [PubMed]
- Varghese, N.; Alyammahi, O.; Nasreddine, S.; Alhassani, A.; Gururani, M.A. Melatonin positively influences the photosynthetic machinery and antioxidant system of avena sativa during salinity stress. Plants 2019, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Abramovitch, K.; Thames, W.; Leon, I.-L.K.; Colosi, D.C.; Goren, A.D. Comparison of the efficacy and technical accuracy of different rectangular collimators for intraoral radiography. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2009, 108, e22–e28. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; Ma, B.-L.; Wu, W.; Ahmad, I.; Zhu, G.; Yan, W.; Jiao, X. Nitrogen application improved photosynthetic productivity, chlorophyll fluorescence, yield and yield components of two oat genotypes under saline conditions. Agronomy 2019, 9, 115. [Google Scholar] [CrossRef]
- Gong, D.; Xiong, Y.; Ma, B.; Wang, T.; Ge, J.; Qin, X.; Li, P.; Kong, H.; Li, Z.; Li, F. Early activation of plasma membrane H+-ATPase and its relation to drought adaptation in two contrasting oat (Avena sativa L.) genotypes. Environ. Exp. Bot. 2010, 69, 1–8. [Google Scholar] [CrossRef]
- Willenborg, C.J.; Wildeman, J.C.; Miller, A.K.; Rossnagel, B.G.; Shirtliffe, S.J. Oat germination characteristics differ among genotypes, seed sizes, and osmotic potentials. Crop Sci. 2005, 45, 2023–2029. [Google Scholar] [CrossRef]
- Oraby, H.F.; Ransom, C.B.; Kravchenko, A.N.; Sticklen, M.B. Barley HVA1 gene confers salt tolerance in R3 transgenic oat. Crop Sci. 2005, 45, 2218–2227. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Heald, J.; Kingston-Smith, A.; Winters, A.; Rubiales, D.; Sanz, M.; Mur, L.A.J.; Prats, E. A Metabolomic Study in Oats (Avena sativa) Highlights A Drought Tolerance Mechanism Based upon Salicylate Signaling Pathways and The Modulation of Carbon, Antioxidant and Photo-Oxidative Metabolism. Plant Cell Environ. 2015, 38, 1434–1452. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, X.; Lu, X.; Zhao, B.; Yang, Y.; Liu, J. Integrative analysis of transcriptome and metabolome reveal mechanism of tolerance to salt stress in oat (Avena sativa L.). Plant Physiol. Biochem. 2021, 160, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, J.; Jia, R.; Bao, S.; Haixia; Chen, X. Physiological and TMT-based proteomic analysis of oat early seedlings in response to alkali stress. J. Proteom. 2019, 193, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, B.; Hu, Y.; Huo, P.; Zhang, Q.; Chen, X.; Zhang, Z. Transcriptome analysis of hexaploid hulless oat in response to salinity stress. PLoS ONE 2017, 12, 0171451. [Google Scholar]
- SánchezMartín, J.; Canales, F.J.; Tweed, J.K.S.; Lee, M.R.F.; Rubiales, D.; Gómez-Cadenas, A.; Arbona, V.; Mur, L.A.J.; Prats, E. Fatty acid profile changes during gradual soil water depletion in oats suggests a role for jasmonates in coping with drought. Front. Plant Sci. 2018, 9, 1077. [Google Scholar]
- Porter, G.A.; Bradbury, W.B.; Sisson, J.A.; Opena, G.B.; McBurnie, J.C. Soil management and supplemental irrigation effects on potato: I. Soil properties, tuber yield, and quality. Agron. J. 1999, 91, 416–425. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, X.; Song, X.; Hu, G.; Zhang, E.; Wang, H.; Vance, M.M. The Optimum Ridge-Furrow Ratio and Suitable Ridge-Covering Material in Rainwater Harvesting for Oats Production in Semiarid Regions of China. Field Crop. Res. 2015, 172, 106–118. [Google Scholar]
- Akura, M.; Eri, S. Evaluation of Drought Tolerance Indices for Selection of Turkish Oat (Avena saliva L.) Landraces under Various Environmental Conditions. Zemdirbyste 2011, 108, e22–e28. [Google Scholar]
- Sánchez Martín, J.; Rispail, N.; Flores, F.; Emeran, A.A.; Sillero, J.C.; Rubiales, D.; Prats, E. Higher rust resistance and similar yield of oat landraces versus cultivars under high temperature and drought. Agron. Sustain. Dev. 2017, 37, 1–14. [Google Scholar] [CrossRef]
- Canales, F.J.; Montilla-Bascón, G.; Rispail, N.; Prats, E. Salicylic acid regulates polyamine biosynthesis during drought responses in oat. Plant Signal. Behav. 2019, 14, 1651183. [Google Scholar] [CrossRef] [PubMed]
- Mut, Z. Effect of Seed Size and Drought Stress on Germination and Seedling Growth of Naked Oat (Avena sativa L.). Afr. J. Agr. Res. 2010, 9, 764–770. [Google Scholar]
- Xie, H.S.; Hsiao, A.I.; Qulck, W.A.; Hume, J.A. Influence of Water Stress on Absorption, Translocation and Phytotoxicity of Fenoxaprop-Ethyl and Imazamethabenz-Methyl in Avena Fatua. J. Plant Growth Regul. 2010, 36, 65–71. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Mur, L.A.J.; Rubiales, D.; Prats, E. Targeting sources of drought tolerance within an Avena spp. collection through multivariate approaches. Planta 2012, 236, 1529–1545. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zeng, Z.; Ren, C.; Yuegao, H.U. Water Use Efficiency and Physiological Responses of Oat under Alternate Partial Root-Zone Irrigation in The Semiarid Areas of Northeast China. Procedia Engineering. 2012, 28, 33–42. [Google Scholar]
- Marcińska, I.; CzyczyłoMysza, I.; Skrzypek, E.; Grzesiak, M.T.; PopielarskaKonieczna, M.; Warchoł, M.; Grzesiak, S. Application of photochemical parameters and several indices based on phenotypical traits to assess intraspecific variation of oat (Avena sativa L.) tolerance to drought. Acta Physiol. Plant. 2017, 39, 1178. [Google Scholar] [CrossRef]
- Asgari, F.; Majd, A.; Jonoubi, P.; Najafi, F. Effects of Silicon Nanoparticles on Molecular, Chemical, Structural and Ultrastructural Characteristics of Oat (Avena sativa L.). Plant Physiol. Bioch. 2018, 127, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Qiang, X.; Xin, X.; Yan, Z.; Jiao, K.; Herbert, S.J.; Lin, H. Salicylic Acid, Hydrogen Peroxide and Calcium-Induced Saline Tolerance Associated with Endogenous Hydrogen Peroxide Homeostasis in Naked Oat Seedlings. Plant Growth Regul. 2007, 54, 249–259. [Google Scholar]
- Chauhan, A.; Abu-Amarah, B.A.; Kumar, A.; Verma, J.S.; Ghramh, H.A.; Khan, K.A.; Ansari, M.J. Influence of gibberellic acid and different salt concentrations on germination percentage and physiological parameters of oat cultivars. Saudi J. Biol. Sci. 2019, 26, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Oraby, H.; Ahmad, R. Physiological and Biochemical Changes of Cbf3 Transgenic Oat in Response to Salinity Stress. Plant Sci. 2012, 185–186, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Qin, Y.; Liu, J.; Wang, Y.; Sa, R.; Zhang, N.; Jia, R. Proteomic Response of Oat Leaves to Long-Term Salinity Stress. Environ. Sci. Pollut. Res. 2017, 24, 3387–3399. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.H.; Liu, J.H.; Zhang, N.; Yang, J.H.; Sa, R.L.; Wu, L. Effect of Alkali Stress on Soluble Sugar, Antioxidant Enzymes and Yield of Oat. J. Integr. Agr. 2013, 12, 1441–1449. [Google Scholar] [CrossRef]
- Gao, Z.; Han, J.; Mu, C.; Lin, J.; Sun, S. Effects Of Saline and Alkaline Stresses on Growth and Physiological Changes in Oat (Avena sativa L.) Seedlings. Not. Bot. Horti Agrobot. 2014, 42, 357–362. [Google Scholar] [CrossRef]
- Fan, Y.; Ge, T.; Zheng, Y.; Li, H.; Cheng, F. Use of Mixed Solid Waste as A Soil Amendment for Saline-Sodic Soil Remediation and Oat Seedling Growth Improvement. Environ. Sci. Pollut. Res. 2016, 23, 21407–21415. [Google Scholar] [CrossRef] [PubMed]
- Din, J.U.; Khan, S.U.; Khan, A.; Qayyum, A.; Abbasi, K.S.; Jenks, M.A. Evaluation of potential morpho-physiological and biochemical indicators in selecting heat-tolerant tomato (Solanum lycopersicum Mill.) genotypes. Hortic. Environ. Biotechnol. 2016, 56, 769–776. [Google Scholar] [CrossRef]
- Kottmann, L.; Wilde, P.; Schittenhelm, S. How do timing, duration, and intensity of drought stress affect the agronomic performance of winter rye? Eur. J. Agron. 2016, 75, 25–32. [Google Scholar] [CrossRef]
- Urano, K.; Yoshiba, Y.; Nanjo, T.; Ito, T.; Yamaguchi Shinozaki, K.; Shinozaki, K. Arabidopsis stress-inducible gene for arginine decarboxylase AtADC2 is required for accumulation of putrescine in salt tolerance. Biochem. Biophys. Res. Commun. 2004, 313, 369–375. [Google Scholar] [CrossRef]
- Song, L.; Li, F.M.; Fan, X.W.; Xiong, Y.C.; Wang, W.Q.; Wu, X.B.; Turner, N.C. Soil water availability and plant competition affect the yield of spring wheat. Eur. J. Agron. 2009, 31, 51–60. [Google Scholar] [CrossRef]
- Rao, P.S.; Mishra, B.; Gupta, S.R.; Rathore, A. Reproductive stage tolerance to salinity and alkalinity stresses in rice genotypes. Plant Breed. 2008, 127, 256–261. [Google Scholar] [CrossRef]
- Cui, Y.; Ouyang, S.; Zhao, Y.; Tie, L.; Shao, C.; Duan, H. Plant responses to high temperature and drought: A bibliometrics analysis. Front. Plant Sci. 2022, 13, 1052660. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.P.; Ma, B.L.; Hu, Y.G.; Liu, J.H. Leaf photosynthesis, biomass production and water and nitrogen use efficiencies of two contrasting naked vs. hulled oat genotypes subjected to water and nitrogen stresses. J. Plant Nutr. 2011, 34, 2139–2157. [Google Scholar] [CrossRef]
- Nogués, S.; Allen, D.; Baker, M. Ultraviolet-B radiation effects on water relations, leaf development, and photosynthesis in droughted pea plants. Plant Physiol. 1998, 117, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, W.; Xin, C.; Tang, W.; Eneji, A.E.; Dong, H. Lint yield and nitrogen use efficiency of field-grown cotton vary with soil salinity and nitrogen application rate. Field Crop. Res. 2012, 138, 63–70. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.J.; Sun, X.F.; Zhao, L.L.; Wang, P.C. Transcriptomic and metabolomic analyses reveal key metabolites, pathways and candidate genes in Sophora davidii (Franch.) Skeels seedlings under drought stress. Front. Plant Sci. 2022, 13, 785702. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, X.F.; Zhao, L.L.; Wang, P.C.; Huang, L.J. Morphological, transcriptomic and metabolomic analyses Sophora davidii mutants for plant height. BMC Plant Biol. 2022, 22, 144. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wang, Y.; Zhou, Y.; Tao, Y.; Mao, W.; Shi, K.; Asami, T.; Chen, Z.; Yu, J. Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Annu. Plant Signal. Behav. 2009, 5, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Huang, L.J.; Sun, X.F.; Zhao, L.L.; Wang, P.C. Differential physiological, transcriptomic, and metabolomic responses of Paspalum wettsteinii under high-temperature stress. Front. Plant Sci. 2022, 13, 865608. [Google Scholar] [CrossRef] [PubMed]
- Singh, K. Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol. 2002, 5, 430–436. [Google Scholar] [CrossRef]
- Yang, T.; Poovaiah, B. Hydrogen peroxide homeostasis: Activation of plant catalase by calcium/calmodulin. Proc. Natl. Acad. Sci. USA 2002, 99, 4097–4102. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kuang, L.; Li, X.; Wu, L.; Wu, D.; Zhang, G. Metabolomic and transcriptomic analyses reveal the reasons why Hordeum marinum has higher salt tolerance than Hordeum vulgare. Environ. Exp. Bot. 2018, 156, 48–61. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, S.; An, L.; Chen, N. NADPH oxidase-dependent hydrogen peroxide production, induced by salinity stress, may be involved in the regulation of total calcium in roots of wheat. J. Plant Physiol. 2007, 164, 1429–1435. [Google Scholar] [CrossRef]
- Gilroy, S.; Suzuki, N.; Mille, G.; Choi, W.; Toyota, M.; Devireddy, A.; Mittler, R. A tidal wave of signals: Calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 2014, 19, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 251–275. [Google Scholar]
- Abbas, T.; Balal, R.M.; Shahid, M.A.; Pervez, M.A.; Ayyub, C.M.; Aqueel, M.A.; Javaid, M.M. Silicon-induced alleviation of NaCl toxicity in okra (Abelmoschus esculentus) is associated with enhanced photosynthesis, osmoprotectants and antioxidant metabolism. Acta Physiol. Plant. 2015, 37, 6. [Google Scholar] [CrossRef]
- Wang, W.C.; Cui, K.H. Abiotic stress on the research progress of plant hole protein expression regulation. J. Plant Physiol. 2016, 52, 423–430. [Google Scholar]
- Sutka, M.; Li, G.; Boudet, J.; Boursiac, Y.; Doumas, P.; Maurel, C. Natural variation of root hydraulics in arabidopsis grown in normal and salt-stressed conditions. Plant Physiol. 2011, 155, 1264–1276. [Google Scholar] [CrossRef] [PubMed]
- Prak, S.; Hem, S.; Boudet, J.; Viennois, G.; Sommerer, N.; Rossignol, M.; Maurel, C.; Santoni, V. Multiple phosphorylations in the c-terminal tail of plant plasma membrane aquaporins. Mol. Cell. Proteom. 2008, 7, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Ben Halima, N.; Khemakhem, B.; Fendri, I.; Ogata, H.; Baril, P.; Pichon, C.; Abdelkafi, S. Identification of a new oat β-amylase by functional proteomics. BBA-Proteins Proteom. 2016, 1864, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Dassanayake, M.; Oh, D.-H.; Haas, J.S.; Hernandez, A.; Hong, H.; Ali, S.; Yun, D.-J.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J.; et al. The genome of the extremophile crucifer Thellungiella parvula. Nat. Genet. 2011, 43, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Ghorbel, M.; Zribi, I.; Chihaoui, M.; Alghamidi, A.; Mseddi, K.; Brini, F. Genome-wide investigation and expression analysis of the catalase gene family in oat plants (Avena sativa L.). Plants 2023, 12, 3694. [Google Scholar] [CrossRef] [PubMed]
- Bekele, W.A.; Wight, C.P.; Chao, S.; Howarth, C.J.; Tinker, N.A. Haplotype-based genotyping-by-sequencing in oat genome research. Plant Biotechnol. J. 2018, 16, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Gigon, A.; Matos, A.; Laffray, D.; Zuily-Fodil, Y.; Pham-Thi, A. Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (Ecotype Columbia). Ann. Bot. 2004, 94, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Quartacci, M.F. Plasma membrane lipids in the resurrection plant Ramonda serbica following dehydration and rehydration. J. Exp. Bot. 2002, 53, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Monteiro de Paula, F.; Pham Thi, A.T.; Zuily-Fodil, Y.; Ferrari-Iliou, R.; Vieira da Silva, J.; Mazliak, P. Effects of water stress on the biosynthesis and degradation of polyunsaturated lipid molecular species in leaves of Vigna unguiculata. Plant Physiol. 1993, 31, 707–715. [Google Scholar]
- Chapman, K.D. Phospholipase activity during plant growth and development and in response to environmental stress. Trends Plant Sci. 1998, 3, 419–426. [Google Scholar] [CrossRef]
- Neffati, M.; Marzouk, B. Changes in essential oil and fatty acid composition in coriander (Coriandrum sativum L.) leaves under saline conditions. Ind. Crop. Prod. 2008, 28, 137–142. [Google Scholar] [CrossRef]
- Xie, H.; Li, M.; Chen, Y.; Zhou, Q.; Liu, W.; Liang, G.; Jia, Z. Important physiological changes due to drought stress on oat. Front. Ecol. Evol. 2021, 9, 644726. [Google Scholar] [CrossRef]
- Yang, C.; Shi, D.; Wang, D. Comparative effects of salt and alkali stresses on growth, osmotic adjustment, and ionic balance of an alkali-resistant Halophyte Suaeda glauca (Bge.). Plant Growth Regul. 2008, 56, 179–190. [Google Scholar] [CrossRef]
- Shukla, P.S.; Agarwal, P.K.; Jha, B. Improved salinity tolerance of Arachis hypogaea (L.) by the interaction of halotolerant plant-growth-promoting rhizobacteria. J. Plant Growth Regul. 2012, 31, 195–206. [Google Scholar] [CrossRef]
- Barnett, N.; Naylor, A. Mino acid and protein metabolism in bermuda grass during water stress. Plant Physiol. 1966, 41, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Zhu, J. Salt, and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.; Mckersie, B.; Fletcher, R. Paclobutrazol-induced tolerance of wheat leaves to paraquat may involve increased antioxidant enzyme activity. J. Plant Physiol. 1995, 145, 570–576. [Google Scholar] [CrossRef]
- Liu, S.C.; Chiang, K.S.; Lin, C.H.; Chung, W.C.; Lin, S.H.; Yang, T.C. Cost analysis in choosing group size when group testing for Potato virus Y in the presence of classification errors. Ann. Appl. Biol. 2011, 159, 491–502. [Google Scholar] [CrossRef]
- Qin, L.; Hui, S. Plant responses to the high temperature and moisture stress. Nat. Sci. 2005, 26, 87745345. [Google Scholar]
- Bérard, A.; Ben Sassi, M.; Kaisermann, A.; Renault, P. Soil microbial community responses to heat wave components: Drought and high temperature. Clim. Res. 2015, 66, 243–264. [Google Scholar] [CrossRef]
- Cohen, I.; Zandalinas, S.I.; Fritschi, F.B.; Sengupta, S.; Fichman, Y.; Azad, R.K.; Mittler, R. The impact of water deficit and heat stress combination on the molecular response, physiology, and seed production of soybean. Physiol. Plant. 2020, 172, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Mirfattahi, Z.; Eshghi, S. Inducing salt tolerance in strawberry (Fragaria × ananassa Duch) plants by acetate application. J. Plant Nutr. 2020, 43, 1780–1793. [Google Scholar] [CrossRef]
- Liu, J. Proline accumulation and salt-stress-induced gene expression in a salt-hypersensitive mutant of Arabidopsis. Plant Physiol. 1997, 114, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Liu, L.; Wang, H.; Li, D.; Bai, Z.; Zhang, Y.; Sun, H.; Zhang, K.; Li, C. Exogenous melatonin accelerates seed germination in cotton (Gossypium hirsutum L.). PLoS ONE 2019, 14, 1101. [Google Scholar] [CrossRef] [PubMed]
- Posmyk, M.M.; Bałabusta, M.; Wieczorek, M.; Sliwinska, E.; Janas, K.M. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J. Pineal Res. 2009, 46, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Zhang, Y.J.; Gao, Y.; Zhao, L.L.; Wang, P.C. Influence of exogenous abscisic acid on germination and physiological traits of Sophora viciifolia seedlings under drought conditions. Appl. Sci. 2024, 14, 4359. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 2015, 66, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Shehzadi, A.; Akram, N.A.; Ali, A.; Ashraf, M. Exogenously applied glycinebetaine induced alteration in some key physio-biochemical attributes and plant anatomical features in water stressed oat (Avena sativa L.) plants. J. Arid Land 2019, 11, 292–305. [Google Scholar] [CrossRef]
- Canales, F.J.; Rispail, N.; García-Tejera, O.; Arbona, V.; Pérez-de-Luque, A.; Prats, E. Drought resistance in oat involves ABA-mediated modulation of transpiration and root hydraulic conductivity. Environ. Exp. Bot. 2021, 182, 104333. [Google Scholar] [CrossRef]
- Sharp, R.E. Interaction with ethylene: Changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell Environ. 2002, 25, 211–222. [Google Scholar] [CrossRef]
- Jiang, G.; Hao, N.; Bai, K.; Zhang, Q.; Sun, J.; Guo, R.; Ge, Q.; Kuang, T. Chain correlation between variables of gas exchangeand yield potential in different winter wheat cultivars. Photosynthetica 2000, 38, 227–232. [Google Scholar] [CrossRef]
- Liang, X.; Shalapy, M.; Zhao, S.; Liu, J.; Wang, J. A stress-responsive transcription factor PeNAC1 regulating beta-d-glucan biosynthetic genes enhances salt tolerance in oat. Planta 2021, 254, 00425. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, X.; Ding, F.; Ma, H.; Zhang, T.; Yang, Y. Salinity induced the changes of root growth and antioxidative responses in two wheat cultivars. Protoplasma 2013, 251, 771–780. [Google Scholar] [CrossRef] [PubMed]

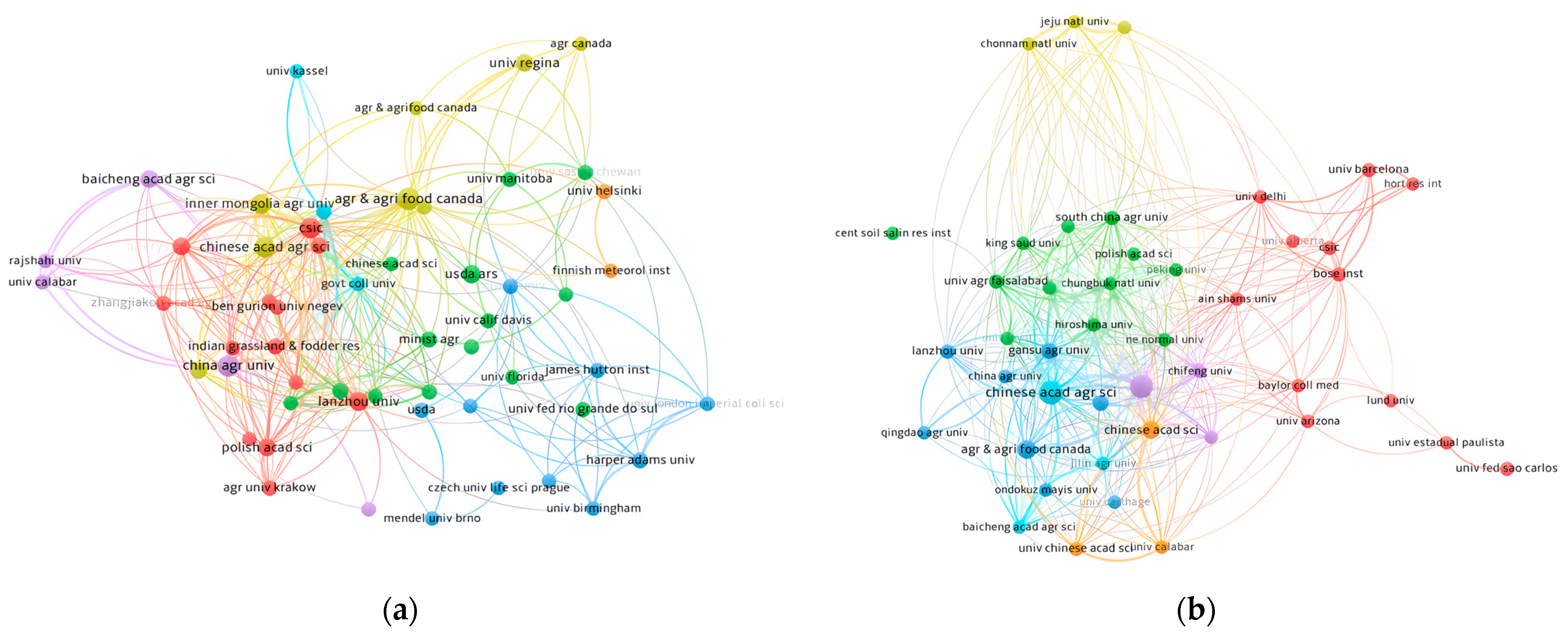
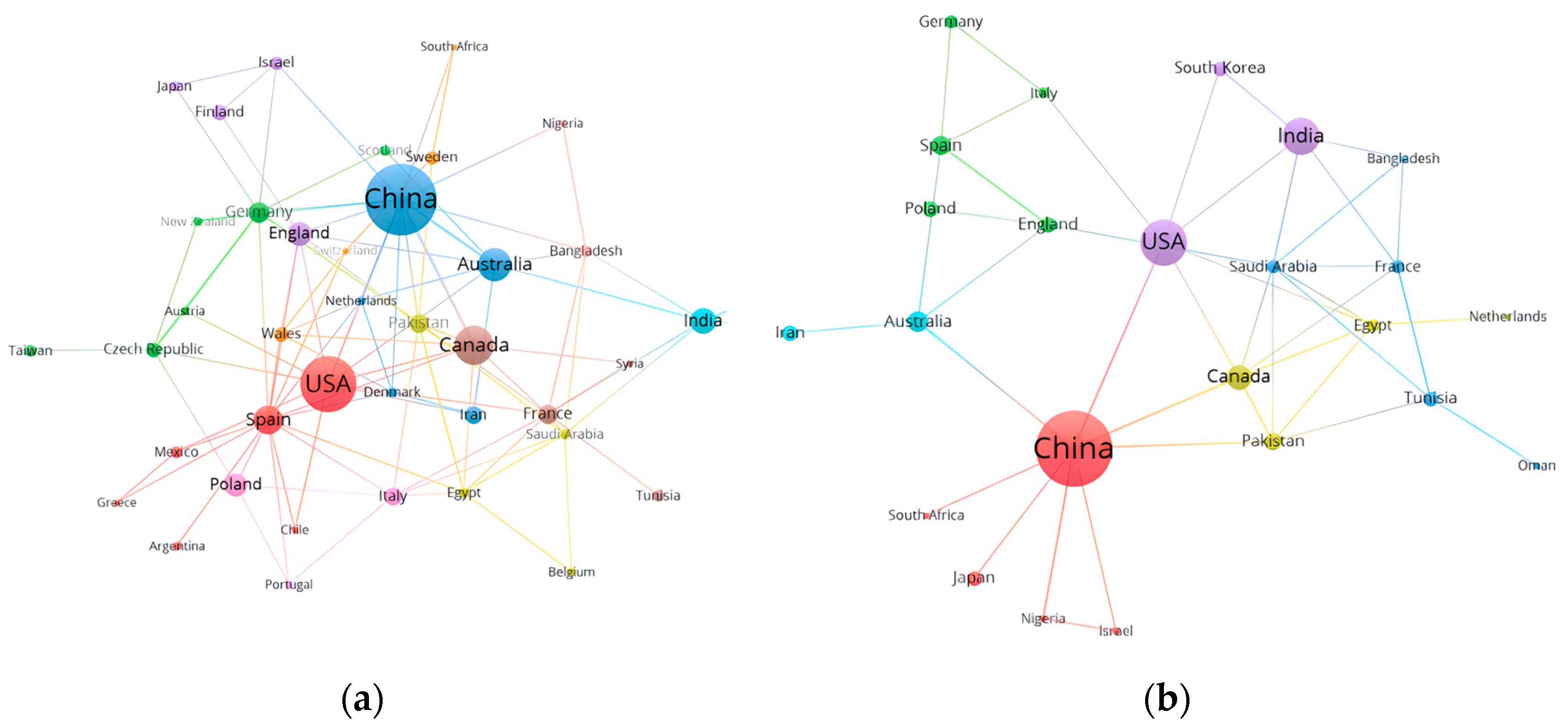
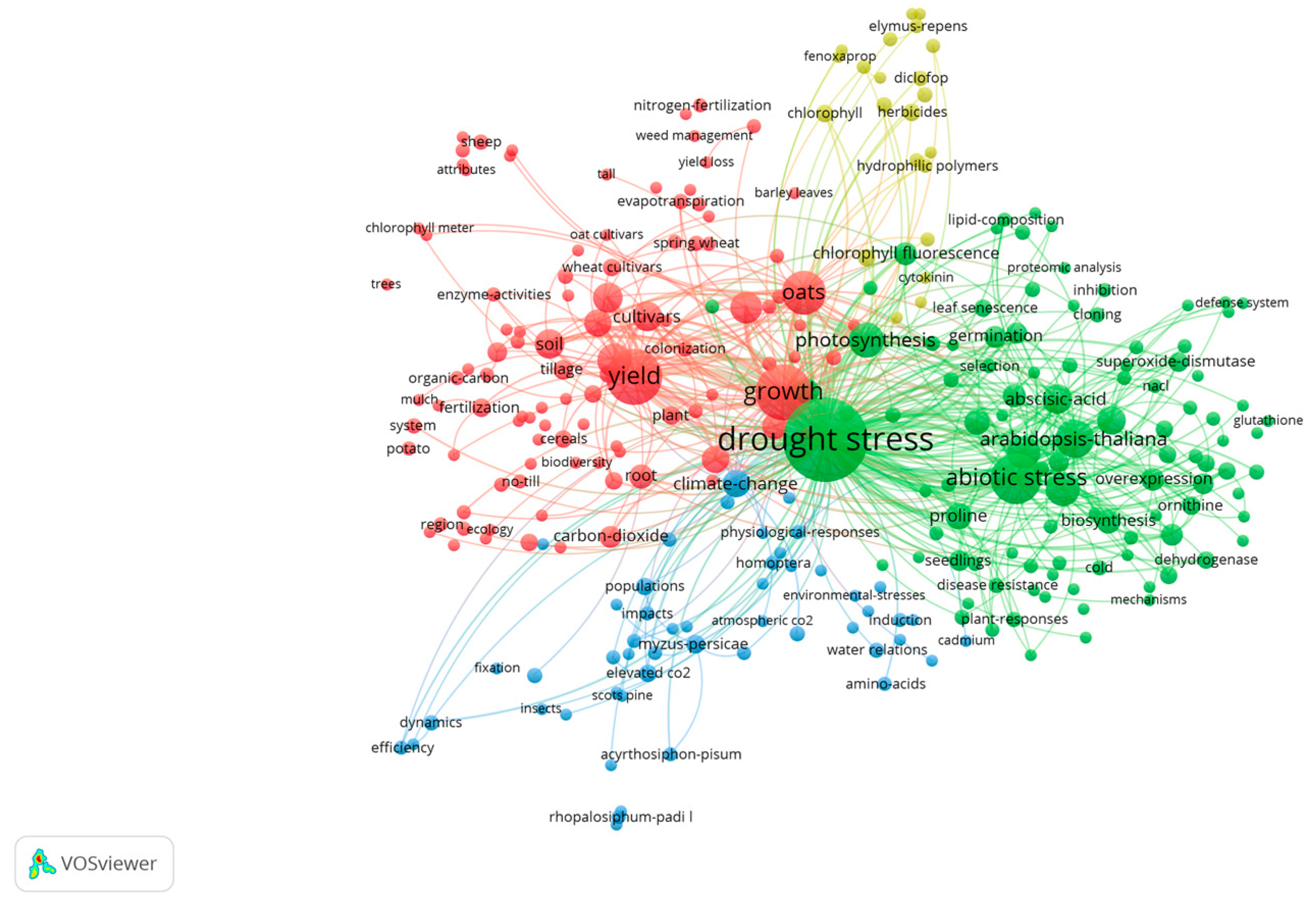


| Ranking | Institutions | TP | TLS | TC | Country |
|---|---|---|---|---|---|
| 1 | Canadian Agricultural Food University | 19 | 1512 | 335 | Canada |
| 2 | China Agricultural University | 15 | 1627 | 183 | China |
| 3 | CSS Heavy Industry Co. Ltd | 14 | 1201 | 330 | China |
| 4 | Agricultural Science of China Academy of Sciences | 13 | 949 | 137 | China |
| 5 | Inner Mongolia Agricultural University | 12 | 1119 | 115 | China |
| 6 | Lanzhou University | 10 | 695 | 112 | China |
| 7 | USDA-ARS | 8 | 41 | 189 | USA |
| 8 | Aberystwyth University | 7 | 1062 | 234 | Britain |
| 9 | Baocheng academic Agricultural Science | 7 | 1006 | 103 | China |
| 10 | Polish Academy of Sciences | 7 | 480 | 41 | Poland |
| Ranking | Institutions | TP | TLS | TC | Country |
|---|---|---|---|---|---|
| 1 | Agricultural Science of China Academy of Sciences | 21 | 2036 | 215 | China |
| 2 | Inner Mongolia Agricultural University | 19 | 2171 | 187 | China |
| 3 | Canadian agricultural Food University | 9 | 728 | 386 | Canada |
| 4 | China Agricultural University | 8 | 946 | 125 | China |
| 5 | Gansu Agricultural University | 5 | 647 | 231 | China |
| 6 | Yangzhou University | 5 | 230 | 42 | China |
| 7 | Bose Inst | 4 | 238 | 289 | USA |
| 8 | CSS Heavy Industry Coltd | 4 | 162 | 183 | China |
| 9 | Lanzhou University | 4 | 322 | 103 | China |
| 10 | South China Agricultural University | 4 | 285 | 31 | China |
| Ranking | Country | TP | TC | AC |
|---|---|---|---|---|
| 1 | China | 91 | 1211 | 13.31 |
| 2 | USA | 63 | 2034 | 32.29 |
| 3 | Canada | 36 | 646 | 17.94 |
| 4 | Australia | 28 | 649 | 23.18 |
| 5 | Spain | 23 | 458 | 19.9 |
| 6 | India | 19 | 603 | 31.74 |
| 7 | England | 17 | 312 | 18.35 |
| 8 | Poland | 16 | 61 | 3.81 |
| 9 | Germany | 14 | 572 | 40.36 |
| 10 | France | 12 | 378 | 31.5 |
| Ranking | Country | TP | TC | AC |
|---|---|---|---|---|
| 1 | China | 97 | 1676 | 17.28 |
| 2 | USA | 45 | 2337 | 51.93 |
| 3 | India | 33 | 1190 | 36.06 |
| 4 | Canada | 18 | 584 | 32.44 |
| 5 | Australia | 13 | 215 | 16.54 |
| 6 | Spain | 12 | 751 | 62.58 |
| 7 | Pakistan | 10 | 122 | 12.20 |
| 8 | England | 9 | 463 | 51.44 |
| 9 | Iran | 9 | 149 | 16.56 |
| 10 | Poland | 9 | 175 | 19.44 |
| Ranking | Element | H_Index | TC | NP | IF/Year |
|---|---|---|---|---|---|
| 1 | Proceedings of the National Academy of Sciences of the United States of America | 2 | 942 | 2 | 11.1 2020–2021 |
| 2 | Physiologia Plantarum | 7 | 711 | 7 | 6.4 2022–2023 |
| 3 | Field Crops Research | 12 | 480 | 16 | 5.8 2022–2023 |
| 4 | Agricultural and Forest Meteorology | 2 | 300 | 2 | 6.2 2021–2022 |
| 5 | Plant Science | 4 | 240 | 4 | 4.3 2020–2021 |
| 6 | Plant Physiology | 2 | 231 | 2 | 7.4 2022–2023 |
| 7 | Plant Cell and Environment | 3 | 230 | 3 | 7.3 2022–2023 |
| 8 | Photosynthetica | 2 | 226 | 2 | 2.7 2022–2023 |
| 9 | Ecological Applications | 1 | 184 | 1 | 5.0 2022–2023 |
| 10 | Canadian journal of Plant Science | 6 | 170 | 9 | 1.2 2022–2023 |
| Ranking | Element | H_Index | TC | NP | IF/Year |
|---|---|---|---|---|---|
| 1 | Plant Science | 5 | 1245 | 5 | 4.3 2020–2021 |
| 2 | Physiologia Plantarum | 8 | 859 | 8 | 6.4 2022–2023 |
| 3 | Proceedings of the National Academy of Sciences of the United States of America | 1 | 529 | 1 | 11.1 2020–2021 |
| 4 | Plant Growth Regulation | 7 | 365 | 7 | 4.2 2022–2023 |
| 5 | Plant Physiology | 3 | 333 | 3 | 7.4 2022–2023 |
| 6 | Journal of Nutrition | 3 | 259 | 4 | 4.2 2016–2017 |
| 7 | Plant Physiology and Biochemistry | 7 | 253 | 8 | 6.5 2022–2023 |
| 8 | Photosynthetica | 1 | 220 | 1 | 2.7 2022–2023 |
| 9 | Plant Journal | 2 | 213 | 2 | 7.2 2022–2023 |
| 10 | Plant Cell and Environment | 3 | 211 | 3 | 7.3 2022–2023 |
| Drought Stress | Salt Stress | ||||
|---|---|---|---|---|---|
| Keywords | Centrality | Count | Keywords | Centrality | Count |
| Drought stress | 0.72 | 280 | Salt stress | 0.64 | 180 |
| Oats | 0.4 | 60 | Avena staiva | 0.26 | 34 |
| Abiotic stress | 0.26 | 81 | abiotic stress | 0.2 | 48 |
| Growth | 0.24 | 77 | Abscisic acid | 0.14 | 15 |
| Yield | 0.24 | 60 | Arginine decarboxylase | 0.13 | 15 |
| Abscisic acid | 0.12 | 22 | Growth | 1 | 47 |
| Climate change | 0.1 | 25 | Biosynthesis | 0.09 | 20 |
| Photosynthesis | 0.1 | 17 | Oxidative stress | 0.08 | 16 |
| Arabidopsis | 0.08 | 15 | Gene | 0.08 | 12 |
| Cultivars | 0.07 | 12 | Arabidopsis | 0.08 | 15 |
| Gene | 0.06 | 16 | Proline | 0.07 | 14 |
| Leaves | 0.06 | 12 | Bacteria | 0.07 | 6 |
| Oxidative stress | 0.05 | 14 | Gene expression | 0.05 | 8 |
| Temperature | 0.04 | 14 | Metabolism | 0.05 | 10 |
| Quality | 0.04 | 17 | Alkali stress | 0.05 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Wang, X.; Li, J.; Gao, Y.; Yang, Y.; Wang, R.; Zhou, Z.; Wang, P.; Zhang, Y. Trends and Directions in Oats Research under Drought and Salt Stresses: A Bibliometric Analysis (1993–2023). Plants 2024, 13, 1902. https://doi.org/10.3390/plants13141902
Huang H, Wang X, Li J, Gao Y, Yang Y, Wang R, Zhou Z, Wang P, Zhang Y. Trends and Directions in Oats Research under Drought and Salt Stresses: A Bibliometric Analysis (1993–2023). Plants. 2024; 13(14):1902. https://doi.org/10.3390/plants13141902
Chicago/Turabian StyleHuang, Haiyan, Xiangtao Wang, Junqin Li, Yang Gao, Yuting Yang, Rui Wang, Zijun Zhou, Puchang Wang, and Yujun Zhang. 2024. "Trends and Directions in Oats Research under Drought and Salt Stresses: A Bibliometric Analysis (1993–2023)" Plants 13, no. 14: 1902. https://doi.org/10.3390/plants13141902
APA StyleHuang, H., Wang, X., Li, J., Gao, Y., Yang, Y., Wang, R., Zhou, Z., Wang, P., & Zhang, Y. (2024). Trends and Directions in Oats Research under Drought and Salt Stresses: A Bibliometric Analysis (1993–2023). Plants, 13(14), 1902. https://doi.org/10.3390/plants13141902






