From ‘Farm to Fork’: Exploring the Potential of Nutrient-Rich and Stress-Resilient Emergent Crops for Sustainable and Healthy Food in the Mediterranean Region in the Face of Climate Change Challenges
Abstract
:1. Introduction
2. Agronomical Aspects Related to NUSs for the Mediterranean Region
2.1. Historical Background
2.2. Importance and Benefits
2.3. Classification and Characteristics
| Common Name | Scientific Name | Botanical Family | Type | Abiotic Stress Tolerance | |||
|---|---|---|---|---|---|---|---|
| Cold | Heat | Drought | Salinity | ||||
| Quinoa | Chenopodium quinoa Willd. | Amaranthaceae | Dicotyledons/Herbaceous/C3 | Medium | Medium/low | High | Medium/high |
| Amaranth | Amaranthus sp. (75 species [71]) | Amaranthaceae | Dicotyledons/Herbaceous/C4 | Medium/low | High | High | Medium |
| Buckwheat | Fagopyrum sp. | Polygonaceae | Dicotyledons/Herbaceous/C3 | Medium | Medium/low | High | Medium |
| Millet/Sorghum | Subfamilies:
| Poaceae | Monocotyledons/Herbaceous/C4 | Low | High | High | High |
| Teff | Eragrostis tef (Zucc.) Trotter | Poaceae | Monocotyledons/Herbaceous/C4 | Medium | High | Medium | Medium/low |
| Chia | Salvia hispanica L. | Lamiaceae | Dicotyledons/Herbaceous/C3 | Low | Medium | Medium | Medium |
| Moringa | Moringa oleifera Lam. | Moringaceae | Dicotyledons/Woody/C3 | Very low | High | High | Medium/high |
| Hemp | Cannabis sativa L. | Cannabaceae | Dicotyledons/Herbaceous/C3 | Low | Medium/high | High | High |
| Desert truffles | Terfezia claveryi Chatin (with Helianthemum almeriense Pau) | Pezizaceae (Cistaceae) | Ascomycete (Dicotyledons/woody/C3) | Medium/high | Medium | Medium | Medium/low |
2.4. Challenges and Limitations
2.5. Genetic and Breeding Advances
3. Nutritional and Technological Aspects Related to NUSs for the Mediterranean Region
3.1. Nutritional Composition
| Nutritional Component | Protein g/100 g | Fat g/100 g | Ash g/100 g | Dietary Fibre g/100 g | Carbohydrates g/100 g | References |
|---|---|---|---|---|---|---|
| Quinoa | 13.1–18.7 | 1.9–9.5 | 2.9–3.8 | 9.4–22.7 | 41.5–77.0 | [156,157,158] |
| Amaranth | 13.0–17.6 | 4.2–8.5 | 2.4–4 | 10.0–25.0 | 48.0–69.0 | [156,159,160] |
| Buckwheat | 8.5–18.8 | 1.5–6.5 | 1.7–2.7 | 20.0–26.0 | 54.5–57.4 | [161,162] |
| Chía | 15–25 | 25–40 | 4–4.8 | 18.0–35.5 | 26.0–42.0 | [156,163] |
| Sorghum | 4.4–21.1 | 1.5–8.9 | 1.1–2.2 | 10.2–19.0 | 63.7–80.0 | [153,164] |
| Teff | 9.1–12.6 | 2.3–3.3 | 2.4–2.5 | 2.4–9.8 | 70.8–73.8 | [165] |
| Moringa | 18.6–39.4 | 1.4–5.2 | 7.9–8.2 | 2.0–10.2 | 29.8–51.6 | [166,167] |
| Hemp | 21–32 | 25.4–35.9 | 3.7–6.3 | 28.8–38.8 | 32.5–38.1 | [168] |
| Desert truffle | 8–29 | 3–7 | 2.6–15 | 1.4–13.2 | 46.0–83.0 | [169] |
3.1.1. Protein and Amino Acids
3.1.2. Oils and Fatty Acids
3.1.3. Ash and Mineral Content
3.1.4. Fibre and Carbohydrates
3.2. Bioactive Compounds
3.2.1. Phenolics
3.2.2. Phytosterols
3.2.3. Betalains
3.3. Antinutritional Factors
| Antinutrient Factor | Phytic Acid | Saponins | Protease Inhibitors | Lectins | Oxalates | Nitrates | References |
|---|---|---|---|---|---|---|---|
| Crop and Usual Treatment | g/100 g | mg/100 g | mg/100 g | g/100 g | mg/100 g | mg/100 g | |
| Quinoa | 0.50–1.39 | 1.90–6.24; 199.07 629.7–1633.3 ** | 2.4 * g/kg | 5.2 HU’; 9.3% | 4.31; 6.17–9.45 | 21.83 | [158,258,266,269] |
| Amaranth | 0.61–1.34 | 5.33–10.66 HU/mg 79–186 | 0.519–1.016 TIU/mg | 0.5–7.3; 11.2–130.6 HAU/mg | 178–278; 46–152 | 48–84 | [258,261,267,270,271] |
| Buckwheat | 0.63; 1.7 | 0.058 | 55 α U/mg protein; 94 α U/mg protein | NYR (+) | 93–155 | NYR (−) | [161,272,273,274] |
| Millet | 0.12–0.92; 0.336–0.649; 0.519–0.784; 0.64–0.81 | 0.039–0.044 | 0.385–0.985 TIU/mg flour | NYR (−) | 11.3–20.0 | NYR (−) | [164,272,275,276,277] |
| Teff | 0.682–1.374; 1.35 | 722 | NYR | NYR (−) | 3.50–5.27 *** | NYR (−) | [278,279,280,281] |
| Chia | 1.5–2.6 | 616 | 445 TIU/mg protein | NYR | NYR (−) | NYR (−) | [163,282] |
| Moringa (seeds) | 0.115 δ;2.54 | 1080 δ | 15.4; 120 δ | NYR (+) | 2.21 δ | NYR | [283,284] |
| Hemp | 2.66–3.08 | 123.54 α | 3.6% β | NYR | NYR | NYR | [285,286,287] |
| Dessert truffles | 0.581 | 12.38 | NYR | 18.11 HU | NYR | NYR | [265,288] |
| Usual Treatment to diminish the antinutrient factor concentration or deactivation. In general, antinutrient levels can be controlled by cultivar selection and breeding, field management, and processing | Soaking, Steeping, Malting, Fermentation, Germination, Extrusion, Popped grains, Phytase activation by sprouting, Germination, or lowering the pH Inclusion of exogenous phytases Agronomic conditions Genetic engineering | Germination Popped seeds Extrusion Sweet varieties (<110 mg/100 g) Agronomic conditions | Cooking around 100 °C or at higher temperatures Popped grains Extrusion | Cooking around 100 °C or more Soaking Extrusion Popped seeds Germination | Water cooking-soluble oxalate, Soaking/Steeping Control of fertilization/soil | Water cooking Soaking/Steeping Control of fertilization/soil |
3.4. Nutritional Challenges and Considerations
3.5. Technological Aspects
3.5.1. Culinary and Processing Aspects
3.5.2. Technological Treatments
4. The Health Benefits of NUSs for the Mediterranean Region
5. Future Prospects
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ulian, T.; Diazgranados, M.; Pironon, S.; Padulosi, S.; Liu, U.; Davies, L.; Howes, M.J.R.; Borrell, J.S.; Ondo, I.; Pérez-Escobar, O.A.; et al. Unlocking plant resources to support food security and promote sustainable agriculture. Plants People Planet 2020, 2, 421–445. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.A.; Mayes, S.; Massawe, F. Crop diversification through a wider use of underutilised crops: A strategy to ensure food and nutrition security in the face of climate change. In Sustainable Solutions for Food Security: Combating Climate Change by Adaptation; Springer: Berlin/Heidelberg, Germany, 2019; pp. 125–149. [Google Scholar] [CrossRef]
- Pingali, P. Agricultural policy and nutrition outcomes—Getting beyond the preoccupation with staple grains. Food Secur. 2015, 7, 583–591. [Google Scholar] [CrossRef]
- Sibhatu, K.T.; Krishna, V.V.; Qaim, M. Production diversity and dietary diversity in smallholder farm households. Proc. Natl. Acad. Sci. USA 2015, 112, 10657–10662. [Google Scholar] [CrossRef] [PubMed]
- Harkness, C.; Areal, F.J.; Semenov, M.A.; Senapati, N.; Shield, I.F.; Bishop, J. Stability of farm income: The role of agricultural diversity and agri-environment scheme payments. Agric. Syst. 2021, 187, 103009. [Google Scholar] [CrossRef]
- Ouda, S.; Zohry, A.E.-H. Climate Extremes and Crops. In Climate-Smart Agriculture; Springer: Berlin/Heidelberg, Germany, 2022; pp. 93–114. [Google Scholar] [CrossRef]
- Liu, C.; Plaza-Bonilla, D.; Coulter, J.A.; Kutcher, H.R.; Beckie, H.J.; Wang, L.; Floc’h, J.-B.; Hamel, C.; Siddique, K.H.M.; Li, L.; et al. Diversifying crop rotations enhances agroecosystem services and resilience. Adv. Agron. 2022, 173, 299–335. [Google Scholar] [CrossRef]
- Hellegers, P. Food security vulnerability due to trade dependencies on Russia and Ukraine. Food Secur. 2022, 14, 1503–1510. [Google Scholar] [CrossRef]
- Kumar, L.; Chhogyel, N.; Gopalakrishnan, T.; Hasan, M.K.; Jayasinghe, S.L.; Kariyawasam, C.S.; Kogo, B.; Ratnayake, S. Climate change and future of agri-food production. In Future Foods: Global Trends, Opportunities, and Sustainability Challenges; Academic Press: Cambridge, MA, USA, 2022; pp. 49–79. [Google Scholar] [CrossRef]
- Sands, D.C.; Morris, C.E.; Dratz, E.A.; Pilgeram, A. Elevating optimal human nutrition to a central goal of plant breeding and production of plant-based foods. Plant Sci. 2009, 177, 377–389. [Google Scholar] [CrossRef]
- Mayes, S.; Massawe, F.J.; Alderson, P.G.; Roberts, J.A.; Azam-Ali, S.N.; Hermann, M. The potential for underutilized crops to improve security of food production. J. Exp. Bot. 2012, 63, 1075–1079. [Google Scholar] [CrossRef]
- Lionello, P.; Malanotte-Rizzoli, P.; Boscolo, R.; Alpert, P.; Artale, V.; Li, L.; Luterbacher, J.; May, W.; Trigo, R.; Tsimplis, M.; et al. The Mediterranean climate: An overview of the main characteristics and issues. Dev. Earth Environ. Sci. 2006, 4, 1–26. [Google Scholar] [CrossRef]
- Del Pozo, A.; Brunel-Saldias, N.; Engler, A.; Ortega-Farias, S.; Acevedo-Opazo, C.; Lobos, G.A.; Jara-Rojas, R.; Molina-Montenegro, M.A. Climate Change Impacts and Adaptation Strategies of Agriculture in Mediterranean-Climate Regions (MCRs). Sustainability 2019, 11, 2769. [Google Scholar] [CrossRef]
- Subedi, B.; Poudel, A.; Aryal, S. The impact of climate change on insect pest biology and ecology: Implications for pest management strategies, crop production, and food security. J. Agric. Food Res. 2023, 14, 100733. [Google Scholar] [CrossRef]
- Cantero-Martínez, C.; Gabiña, D. Evaluation of agricultural practices to improve efficiency and environment conservation in Mediterranean arid and semi-arid production systems: MEDRATE project. In Options Méditerranéennes: Série A Séminaires Méditerranéens; CIHEAM: Zaragoza, Spain, 2004; pp. 21–31. [Google Scholar]
- Grigorieva, E.; Livenets, A.; Stelmakh, E. Adaptation of Agriculture to Climate Change: A Scoping Review. Climate 2023, 11, 202. [Google Scholar] [CrossRef]
- Sood, S.; Malhotra, N.; Tripathi, K.; Laibach, N.; Rosero, A. Editorial: Food of the future: Underutilized foods. Front. Nutr. 2023, 10, 1307856. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, G.; Bommarco, R.; Wanger, T.C.; Kremen, C.; van der Heijden, M.G.A.; Liebman, M.; Hallin, S. Agricultural diversification promotes multiple ecosystem services without compromising yield. Sci. Adv. 2020, 6, eaba1715. [Google Scholar] [CrossRef] [PubMed]
- Raneri, J.E.; Padulosi, S.; Meldrum, G.; King, O.I. Promoting Neglected and Underutilized Species to Boost Nutrition in LMICs. 2019. Available online: https://cgspace.cgiar.org/server/api/core/bitstreams/b215c446-2370-481f-8876-7580640b26b0/content (accessed on 9 March 2024).
- Zeder, M.A.; Zeder, A. Domestication and early agriculture in the Mediterranean Basin: Origins, diffusion, and impact. Proc. Natl. Acad. Sci. USA 2008, 105, 11597–11604. [Google Scholar] [CrossRef] [PubMed]
- Harlan, J.R. Crops and Man; American Society of Agronomy and Crop Science Society of America: Madison, WI, USA, 1975. [Google Scholar]
- Doebley, J.F.; Gaut, B.S.; Smith, B.D. The molecular genetics of crop domestication. Cell 2006, 127, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.; Amri, A.; Castañeda-Álvarez, N.P.; Dempewolf, H.; Dullo, E.; Guarino, L.; Hole, D.; Mba, C.; Toledo, A.; Maxted, N. Modeling of crop wild relative species identifies areas globally for in situ conservation. Commun. Biol. 2019, 2, 136. [Google Scholar] [CrossRef] [PubMed]
- Behre, K.-E. The history of rye cultivation in Europe. Veg. Hist. Archaeobot. 1992, 1, 141–156. [Google Scholar] [CrossRef]
- Hancock, J.F. Evolution of European Agriculture. In World Agriculture before and after 1492; Hancock, J.F., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 19–29. [Google Scholar] [CrossRef]
- Padulosi, S.; Heywood, V.; Hunter, D.; Jarvis, A. Underutilized species and climate change: Current status and outlook. In Crop Adaptation to Climate Change; Yadav, S.Y., Redden, R.J., Hatfield, J.L., Lotze-Campen, H., Hall, A.E., Eds.; Wiley: Hoboken, NJ, USA, 2011; pp. 507–521. [Google Scholar] [CrossRef]
- ECPGR. Minor Cereals and Pseudocereals in Europe. Report of a Network Coordinating Group on Minor Crops. 1999. Available online: https://www.ecpgr.org/fileadmin/bioversity/publications/pdfs/607_Report_of_a_network_coordinating_group_on_minor_crops-1.pdf (accessed on 30 January 2024).
- Wanner, H.; Pfister, C.; Neukom, R. The variable European Little Ice Age. Quat. Sci. Rev. 2022, 287, 107531. [Google Scholar] [CrossRef]
- Degroot, D.; Anchukaitis, K.; Bauch, M.; Burnham, J.; Carnegy, F.; Cui, J.; de Luna, K.; Guzowski, P.; Hambrecht, G.; Huhtamaa, H.; et al. Towards a rigorous understanding of societal responses to climate change. Nature 2021, 591, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Xu, J.P.; Callac, P.; Chen, M.Y.; Wu, Q.; Wach, M.; Mata, G.; Zhao, R.L. Insight into the evolutionary and domesticated history of the most widely cultivated mushroom Agaricus bisporus via mitogenome sequences of 361 global strains. BMC Genom. 2023, 24, 182. [Google Scholar] [CrossRef] [PubMed]
- Shavit, E. The History of Desert Truffle Use. In Desert Truffles; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2014; pp. 217–241. [Google Scholar] [CrossRef]
- Morte, A.; Kagan-Zur, V.; Navarro-Ródenas, A.; Sitrit, Y. Cultivation of Desert Truffles—A Crop Suitable for Arid and Semi-Arid Zones. Agronomy 2021, 11, 1462. [Google Scholar] [CrossRef]
- Morte, A.; Pérez-Gilabert, M.; Gutiérrez, A.; Arenas, F.; Marqués-Gálvez, J.E.; Bordallo, J.J.; Rodríguez, A.; Berná, L.M.; Lozano-Carrillo, C.; Navarro-Ródenas, A. Basic and applied research for desert truffle cultivation. In Mycorrhiza—Eco-Physiology, Secondary Metabolites, Nanomaterials, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 23–42. [Google Scholar] [CrossRef]
- Fischer, A.R.H.; Beers, P.J.; Van Latesteijn, H.; Andeweg, K.; Jacobsen, E.; Mommaas, H.; Van Trijp, H.C.M.; Veldkamp, A. Transforum system innovation towards sustainable food. A review. Agron. Sustain. Dev. 2012, 32, 595–608. [Google Scholar] [CrossRef]
- Kakabouki, I.; Tataridas, A.; Mavroeidis, A.; Kousta, A.; Roussis, I.; Katsenios, N.; Efthimiadou, A.; Papastylianou, P. Introduction of alternative crops in the Mediterranean to satisfy EU Green Deal goals. A review. Agron. Sustain. Dev. 2021, 41, 71. [Google Scholar] [CrossRef]
- Gitz, V.; Meybeck, A.; Lipper, L.; Young, C.D.; Braatz, S. Climate Change and Food Security: Risks and Responses; Food and Agriculture Organization of the United Nations (FAO) Report; FAO: Rome, Italy, 2016; Volume 110. [Google Scholar]
- Lin, P.H.; Chao, Y.Y. Different Drought-Tolerant Mechanisms in Quinoa (Chenopodium quinoa Willd.) and Djulis (Chenopodium formosanum Koidz.) Based on Physiological Analysis. Plants 2021, 10, 2279. [Google Scholar] [CrossRef]
- Assefa, Y.; Staggenborg, S.A.; Prasad, V.P.V. Grain Sorghum Water Requirement and Responses to Drought Stress: A Review. Crop Manag. 2010, 9, 1–11. [Google Scholar] [CrossRef]
- Gill, A.R.; Loveys, B.R.; Cavagnaro, T.R.; Burton, R.A. The potential of industrial hemp (Cannabis sativa L.) as an emerging drought resistant fibre crop. Plant Soil 2023, 493, 7–16. [Google Scholar] [CrossRef]
- Morte, A.; Navarro-Ródenas, A.; Nicolás, E. Physiological parameters of desert truffle mycorrhizal Helianthemun almeriense plants cultivated in orchards under water deficit conditions. Symbiosis 2010, 52, 133–139. [Google Scholar] [CrossRef]
- Henzell, R.G.; Jordan, D.R. Grain Sorghum. In Cereals; Carena, M.J., Ed.; Springer: New York, NY, USA, 2009; pp. 183–197. [Google Scholar] [CrossRef]
- Goel, K.; Kundu, P.; Gahlaut, V.; Sharma, P.; Kumar, A.; Thakur, S.; Verma, V.; Bhargava, B.; Chandora, R.; Zinta, G. Functional divergence of Heat Shock Factors (Hsfs) during heat stress and recovery at the tissue and developmental scales in C4 grain amaranth (Amaranthus hypochondriacus). Front. Plant Sci. 2023, 14, 1151057. [Google Scholar] [CrossRef]
- Iqbal, S.; Basra, S.M.A.; Saddiq, M.S.; Yang, A.; Akhtar, S.S.; Jacobsen, S.-E. The Extraordinary Salt Tolerance of Quinoa. In Emerging Research in Alternative Crops; Hirich, A., Choukr-Allah, R., Ragab, R., Eds.; Springer: Cham, Switzerland, 2020; pp. 125–143. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Emam, M.M.; Salama, K.H.A.; Morsy, A.A. Sorghum under saline conditions: Responses, tolerance mechanisms, and management strategies. Planta 2021, 254, 24. [Google Scholar] [CrossRef] [PubMed]
- Kamenya, S.N.; Mikwa, E.O.; Song, B.; Odeny, D.A. Genetics and breeding for climate change in Orphan crops. Theor. Appl. Genet. 2021, 134, 1787. [Google Scholar] [CrossRef] [PubMed]
- Geisen, S.; Krishnaswamy, K.; Myers, R. Physical and Structural Characterization of Underutilized Climate-Resilient Seed Grains: Millets, Sorghum, and Amaranth. Front. Sustain. Food Syst. 2021, 5, 599656. [Google Scholar] [CrossRef]
- Pathan, S.; Ndunguru, G.; Clark, K.; Ayele, A.G. Yield and nutritional responses of quinoa (Chenopodium quinoa Willd.) genotypes to irrigated, rainfed, and drought-stress environments. Front. Sustain. Food Syst. 2023, 7, 1242187. [Google Scholar] [CrossRef]
- Knodel, N. A Framework for Agricultural Decarbonisation: Environmental Assessment from Seed to Soil of a Cradle-To-Cradle Farm System with Industrial Hemp and Pyrogenic Carbon Capture & Storage. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Selwal, N.; Bedi, M.; Hamid, S.; Pujari, M. Buckwheat (Fagopyrum esculentum) Response and Tolerance to Abiotic Stress. In Omics Approach to Manage Abiotic Stress in Cereals; Springer: Singapore, 2022; pp. 575–597. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Gerhardt, K.; Knicky, M.; Wendin, K. Buckwheat: An underutilized crop with attractive sensory qualities and health benefits. Crit. Rev. Food Sci. Nutr. 2023, 1–16. [Google Scholar] [CrossRef]
- Horn, L.; Shakela, N.; Mutorwa, M.K.; Naomab, E.; Kwaambwa, H.M. Moringa oleifera as a sustainable climate-smart solution to nutrition, disease prevention, and water treatment challenges: A review. J. Agric. Food Res. 2022, 10, 100397. [Google Scholar] [CrossRef]
- Acevedo, M.; Pixley, K.; Zinyengere, N.; Meng, S.; Tufan, H.; Cichy, K.; Bizikova, L.; Isaacs, K.; Ghezzi-Kopel, K.; Porciello, J. A scoping review of adoption of climate-resilient crops by small-scale producers in low- and middle-income countries. Nat. Plants 2020, 6, 1231–1241. [Google Scholar] [CrossRef]
- Angeli, V.; Silva, P.M.; Massuela, D.C.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa Willd.): An Overview of the Potentials of the “Golden Grain” and Socio-Economic and Environmental Aspects of Its Cultivation and Marketization. Foods 2020, 9, 216. [Google Scholar] [CrossRef]
- Ahmadi, S.H.; Solgi, S.; Sepaskhah, A.R. Quinoa: A super or pseudo-super crop? Evidences from evapotranspiration, root growth, crop coefficients, and water productivity in a hot and semi-arid area under three planting densities. Agric. Water Manag. 2019, 225, 105784. [Google Scholar] [CrossRef]
- Herman, S.; Marco, G.; Cecilia, B.; Alfonso, V.; Luis, M.; Cristián, V.; Sebastián, P.; Sebastián, A. Effect of water availability on growth, water use efficiency and omega 3 (ALA) content in two phenotypes of chia (Salvia hispanica L.) established in the arid Mediterranean zone of Chile. Agric. Water Manag. 2016, 173, 67–75. [Google Scholar] [CrossRef]
- Rastogi, A.; Shukla, S. Amaranth: A new millennium crop of nutraceutical values. Crit. Rev. Food Sci. Nutr. 2013, 53, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Owaid, M.N. Bioecology and Uses of Desert Truffles (Pezizales) in the Middle East. Walailak J. Sci. Technol. (WJST) 2017, 15, 179–188. [Google Scholar] [CrossRef]
- Hordofa, T.; Dirirsa, G.; Wondimu, T.; Tesfaye, A. Crop Water Requirement and Crop Coefficient of Tef (Eragrostis tef) in Central Rift Valley of Ethiopia. J. Nat. Sci. Res. 2020, 11, 34–39. [Google Scholar] [CrossRef]
- Godino, M.; Arias, C.; Izquierdo, M.I. Moringa oleifera: Potential areas of cultivation on the Iberian Peninsula. Acta Hortic. 2017, 1158, 405–412. [Google Scholar] [CrossRef]
- Iqbal, S.; Basra, S.M.A.; Afzal, I.; Wahid, A. Exploring potential of well adapted quinoa lines for salt tolerance. Int. J. Agric. Biol. 2017, 19, 933–940. [Google Scholar] [CrossRef]
- Dixit, N. Salinity Induced Antioxidant Defense in Roots of Industrial Hemp (IH: Cannabis sativa L.) for Fiber during Seed Germination. Antioxidants 2022, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Estrada, Y.; Fernández-Ojeda, A.; Morales, B.; Egea-Fernández, J.M.; Flores, F.B.; Bolarín, M.C.; Egea, I. Unraveling the Strategies Used by the Underexploited Amaranth Species to Confront Salt Stress: Similarities and Differences with Quinoa Species. Front. Plant Sci. 2021, 12, 604481. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.K. Buckwheat (Fagopyrum esculentum Moench and F. tataricum Gaertn.). In Neglected and Underutilized Crops: Future Smart Food; Elservier: Amsterdam, The Netherlands, 2023; pp. 151–200. [Google Scholar] [CrossRef]
- Afzal, I.; Haq, M.Z.U.; Ahmed, S.; Hirich, A.; Bazile, D. Challenges and Perspectives for Integrating Quinoa into the Agri-Food System. Plants 2023, 12, 3361. [Google Scholar] [CrossRef]
- Christiansen, J.L.; Jacobsen, S.E.; Jørgensen, S.T. Photoperiodic effect on flowering and seed development in quinoa (Chenopodium quinoa Willd.). Acta Agric. Scand. B Soil Plant Sci. 2010, 60, 539–544. [Google Scholar] [CrossRef]
- Rodríguez-Abello, D.C.; Navarro-Alberto, J.A.; Ramírez-Avilés, L.; Zamora-Bustillos, R. The effect of sowing time on the growth of chia (Salvia hispanica L.): What do nonlinear mixed models tell us about it? PLoS ONE 2018, 13, e0206582. [Google Scholar] [CrossRef] [PubMed]
- Matías, J.; Rodríguez, M.J.; Cruz, V.; Calvo, P.; Reguera, M. Heat stress lowers yields, alters nutrient uptake and changes seed quality in quinoa grown under Mediterranean field conditions. J. Agron. Crop Sci. 2021, 207, jac.12495. [Google Scholar] [CrossRef]
- Ben-Zeev, S.; Kirby, N.; Diehn, S.; Shtein, I.; Elbaum, R.; Saranga, Y. Unraveling the central role of root morphology and anatomy in lodging of tef (Eragrostis tef). Plants People Planet 2023. early access. [Google Scholar] [CrossRef]
- Todaro, V.; D’Oria, M.; Secci, D.; Zanini, A.; Tanda, M.G. Climate Change over the Mediterranean Region: Local Temperature and Precipitation Variations at Five Pilot Sites. Water 2022, 14, 2499. [Google Scholar] [CrossRef]
- Wrigley, C.W.; Corke, H.; Walker, C.E. Encyclopedia of Grain Science; Elservier: Oxford, UK, 2004; pp. 1–10. [Google Scholar] [CrossRef]
- Taylor, J.R.N. Sorghum and Millets: Taxonomy, History, Distribution, and Production. In Sorghum and Millets: Chemistry, Technology, and Nutritional Attributes; Woodhead Publishing: Duxford, UK, 2019; pp. 1–21. [Google Scholar] [CrossRef]
- Lutz, W.; Kc, S. Dimensions of global population projections: What do we know about future population trends and structures? Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2779. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Wang, J.W.; Li, J.; Han, B. Designing future crops: Challenges and strategies for sustainable agriculture. Plant J. 2021, 105, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Dugan, F.M.; Lupien, S.L.; Hu, J. Fungal plant pathogens associated with emerging crops in North America: A challenge for plant health professionals. Plant Health Prog. 2017, 18, 221–229. [Google Scholar] [CrossRef]
- Tyl, C.; DeHaan, L.; Frels, K.; Bajgain, P.; Marks, M.D.; Anderson, J.A. Emerging Crops with Enhanced Ecosystem Services: Progress in Breeding and Processing for Food Use. Cereal Foods World 2020, 65, 1–8. [Google Scholar] [CrossRef]
- Pais, D.F.; Marques, A.C.; Fuinhas, J.A. How to Promote Healthier and More Sustainable Food Choices: The Case of Portugal. Sustainability 2023, 15, 3868. [Google Scholar] [CrossRef]
- Jordan, N.R.; Dorn, K.; Runck, B.; Ewing, P.; Williams, A.; Anderson, K.A.; Felice, L.; Haralson, K.; Goplen, J.; Altendorf, K.; et al. Sustainable commercialization of new crops for the agricultural bioeconomy. Elementa 2016, 2016, 81. [Google Scholar] [CrossRef]
- Anuradha Kumari, M.; Zinta, G.; Chauhan, R.; Kumar, A.; Singh, S.; Singh, S. Genetic resources and breeding approaches for improvement of amaranth (Amaranthus spp.) and quinoa (Chenopodium quinoa). Front. Nutr. 2023, 10, 1129723. [Google Scholar] [CrossRef] [PubMed]
- Calderón-González, Á.; Matías, J.; Cruz, V.; Molinero-Ruiz, L.; Fondevilla, S. Identification and Characterization of Sources of Resistance to Peronospora variabilis in Quinoa. Agronomy 2023, 13, 284. [Google Scholar] [CrossRef]
- Kučka, M.; Ražná, K.; Čerteková, S.; Chňapek, M.; Mikolášová, L.; Gálová, Z.; Balážová, Z. Buckwheat—A Genomic, Transcriptomic and Proteomic View. J. Microbiol. Biotechnol. Food Sci. 2023, 13, e10059. [Google Scholar] [CrossRef]
- Hassani, M.; Piechota, T.; Atamian, H.S. Prediction of Cultivation Areas for the Commercial and an Early Flowering Wild Accession of Salvia hispanica L. in the United States. Agronomy 2022, 12, 1651. [Google Scholar] [CrossRef]
- Flajšman, M.; Ačko, D.K. Industrial hemp breeding and genetics. In Industrial Hemp; Academic Press: Cambridge, MA, USA, 2022; pp. 37–57. [Google Scholar]
- Craine, E.B.; Davies, A.; Packer, D.; Miller, N.D.; Schmöckel, S.M.; Spalding, E.P.; Tester, M. A comprehensive characterization of agronomic and end-use quality phenotypes across a quinoa world core collection. Front. Plant Sci. 2023, 14, 1101547. [Google Scholar] [CrossRef]
- Zurita-Silva, A.; Fuentes, F.; Zamora, P.; Jacobsen, S.E.; Schwember, A.R. Breeding quinoa (Chenopodium quinoa Willd.): Potential and perspectives. Mol. Breed. 2014, 34, 13–30. [Google Scholar] [CrossRef]
- Jarvis, D.E.; Hoy, Y.S.; Lightfoot, D.J.; Schmöckel, S.M.; Li, B.; Borm, T.J.A.; Ohyanagi, K.; Mineta, K.; Michell, C.T.; Saber, N. The genome of Chenopodium quinoa. Nature 2017, 542, 307–312. [Google Scholar] [CrossRef]
- Li, L.; Nie, M.; Lu, J.; He, C.; Wu, Q. Genome-wide identification, expression analysis and gene duplication analysis of Dof transcription factors between quinoa and its ancestral diploid sub-genome species reveal key CDF homologs involved in photoperiodic flowering regulation. S. Afr. J. Bot. 2023, 162, 545–558. [Google Scholar] [CrossRef]
- Patiranage, D.S.R.; Rey, E.; Emrani, N.; Wellman, G.; Schmid, K.; Schmöckel, S.M.; Tester, M. Genome-wide association study in quinoa reveals selection pattern typical for crops with a short breeding history. eLife 2022, 11, e66873. [Google Scholar] [CrossRef] [PubMed]
- Sandell, F.L.; Holzweber, T.; Street, N.R.; Dohm, J.C.; Himmelbauer, H. Genomic basis of seed colour in quinoa inferred from variant patterns using extreme gradient boosting. Plant Biotechnol. J. 2024, 22, 1312–1324. [Google Scholar] [CrossRef]
- Bochicchio, R.; Philips, T.; Lovelli, S.; Labella, R.; Galgano, F.; Di Marisco, A.; Perniola, M.; Amato, M. Innovative Crop Productions for Healthy Food: The Case of Chia (Salvia hispanica L.). In The Sustainability of Agro-Food and Natural Resource Systems in the Mediterranean Basin; Springer: Berlin/Heidelberg, Germany, 2015; pp. 29–45. [Google Scholar] [CrossRef]
- Cahill, J.P.; Provance, M.C. Genetics of qualitative traits in domesticated chia (Salvia hispanica L.). J. Hered. 2002, 93, 52–55. [Google Scholar] [CrossRef]
- Zare, T.; Paril, J.F.; Barnett, E.M.; Kaur, P.; Appels, R.; Ebert, B.; Roessner, U.; Fournier-Level, A. Comparative genomics points to tandem duplications of SAD gene clusters as drivers of increased α-linolenic (ω-3) content in S. hispanica seeds. Plant Genome 2024, 17, e20430. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Geniza, M.; Elser, J.; Al-Bader, N.; Baschieri, R.; Phillips, J.L.; Haq, E.; Preece, J.; Naithani, S.; Jaiswal, P. Reference genome of the nutrition-rich orphan crop chia (Salvia hispanica) and its implications for future breeding. Front. Plant Sci. 2023, 14, 1272966. [Google Scholar] [CrossRef] [PubMed]
- Ponnam, N.; Kumari, M.; Ganesan, S.; Barik, S.; Pattanaik, M.; Adamala, A.K.; Rao, V.K.; Rupa, T.R.; Varalakshmi, B.; Acharya, G.C. Breeding leafy amaranth (Amaranthus spp.) for white rust resistance. S. Afr. J. Bot. 2023, 163, 794–804. [Google Scholar] [CrossRef]
- Dharajiya, D.T.; Trivedi, G.N.; Thakkar, N.J.; Pachchigar, K.P.; Teli, B.; Tiwari, K.K.; Blair, M.W. Genomics-Assisted Design of Biotic Stress Resistant Vegetable Amaranths. In Genomic Designing for Biotic Stress Resistant Vegetable Crops; Springer: Heidelberg, Germany, 2022; pp. 261–300. [Google Scholar] [CrossRef]
- Lightfoot, D.J.; Jarvis, D.E.; Ramaraj, T.; Lee, R.; Jellen, E.N.; Maughan, P.J. Single-molecule sequencing and Hi-C-based proximity-guided assembly of amaranth (Amaranthus hypochondriacus) chromosomes provide insights into genome evolution. BMC Biol. 2017, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Jamalluddin, N. Genetic Diversity Analysis and Trait Phenotyping for Drought Tolerance in Amaranth (Amaranthus spp.) Germplasm. Ph.D. Thesis, University of Nottingham Malaysia, Selangor, Malaysia, 2020. [Google Scholar]
- Chen, J.; Liu, Y.; Liu, M.; Guo, W.; Wang, Y.; He, Q.; Chen, W.; Liao, Y.; Zhang, W.; Gao, Y.; et al. Pangenome analysis reveals genomic variations associated with domestication traits in broomcorn millet Check for updates. Nat. Genet. 2023, 55, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Luthar, Z.; Fabjan, P.; Mlinarič, K. Biotechnological methods for buckwheat breeding. Plants 2021, 10, 1547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; He, M.; Fan, Y.; Zhao, H.; Gao, B.; Yang, K.; Li, F.; Tang, Y.; Gao, Q.; Lin, T.; et al. Resequencing of global Tartary buckwheat accessions reveals multiple domestication events and key loci associated with agronomic traits. Genome Biol. 2021, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Ma, B.; Gao, Q.; Du, H.; Han, Y.; Li, Y.; Cao, Y.; Qi, M.; Zhu, Y.; et al. The Tartary Buckwheat Genome Provides Insights into Rutin Biosynthesis and Abiotic Stress Tolerance. Mol. Plant 2017, 10, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Sun, Z.; Ding, M.; Logacheva, M.D.; Kreft, I.; Wang, D.; Yan, M.; Shao, J.; Tang, Y.; Wu, Y.; et al. FtSAD2 and FtJAZ1 regulate activity of the FtMYB11 transcription repressor of the phenylpropanoid pathway in Fagopyrum tataricum. New Phytol. 2017, 216, 814–828. [Google Scholar] [CrossRef]
- Shi, T.X.; Li, R.Y.; Zheng, R.; Chen, Q.F.; Li, H.Y.; Huang, J.; Zhu, L.W.; Liang, G.C. Mapping QTLs for 1000-grain weight and genes controlling hull type using SNP marker in Tartary buckwheat (Fagopyrum tataricum). BMC Genom. 2021, 22, 142. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, L.; Jia, Y.; Zhao, J.; Li, C.; Chen, H.; Wu, H.; Wu, Q. Accumulation of the bitter substance quercetin mediated by the overexpression of a novel seed-specific gene FtRDE2 in Tartary buckwheat. Plant Physiol. Biochem. 2024, 207, 108402. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Sudan, J.; Urwat, U.; Pakhtoon, M.M.; Bhat, B.; Sharma, V.; Sofi, P.A.; Shikari, A.B.; Bhat, B.A.; Sofi, N.R.; et al. Genome-wide SNP discovery and genotyping delineates potential QTLs underlying major yield-attributing traits in buckwheat. Plant Genome 2024, 17, e20427. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Zhang, K.; He, Y.; Fan, Y.; Li, W.; Shi, Y.; Gao, Y.; Huang, X.; He, J.; Zhao, H.; et al. Multi-omics identification of a key glycosyl hydrolase gene FtGH1 involved in rutin hydrolysis in Tartary buckwheat (Fagopyrum tataricum). Plant Biotechnol. J. 2023, 22, 1206–1223. [Google Scholar] [CrossRef]
- Santra, D.K.; Khound, R.; Das, S. Proso millet (Panicum miliaceum L.) breeding: Progress, challenges and opportunities. Adv. Plant Breed. Strateg. Cereals 2019, 5, 223–257. [Google Scholar] [CrossRef]
- Nagaraja, T.E.; Nandini, C.; Bhat, S.; Parveen, S.G. Artificial hybridization techniques in small millets—A review. Front. Plant Sci. 2023, 14, 1112117. [Google Scholar] [CrossRef]
- Fukunaga, K.; Kawase, M. Crop Evolution of Foxtail Millet. Plants 2024, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, C.; He, Q.; Zhang, J.; Liang, H.; Lu, Z.; Kie, K.; Tang, S.; Zhou, Y.; Liu, B.; et al. A complete reference genome assembly for foxtail millet and Setaria-db, a comprehensive database for Setaria. Mol. Plant 2024, 17, 219–222. [Google Scholar] [CrossRef]
- He, Q.; Tang, S.; Zhi, H.; Chen, J.; Zhang, J.; Liang, H.; Xie, K.; Tang, S.; Zhou, Y.; Liu, B.; et al. A graph-based genome and pan-genome variation of the model plant Setaria. Nat. Genet. 2023, 55, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, A.; Mallikarjuna, M.G.; Bansal, S.; Nayaka, S.C.; Rajashekara, H.; Chellapilla, T.S.; Prakash, G. Genome-wide identification and characterization of NBLRR genes in finger millet (Eleusine coracana L.) and their expression in response to Magnaporthe grisea infection. BMC Plant Biol. 2024, 24, 75. [Google Scholar] [CrossRef]
- Habiyaremye, C.; Barth, V.; Highet, K.; Coffey, T.; Murphy, K.M. Phenotypic Responses of Twenty Diverse Proso Millet (Panicum miliaceum L.) Accessions to Irrigation. Sustainability 2017, 9, 389. [Google Scholar] [CrossRef]
- Khound, R.; Sun, G.; Mural, R.V.; Schnable, J.C.; Santra, D.K. SNP discovery in proso millet (Panicum miliaceum L.) using low-pass genome sequencing. Plant Direct 2022, 6, e447. [Google Scholar] [CrossRef] [PubMed]
- Magris, G.; Foria, S.; Ciani, S.; Santra, D.K.; Polenghi, O.; Cerne, V.; Morgante, M.; Di Gaspero, G. Targeted sequencing of the Panicum miliaceum gene space and genotyping of variant sites from population genetics studies, combined in a single assay, as a tool for broomcorn millet assisted breeding. Euphytica 2023, 219, 102. [Google Scholar] [CrossRef]
- Boukail, S.; Macharia, M.; Miculan, M.; Masoni, A.; Calamai, A.; Palchetti, E.; Dell’Acqua, M. Genome wide association study of agronomic and seed traits in a world collection of proso millet (Panicum miliaceum L.). BMC Plant Biol. 2021, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, N.U.; Alghamdi, K.M.; Saleem, S.; Shajar, F.; Tahir, I.; Bahieldin, A.; Rehman, R.U.; Hakeem, K.R. Selenate and selenite transporters in proso millet: Genome extensive detection and expression studies under salt stress and selenium. Front. Plant Sci. 2022, 13, 1060154. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Liu, J.; Ma, Q.; Wu, E.; Gao, J.; Yang, Q.; Feng, B. Transcriptome analysis reveals the mechanism of nitrogen fertilizers in starch synthesis and quality in waxy and non-waxy proso millet. Carbohydr. Polym. 2024, 323, 121372. [Google Scholar] [CrossRef]
- Beyene, G.; Chauhan, R.D.; Villmer, J.; Husic, N.; Wang, N.; Gebre, E.; Girma, D.; Chanyalew, S.; Assefa, K.; Tabor, G.; et al. CRISPR/Cas9-mediated tetra-allelic mutation of the ‘Green Revolution’ SEMIDWARF-1 (SD-1) gene confers lodging resistance in tef (Eragrostis tef). Plant Biotechnol. J. 2022, 20, 1716. [Google Scholar] [CrossRef]
- Woldeyohannes, A.B.; Desta, E.A.; Fadda, C.; Pè, M.E.; Dell’Acqua, M. Value of teff (Eragrostis tef) genetic resources to support breeding for conventional and smallholder farming: A review. CABI Agric. Biosci. 2022, 3, 27. [Google Scholar] [CrossRef]
- VanBuren, R.; Man Wai, C.; Wang, X.; Pardo, J.; Yocca, A.E.; Wang, H.; Chaluvadi, S.R.; Han, G.; Bryant, D.; Edger, P.P.; et al. Exceptional subgenome stability and functional divergence in the allotetraploid Ethiopian cereal teff. Nat. Commun. 2020, 11, 884. [Google Scholar] [CrossRef] [PubMed]
- Chanyalew, S.; Singh, H.; Tefera, H.; Sorrels, M. Molecular genetic map and QTL analysis of agronomic traits based on a Eragrostis tef × E. pilosa recombinant inbred population. J. Genet. Breed. 2005, 59, 53–66. [Google Scholar]
- Yu, J.K.; Graznak, E.; Breseghello, F.; Tefera, H.; Sorrells, M.E. QTL mapping of agronomic traits in tef [Eragrostis tef (Zucc) Trotter]. BMC Plant Biol. 2007, 7, 30. [Google Scholar] [CrossRef]
- Ligaba-Osena, A.; Salehin, M.; Numan, M.; Wang, X.; Choi, S.C.; Jima, D.; Bobay, L.M.; Guo, W. Genome-wide transcriptome analysis of the orphan crop tef (Eragrostis tef (Zucc.) Trotter) under long-term low calcium stress. Sci. Rep. 2022, 12, 19552. [Google Scholar] [CrossRef] [PubMed]
- Haddadi, B.S.; Fang, R.; Girija, A.; Kattupalli, D.; Widdowson, E.; Beckmann, M.; Yadav, R.; Mur, L.A.J. Metabolomics targets tissue-specific responses in alleviating the negative effects of salinity in tef (Eragrostis tef) during germination. Planta 2023, 258, 67. [Google Scholar] [CrossRef]
- Girija, A.; Han, J.; Corke, F.; Brook, J.; Doonan, J.; Yadav, R.; Jifar, H.; Mur, L.A.J. Elucidating drought responsive networks in tef (Eragrostis tef) using phenomic and metabolomic approaches. Physiol. Plant 2022, 174, e13597. [Google Scholar] [CrossRef]
- Berenji, J.; Sikora, V.; Fournier, G.; Beherec, O. Genetics and selection of hemp. In Hemp: Industrial Production and Uses; CABI: London, UK, 2013; pp. 48–71. [Google Scholar] [CrossRef]
- Barcaccia, G.; Palumbo, F.; Scariolo, F.; Vannozzi, A.; Borin, M.; Bona, S. Potentials and Challenges of Genomics for Breeding Cannabis Cultivars. Front. Plant Sci. 2020, 11, 573299. [Google Scholar] [CrossRef] [PubMed]
- Weiblen, G.D.; Wenger, J.P.; Craft, K.J.; ElSohly, M.A.; Mehmedic, Z.; Treiber, E.L.; Marks, M.D. Gene duplication and divergence affecting drug content in Cannabis sativa. New Phytol. 2015, 208, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Laverty, K.U.; Stout, J.M.; Sullivan, M.J.; Shah, H.; Gill, N.; Holbrook, L.; Deikus, G.; Sebra, R.; Hughes, T.R.; Page, J.E.; et al. A physical and genetic map of Cannabis sativa identifies extensive rearrangements at the THC/CBD acid synthase loci. Genome Res. 2019, 29, 146–156. [Google Scholar] [CrossRef]
- Soorni, A.; Fatahi, R.; Haak, D.C.; Salami, S.A.; Bombarely, A. Assessment of Genetic Diversity and Population Structure in Iranian Cannabis Germplasm. Sci. Rep. 2017, 7, 15668. [Google Scholar] [CrossRef] [PubMed]
- Stack, G.M.; Cala, A.R.; Quade, M.A.; Toth, J.A.; Monserrate, L.A.; Wilkerson, D.G.; Carlson, C.H.; Mamerto, A.; Michael, T.P.; Crawford, S.; et al. Genetic mapping, identification, and characterization of a candidate susceptibility gene for powdery mildew in Cannabis sativa L. Mol. Plant-Microbe Interact. 2023, 37, 51–61. [Google Scholar] [CrossRef]
- Wei, H.; Yang, Z.; Niyitanga, S.; Tao, A.; Xu, J.; Fang, P.; Lin, L.; Zhang, L.; Qi, J.; Ming, R.; et al. The reference genome of seed hemp (Cannabis sativa) provides new insights into fatty acid and vitamin E synthesis. Plant Commun. 2024, 5, 100718. [Google Scholar] [CrossRef]
- Alavilli, H.; Poli, Y.; Verma, K.S.; Kumar, V.; Gupta, S.; Chaudhary, V.; Jyoti, A.; Sahi, S.V.; Kothari, S.L. Miracle Tree Moringa oleifera: Status of the Genetic Diversity, Breeding, In Vitro Propagation, and a Cogent Source of Commercial Functional Food and Non-Food Products. Plants 2022, 11, 3132. [Google Scholar] [CrossRef]
- Shyamli, P.S.; Pradhan, S.; Panda, M.; Parida, A. De novo Whole-Genome Assembly of Moringa oleifera Helps Identify Genes Regulating Drought Stress Tolerance. Front. Plant Sci. 2021, 12, 766999. [Google Scholar] [CrossRef] [PubMed]
- Shankar, R.; Mandal, S.; Rao, K.; Kalaivanan, D. Moringa Breeding: International Scenario. In Proceedings of the International Seminar on Exotic and Underutilized Horticultural Crops: Priorities & Emerging Trends, Bengaluru, India, 17–19 October 2023. [Google Scholar]
- Pasha, S.N.; Shafi, K.M.; Joshi, A.G.; Meenakshi, I.; Harini, K.; Mahita, J.; Sajeevan, R.S.; Karpe, S.D.; Ghosh, P.; Nitish, S.; et al. The transcriptome enables the identification of candidate genes behind medicinal value of Drumstick tree (Moringa oleifera). Genomics 2020, 112, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zou, Q.; Wang, J.; Zhang, J.; Liu, Z.; Chen, X. Proteomic Profiles Reveal the Function of Different Vegetative Tissues of Moringa oleifera. Protein J. 2016, 35, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Burchi, F.; Fanzo, J.; Frison, E. The role of food and nutrition system approaches in tackling hidden hunger. Int. J. Environ. Res. Public Health 2011, 8, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Titcomb, T.J.; Tanumihardjo, S.A. Global Concerns with B Vitamin Statuses: Biofortification, Fortification, Hidden Hunger, Interactions, and Toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1968–1984. [Google Scholar] [CrossRef] [PubMed]
- El Bilali, H. Research on agro-food sustainability transitions: Where are food security and nutrition? Food Secur. 2019, 11, 559–577. [Google Scholar] [CrossRef]
- Tadele, Z. Orphan crops: Their importance and the urgency of improvement. Planta 2019, 250, 677–694. [Google Scholar] [CrossRef]
- Assogbadjo, A.E.; Chadare, F.J.; Manda, L.; Sinsin, B. A 20-Year Journey Through an Orphan African Baobab (Adansonia digitata L.) Towards Improved Food and Nutrition Security in Africa. Front. Sustain. Food Syst. 2021, 5, 675382. [Google Scholar] [CrossRef]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef] [PubMed]
- Janssen, F.; Pauly, A.; Rombouts, I.; Jansens, K.J.A.; Deleu, L.J.; Delcour, J.A. Proteins of Amaranth (Amaranthus spp.), Buckwheat (Fagopyrum spp.), and Quinoa (Chenopodium spp.): A Food Science and Technology Perspective. Compr. Rev. Food Sci. Food Saf. 2017, 16, 39–58. [Google Scholar] [CrossRef]
- Palombini, S.V.; Claus, T.; Maruyama, S.A.; Gohara, A.K.; Souza, A.H.P.; de Souza, N.E.; Visentainer, J.V.; Gomes, S.T.M.; Matsushita, M. Evaluation of nutritional compounds in new amaranth and quinoa cultivars. Food Sci. Technol. 2013, 33, 339–344. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Liu, R.; Hernandez, M.; Draves, J.; Marcone, M.F.; Tsao, R. Assessing the fatty acid, carotenoid, and tocopherol compositions of amaranth and quinoa seeds grown in Ontario and their overall contribution to nutritional quality. J. Agric. Food Chem. 2016, 64, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martínez, E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef]
- Jin, J.; Ohanenye, I.C.; Udenigwe, C.C. Buckwheat proteins: Functionality, safety, bioactivity, and prospects as alternative plant-based proteins in the food industry. Crit. Rev. Food Sci. Nutr. 2022, 62, 1752–1764. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Rizwana Tripathi, A.D.; Kumar, T.; Sharma, K.P.; Patel, S.K.S. Nutritional and Functional New Perspectives and Potential Health Benefits of Quinoa and Chia Seeds. Antioxidants 2023, 12, 1413. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.P.; Anunciação, P.C.; da Matyelka, J.C.S.; Della Lucia, C.M.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Chemical composition of Brazilian chia seeds grown in different places. Food Chem. 2017, 221, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Awol, S.M.; Kuyu, C.G.; Bereka, T.Y. Physicochemical stability, microbial growth, and sensory quality of teff flour as affected by packaging materials during storage. LWT 2023, 189, 115488. [Google Scholar] [CrossRef]
- Awika, J.M. Sorghum: Its Unique Nutritional and Health-Promoting Attributes. In Gluten-Free Ancient Grains: Cereals, Pseudocereals, and Legumes: Sustainable, Nutritious, and Health-Promoting Foods for the 21st Century; Elsevier: Amsterdam, The Netherlands, 2017; pp. 21–54. [Google Scholar] [CrossRef]
- Gharsallah, K.; Rezig, L.; Rajoka, M.S.R.; Mehwish, H.M.; Ali, M.A.; Chew, S.C. Moringa oleifera: Processing, phytochemical composition, and industrial applications. S. Afr. J. Bot. 2023, 160, 180–193. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Farag, M.A.; Yosri, N.; Sabir, J.S.M.; Saeed, A.; Al-Mousawi, S.M.; Taha, W.; Musharraf, S.G.; Patel, S.; El-Seedi, H.R.; et al. Truffles: From Islamic culture to chemistry, pharmacology, and food trends in recent times. Trends Food Sci. Technol. 2019, 91, 193–218. [Google Scholar] [CrossRef]
- Olmos, E.; Roman-Garcia, I.; Reguera, M.; Mestanza, C.; Fernandez-Garcia, N. An update on the nutritional profiles of quinoa (Chenopodium quinoa Willd.), amaranth (Amaranthus spp.), and chia (Salvia hispanica L.), three key species with the potential to contribute to food security worldwide. JSFA Rep. 2022, 2, 591–602. [Google Scholar] [CrossRef]
- Navruz-Varli, S.; Sanlier, N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.). J. Cereal Sci. 2016, 69, 371–376. [Google Scholar] [CrossRef]
- Villacrés, E.; Quelal, M.; Galarza, S.; Iza, D.; Silva, E. Nutritional Value and Bioactive Compounds of Leaves and Grains from Quinoa (Chenopodium quinoa Willd.). Plants 2022, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Soriano-García, M.; Aguirre-Díaz, I.S.; Soriano-García, M.; Aguirre-Díaz, I.S. Nutritional Functional Value and Therapeutic Utilization of Amaranth. In Nutritional Value of Amaranth; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.E. Nutritional value and use of the andean crops quinoa (Chenopodium quinoa) and kañiwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Dogra, D. Common Buckwheat: Nutritional Profiling of Grains. Asian J. Dairy Food Res. 2019, 38, 333–340. [Google Scholar] [CrossRef]
- Sofi, S.A.; Ahmed, N.; Farooq, A.; Rafiq, S.; Zargar, S.M.; Kamran, F.; Dar, T.A.; Mir, S.A.; Dar, B.N.; Khaneghah, A.M. Nutritional and bioactive characteristics of buckwheat, and its potential for developing gluten-free products: An updated overview. Food Sci. Nutr. 2023, 11, 2256. [Google Scholar] [CrossRef] [PubMed]
- Vera-Cespedes, N.; Muñoz, L.A.; Rincón, M.Á.; Haros, C.M. Physico-Chemical and Nutritional Properties of Chia Seeds from Latin American Countries. Foods 2023, 12, 3013. [Google Scholar] [CrossRef]
- Ravindran, G. Seed protein of millets: Amino acid composition, proteinase inhibitors and in-vitro protein digestibility. Food Chem. 1992, 44, 13–17. [Google Scholar] [CrossRef]
- Teff: Nutrient Composition and Health Benefits. Ethiopia Strategy Support Program Working Paper 67. Nutritional Composition of Teff Grain. Available online: https://ebrary.ifpri.org/digital/api/collection/p15738coll2/id/128374/download+of+Teff+Grain%2C&rlz=1C1GCEU_esES1095ES1095&oq=Teff%3A+nutrient+composition+and+health+benefits.+Ethiopia+Strategy+Support+Program+Working+Paper+67%2C+Nutritional+Composition+of+Teff+Grain%2C&gs_lcrp=EgZjaHJvbWUyBggAEEUYOTIGCAEQRRg60gEHMjQ5ajBqNKgCALACAA&sourceid=chrome&ie=UTF-8 (accessed on 22 February 2024).
- Moyo, B.; Masika, P.J.; Hugo, A.; Muchenje, V. Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. Afr. J. Biotechnol. 2011, 10, 12925–12933. [Google Scholar] [CrossRef]
- Oyeyinka, A.T.; Oyeyinka, S.A. Moringa oleifera as a food fortificant: Recent trends and prospects. J. Saudi Soc. Agric. Sci. 2018, 17, 127–136. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, D.; Wang, W.; Li, Y.; Sun, X. Nutritional and chemical composition of industrial hemp seeds. In Industrial Hemp: Food and Nutraceutical Applications; Academic Press: Cambridge, MA, USA, 2022; pp. 73–93. [Google Scholar] [CrossRef]
- Gadallah, M.G.E.; Ashoush, I.S.; Gadallah, M.G.E.; Ashoush, I.S. Value Addition on Nutritional and Sensory Properties of Biscuit Using Desert Truffle (Terfezia claveryi) Powder. Food Nutr. Sci. 2016, 7, 1171–1181. [Google Scholar] [CrossRef]
- Bobkov, S. Biochemical and Technological Properties of Buckwheat Grains. In Molecular Breeding and Nutritional Aspects of Buckwheat; Elsevier: Amsterdam, The Netherlands, 2016; pp. 423–440. [Google Scholar] [CrossRef]
- Rodríguez Gómez, M.J.; Maestro-Gaitán, I.; Calvo Magro, P.; Cruz Sobrado, V.; Reguera Blázquez, M.; Matías Prieto, J. Unique nutritional features that distinguish Amaranthus cruentus L. and Chenopodium quinoa Willd seeds. Food Res. Int. 2023, 164, 112160. [Google Scholar] [CrossRef] [PubMed]
- Graziano, S.; Agrimonti, C.; Marmiroli, N.; Gullì, M. Utilisation and limitations of pseudocereals (quinoa, amaranth, and buckwheat) in food production: A review. Trends Food Sci. Technol. 2022, 125, 154–165. [Google Scholar] [CrossRef]
- USDA—United States Department of Agriculture. National Nutrient Database for Standard Reference Release; USDA: Washington, DC, USA, 2015; p. 28. [Google Scholar]
- Mattila, P.H.; Pihlava, J.M.; Hellström, J.; Nurmi, M.; Eurola, M.; Mäkinen, S.; Jalava, T.; Pihlanto, A. Contents of phytochemicals and antinutritional factors in commercial protein-rich plant products. Food Qual. Saf. 2018, 2, 213–219. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Pastucha, E.; Piątkowska, E.; Leszczyńska, T.; Pisulewska, E.; Witkowicz, R.; Francik, R. Basic chemical composition and bioactive compounds content in selected cultivars of buckwheat whole seeds, dehulled seeds and hulls. J. Cereal Sci. 2016, 69, 1–8. [Google Scholar] [CrossRef]
- Khan, A.; Khan, N.A.; Bean, S.R.; Chen, J.; Xin, Z.; Jiao, Y. Variations in Total Protein and Amino Acids in the Sequenced Sorghum Mutant Library. Plants 2023, 12, 1662. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.; Zhang, L.; Chang, W.; Ding, X.; Qian, J.; Tang, J. Determination and Analysis of Composition, Structure, and Properties of Teff Protein Fractions. Foods 2023, 12, 3965. [Google Scholar] [CrossRef] [PubMed]
- Tejedor-Calvo, E.; Amara, K.; Reis, F.S.; Barros, L.; Martins, A.; Calhelha, R.C.; Venturini, M.E.; Blanco, D.; Redondo, D.; Marco, P.; et al. Chemical composition and evaluation of antioxidant, antimicrobial and antiproliferative activities of Tuber and Terfezia truffles. Food Res. Int. 2021, 140, 110071. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Gómez, M.J.; Matías Prieto, J.; Cruz Sobrado, V.; Calvo Magro, P. Nutritional characterization of six quinoa (Chenopodium quinoa Willd) varieties cultivated in Southern Europe. J. Food Compos. Anal. 2021, 99, 103876. [Google Scholar] [CrossRef]
- Abugoch James, L.E. Quinoa (Chenopodium quinoa Willd.): Composition, chemistry, nutritional, and functional properties. Adv. Food Nutr. Res. 2009, 58, 1–31. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzales, R.; Ricci, A.; Fontecha, J.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd.) seeds. Ind. Crops Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Hernandez, M.; Zhang, H.; Marcone, M.F.; Liu, R.; Tsao, R. Characterisation of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 174, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Ruan, J.; Zhou, Y.; Yan, J.; Zhou, M.; Woo, S.H.; Weng, W.; Cheng, J.; Zhang, K. Tartary Buckwheat: An Under-utilized Edible and Medicinal Herb for Food and Nutritional Security. Food Rev. Int. 2022, 38, 440–454. [Google Scholar] [CrossRef]
- Gulpinar, A.R.; Erdogan Orhan, I.; Kan, A.; Senol, F.S.; Celik, S.A.; Kartal, M. Estimation of in vitro neuroprotective properties and quantification of rutin and fatty acids in buckwheat (Fagopyrum esculentum Moench) cultivated in Turkey. Food Res. Int. 2012, 46, 536–543. [Google Scholar] [CrossRef]
- Shen, Y.; Zheng, L.; Jin, J.; Li, X.; Fu, J.; Wang, M.; Guan, Y.; Song, X. Phytochemical and biological characteristics of Mexican chia seed oil. Molecules 2018, 23, 3219. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Kobus-Cisowska, J.; Taczanowski, M.; Kmiecik, D.; Gramza-Michałowska, A. The chemical composition and nutritional value of chia seeds—Current state of knowledge. Nutrients 2019, 11, 1242. [Google Scholar] [CrossRef]
- Mehmood, S.; Orhan, I.; Ahsan, Z.; Aslan, S.; Gulfraz, M. Fatty acid composition of seed oil of different Sorghum bicolor varieties. Food Chem. 2008, 109, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Desta, K.T.; Choi, Y.M.; Shin, M.J.; Yoon, H.; Wang, X.; Lee, Y.; Yi, J.; Jeon, Y.A.; Lee, S. Comprehensive evaluation of nutritional components, bioactive metabolites, and antioxidant activities in diverse sorghum (Sorghum bicolor (L.) Moench) landraces. Food Res. Int. 2023, 173, 113390. [Google Scholar] [CrossRef]
- Liu, K.S. Comparison of Lipid Content and Fatty Acid Composition and Their Distribution within Seeds of 5 Small Grain Species. J. Food Sci. 2011, 76, C334–C342. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J.; Bennetzen, J.L.; Messing, J. Teff, an orphan cereal in the chloridoideae, provides insights into the evolution of storage proteins in grasses. Genome Biol. Evol. 2016, 8, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Yisak, H.; Yaya, E.E.; Chandravanshi, B.S.; Redi-Abshiro, M. GC-MS profiling of fatty acids and nutritional properties of the white and brown teff [Eragrostis tef (Zuccagni) Trotter] varieties cultivated in different parts of Ethiopia. J. Food Compos. Anal. 2022, 107, 104405. [Google Scholar] [CrossRef]
- Ruttarattanamongkol, K.; Siebenhandl-Ehn, S.; Schreiner, M.; Petrasch, A.M. Pilot-scale supercritical carbon dioxide extraction, physico-chemical properties and profile characterization of Moringa oleifera seed oil in comparison with conventional extraction methods. Ind. Crops Prod. 2014, 58, 68–77. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.; Nam, K.; Zahra, Z.; Zahra, Z.; Farooqi, M.Q.U. Potentials of truffles in nutritional and medicinal applications: A review. Fungal Biol. Biotechnol. 2020, 7, 9. [Google Scholar] [CrossRef]
- Akyüz, M.; Kırbağ, S. Nutritive Value of Desert Truffles Species of Genera Terfezia and Picoa (Ascomycetes) from Arid and Semiarid Regions of Eastern Turkey. Int. J. Med. Mushrooms 2018, 20, 1097–1106. [Google Scholar] [CrossRef]
- Ghazali, H.M.; Mohammed, A.S. Moringa (Moringa oleifera) Seed Oil: Composition, Nutritional Aspects, and Health Attributes. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2011; pp. 787–793. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Hossini, A.M.; Hou, X.; Wang, C.; Weylandt, K.H.; Pietzner, A. Effects of Moringa oleifera Seed Oil on Cultured Human Sebocytes In Vitro and Comparison with Other Oil Types. Int. J. Mol. Sci. 2023, 24, 332. [Google Scholar] [CrossRef]
- Ahmadou, F.; Bourais, I.; Aqil, Y.; Shariati, M.A.; Hlebová, M.; Martsynkovsky, S.; Nagdalian, A.; El Hajjaji, S. Physiochemical Characteristics and Fatty Acids Composition of Moringa Oleifera Oil of Far North Cameroon. J. Microbiol. Biotechnol. Food Sci. 2023, 13, e10250. [Google Scholar] [CrossRef]
- Pereira, E.; Encina-Zelada, C.; Barros, L.; Gonzales-Barron, U.; Cadavez, V.; Ferreira, I.C. Chemical and nutritional characterization of Chenopodium quinoa Willd (quinoa) grains: A good alternative to nutritious food. Food Chem. 2019, 280, 110–114. [Google Scholar] [CrossRef]
- Hathcock, J.N. Vitamin and Mineral Safety, 2nd ed.; Council for Responsible Nutrition (CRN): Washington, DC, USA, 2004. [Google Scholar]
- Latham, M.C.; FAO. Nutrición humana en el mundo en desarrollo. In De las Naciones Unidas para la Agricultura y la Alimentación; FAO: Rome, Italy, 2002. [Google Scholar]
- Schmidt, D.; Verruma-Bernardi, M.R.; Forti, V.A.; Borges, M.T.M.R. Quinoa and Amaranth as Functional Foods: A Review. Food Rev. Int. 2023, 39, 2277–2296. [Google Scholar] [CrossRef]
- Ramírez-Ojeda, A.M.; Moreno-Rojas, R.; Cámara-Martos, F. Mineral and trace element content in legumes (lentils, chickpeas and beans): Bioaccesibility and probabilistic assessment of the dietary intake. J. Food Compos. Anal. 2018, 73, 17–28. [Google Scholar] [CrossRef]
- Zhu, F. Chemical composition and health effects of Tartary buckwheat. Food Chem. 2016, 203, 231–245. [Google Scholar] [CrossRef]
- Zhao, M.; Gou, J.; Zhang, K.; Ruan, J. Principal Components and Cluster Analysis of Trace Elements in Buckwheat Flour. Foods 2023, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Zeller, F.J.; Huang, K.F.; Shi, T.X.; Chen, Q.F. Variation of major minerals and trace elements in seeds of tartary buckwheat (Fagopyrum tataricum Gaertn.). Genet. Resour. Crop Evol. 2014, 61, 567–577. [Google Scholar] [CrossRef]
- Ullah, R.; Nadeem, M.; Khalique, A.; Imran, M.; Mehmood, S.; Javid, A.; Hussain, J. Nutritional and therapeutic perspectives of Chia (Salvia hispanica L.): A review. J. Food Sci. Technol. 2016, 53, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, L.A.; Cobos, A.; Diaz, O.; Aguilera, J.M. Chia Seed (Salvia hispanica): An Ancient Grain and a New Functional Food. Food Rev. Int. 2013, 29, 394–408. [Google Scholar] [CrossRef]
- Adebo, J.A.; Kesa, H. Evaluation of nutritional and functional properties of anatomical parts of two sorghum (Sorghum bicolor) varieties. Heliyon 2023, 9, e17296. [Google Scholar] [CrossRef] [PubMed]
- Espitia-Hernández, P.; González, M.L.C.; Ascacio-Valdés, J.A.; Dávila-Medina, D.; Flores-Naveda, A.; Silva, T.; Chacón, X.R.; Sepúlveda, L. Sorghum (Sorghum bicolor L.) as a potential source of bioactive substances and their biological properties. Crit. Rev. Food Sci. Nutr. 2022, 62, 2269–2280. [Google Scholar] [CrossRef]
- Akansha, K.S.; Chauhan, E.S. Nutritional composition, physical characteristics and health benefits of Teff grain for human consumption: A critical review. Pharma Innov. J. 2018, 7, 03–07. [Google Scholar]
- Reta, C.; Asmellash, T.; Atlabachew, M.; Mehari, B. Multielement analysis coupled with chemometrics modelling for geographical origin classification of teff [Eragrostis tef (Zuccagni) Trotter] grains from Amhara region. BMC Chem. 2023, 17, 50. [Google Scholar] [CrossRef]
- Abebe, A.; Chandravanshi, B.S. Levels of essential and non-essential metals in the raw seeds and processed food (roasted seeds and bread) of maize/corn (Zea mays L.) cultivated in selected areas of Ethiopia. Bull. Chem. Soc. Ethiop. 2017, 31, 185–199. [Google Scholar] [CrossRef]
- Assefa, K.; Cannarozzi, G.; Girma, D.; Kamies, R.; Chanyalew, S.; Plaza-Wüthrich, S.; Biosch, R.; Rindisbacher, A.; Rafudeen, S.; Tadele, Z. Genetic diversity in tef [Eragrostis tef (Zucc.) Trotter]. Front. Plant Sci. 2015, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Ntshambiwa, K.T.; Seifu, E.; Mokhawa, G. Nutritional composition, bioactive components and antioxidant activity of Moringa stenopetala and Moringa oleifera leaves grown in Gaborone, Botswana. Food Prod. Process. Nutr. 2023, 5, 7. [Google Scholar] [CrossRef]
- Agamou, A.; Armel, J.; Edith, F.N.; Moses, M.C. Influence of compounds contents and particle size on some functional properties of moringa oleifera leaves powders. Asian Food Sci. J. 2021, 20, 60–71. [Google Scholar]
- Zhu, F. Dietary fiber polysaccharides of amaranth, buckwheat and quinoa grains: A review of chemical structure, biological functions and food uses. Carbohydr. Polym. 2020, 248, 116819. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, K.; Kurek, S.; Mildner–Szkudlarz, S.; Kreft, I.; Walkowiak, J. Fatty acids profile, sterols, tocopherol and squalene content in Fagopyrum tataricum seed milling fractions. J. Cereal Sci. 2020, 96, 103118. [Google Scholar] [CrossRef]
- Awulachew, M.T. Investigating the Effects of Teff-Sorghum-Fenugreek Flour Blending Ratios on Quality Attributes of Injera; Addis Ababa University: Addis Ababa, Ethiopia, 2022. [Google Scholar]
- Sultana, S. Nutritional and functional properties of Moringa oleifera. Metabol. Open 2020, 8, 100061. [Google Scholar] [CrossRef]
- Zniber, I.; Boukcim, H.; Khabar, L.; Ducousso, M.; Henkrar, F.; El Mouttaqi, A.; Hirich, A. Characterization of Desert Truffles in the Great Moroccan Sahara: A Review. Environ. Sci. Proc. 2022, 16, 55. [Google Scholar] [CrossRef]
- Venskutonis, P.R.; Kraujalis, P. Nutritional Components of Amaranth Seeds and Vegetables: A Review on Composition, Properties, and Uses. Compr. Rev. Food Sci. Food Saf. 2013, 12, 381–412. [Google Scholar] [CrossRef]
- Hrnčič, M.K.; Ivanovski, M.; Cör, D.; Knez, Ž. Chia Seeds (Salvia hispanica L.): An overview-phytochemical profile, isolation methods, and application. Molecules 2020, 25, 11. [Google Scholar] [CrossRef]
- Sidorova, Y.S.; Shipelin, V.A.; Petrov, N.A.; Zorin, S.N.; Mazo, V.K. Anxiolytic and Antioxidant Effect of Phytoecdysteroids and Polyphenols from Chenopodium quinoa on an In Vivo Restraint Stress Model. Molecules 2022, 27, 9003. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, X.; Zheng, S.; Lu, K.; Zhao, G.; Ming, J. The composition, antioxidant and antiproliferative capacities of phenolic compounds extracted from tartary buckwheat bran [Fagopyrum tartaricum (L.) Gaerth]. J. Funct. Foods 2016, 22, 145–155. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzalez, R.; Sayas-Barberá, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Bioaccessibility of Phenolic Compounds and Antioxidant Capacity of Chia (Salvia hispanica L.) Seeds. Plant Foods Hum. Nutr. 2018, 73, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Apea-Bah, F.B.; Li, X.; Beta, T. Phenolic composition and antioxidant properties of cooked rice dyed with sorghum-leaf bio-colorants. Foods 2021, 10, 2058. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, R.; Panghal, A.; Chaudhary, G.; Kumari, A.; Chhikara, N. Nutritional, phytochemical and functional potential of sorghum: A review. Food Chem. Adv. 2023, 3, 100501. [Google Scholar] [CrossRef]
- Ravisankar, S.; Abegaz, K.; Awika, J.M. Structural profile of soluble and bound phenolic compounds in teff (Eragrostis tef) reveals abundance of distinctly different flavones in white and brown varieties. Food Chem. 2018, 263, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ramírez, A.; Pavo-Caballero, C.; Baeza, E.; Baenas, N.; Garcia-Viguera, C.; Marín, F.R.; Soler-Rivas, C. Mushrooms do not contain flavonoids. J. Funct. Foods 2016, 25, 1–13. [Google Scholar] [CrossRef]
- Gil, J.V.; Esteban-Muñoz, A.; Fernández-Espinar, M.T. Changes in the polyphenolic profile and antioxidant activity of wheat bread after incorporating quinoa flour. Antioxidants 2022, 11, 33. [Google Scholar] [CrossRef]
- Lis, B.; Jedrejek, D.; Rywaniak, J.; Soluch, A.; Stochmal, A.; Olas, B. Flavonoid Preparations from Taraxacum officinale L. Fruits—A Phytochemical, Antioxidant and Hemostasis Studies. Molecules 2020, 25, 5402. [Google Scholar] [CrossRef]
- Rani, N.Z.A.; Husain, K.; Kumolosasi, E. Moringa genus: A review of phytochemistry and pharmacology. Front. Pharmacol. 2018, 9, 108. [Google Scholar] [CrossRef]
- Pakade, V.; Cukrowska, E.; Chimuka, L. Comparison of antioxidant activity of Moringa oleifera and selected vegetables in South Africa. S. Afr. J. Sci. 2013, 109, 1–5. [Google Scholar] [CrossRef]
- Ryan, E.; Galvin, K.; O’Connor, T.P.; Maguire, A.R.; O’Brien, N.M. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods Hum. Nutr. 2007, 62, 85–91. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, A.N.G.; Pistelli, L.; Omer, E.A.; Elsayed, S.I.M.; Elshamy, A.I.; Hendawy, S.F. Growth, yield productivity, and oil composition of two Amaranthus species grown under two different environmental conditions in Egypt. Crop Sci. 2023, 63, 1472–1480. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, A.K.; Dobhal, M.P.; Sharma, M.C.; Gupta, R.S. Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J. Diabetes 2011, 3, 29–37. [Google Scholar] [CrossRef]
- Dziedzic, K.; Szwengiel, A.; Górecka, D.; Rudzińska, M.; Korczak, J.; Walkowiak, J. The effect of processing on the phytosterol content in buckwheat groats and by-products. J. Cereal Sci. 2016, 69, 25–31. [Google Scholar] [CrossRef]
- Motyka, S.; Skała, E.; Ekiert, H.; Szopa, A. Health-promoting approaches of the use of chia seeds. J. Funct. Foods 2023, 103, 105480. [Google Scholar] [CrossRef]
- El-Alfy, T.S.; Ezzat, S.M.; Sleem, A.A. Chemical and biological study of the seeds of Eragrostis tef (Zucc.) Trotter. Nat. Prod. Res. 2012, 26, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Weete, J.D.; Kulifaj, M.; Montant, C. Distribution of sterols in fungi. II. Brassicasterol in Tuber and Terfezia species. Can. J. Microbiol. 2011, 31, 1127–1130. [Google Scholar] [CrossRef]
- Escribano, J.; Cabanes, J.; Atienzar, M.J.; Tremolada, M.I.; Pando, L.R.G.; Carmona, F.G.; Herrero, F.G. Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties. Food Chem. 2017, 234, 285–294. [Google Scholar] [CrossRef]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. Betalains: Natural plant pigments with potential application in functional foods. LWT 2015, 64, 645–649. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; García-Carmona, F. Biosynthesis of betalains: Yellow and violet plant pigments. Trends Plant Sci. 2013, 18, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Wagner, A.E.; Schini-Kerth, V.B.; Rimbach, G. Betanin—A food colorant with biological activity. Mol. Nutr. Food Res. 2015, 59, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.I.; Farooq, M.; Syed, Q.A.; Ishaq, A.; Al-Ghamdi, A.A.; Hatamleh, A.A. Botany, nutritional value, phytochemical composition and biological activities of quinoa. Plants 2021, 10, 2258. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Tsao, R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: A review. Mol. Nutr. Food Res. 2017, 61, 1600767. [Google Scholar] [CrossRef] [PubMed]
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.M.; Mattila, P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Haros, M.; Carlsson, N.G.; Almgren, A.; Larsson-Alminger, M.; Sandberg, A.S.; Andlid, T. Phytate degradation by human gut isolated Bifidobacterium pseudocatenulatum ATCC27919 and its probiotic potential. Int. J. Food Microbiol. 2009, 135, 7–14. [Google Scholar] [CrossRef]
- Hurrell, R.F.; Reddy, M.B.; Juillerat, M.A.; Cook, J.D. Degradation of phytic acid in cereal porridges improves iron absorption by human subjects. Am. J. Clin. Nutr. 2003, 77, 1213–1219. [Google Scholar] [CrossRef]
- Lopez, H.W.; Krespine, V.; Guy, G.; Messager, A.; Demigne, C.; Remesy, C. Prolonged fermentation of whole wheat sourdough reduces phytate level and increases soluble magnesium. J. Agric. Food Chem. 2001, 49, 2657–2662. [Google Scholar] [CrossRef]
- Vashishth, A.; Ram, S.; Beniwal, V. Cereal phytases and their importance in improvement of micronutrients bioavailability. 3 Biotech 2017, 7, 42. [Google Scholar] [CrossRef]
- García-Mantrana, I.; Monedero, V.; Haros, M. Application of phytases from bifidobacteria in the development of cereal-based products with amaranth. Eur. Food Res. Technol. 2014, 238, 853–862. [Google Scholar] [CrossRef]
- Ruales, J.; Nair, B.M. Saponins, phytic acid, tannins and protease inhibitors in quinoa (Chenopodium quinoa, Willd) seeds. Food Chem. 1993, 48, 137–143. [Google Scholar] [CrossRef]
- Gee, J.M.; Price, K.R.; Ridout, C.L.; Wortley, G.M.; Hurrell, R.F.; Johnson, I.T. Saponins of quinoa (Chenopodium quinoa): Effects of processing on their abundance in quinoa products and their biological effects on intestinal mucosal tissue. JSFA 1993, 63, 201–209. [Google Scholar] [CrossRef]
- Haros, C.M.; Schönlechner, R. Pseudocereals: Chemistry and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Kårlund, A.; Paukkonen, I.; Gómez-Gallego, C.; Kolehmainen, M. Intestinal Exposure to Food-Derived Protease Inhibitors: Digestion Physiology- and Gut Health-Related Effects. Healthcare 2021, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Vorster, J.; van der Westhuizen, W.; du Plessis, G.; Marais, D.; Sparvoli, F.; Cominelli, E.; Camilli, E.; Ferrari, M.; Le Donne, C.; Marconi, S.; et al. In order to lower the antinutritional activity of serine protease inhibitors, we need to understand their role in seed development. Front. Plant Sci. 2023, 14, 1252223. [Google Scholar] [CrossRef]
- Gélinas, B.; Seguin, P. Oxalate in grain amaranth. J. Agric. Food Chem. 2007, 55, 4789–4794. [Google Scholar] [CrossRef] [PubMed]
- Siener, R.; Hönow, R.; Seidler, A.; Voss, S.; Hesse, A. Oxalate contents of species of the Polygonaceae, Amaranthaceae and Chenopodiaceae families. Food Chem. 2006, 98, 220–224. [Google Scholar] [CrossRef]
- Chai, W.; Liebman, M. Effect of Different Cooking Methods on Vegetable Oxalate Content. J. Agric. Food Chem. 2005, 53, 3027–3030. [Google Scholar] [CrossRef] [PubMed]
- Miyake, K.; Tanaka, T.; McNeil, P.L. Lectin-based food poisoning: A new mechanism of protein toxicity. PLoS ONE 2007, 2. [Google Scholar] [CrossRef] [PubMed]
- Hamid, L.L.; Al-Meani, S.A.L. Extraction and Purification of a Lectins from Iraqi Truffle (Terfezia sp.). Egypt J. Chem. 2021, 64, 2983–2987. [Google Scholar] [CrossRef]
- Pompeu, D.G.; Mattioli, M.A.; Ribeiro, R.; Gonçalves, D.B.; Magalhães, J.; Marangoni, S.; Da Silva, J.A.; Granjeiro, P.A. Purification, partial characterization and antimicrobial activity of Lectin from Chenopodium quinoa seeds. Food Sci. Technol. 2015, 35, 696–703. [Google Scholar] [CrossRef]
- Valadez-Vega, C.; Lugo-Magaña, O.; Figueroa-Hernández, C.; Bautista, M.; Betanzos-Cabrera, G.; Bernardino-Nicanor, A.; González-Amaro, R.M.; Alonso-Villegas, R.; Morales-González, J.A.; González-Cruz, L. Effects of Germination and Popping on the Anti-Nutritional Compounds and the Digestibility of Amaranthus hypochondriacus Seeds. Foods 2022, 11, 2075. [Google Scholar] [CrossRef] [PubMed]
- Clements, W.T.; Lee, S.R.; Bloomer, R.J. Nitrate Ingestion: A Review of the Health and Physical Performance Effects. Nutrients 2014, 6, 5224–5264. [Google Scholar] [CrossRef] [PubMed]
- Srujana, M.N.S.; Kumari, B.A.; Maheswari, K.U.; Devi, K.B.S.; Suneetha, W.J. Antioxidant and Anti Nutritional Composition of Germinated Quinoa (Chenopodium quinoa Willd). Curr. J. Appl. Sci. Technol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Oleszek, W.; Junkuszew, M.; Stochmal, A. Determination and Toxicity of Saponins from Amaranthus cruentus Seeds. J. Agric. Food Chem. 1999, 47, 3685–3687. [Google Scholar] [CrossRef] [PubMed]
- Sriraj, P.; Toomsan, B.; Butnan, S. Effects of Neem Seed Extract on Nitrate and Oxalate Contents in Amaranth Fertilized with Mineral Fertilizer and Cricket Frass. Horticulturae 2022, 8, 898. [Google Scholar] [CrossRef]
- Kumari, S.; Bhinder, S.; Singh, B.; Kaur, A. Physicochemical properties, non-nutrients and phenolic composition of germinated freeze-dried flours of foxtail millet, proso millet and common buckwheat. J. Food Compos. Anal. 2023, 115, 105043. [Google Scholar] [CrossRef]
- Dunaevsky, Y.E.; Pavlukova, E.B.; Belozersky, M.A. Isolation and properties of anionic protease inhibitors from buckwheat seeds. Biochem. Mol. Biol. Int. 1996, 40, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Tsybina, T.A.; Dunaevsky, Y.E.; Musolyamov, A.K.; Egorov, T.A.; Belozersky, M.A. Cationic inhibitors of serine proteinases from buckwheat seeds. Biochemistry 2001, 66, 941–947. [Google Scholar] [CrossRef]
- Dey, S.; Saxena, A.; Kumar, Y.; Maity, T.; Tarafdar, A. Understanding the Antinutritional Factors and Bioactive Compounds of Kodo Millet (Paspalum scrobiculatum) and Little Millet (Panicum sumatrense). J. Food Qual. 2022, 2022, 1578448. [Google Scholar] [CrossRef]
- Udeh, H.O.; Duodu, K.G.; Jideani, A.I.O. Effect of malting period on physicochemical properties, minerals, and phytic acid of finger millet (Eleusine coracana) flour varieties. Food Sci. Nutr. 2018, 6, 1858–1869. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A. Influence of Oxalate, Phytate, Tannin, Dietary Fiber, and Cooking on Calcium Bioavailability of Commonly Consumed Cereals and Millets in India. Cereal Chem. 2015, 92, 389–394. [Google Scholar] [CrossRef]
- Baye, K. Teff: Nutrient composition and health benefits. Int. Food Policy Res. Inst. 2014, 67, 1–17. [Google Scholar]
- Karaçoban, İ.; Bilgiçli, N.; Yaver, E. Impact of Fermentation, Autoclaving and Phytase Treatment on the Antioxidant Properties and Quality of Teff Cookies. Food Technol. Biotechnol. 2023, 61, 328. [Google Scholar] [CrossRef]
- Kataria, A.; Sharma, S.; Dar, B.N. Changes in phenolic compounds, antioxidant potential and antinutritional factors of Teff (Eragrostis tef) during different thermal processing methods. Int. J. Food Sci. Technol. 2021, 57, 6893–6902. [Google Scholar] [CrossRef]
- Tiruneh, R. Minerals and Oxalate content of feed and water in relation with ruminant urolithiasis in Adea district, central Ethiopia. Rev. Med. Vet. 2004, 155, 272–277. [Google Scholar]
- Kataria, A.; Sharma, S.; Singh, A.; Singh, B. Effect of hydrothermal and thermal processing on the antioxidative, antinutritional and functional characteristics of Salvia hispanica. J. Food Meas. Charact. 2022, 16, 332–343. [Google Scholar] [CrossRef]
- Rayan, A.M. EHE Effects of dehulling, soaking, and cooking on the nutritional quality of Moringa oleifera seeds. J. Agroaliment. Process. Technol. 2016, 22, 156–165. [Google Scholar]
- Saa, R.W.; Fombang Nig, E.; Radha, C.; Ndjantou, E.B.; Njintang Yanou, N. Effect of soaking, germination, and roasting on the proximate composition, antinutrient content, and some physicochemical properties of defatted Moringa oleifera seed flour. J. Food Process Preserv. 2022, 46, e16329. [Google Scholar] [CrossRef]
- Aloo, S.O.; Kwame, F.O.; Oh, D.H. Identification of possible bioactive compounds and a comparative study on in vitro biological properties of whole hemp seed and stem. Food Biosci. 2023, 51, 102329. [Google Scholar] [CrossRef]
- Alonso-Esteban, J.I.; Torija-Isasa, M.E.; de Sánchez-Mata, M.C. Mineral elements and related antinutrients, in whole and hulled hemp (Cannabis sativa L.) seeds. J. Food Compos. Anal. 2022, 109, 104516. [Google Scholar] [CrossRef]
- Zhang, M.; Li, T.; Guo, G.; Liu, Z.; Hao, N. Production of Nattokinase from Hemp Seed Meal by Solid-State Fermentation and Improvement of Its Nutritional Quality. Fermentation 2023, 9, 469. [Google Scholar] [CrossRef]
- Bouyahya Idrissi, O. Analysis of nutrient and antinutrient content of the truffle (Tirmania pinoyi) from Morocco. Int. Food Res. J. 2018, 25, 174–178. [Google Scholar]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2023; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2023—Revealing the True Cost of Food to Transform Agrifood Systems; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Hendrawan, V.S.A.; Komori, D.; Kim, W. Possible factors determining global-scale patterns of crop yield sensitivity to drought. PLoS ONE 2023, 18, e0281287. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, B.; Wang, Y.; Li, W.; He, D.; Zhang, Y.; Zhang, X.; Xing, X. Emerging natural hemp seed proteins and their functions for nutraceutical applications. Food Sci. Hum. Wellness 2023, 12, 929–941. [Google Scholar] [CrossRef]
- López, D.N.; Galante, M.; Robson, M.; Boeris, V.; Spelzini, D. Amaranth, quinoa and chia protein isolates: Physicochemical and structural properties. Int. J. Biol. Macromol. 2018, 109, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Le, T.D.; Suttikhana, I. Stability and bioactivity of peptides in food matrices based on processing conditions. Food Res. Int. 2023, 168, 112786. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, Q.; Yang, Y.; Zhong, H. The effects of high-pressure treatment on the structure, physicochemical properties and digestive property of starch—A review. Int. J. Biol. Macromol. 2023, 244, 125376. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; Tiwari, B.K. Innovative extraction technologies for high-value compounds. In Pulse Foods: Processing, Quality and Nutraceutical Applications; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Taco, V.; Savarino, P.; Benali, S.; Villacrés, E.; Raquez, J.M.; Gerbaux, P.; Duez, P.; Nachtergael, A. Deep eutectic solvents for the extraction and stabilization of Ecuadorian quinoa (Chenopodium quinoa Willd.) saponins. J. Clean. Prod. 2022, 363, 132609. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Z.; Zheng, C.; Liu, C. A comprehensive review of bioactive compounds and processing technology of sesame seed. Oil Crop Sci. 2022, 7, 88–94. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Rashwan, A.K.; Osman, A.I. Potential food applications and biological activities of fermented quinoa: A review. Trends Food Sci. Technol. 2024, 144, 104339. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, K.; Ahmed, N.; Chauhan, D.; Rizvi, Q.U.E.H.; Jan, S.; Singh, T.P.; Dhaliwal, H.S. Effect of soaking and germination treatments on nutritional, anti-nutritional, and bioactive properties of amaranth (Amaranthus hypochondriacus L.), quinoa (Chenopodium quinoa L.), and buckwheat (Fagopyrum esculentum L.). Curr. Res. Food Sci. 2021, 4, 917–925. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, C.T.S.; Mameri, H.; Menezes, N.S.; Carvalho, C.W.P.; Queiroz, V.A.V.; Cameron, L.C.; Morel, M.-H.; Takeiti, C.Y.; Ferreira, M.S.L. Effect of extrusion and turmeric addition on phenolic compounds and kafirin properties in tannin and tannin-free sorghum. Food Res. Int. 2021, 149, 110663. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Gujral, H.S. Influence of nutritional and antinutritional components on dough rheology and in vitro protein & starch digestibility of minor millets. Food Chem. 2019, 299, 125115. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.-Q.; Gao, H.; Song, L.-M.; Tao, F.-Y.; Ji, X.-Y.; Yu, Y.; Cao, Y.; Tang, S.; Xue, P. Optimization of green deep eutectic solvent (DES) extraction of Chenopodium quinoa Willd. husks saponins by response surface methodology and their antioxidant activities. RSC Adv. 2023, 13, 29408–29418. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, W.; Xu, Q.; Reddy, M.B. Neuroprotection of phytic acid in Parkinson’s and Alzheimer’s disease. J. Funct. Foods 2023, 110, 105856. [Google Scholar] [CrossRef]
- Paes, L.T.; D’Almeida, C.T.d.S.; do Carmo, M.A.V.; da Silva Cruz, L.; Bubula de Souza, A.; Viana, L.M.; Gonçalves Maltarollo, V.; Martino, H.S.D.; Domingues de Almeida Lima, G.; Larraz Ferreira, M.S.; et al. Phenolic-rich extracts from toasted white and tannin sorghum flours have distinct profiles influencing their antioxidant, antiproliferative, anti-adhesive, anti-invasive, and antimalarial activities. Food Res. Int. 2024, 176, 113739. [Google Scholar] [CrossRef] [PubMed]
- McDonell, E. La despensa nacional: Quinoa and the Spatial Contradictions of Peru’s Gastronomic Revolution. Lat. Am. Res. Rev. 2023, 59, 84–104. [Google Scholar] [CrossRef]
- Córdoba-Cerón, D.M.; Carranza-Saavedra, D.; Roa-Acosta, D.F.; Hoyos-Concha, J.L.; Solanilla-Duque, J.F. Physical and culinary analysis of long gluten-free extruded pasta based on high protein quinoa flour. Front. Sustain. Food Syst. 2022, 6, 1017324. [Google Scholar] [CrossRef]
- Badia-Olmos, C.; Sánchez-García, J.; Laguna, L.; Zúñiga, E.; Mónika Haros, C.; Maria Andrés, A.; Tarrega, A. Flours from fermented lentil and quinoa grains as ingredients with new techno-functional properties. Food Res. Int. 2024, 177, 113915. [Google Scholar] [CrossRef]
- Kierulf, A.; Whaley, J.; Liu, W.; Enayati, M.; Tan, C.; Perez-Herrera, M.; You, Z.; Abbaspourrad, A. Protein content of amaranth and quinoa starch plays a key role in their ability as Pickering emulsifiers. Food Chem. 2020, 315, 126246. [Google Scholar] [CrossRef]
- Yalcin, S.; Atik, İ.; Atik, A. Effects of chia flour as a fat substitute on the physicochemical, nutritional and sensory properties of biscuits. Int. J. Food Sci. Technol. 2023, 58, 3760–3768. [Google Scholar] [CrossRef]
- Momchilova, M.M.; Gradinarska-Ivanova Yordanov, D.G.; Zsivanovits, G.I. Microstructure and technological properties of cooked meat sausages prepared with emulsions of vegetable oils as substitutes for animal fat. Food Res. 2023, 7, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Chang, L.; Ohm, J.B.; Chen, B.; Rao, J. Structural, functional properties, and volatile profile of hemp protein isolate as affected by extraction method: Alkaline extraction-isoelectric precipitation vs. salt extraction. Food Chem. 2023, 405, 135001. [Google Scholar] [CrossRef] [PubMed]
- Zahari, I.; Ferawati, F.; Helstad, A.; Ahlström, C.; Östbring, K.; Rayner, M.; Purhagen, J.K. Development of High-Moisture Meat Analogues with Hemp and Soy Protein Using Extrusion Cooking. Foods 2020, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- George, T.T.; Oyenihi, A.B.; Rautenbach, F.; Obilana, A.O. Characterization of Moringa oleifera Leaf Powder Extract Encapsulated in Maltodextrin and/or Gum Arabic Coatings. Foods 2021, 10, 3044. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Dias, T.; Mouazen, A.M.; Cruz, C. Using Science and Technology to Unveil the Hidden Delicacy Terfezia arenaria, a Desert Truffle. Foods 2023, 12, 3527. [Google Scholar] [CrossRef] [PubMed]
- Agregán, R.; Guzel, N.; Guzel, M.; Bangar, S.P.; Zengin, G.; Kumar, M.; Lorenzo, J.M. The Effects of Processing Technologies on Nutritional and Anti-nutritional Properties of Pseudocereals and Minor Cereal. Food Bioproc. Technol. 2023, 16, 961–986. [Google Scholar] [CrossRef]
- Bruno, M.C. Quinoa: Origins and Development. In Encyclopedia of Global Archaeology; Smith, C., Ed.; Springer: New York, NY, USA, 2014; pp. 6215–6220. [Google Scholar] [CrossRef]
- Tanwar, B.; Goyal, A.; Irshaan, S.; Kumar, V.; Sihag, M.K.; Patel, A.; Kaur, I. Whole Grains and Their Bioactives; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Zettel, V.; Hitzmann, B. Applications of chia (Salvia hispanica L.) in food products. Trends Food Sci. Technol. 2018, 80, 43–50. [Google Scholar] [CrossRef]
- Levent, H. Effect of partial substitution of gluten-free flour mixtures with chia (Salvia hispanica L.) flour on quality of gluten-free noodles. J. Food Sci. Technol. 2017, 54, 1971–1978. [Google Scholar] [CrossRef]
- Zettel, V.; Hitzmann, B. Chia (Salvia hispanica L.) as fat replacer in sweet pan breads. Int. J. Food Sci. Technol. 2016, 51, 1425–1432. [Google Scholar] [CrossRef]
- Coelho, M.S.; de las Salas-Mellado, M.M. Effects of substituting chia (Salvia hispanica L.) flour or seeds for wheat flour on the quality of the bread. LWT Food Sci. Technol. 2015, 60, 729–736. [Google Scholar] [CrossRef]
- Felisberto, M.H.F.; Wahanik, A.L.; Gomes-Ruffi, C.R.; Clerici, M.T.P.S.; Chang, Y.K.; Steel, C.J. Use of chia (Salvia hispanica L.) mucilage gel to reduce fat in pound cakes. LWT Food Sci. Technol. 2015, 63, 1049–1055. [Google Scholar] [CrossRef]
- Senna, C.; Soares, L.; Egea, M.B.; Fernandes, S.S. The Techno-Functionality of Chia Seed and Its Fractions as Ingredients for Meat Analogs. Molecules 2024, 29, 440. [Google Scholar] [CrossRef]
- Fernández-López, J.; Viuda-Martos, M.; Sayas-Barberá, M.E.; de Vera, C.N.-R.; Lucas-González, R.; Roldán-Verdú, A.; Botella-Martínez, C.; Pérez-Alvarez, J.A. Chia, quinoa, and their coproducts as potential antioxidants for the meat industry. Plants 2020, 9, 359. [Google Scholar] [CrossRef]
- Nehra, M.; Gahlawat, S.K. Chia and Quinoa: Superfoods for Health; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Arendt, E.K.; Zannini, E. 13—Amaranth. In Cereal Grains for the Food and Beverage Industries; Arendt, E.K., Zannini, E., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 439–473. [Google Scholar] [CrossRef]
- Bvenura, C.; Kambizi, L. Future grain crops. In Future Foods: Global Trends, Opportunities, and Sustainability Challenges; Academic Press: Cambridge, MA, USA, 2022; pp. 81–105. [Google Scholar] [CrossRef]
- Ma, Z.F.; Sun, Q.; Yap, P.S.; Khoo, H.E. Moringa: Phytopharmacological Properties and Its Potential as a Functional Food Ingredient. Sustain. Food Sci. Compr. Approach 2023, 1–4, 102–106. [Google Scholar] [CrossRef]
- Wong, S.E.; Illingworth, K.A.; Siow, L.F. Moringa Proteins: Nutrition, Functionality, and Applications. In Sustainable Protein Sources; Academic Press: Cambridge, MA, USA, 2024; pp. 493–513. [Google Scholar] [CrossRef]
- D’Auria, G.; Nitride, C.; Ferranti, P. Moringa oleifera Lam. Proteins: Properties and Food Applications. Sustain. Food Sci. Compr. Approach 2023, 1–4, 89–101. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Ioactive Components in Moringa oleifera Leaves Protect against Chronic Disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef]
- Milla, P.G.; Peñalver, R.; Nieto, G. Health Benefits of Uses and Applications of Moringa oleifera in Bakery Products. Plants 2021, 10, 318. [Google Scholar] [CrossRef]
- Ibrahim, I.; Eldi, E.; Amir, A.; Bunyamin, B. Moderation of Good Corporate Governance: Earnings Management and Firm Value Against Company Performance. Atestasi J. Ilm. Akunt. 2022, 5, 496–510. [Google Scholar] [CrossRef]
- Mohamed, F.A.E.-F.; Salama, H.H.; El-Sayed, S.M.; El-Sayed, H.S.; Zahran, H.A.-H. Utilization of Natural Antimicrobial and Antioxidant of Moringa oleifera Leaves Extract in Manufacture of Cream Cheese; CABI: Wallingford, UK, 2019. [Google Scholar]
- Mashau, M.E.; Munandi, M.; Ramashia, S.E. Exploring the influence of Moringa oleifera leaves extract on the nutritional properties and shelf life of mutton patties during refrigerated storage. CyTA J. Food 2021, 19, 389–398. [Google Scholar] [CrossRef]
- Cardines, P.H.F.; Baptista, A.T.A.; Gomes, R.G.; Bergamasco, R.; Vieira, A.M.S. Moringa oleifera seed extracts as promising natural thickening agents for food industry: Study of the thickening action in yogurt production. LWT 2018, 97, 39–44. [Google Scholar] [CrossRef]
- Ogunsina, B.S.; Radha, C.; Indrani, D. Quality characteristics of bread and cookies enriched with debittered Moringa oleifera seed flour. Int. J. Food Sci. Nutr. 2011, 62, 185–194. [Google Scholar] [CrossRef]
- Coello, K.E.; Peñas, E.; Martinez-Villaluenga, C.; Cartea, M.E.; Velasco, P.; Frias, J. Pasta products enriched with moringa sprout powder as nutritive dense foods with bioactive potential. Food Chem. 2021, 360, 130032. [Google Scholar] [CrossRef]
- Aoki, H.; Nakatsuka-Mori, T.; Ueno, Y.; Nabeshima, Y.; Oyama, H. Analysis of functional ingredients of tempe-like fermented Moringa oleifera seeds (Moringa tempe) prepared with Rhizopus species. J. Biosci. Bioeng. 2023, 135, 306–312. [Google Scholar] [CrossRef]
- Bankole, M.; Bodjrènou, S.; Honfo, F.; Codo, G.; Bodecker, J.; Termote, C.; Chadare, F. Hounkpatin W.A.Valorization of Vigna radiata (L.) Wilczek. and Moringa oleifera to improve food recipes of 6–23-month-old children in northern Benin. J. Agric. Food Res. 2023, 13, 100639. [Google Scholar] [CrossRef]
- Affonfere, M.; Madode, Y.E.; Chadare, F.J.; Azokpota, P.; Hounhouigan, D.J. A dual food-to-food fortification with moringa (Moringa oleifera Lam.) leaf powder and baobab (Adansonia digitata L.) fruit pulp increases micronutrients solubility in sorghum porridge. Sci. Afr. 2022, 16, e01264. [Google Scholar] [CrossRef]
- Anberbir, S.M.; Satheesh, N.; Abera, A.A.; Kassa, M.G.; Tenagashaw, M.W.; Asres, D.T.; Tiruneh, A.T.; Habtu, T.A.; Sadik, J.A.; Wudineh, T.A.; et al. Evaluation of nutritional composition, functional and pasting properties of pearl millet, teff, and buckwheat grain composite flour. Appl. Food Res. 2024, 4, 100390. [Google Scholar] [CrossRef]
- Kumari, S.; Singh, B.; Kaur, A. Influence of malted buckwheat, foxtail and proso millet flour incorporation on the physicochemical, protein digestibility and antioxidant properties of gluten-free rice cookies. Food Chem. Adv. 2023, 3, 100557. [Google Scholar] [CrossRef]
- Nalbandian, E.; Karkle, E.; Ganjyal, G.M. Physicochemical properties of buckwheat flours and their influence on focaccia bread quality. Cereal Chem. 2024, 101, 220–230. [Google Scholar] [CrossRef]
- Auyeskhan, U.; Azgbagambetov, A.; Sadykov, T.; Dairabayeva, D.; Talamona, D.; Chan, M.Y. Reducing meat consumption in Central Asia through 3D printing of plant-based protein-enhanced alternatives—A mini review. Front. Nutr. 2024, 10, 1308836. [Google Scholar] [CrossRef]
- Passi, P.; Kaur, K.; Kaur, J.; Kaul, S. Quality characterization, bread making properties and storage stability of foxtail millet incorporated bread ready-mix. J. Stored Prod. Res. 2023, 104, 102208. [Google Scholar] [CrossRef]
- Divyashree, S.; Ramu, R.; Sreenivasa, M.Y. Evaluation of new candidate probiotic lactobacillus strains isolated from a traditional fermented food—Multigrain-millet dosa batter. Food Biosci. 2024, 57, 103450. [Google Scholar] [CrossRef]
- Rani, M.; Dabur, R.S.; Bishnoi, S.; Jairath, G. Influence of storage on physico-chemical physiognomies of fermented whey cereal (pearl millet and moth bean) beverage. J. Food Sci. Technol. 2024, 61, 117–128. [Google Scholar] [CrossRef]
- Naumenko, O.; Hetman, I.; Chyzh, V.; Gunko, S.; Bal-Prylypko, L.; Bilko, M.; Tsentylo, L.; Lialyk, A.; Ivanytska, A.; Liashenko, S. Improving the Quality of Wheat Bread by Enriching Teff Flour. East.-Eur. J. Enterp. Technol. 2023, 3, 33–41. [Google Scholar] [CrossRef]
- Hasani, Z.; Hosseini, H.; Shojaee-Aliabadi, S.; Moslehishad, M. Effects of Replacing Breadcrumbs and Soy Protein with Teff (Eragrostis tef) Flour on Physicochemical and Sensory Characteristics of Typical Hamburgers. Iran. J. Nutr. Sci. Food Technol. 2023, 18, 55–63. [Google Scholar]
- Kebede Ali, M.; Abera, S.; Neme Tolesa, G. Optimization of extrusion cooking process parameters to develop teff (Eragrostis (Zucc) Trotter)-based products: Physical properties, functional properties, and sensory quality. Cogent Food Agric. 2023, 9, 2279705. [Google Scholar] [CrossRef]
- Burton, R.A.; Andres, M.; Cole, M.; Cowley, J.M.; Augustin, M.A. Industrial hemp seed: From the field to value-added food ingredients. J. Cannabis Res. 2022, 4, 45. [Google Scholar] [CrossRef]
- Korus, J.; Witczak, M.; Ziobro, R.; Juszczak, L. Hemp (Cannabis sativa subsp. sativa) flour and protein preparation as natural nutrients and structure forming agents in starch based gluten-free bread. LWT 2017, 84, 143–150. [Google Scholar] [CrossRef]
- Ertaş, N.; Aslan, M. Antioxidant and physicochemical properties of cookies containing raw and roasted hemp flour. Acta Sci. Pol. Technol. Aliment. 2020, 19, 177–184. [Google Scholar] [CrossRef]
- Nissen, L.; di Carlo, E.; Gianotti, A. Prebiotic potential of hemp blended drinks fermented by probiotics. Food Res. Int. 2020, 131, 109029. [Google Scholar] [CrossRef]
- Norajit, K.; Gu, B.-J.; Ryu, G.-H. Effects of the addition of hemp powder on the physicochemical properties and energy bar qualities of extruded rice. Food Chem. 2011, 129, 1919–1925. [Google Scholar] [CrossRef]
- Benucci, G.M.N.; Bonito, G.; Falini, L.B.; Bencivenga, M.; Donnini, D. Truffles, timber, food, and fuel: Sustainable approaches for multi-cropping truffles and economically important plants. In Edible Ectomycorrhizal Mushrooms: Current Knowledge and Future Prospects; Springer: Berlin/Heidelberg, Germany, 2012; pp. 265–280. [Google Scholar]
- Liu, H.; Lv, M.; Peng, Q.; Shan, F.; Wang, M. Physicochemical and textural properties of tartary buckwheat starch after heat–moisture treatment at different moisture levels. Starch Stärke 2015, 67, 276–284. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, H.; Wei, T.; Shen, J.; Wang, M. Differences in physicochemical properties and in vitro digestibility between tartary buckwheat flour and starch modified by heat-moisture treatment. LWT 2017, 86, 285–292. [Google Scholar] [CrossRef]
- Almeida, R.L.J.; Santos, N.C.; Feitoza, J.V.F.; da Silva, G.M.; de Muniz, C.E.S.; da Eduardo, R.S.; de Ribeiro, V.H.A.; de Silva, V.M.A.; de Mota, M.M.A. Effect of heat-moisture treatment on the thermal, structural and morphological properties of Quinoa starch. Carbohydr. Polym. Technol. Appl. 2022, 3, 100192. [Google Scholar] [CrossRef]
- Dong, J.; Huang, L.; Chen, W.; Zhu, Y.; Dun, B.; Shen, R. Effect of Heat-Moisture Treatments on Digestibility and Physicochemical Property of Whole Quinoa Flour. Foods 2021, 10, 3042. [Google Scholar] [CrossRef]
- Vicente, A.; Villanueva, M.; Caballero, P.A.; Muñoz, J.M.; Ronda, F. Buckwheat grains treated with microwave radiation: Impact on the techno-functional, thermal, structural, and rheological properties of flour. Food Hydrocoll. 2023, 137, 108328. [Google Scholar] [CrossRef]
- Vicente, A.; Villanueva, M.; Caballero, P.A.; Lazaridou, A.; Biliaderis, C.G.; Ronda, F. Flours from microwave-treated buckwheat grains improve the physical properties and nutritional quality of gluten-free bread. Food Hydrocoll. 2024, 149, 109644. [Google Scholar] [CrossRef]
- Vicente, A.; Villanueva, M.; Caballero, P.A.; Muñoz, J.M.; Ronda, F. Microwave Modification of Quinoa Grains at Constant and Varying Water Content Modulates Changes in Structural and Physico-Chemical Properties of the Resulting Flours. Foods 2023, 12, 1421. [Google Scholar] [CrossRef]
- Liu, H.; Guo, X.; Li, W.; Wang, X.; Lv, M.; Peng, Q.; Wang, M. Changes in physicochemical properties and in vitro digestibility of common buckwheat starch by heat-moisture treatment and annealing. Carbohydr. Polym. 2015, 132, 237–244. [Google Scholar] [CrossRef]
- Siwatch, M.; Yadav, R.B.; Yadav, B.S. Annealing and heat-moisture treatment of amaranth starch: Effect on structural, pasting, and rheological properties. J. Food Meas. Charact. 2022, 16, 2323–2334. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Villanueva, M.; Ronda, F. Impact of lipids on the functional, rheological, pasting and thermal properties of ultrasound-processed canary seed flours. Food Hydrocoll. 2024, 150, 109727. [Google Scholar] [CrossRef]
- Ahmed, J.; Thomas, L.; Arfat, Y.A.; Joseph, A. Rheological, structural and functional properties of high-pressure treated quinoa starch in dispersions. Carbohydr. Polym. 2018, 197, 649–657. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Cao, R.; Fan, H.; Wang, M. In vitro digestibility and changes in physicochemical and structural properties of common buckwheat starch affected by high hydrostatic pressure. Carbohydr. Polym. 2016, 144, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, F. Effect of high pressure on rheological and thermal properties of quinoa and maize starches. Food Chem. 2018, 241, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Vallons, K.J.R.; Ryan, L.A.M.; Arendt, E.K. Promoting structure formation by high pressure in gluten-free flours. LWT Food Sci. Technol. 2011, 44, 1672–1680. [Google Scholar] [CrossRef]
- Gutiérrez, Á.L.; Rico, D.; Ronda, F.; Martín-Diana, A.B.; Caballero, P.A. Development of a gluten-free whole grain flour by combining soaking and high hydrostatic pressure treatments for enhancing functional, nutritional and bioactive properties. J. Cereal Sci. 2022, 105, 103458. [Google Scholar] [CrossRef]
- Newman, T.V.; San-Juan-Rodriguez, A.; Parekh, N.; Swart, E.C.S.; Klein-Fedyshin, M.; Shrank, W.H.; Hernández, I. Impact of community pharmacist-led interventions in chronic disease management on clinical, utilization, and economic outcomes: An umbrella review. Res. Social. Adm. Pharm. 2020, 16, 1155–1165. [Google Scholar] [CrossRef]
- Jan, N.; Hussain, S.Z.; Naseer, B.; Bhat, T.A. Amaranth and quinoa as potential nutraceuticals: A review of anti-nutritional factors, health benefits and their applications in food, medicinal and cosmetic sectors. Food Chem. X 2023, 18, 100687. [Google Scholar] [CrossRef]
- Cox, A.L.; Eigenmann, P.A.; Sicherer, S.H. Clinical Relevance of Cross-Reactivity in Food Allergy. J. Allergy Clin. Immunol. Pract. 2021, 9, 82–99. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Zhou, N.; Shen, Y.; Li, B.; Chen, B.E.; Li, X. Association Between Omega-3 Fatty Acid Intake and Dyslipidemia: A Continuous Dose-Response Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2023, 12, e029512. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Su, X.; Lu, G.; Ye, L.; Shi, R.; Zhu, M.; Yu, X.; Li, Z.; Jia, X.; Feng, L. Moringa oleifera Lam.: A comprehensive review on active components, health benefits and application. RSC Adv. 2023, 13, 24353–24384. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Ghafoor, K.; Ahmed, I.A.M.; Babiker, E.E.; Özcan, M.M. Comparative study of mineral and oxidative status of Sonchus oleraceus, Moringa oleifera and Moringa peregrina leaves. J. Food Meas. Charact. 2017, 11, 1745–1751. [Google Scholar] [CrossRef]
- Do, B.H.; Hoang, N.S.; Nguyen, T.P.T.; Ho, N.Q.C.; Le, T.L.; Doan, C.C. Phenolic Extraction of Moringa oleifera Leaves Induces Caspase-Dependent and Caspase-Independent Apoptosis through the Generation of Reactive Oxygen Species and the Activation of Intrinsic Mitochondrial Pathway in Human Melanoma Cells. Nutr. Cancer 2021, 73, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Kerdsomboon, K.; Chumsawat, W.; Auesukaree, C. Effects of Moringa oleifera leaf extracts and its bioactive compound gallic acid on reducing toxicities of heavy metals and metalloid in Saccharomyces cerevisiae. Chemosphere 2021, 270, 128659. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bao, Y.; Zhang, C.; Zhan, L.; Khan, W.; Siddiqua, S.; Ahmad, S.; Capanoglu, E.; Skalicka-Woźniak, K.; Zou, L. Bioactive components and anti-diabetic properties of Moringa oleifera Lam. Crit. Rev. Food Sci. Nutr. 2022, 62, 3873–3897. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ouyang, Q.; Chang, X.; Yang, M.; He, J.; Tian, Y.; Sheng, J. Anti-photoaging effects of flexible nanoliposomes encapsulated Moringa oleifera Lam. isothiocyanate in UVB-induced cell damage in HaCaT cells. Drug Deliv. 2022, 29, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.K.; Jarald, E.E.; Showkat, A.; Daud, A. In vitro evaluation of Moringa oleifera gum for colon-specific drug delivery. Int. J. Pharm. Investig. 2012, 2, 48–51. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hempseed in food industry: Nutritional value, health benefits, and industrial applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 282–308. [Google Scholar] [CrossRef] [PubMed]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef]
- Pojić, M.; Mišan, A.; Sakač, M.; Dapčević Hadnađev, T.; Šarić, B.; Milovanović, I.; Hadnađev, M. Characterization of byproducts originating from hemp oil processing. J. Agric. Food Chem. 2014, 62, 12436–12442. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, H.; Ollilainen, V.; Piironen, V.; Lampi, A.-M. Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats. J. Food Compos. Anal. 2008, 21, 152–161. [Google Scholar] [CrossRef]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Liu, Y.H.; Wang, B.; Chen, C.Y.; Zhang, H.M.; Kang, J.X. Identification of a sustainable two-plant diet that effectively prevents age-related metabolic syndrome and extends lifespan in aged mice. J. Nutr. Biochem. 2018, 51, 16–26. [Google Scholar] [CrossRef]
- Frankowski, J.; Przybylska-Balcerek, A.; Stuper-Szablewska, K. Concentration of Pro-Health Compound of Sorghum Grain-Based Foods. Foods 2022, 11, 216. [Google Scholar] [CrossRef]
- Amarakoon, D.; Lou, Z.; Lee, W.J.; Smolensky, D.; Lee, S.H. A mechanistic review: Potential chronic disease-preventive properties of sorghum. J. Sci. Food Agric. 2021, 101, 2641–2649. [Google Scholar] [CrossRef] [PubMed]
- Berenji, J.; Dahlberg, J.A. Perspectives of Sorghum in Europe. J. Agron. Crop Sci. 2004, 190, 332–338. [Google Scholar] [CrossRef]
- Luthar, Z.; Germ, M.; Likar, M.; Golob, A.; Vogel-Mikuš, K.; Pongrac, P.; Kušar, A.; Pravst, I.; Kreft, I. Breeding Buckwheat for Increased Levels of Rutin, Quercetin and Other Bioactive Compounds with Potential Antiviral Effects. Plants 2020, 9, 1638. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, D.; Turemko, M.; Kwiatkowski, J.; Zielinski, H. Evaluation of flavonoid contents and antioxidant capacity of the aerial parts of common and tartary buckwheat plants. Molecules 2012, 17, 9668–9682. [Google Scholar] [CrossRef]
- Kalinova, J.P.; Vrchotova, N.; Triska, J. Contribution to the study of rutin stability in the achenes of Tartary buckwheat (Fagopyrum tataricum). Food Chem. 2018, 258, 314–320. [Google Scholar] [CrossRef]
- Klepacka, J.; Najda, A. Effect of commercial processing on polyphenols and antioxidant activity of buckwheat seeds. Int. J. Food Sci. Technol. 2020. [CrossRef]
- Llanaj, E.; Ahanchi, N.S.; Dizdari, H.; Taneri, P.E.; Niehot, C.D.; Wehrli, F.; Khatami, F.; Raeisi-Dehkordi, H.; Kastrati, L.; Bano, A.; et al. Buckwheat and Cardiometabolic Health: A Systematic Review and Meta-Analysis. J. Pers. Med. 2022, 12, 1940. [Google Scholar] [CrossRef] [PubMed]
- Gebru, Y.A.; Sbhatu, D.B.; Kim, K.-P. Nutritional Composition and Health Benefits of Teff (Eragrostis tef (Zucc.) Trotter). J. Food Qual. 2020, 2020, 9595086. [Google Scholar] [CrossRef]
- Barretto, R.; Buenavista, R.M.; Rivera, J.L.; Wang, S.; Prasad, P.V.V.; Siliveru, K. Teff (Eragrostis tef) processing, utilization and future opportunities: A review. Int. J. Food Sci. Technol. 2021, 56, 3125–3137. [Google Scholar] [CrossRef]
- Santos-Sánchez, G.; Álvarez-López, A.I.; Ponce-España, E.; Carrillo-Vico, A.; Bollati, C.; Bartolomei, M.; Lammi, C.; Chamorro, I.C. Hempseed (Cannabis sativa) protein hydrolysates: A valuable source of bioactive peptides with pleiotropic health-promoting effects. Trends Food Sci. Technol. 2022, 127, 303–318. [Google Scholar] [CrossRef]
- Cruz-Chamorro, I.; Álvarez-Sánchez, N.; Santos-Sánchez, G.; Pedroche, J.; Fernández-Pachón, M.-S.; Millán, F.; Millán-Linares, M.C.; Lardone, P.J.; Bejarano, I.; Guerrero, J.M.; et al. Immunomodulatory and Antioxidant Properties of Wheat Gluten Protein Hydrolysates in Human Peripheral Blood Mononuclear Cells. Nutrients 2020, 12, 1673. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Chamorro, I.; Álvarez-Sánchez, N.; Álvarez-Ríos, A.I.; Santos-Sánchez, G.; Pedroche, J.; Millán, F.; Carrera Sánchez, C.; Fernández-Pachón, M.S.; Millán-Linares, M.C.; Martínez-López, A.; et al. Safety and Efficacy of a Beverage Containing Lupine Protein Hydrolysates on the Immune, Oxidative and Lipid Status in Healthy Subjects: An Intervention Study (the Lupine-1 Trial). Mol. Nutr. Food Res. 2021, 65, e2100139. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Chamorro, I.; Santos-Sánchez, G.; Álvarez-López, A.I.; Pedroche, J.; Lardone, P.J.; Arnoldi, A.; Carrillo-Vico, A. Pleiotropic biological effects of Lupinus spp. protein hydrolysates. Trends Food Sci. Technol. 2023, 133, 244–266. [Google Scholar] [CrossRef]
- Gonzalez, C.G. Climate Change, Food Security, and Agrobioversity: Toward a Just, Resilient, and Sustainable Food System. Fordham Environ. Law Rev. 2010, 22, 493–522. [Google Scholar]
- Singh, R.K.; Sreenivasulu, N.; Prasad, M. Potential of underutilized crops to introduce the nutritional diversity and achieve zero hunger. Funct. Integr. Genom. 2022, 22, 1459–1465. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Biondi, S.; Martínez, E.A.; Orsini, F.; Antognoni, F.; Jacobsen, S.E. Quinoa—A Model Crop for Understanding Salt-tolerance Mechanisms in Halophytes. Plant Biosyst. 2016, 150, 357–371. [Google Scholar] [CrossRef]
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa abiotic stress responses: A review. Plants 2018, 7, 106. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A.; et al. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron. Sustain. Dev. 2014, 34, 349–359. [Google Scholar] [CrossRef]
- Adolf, V.I.; Jacobsen, S.E.; Shabala, S. Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd. ). Environ. Exp. Bot. 2013, 92, 43–54. [Google Scholar] [CrossRef]
- Hussain, M.I.; Farooq, M.; Muscolo, A.; Rehman, A. Crop diversification and saline water irrigation as potential strategies to save freshwater resources and reclamation of marginal soils—A review. Environ. Sci. Pollut. Res. 2020, 27, 28695–28729. [Google Scholar] [CrossRef] [PubMed]
- Harisha, C.B.; Narayanpur, V.B.; Rane, J.; Ganiger, V.M.; Prasanna, S.M.; Vishwanath, Y.C.; Reddi, S.G.; Halli, H.M.; Boraiah, K.M.; Basavaraj, P.S.; et al. Promising Bioregulators for Higher Water Productivity and Oil Quality of Chia under Deficit Irrigation in Semiarid Regions. Plants 2023, 12, 662. [Google Scholar] [CrossRef] [PubMed]
- Zamani, Z.; Zeidali, E.; Alizadeh, H. Effect of drought stress and nitrogen chemical fertilizer on root properties and yield in three quinoa cultivars (Chenopodium quinoa Willd). Crop Sci. Res. Arid Reg. 2024, 5, 487–500. [Google Scholar] [CrossRef]
- Shabala, S.; Hariadi, Y.; Jacobsen, S.E. Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. J. Plant Physiol. 2013, 170, 906–914. [Google Scholar] [CrossRef]
- Sarkar, D.; Kar, S.K.; Chattopadhyay, A.; Rakshit, A.; Tripathi, V.K.; Dubey, P.K.; Abhilash, P.C. Low input sustainable agriculture: A viable climate-smart option for boosting food production in a warming world. Ecol. Indic. 2020, 115, 106412. [Google Scholar] [CrossRef]
- Haseeb, M.; Iqbal, S.; Hafeez, M.B.; Baloch, H.; Zahra, N.; Mumtaz, M.; Ahmad, G.; Fatima, E.M.; Raza, S.; Raza, A.; et al. Characterizing of heavy metal accumulation, translocation and yield response traits of Chenopodium quinoa. J. Agric. Food Res. 2023, 14, 100741. [Google Scholar] [CrossRef]
- Haller, H.; Pronoza, L.; Dyer, M.; Ahlgren, M.; Bergqvist, L.; Flores-Carmenate, G.; Jonsson, A. Phytoremediation of Heavy-Metal-Contaminated Soils: Capacity of Amaranth Plants to Extract Cadmium from Nutrient-Poor, Acidic Substrates. Challenges 2023, 14, 28. [Google Scholar] [CrossRef]
- Nazeer, S.; Yaman Firincioglu, S. Amaranth in Animal Nutrition. J. Agric. Food Environ. Anim. Sci. 2022, 3, 195–211. [Google Scholar]
- Aderibigbe, O.R.; Ezekiel, O.O.; Owolade, S.O.; Korese, J.K.; Sturm, B.; Hensel, O. Exploring the potentials of underutilized grain amaranth (Amaranthus spp.) along the value chain for food and nutrition security: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Ngugi, C.C.; Oyoo-Okoth, E.; Manyala, J.O.; Fitzsimmons, K.; Kimotho, A. Characterization of the nutritional quality of amaranth leaf protein concentrates and suitability of fish meal replacement in Nile tilapia feeds. Aquac. Rep. 2017, 5, 62–69. [Google Scholar] [CrossRef]
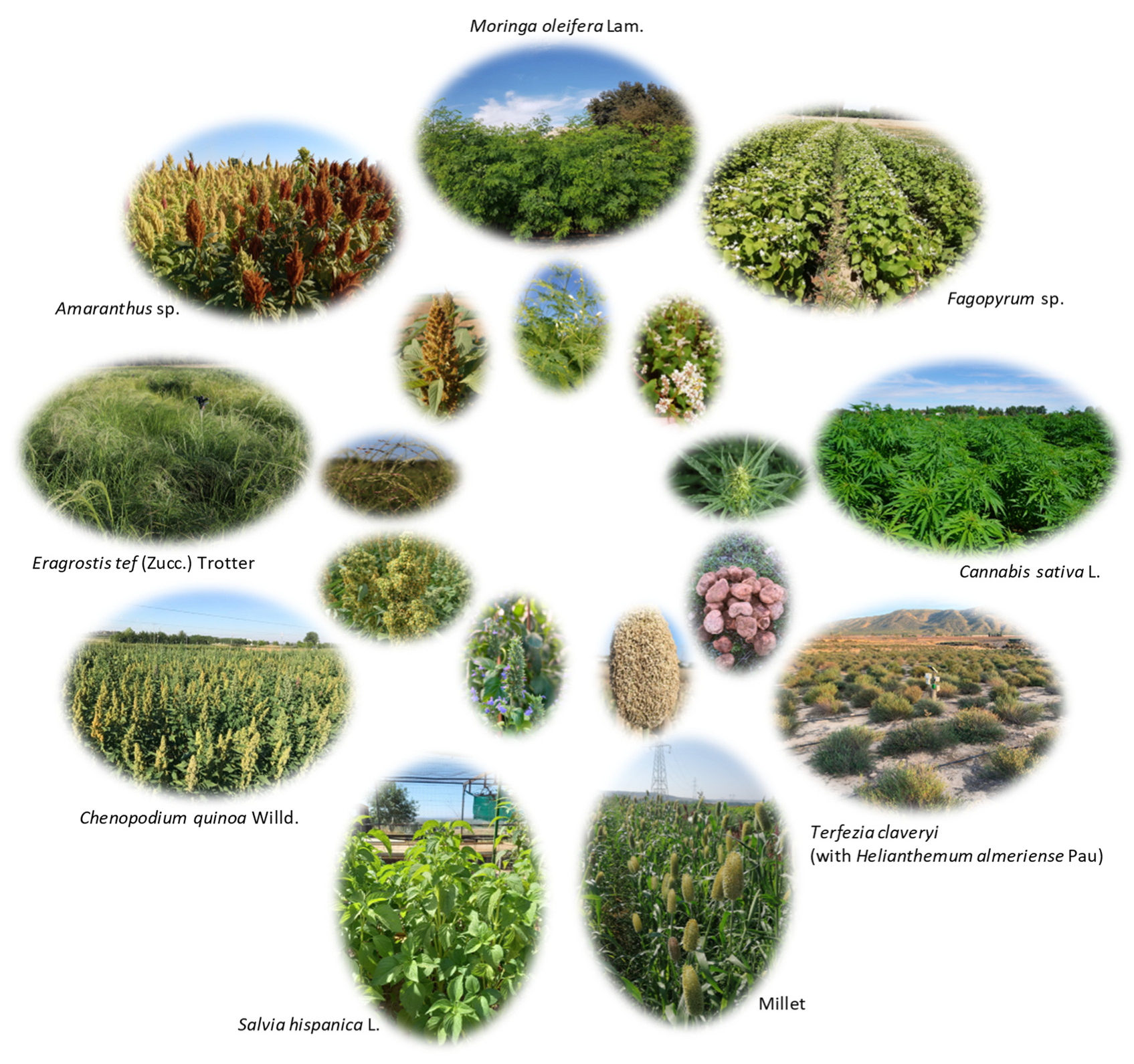
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matías, J.; Rodríguez, M.J.; Carrillo-Vico, A.; Casals, J.; Fondevilla, S.; Haros, C.M.; Pedroche, J.; Aparicio, N.; Fernández-García, N.; Aguiló-Aguayo, I.; et al. From ‘Farm to Fork’: Exploring the Potential of Nutrient-Rich and Stress-Resilient Emergent Crops for Sustainable and Healthy Food in the Mediterranean Region in the Face of Climate Change Challenges. Plants 2024, 13, 1914. https://doi.org/10.3390/plants13141914
Matías J, Rodríguez MJ, Carrillo-Vico A, Casals J, Fondevilla S, Haros CM, Pedroche J, Aparicio N, Fernández-García N, Aguiló-Aguayo I, et al. From ‘Farm to Fork’: Exploring the Potential of Nutrient-Rich and Stress-Resilient Emergent Crops for Sustainable and Healthy Food in the Mediterranean Region in the Face of Climate Change Challenges. Plants. 2024; 13(14):1914. https://doi.org/10.3390/plants13141914
Chicago/Turabian StyleMatías, Javier, María José Rodríguez, Antonio Carrillo-Vico, Joan Casals, Sara Fondevilla, Claudia Mónika Haros, Justo Pedroche, Nieves Aparicio, Nieves Fernández-García, Ingrid Aguiló-Aguayo, and et al. 2024. "From ‘Farm to Fork’: Exploring the Potential of Nutrient-Rich and Stress-Resilient Emergent Crops for Sustainable and Healthy Food in the Mediterranean Region in the Face of Climate Change Challenges" Plants 13, no. 14: 1914. https://doi.org/10.3390/plants13141914
APA StyleMatías, J., Rodríguez, M. J., Carrillo-Vico, A., Casals, J., Fondevilla, S., Haros, C. M., Pedroche, J., Aparicio, N., Fernández-García, N., Aguiló-Aguayo, I., Soler-Rivas, C., Caballero, P. A., Morte, A., Rico, D., & Reguera, M., on behalf of the NutriCrop Spanish Consortium/Group. (2024). From ‘Farm to Fork’: Exploring the Potential of Nutrient-Rich and Stress-Resilient Emergent Crops for Sustainable and Healthy Food in the Mediterranean Region in the Face of Climate Change Challenges. Plants, 13(14), 1914. https://doi.org/10.3390/plants13141914









