Genetic Characterization of Rhizobium spp. Strains in an Organic Field Pea (Pisum sativum L.) Field in Lithuania
Abstract
:1. Introduction
2. Results
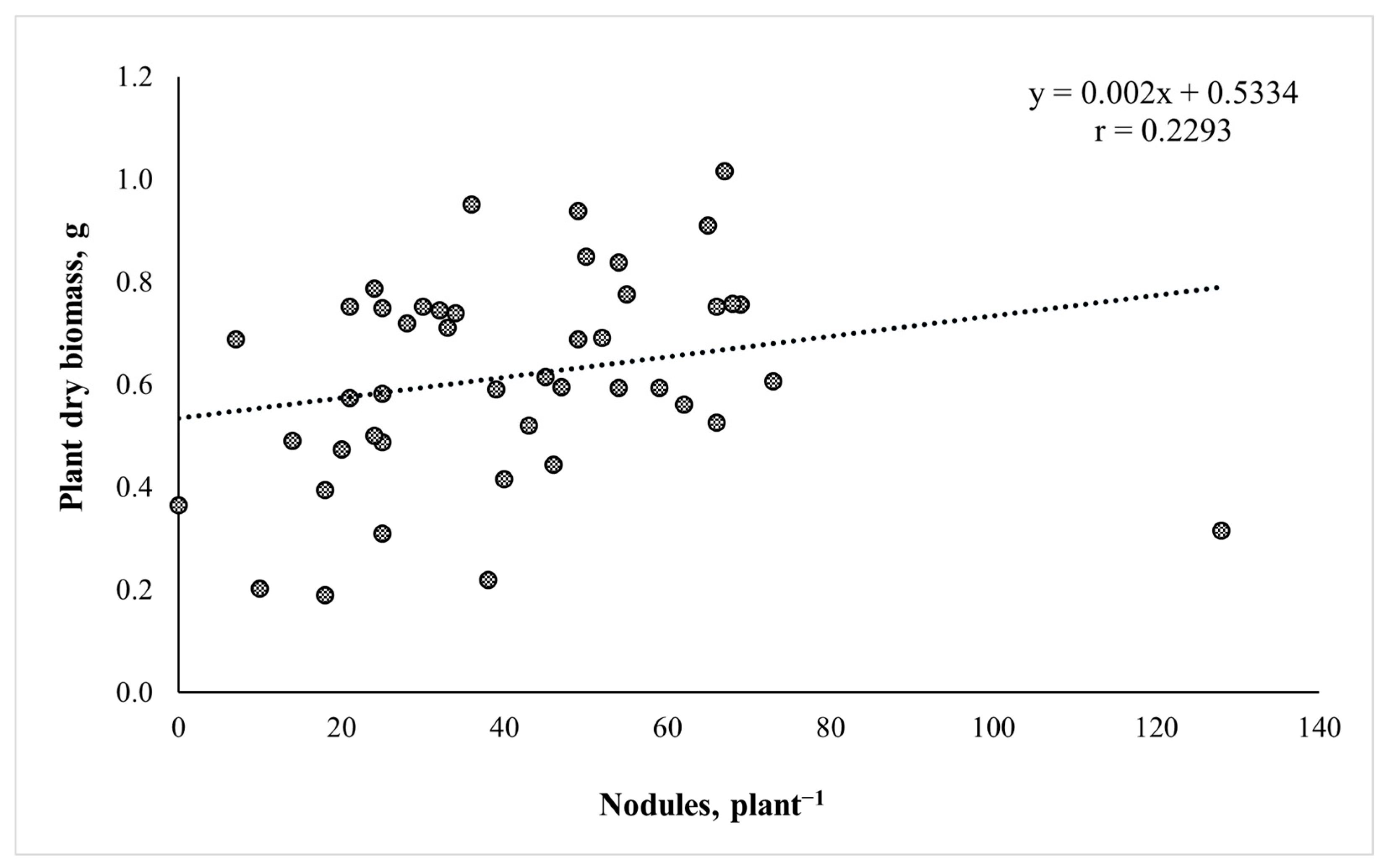
2.1. Rhizobial Strain Isolation, Screening and Evaluation of Nodulation and Plant Biomass Accumulation
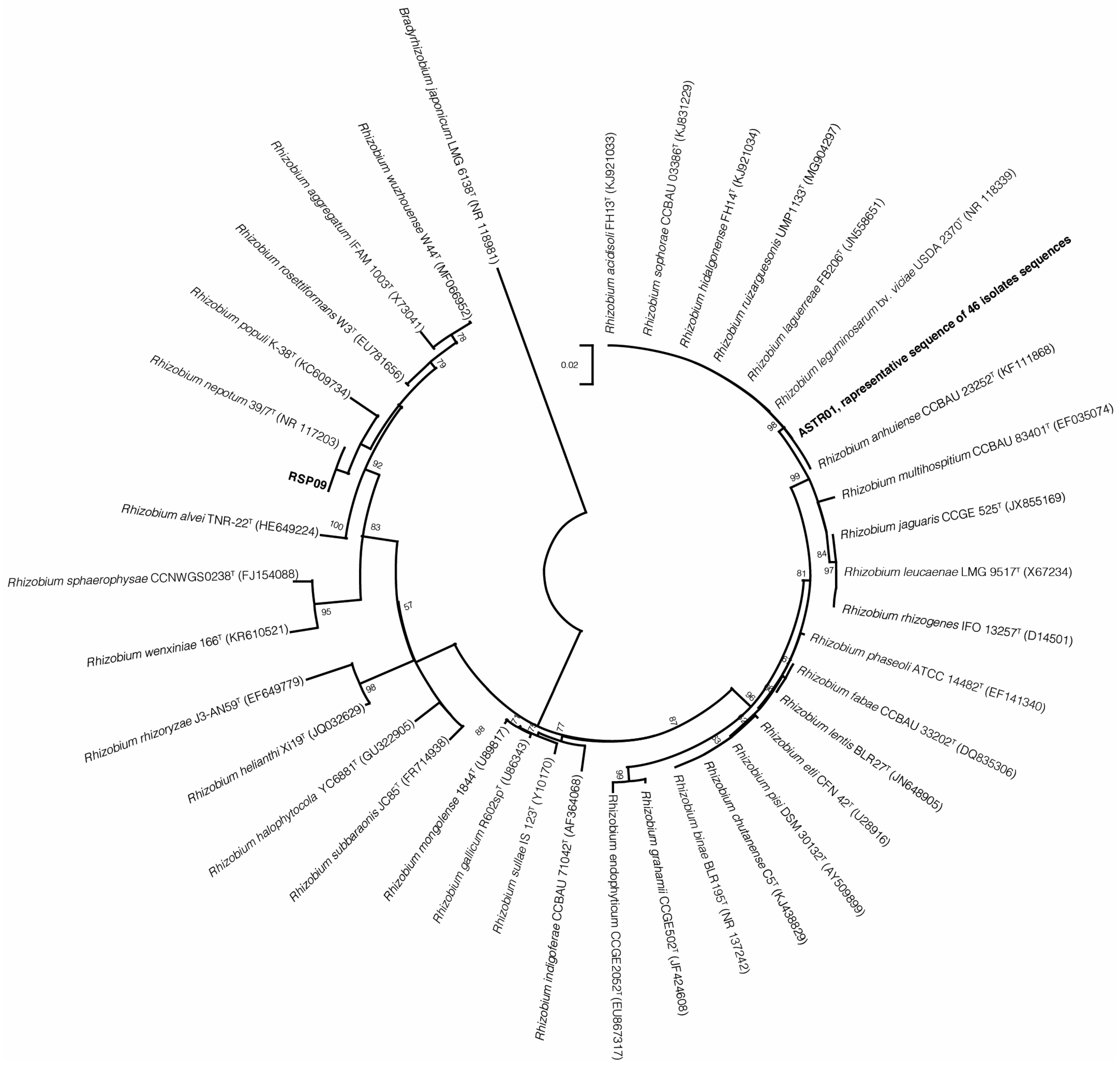
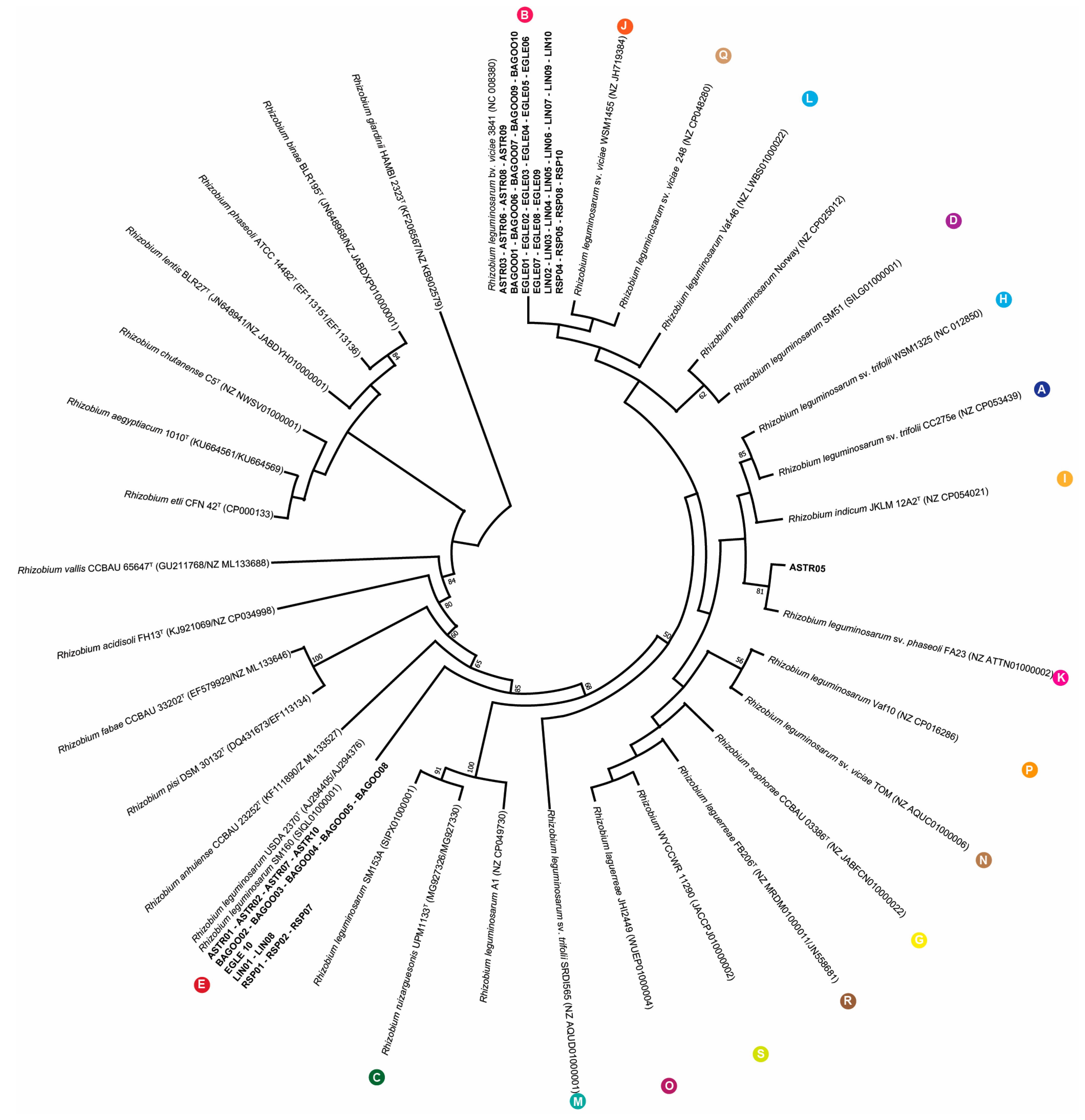
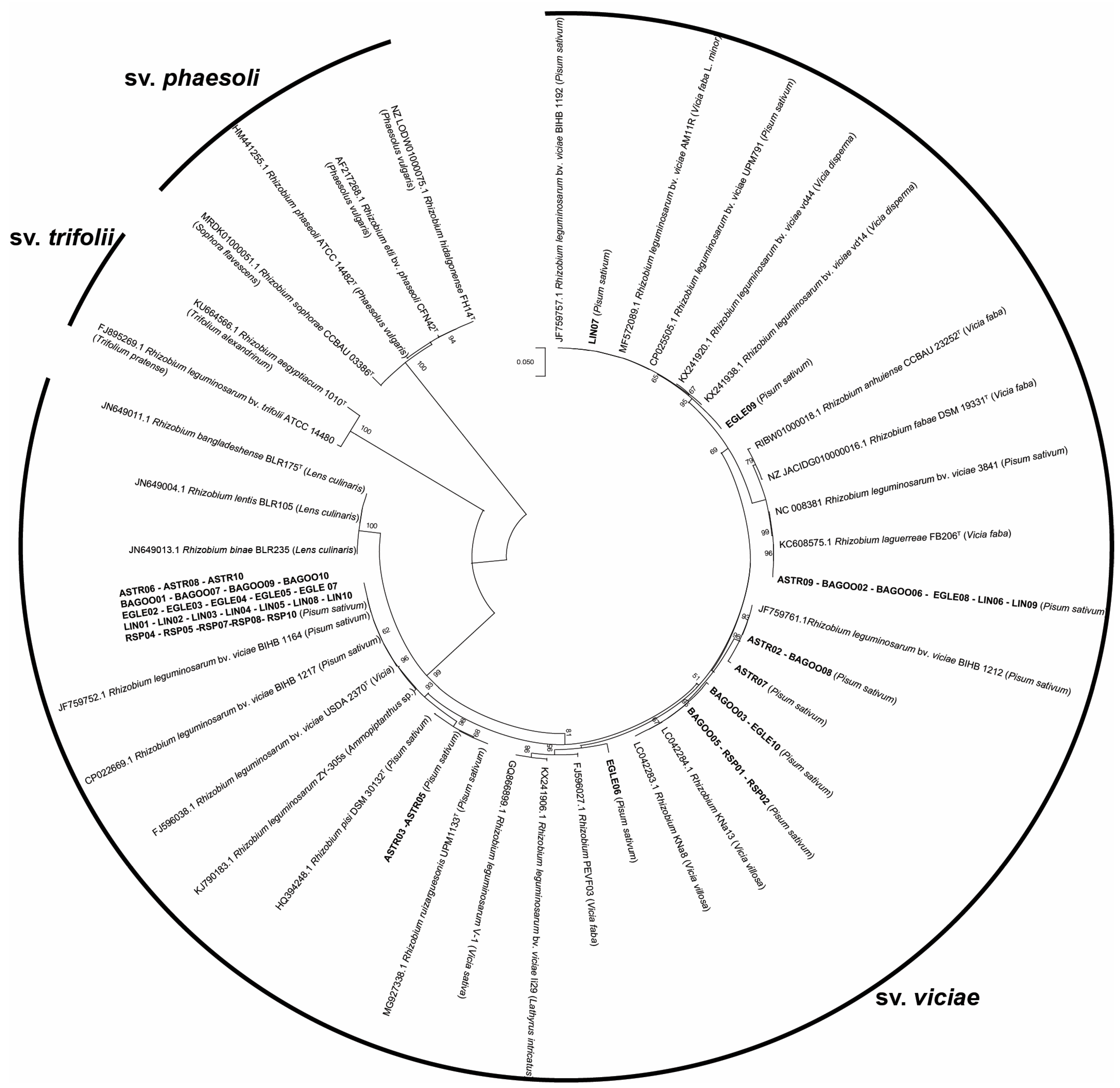
2.2. Phylogenetic Analysis of 16s rRNA, recA, atpD and NodC Gene Sequences
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Isolation and Screening of Rhizobia
4.3. Pea Analysis with Different Rhizobia Isolates
4.4. 16s rRNA and recA, atpD, nodC Genes Amplification, Sequencing and Phylogenetic Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Semaškienė, R.; Jonavičienė, A.; Razbadauskienė, K.; Deveikytė, I.; Sabeckis, A.; Supronienė, S.; Šarūnaitė, L.; Kadžiulienė, Ž. The response to crop health and productivity of field pea (Pisum sativum L.) at different growing conditions. Acta Agric. Scand. B Soil Plant Sci. 2022, 72, 923–930. [Google Scholar] [CrossRef]
- Suproniene, S.; Decorosi, F.; Pini, F.; Bellabarba, A.; Calamai, L.; Giovannetti, L.; Bussotti, F.; Kadziuliene, Z.; Razbadauskiene, K.; Toleikiene, M.; et al. Selection of Rhizobium strains for inoculation of Lithuanian Pisum sativum breeding lines. Symbiosis 2021, 83, 193–208. [Google Scholar] [CrossRef]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple benefits of legumes for agriculture sustainability: An overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Soon, Y.K. Nitrogen release from field pea residues and soil inorganic N in a pea wheat crop rotation in northwestern Canada. Can. J. Plant Sci. 2009, 89, 239–246. [Google Scholar] [CrossRef]
- Kebede, E. Contribution, Utilization, and Improvement of Legumes-Driven Biological Nitrogen Fixation in Agricultural Systems. Front. Sustain. Food Syst. 2021, 5, 767998. [Google Scholar] [CrossRef]
- Chen, C.; Neill, K.; Burgess, M.; Bekkerman, A. Agronomic benefit and economic potential of introducing fall-seeded pea and lentil into conventional wheat-based crop rotations. Agron. J. 2012, 104, 215–224. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Lafond, G.P.; May, W.E.; Holzapfel, C.B.; Lemke, R.L. Intensification of field pea production: Impact on soil microbiology. Agron. J. 2012, 104, 1189–1196. [Google Scholar] [CrossRef]
- Jalal, A.; Teixeira Filho MC, M.; da Silva Oliveira, C.E.; Khan, A.; Boleta, E.H.M.; da Silva, A.L.M.; Shah, T. Legumes effect on nitrogen mineralization and microbial biomass potential in organic farming. Adv. Legumes Sustain. Intensif. 2022, 1, 281–306. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, A.M.; Azirun, S.M.; Boyce, A.N. Tropical legume crop rotation and nitrogen fertilizer effects on agronomic and nitrogen efficiency of rice. Sci. World J. 2014, 2014, 490841. [Google Scholar] [CrossRef]
- European Union. EU 2030 Biodiversity Strategy: Bringing Nature back into Our Lives; EU Green Deal; European Union: Brussels, Belgium, 2020. [Google Scholar]
- European Commission. EU Biodiversity Strategy for 2030. Bringing nature back into our lives, 20,5,2020 COM (2020) final. In Communication for the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; European Commission: Brussels, Belgium, 2020; p. 380. Available online: https://ec.europa.eu/research/environment/index.cfm?pg=nbs (accessed on 9 January 2024).
- Dhillon, L.K.; Lindsay, D.; Yang, T.; Zakeri, H.; Tar’an, B.; Knight, J.D.; Warkentin, T.D. Biological nitrogen fixation potential of pea lines derived from crosses with nodulation mutants. Field Crop. Res. 2022, 289, 108731. [Google Scholar] [CrossRef]
- Raza, A.; Zahra, N.; Hafeez, M.B.; Ahmad, M.; Iqbal, S.; Shaukat, K.; Ahmad, G. Nitrogen Fixation of Legumes: Biology and Physiology. In The Plant Family Fabaceae: Biology and Physiological Responses to Environmental Stresses; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting biological nitrogen fixation: A route towards a sustainable agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Farm to Fork Strategy. In DG SANTE/Unit ‘Food Information and Composition, Food Waste’; no. DG SANTE/Unit ‘Food information and composition, food waste’; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Verma, R.; Annapragada, H.; Katiyar, N.; Shrutika, N.; Das, K.; Murugesan, S. Chapter 4—Rhizobium. In Beneficial Microbes in Agro-Ecology; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Verma, V.C.; Acharya, S.; Kumar, R.; Verma, B.C.; Singh, A.; Tiwari, V.K. Rhizobium as soil health engineer. In Rhizosphere Engineering; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Rogel, M.A.; Ormeño-Orrillo, E.; Martinez Romero, E. Symbiovars in rhizobia reflect bacterial adaptation to legumes. Syst. Appl. Microbiol. 2011, 34, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.F.; Wang, E.T.; Wu, L.J.; Han, T.X.; Gu, C.T.; Gu, J.G.; Chen, W.X. Rhizobium fabae sp. nov., a bacterium that nodulates Vicia faba. Int. J. Syst. Evol. Microbiol. 2008, 58, 2871–2875. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Babena, M.H.; García-Fraile, P.; Peix, A.; Valverde, A.; Rivas, R.; Igual, J.M.; Mateos, P.F.; Martínez-Molina, E.; Velázquez, E. Revision of the taxonomic status of the species Rhizobium leguminosarum (Frank 1879) Frank 1889AL, Rhizobium phaseoli Dangeard 1926AL and Rhizobium trifolii Dangeard 1926AL. R. trifolii is a later synonym of R. leguminosarum. Reclassification of the strain R. leguminosarum DSM 30132 (=NCIMB 11478) as Rhizobium pisi sp. Nov. Int. J. Syst. Evol. Microbiol. 2008, 58, 2484–2490. [Google Scholar] [CrossRef]
- Rashid, M.H.-O.; Young, J.P.W.; Everall, I.; Clercx, P.; Willems, A.; Braun, M.S.; Wink, M. Average nucleotide identity of genome sequences supports the description of Rhizobium lentis sp. nov., Rhizobium bangladeshense sp. nov. and Rhizobium binae sp. nov. from lentil (Lens culinaris) nodules. Int. J. Syst. Evol. Microbiol. 2015, 65, 3037–3045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Zheng, W.T.; Everall, I.; Young, J.P.W.; Zhang, X.X.; Tian, C.F.; Sui, X.H.; Wang, E.T.; Chen, W.X. Rhizobium anhuiense sp. nov., isolated from effective nodules of Vicia faba and Pisum sativum. Int. J. Syst. Evol. Microbiol. 2015, 65, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Kogut, B.M. Assessment of the humus content in arable soils of russia. Eurasian Soil Sci. 2012, 45, 843–851. [Google Scholar] [CrossRef]
- Lebedev, S.A.; Gunina, G.N.; Ashinov, Y.N.; Kravchenko, P.N. Ecological conditions of soils in the Republic of Adygea. In Handbook of Environmental Chemistry; Springer: Cham, Switzerland, 2020; Volume 106. [Google Scholar] [CrossRef]
- Rawal, N.; Pande, K.R.; Shrestha, R.; Vista, S.P. Phosphorus and potassium mineralization as affected by phosphorus levels and soil types under laboratory condition. Agrosystems Geosci. Environ. 2022, 5, e20229. [Google Scholar] [CrossRef]
- Vigliotti, M.; Busico, G.; Ruberti, D. Assessment of the vulnerability to agricultural nitrate in two highly diversified environmental settings. Environments 2020, 7, 80. [Google Scholar] [CrossRef]
- Vyas, P.; Joshi, R.; Sharma, K.C.; Rahi, P.; Gulati, A.; Gulati, A. Cold-adapted and rhizosphere-competent strain of Rahnella sp. with broad-spectrum plant growth-promotion potential. J. Microbiol. Biotechnol. 2010, 20, 1724–1734. [Google Scholar] [CrossRef]
- Schwartz, A.R.; Ortiz, I.; Maymon, M.; Herbold, C.W.; Fujishige, N.A.; Vijanderan, J.A.; Villella, W.; Hanamoto, K.; Diener, A.; Sanders, E.R.; et al. Bacillus simplex—A Little Known PGPB with Anti-Fungal Activity—Alters Pea Legume Root Architecture and Nodule Morphology When Coinoculated with Rhizobium leguminosarum bv. viciae. Agronomy 2013, 3, 595–620. [Google Scholar] [CrossRef]
- Sherpa, M.T.; Bag, N.; Das, S.; Haokip, P.; Sharma, L. Isolation and characterization of plant growth promoting rhizobacteria isolated from organically grown high yielding pole type native pea (Pisum sativum L.) variety Dentami of Sikkim, India. Curr. Res. Microb. Sci. 2021, 2, 100068. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.A.; Ormeño-Orrillo, E.; Dall’Agnol, R.F.; Graham, P.H.; Martinez-Romero, E.; Hungria, M. Novel Rhizobium lineages isolated from root nodules of the common bean (Phaseolus vulgaris L.) in Andean and Mesoamerican areas. Res. Microbiol. 2013, 164, 740–748. [Google Scholar] [CrossRef]
- Qaralleh, H.; Khleifat, K.M.; Abu Hajleh, M.N.; Al-Limoun, M.O.; Alshawawreh, R.; Magharbeh, M.K.; Al-Qaisi, T.S.; Farah, H.S.; El-Hasan, T.; Al-Tarawneh, A.; et al. Plant Growth-Promoting Rhizobium Nepotum Phenol Utilization: Characterization and Kinetics. J. Hunan Univ. Nat. Sci. 2022, 49, 94–107. [Google Scholar] [CrossRef]
- Ghorpade, V.M.; Gupta, S.G. Siderophore Production by Rhizobium nepotum isolated from ‘Stem nodule of Aeschynomene indica’. Int. J. Adv. Res. Biol. Sci. 2016, 3, 105–108. [Google Scholar]
- Herliana, O.; Harjoso, T.; Anwar, A.H.S.; Fauzi, A. The Effect of Rhizobium and N Fertilizer on Growth and Yield of Black Soybean (Glycine max (L) Merril). Proc. IOP Conf. Ser. Earth Environ. Sci. 2019, 255, 012015. [Google Scholar] [CrossRef]
- Rahi, P.; Giram, P.; Chaudhari, D.; Dicenzo, G.C.; Kiran, S.; Khullar, A.; Chandel, M.; Gawari, S.; Mohan, A.; Chavan, S.; et al. Rhizobium indicum sp. nov., isolated from root nodules of pea (Pisum sativum) cultivated in the Indian trans-Himalayas. Syst. Appl. Microbiol. 2020, 43, 126127. [Google Scholar] [CrossRef] [PubMed]
- Young, J.P.W.; Moeskjær, S.; Afonin, A.; Rahi, P.; Maluk, M.; James, E.K.; Cavassim, M.I.A.; Rashid, M.H.; Aserse, A.A.; Perry, B.J.; et al. Defining the Rhizobium leguminosarum Species Complex. Genes 2021, 12, 111. [Google Scholar] [CrossRef]
- Kumar, N.; Lad, G.; Giuntini, E.; Kaye, M.E.; Udomwong, P.; Shamsani, N.J.; Young, J.P.W.; Bailly, X. Bacterial genospecies that are not ecologically coherent: Population genomics of Rhizobium leguminosarum. Open Biol. 2015, 5, 140133. [Google Scholar] [CrossRef]
- Arahal, D.R. Whole-genome analyses: Average nucleotide identity. Methods Microbiol. 2014, 41, 103–122. [Google Scholar] [CrossRef]
- Yang, C.; Bueckert, R.; Schoenau, J.; Diederichsen, A.; Zakeri, H.; Warkentin, T. Symbiosis of selected Rhizobium leguminosarum bv. viciae strains with diverse pea genotypes: Effects on biological nitrogen fixation. Can. J. Microbiol. 2017, 63, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, K.; Dudeja, S.S.; Yadav, R.K. Molecular diversity of native rhizobia trapped by five field pea genotypes in Indian soils. J. Basic Microbiol. 2011, 51, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Abi-Ghanem, R.; Carpenter-Boggs, L.; Smith, J.L. Cultivar effects on nitrogen fixation in peas and lentils. Biol. Fertil. Soils 2011, 47, 115–120. [Google Scholar] [CrossRef]
- Buhian, W.P.; Bensmihen, S. Mini-review: Nod factor regulation of phytohormone signaling and homeostasis during rhizobia-legume symbiosis. Front. Plant Sci. 2018, 9, 1247. [Google Scholar] [CrossRef] [PubMed]
- Bourion, V.; Heulin-Gotty, K.; Aubert, V.; Tisseyre, P.; Chabert-Martinello, M.; Pervent, M.; Delaitre, C.; Vile, D.; Siol, M.; Duc, G.; et al. Co-inoculation of a pea core-collection with diverse rhizobial strains shows competitiveness for nodulation and efficiency of nitrogen fixation are distinct traits in the interaction. Front. Plant Sci. 2018, 8, 2249. [Google Scholar] [CrossRef] [PubMed]
- Peix, A.; Ramírez-Bahena, M.H.; Velázquez, E.; Bedmar, E.J. Bacterial Associations with Legumes. CRC Crit. Rev. Plant Sci. 2015, 34, 17–42. [Google Scholar] [CrossRef]
- Wang, D.; Yang, S.; Tang, F.; Zhu, H. Symbiosis specificity in the legume-rhizobial mutualism. Cell. Microbiol. 2012, 14, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.F.; Wang, E.T.; Ji, Z.J.; Zhang, J.J. Recent development and new insight of diversification and symbiosis specificity of legume rhizobia: Mechanism and application. J. Appl. Microbiol. 2021, 131, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Paulitsch, F.; Delamuta, J.R.M.; Ribeiro, R.A.; da Silva Batista, J.S.; Hungria, M. Phylogeny of symbiotic genes reveals symbiovars within legume-nodulating Paraburkholderia species. Syst. Appl. Microbiol. 2020, 43, 126151. [Google Scholar] [CrossRef]
- Wdowiak-Wróbel, S.; Marek-Kozaczuk, M.; Kalita, M.; Karaś, M.; Wójcik, M.; Małek, W. Diversity and plant growth promoting properties of rhizobia isolated from root nodules of Ononis arvensis. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2017, 110, 1087–1103. [Google Scholar] [CrossRef]
- Wang, F.Q.; Wang, E.T.; Liu, J.; Chen, Q.; Sui, X.H.; Chen, W.F. Mesorhizobium albiziae sp. nov., a novel bacterium that nodulates Albizia kalkora in a subtropical region of China. Int. J. Syst. Evol. Microbiol. 2007, 57, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Kalita, M.; Małek, W. Root nodules of Genista germanica harbor Bradyrhizobium and Rhizobium bacteria exchanging nodC and nodZ genes. Syst. Appl. Microbiol. 2020, 43, 126026. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Zaidi, A.; Musarrat, J. Microbes for Legume Improvement; Springer Nature: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Reinprecht, Y.; Schram, L.; Marsolais, F.; Smith, T.H.; Hill, B.; Pauls, K.P. Effects of Nitrogen Application on Nitrogen Fixation in Common Bean Production. Front. Plant Sci. 2020, 11, 534817. [Google Scholar] [CrossRef] [PubMed]
- Jēkabsone, A.; Andersone-Ozola, U.; Karlsons, A.; Neiceniece, L.; Romanovs, M.; Ievinsh, G. Dependence on Nitrogen Availability and Rhizobial Symbiosis of Different Accessions of Trifolium fragiferum, a Crop Wild Relative Legume Species, as Related to Physiological Traits. Plants 2022, 11, 1141. [Google Scholar] [CrossRef]
- Lagunas, B.; Richards, L.; Sergaki, C.; Burgess, J.; Pardal, A.J.; Hussain, R.M.F.; Richmond, B.L.; Baxter, L.; Roy, P.; Pakidi, A.; et al. Rhizobial nitrogen fixation efficiency shapes endosphere bacterial communities and Medicago truncatula host growth. Microbiome 2023, 11, 146. [Google Scholar] [CrossRef]
- Khaitov, B.; Kurbonov, A.; Abdiev, A.; Adilov, M. Effect of chickpea in association with Rhizobium to crop productivity and soil fertility. Eurasian J. Soil Sci. (EJSS) 2016, 5, 105. [Google Scholar] [CrossRef]
- Morel Revetria, M.A.; Berais-Rubio, A.; Giménez, M.; Sanjuán, J.; Signorelli, S.; Monza, J. Competitiveness and Phylogenetic Relationship of Rhizobial Strains with Different Symbiotic Efficiency in Trifolium repens: Conversion of Parasitic into Non-Parasitic Rhizobia by Natural Symbiotic Gene Transfer. Biology 2023, 12, 243. [Google Scholar] [CrossRef] [PubMed]
- Irisarri, P.; Cardozo, G.; Tartaglia, C.; Reyno, R.; Gutiérrez, P.; Lattanzi, F.A.; Rebuffo, M.; Monza, J. Selection of competitive and efficient rhizobia strains for white clover. Front. Microbiol. 2019, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, Y.; Hemissi, I.; Ben Salem, I.; Mejri, S.; Saidi, M.; Belhadj, O. Potential of Rhizobia in Improving Nitrogen Fixation and Yields of Legumes. Symbiosis 2018, 107, 1–16. [Google Scholar] [CrossRef]
- Kandil, A.E.; Özdamar Ünlü, H. Effect of rhizobium inoculation on yield and some quality properties of fresh cowpea. Cogent Food Agric. 2023, 9, 2275410. [Google Scholar] [CrossRef]
- Fahde, S.; Boughribil, S.; Sijilmassi, B.; Amri, A. Rhizobia: A Promising Source of Plant Growth-Promoting Molecules and Their Non-Legume Interactions: Examining Applications and Mechanisms. Agriculture 2023, 13, 1279. [Google Scholar] [CrossRef]
- Buernor, A.B.; Kabiru, M.R.; Bechtaoui, N.; Jibrin, J.M.; Asante, M.; Bouraqqadi, A.; Dahhani, S.; Ouhdouch, Y.; Hafidi, M.; Jemo, M. Grain Legume Yield Responses to Rhizobia Inoculants and Phosphorus Supplementation under Ghana Soils: A Meta-Synthesis. Front. Plant Sci. 2022, 13, 877433. [Google Scholar] [CrossRef]
- Gedamu, S.A.; Tsegaye, E.A.; Beyene, T.F. Effect of rhizobial inoculants on yield and yield components of faba bean (Vicia fabae L.) on vertisol of Wereillu District, South Wollo, Ethiopia. CABI Agric. Biosci. 2021, 2, 8. [Google Scholar] [CrossRef]
- Wilson, G.; Ulzen, J.; Abaidoo, R.C.; Opoku, A.; Adjei-Nsiah, S.; Osei, O. Native Rhizobia Strains Enhance Seed Yield of Groundnut Varieties in Northern Ghana. Front. Agron. 2021, 3, 653044. [Google Scholar] [CrossRef]
- Louvrier, P.; Laguerre, G.; Amarger, N. Distribution of symbiotic genotypes in Rhizobium leguminosarum biovar viciae populations isolated directly from soils. Appl. Environ. Microbiol. 1996, 62, 4202–4205. [Google Scholar] [CrossRef]
- Vincent, J.M. A Manual for the Practical Study of Root-Nodule Bacteria. In I.B.P. Handbook; Blackwell Scientific Publisher: Oxford, UK, 1970; Volume 8. [Google Scholar]
- Somasegaran, P.; Hoben, H.J. Handbook for Rhizobia: Methods in Legumes-Rhizobium Technology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Muthini, M. Morphological Assessment and Effectiveness of Indigenous Rhizobia Isolates that Nodulate P. vulgaris in Water Hyacinth Compost Testing Field in Lake Victoria Basin. Br. J. Appl. Sci. Technol. 2014, 4, 718–738. [Google Scholar] [CrossRef]
- Beck, D.P.; Materon, L.A.; Afandi, F. Practical Rhizobium-Legume Technology Manual; International Center for Agricultural Research in the Dry Areas (ICARDA): Beirut, Lebanon, 1993. [Google Scholar]
- Ramachandran, V.K.; East, A.K.; Karunakaran, R.; Downie, J.A.; Poole, P.S. Adaptation of Rhizobium leguminosarum to pea, alfalfa and sugar beet rhizospheres investigated by comparative transcriptomics. Genome Biol. 2011, 12, R106. [Google Scholar] [CrossRef] [PubMed]
- Beringer, J.E. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 1974, 84, 188–198. [Google Scholar] [CrossRef]
- Couchoud, M.; Salon, C.; Girodet, S.; Jeudy, C.; Vernoud, V.; Prudent, M. Pea Efficiency of Post-drought Recovery Relies on the Strategy to Fine-Tune Nitrogen Nutrition. Front. Plant Sci. 2020, 11, 204. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA Sequencing. In Nucleic Acid Techniques in Bacterial Systematics; John Wiley and Sons: New York, NY, USA, 1991. [Google Scholar]
- Gaunt, M.W.; Turner, S.L.; Rigottier-Gois, L.; Lloyd-Macgilp, S.A.; Young, J.P.W. Phylogenies of atpD and recA support the small subunit rRNA-based classification of rhizobia. Int. J. Syst. Evol. Microbiol. 2001, 51, 2037–2048. [Google Scholar] [CrossRef]
- Laguerre, G.; Nour, S.M.; Macheret, V.; Sanjuan, J.; Drouin, P.; Amarger, N. Classification of rhizobia based on nodC and nifH gene analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbionts. Microbiology 2001, 147, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BIOEDIT: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/ NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular evolutionary genetics analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A Simple Method for Estimating Evolutionary Rates of Base Substitutions through Comparative Studies of Nucleotide Sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS Software, version 9.4; SAS Institute Inc.: Cary, NC, USA, 2014. [Google Scholar]

| Properties | ||||||
|---|---|---|---|---|---|---|
| Sample | Granulometric Composition | Humus, % | pH | Nmin, mg kg−1 | Mobile Phosphorus P2O5, mg kg−1 | Mobile Potassium K2O, mg kg−1 |
| SOIL15, 0–30 cm | Heavy loam | 2.84 | 6.8 | 9.82 | 250 | 240 |
| Gene | Primers | Reference | |
|---|---|---|---|
| Forward | Reverse | ||
| 16 rRNA | 27F 5′-(AGAGTTGATCMTGGCTCAG)-3 | 1387R 5′-(GGGCGGWGTGTACAAG GC)-3′ | [71] |
| RecA | 5′-(CGKCTSGTAGAGGAYAAATCGGTGGA)-3′ | RecA555r 5′-(CGRATCTGGTTGATGAAGATCACCAT)-3′ | [72] |
| AtpD | AtpD273f 5′-(SCTGGGSCGYATCMTGAACGY)-3′ | AtpD771r 5′-(GCCGACACTTCCGAACCNGCCTG)-3′ | [72] |
| NodC | NodCF 5′-(AYGTHGTYGAYGACGGTTC)-3′ | NodCI 5′-(CGYGACAGCCANTCKCTATTG)-3′ | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaziūnienė, J.; Pini, F.; Shamshitov, A.; Razbadauskienė, K.; Frercks, B.; Gegeckas, A.; Mažylytė, R.; Lapinskienė, L.; Supronienė, S. Genetic Characterization of Rhizobium spp. Strains in an Organic Field Pea (Pisum sativum L.) Field in Lithuania. Plants 2024, 13, 1888. https://doi.org/10.3390/plants13141888
Kaziūnienė J, Pini F, Shamshitov A, Razbadauskienė K, Frercks B, Gegeckas A, Mažylytė R, Lapinskienė L, Supronienė S. Genetic Characterization of Rhizobium spp. Strains in an Organic Field Pea (Pisum sativum L.) Field in Lithuania. Plants. 2024; 13(14):1888. https://doi.org/10.3390/plants13141888
Chicago/Turabian StyleKaziūnienė, Justina, Francesco Pini, Arman Shamshitov, Kristyna Razbadauskienė, Birutė Frercks, Audrius Gegeckas, Raimonda Mažylytė, Laura Lapinskienė, and Skaidrė Supronienė. 2024. "Genetic Characterization of Rhizobium spp. Strains in an Organic Field Pea (Pisum sativum L.) Field in Lithuania" Plants 13, no. 14: 1888. https://doi.org/10.3390/plants13141888






