Functional, Chemical, and Phytotoxic Characteristics of Cestrum parqui L’Herit: An Overview
Abstract
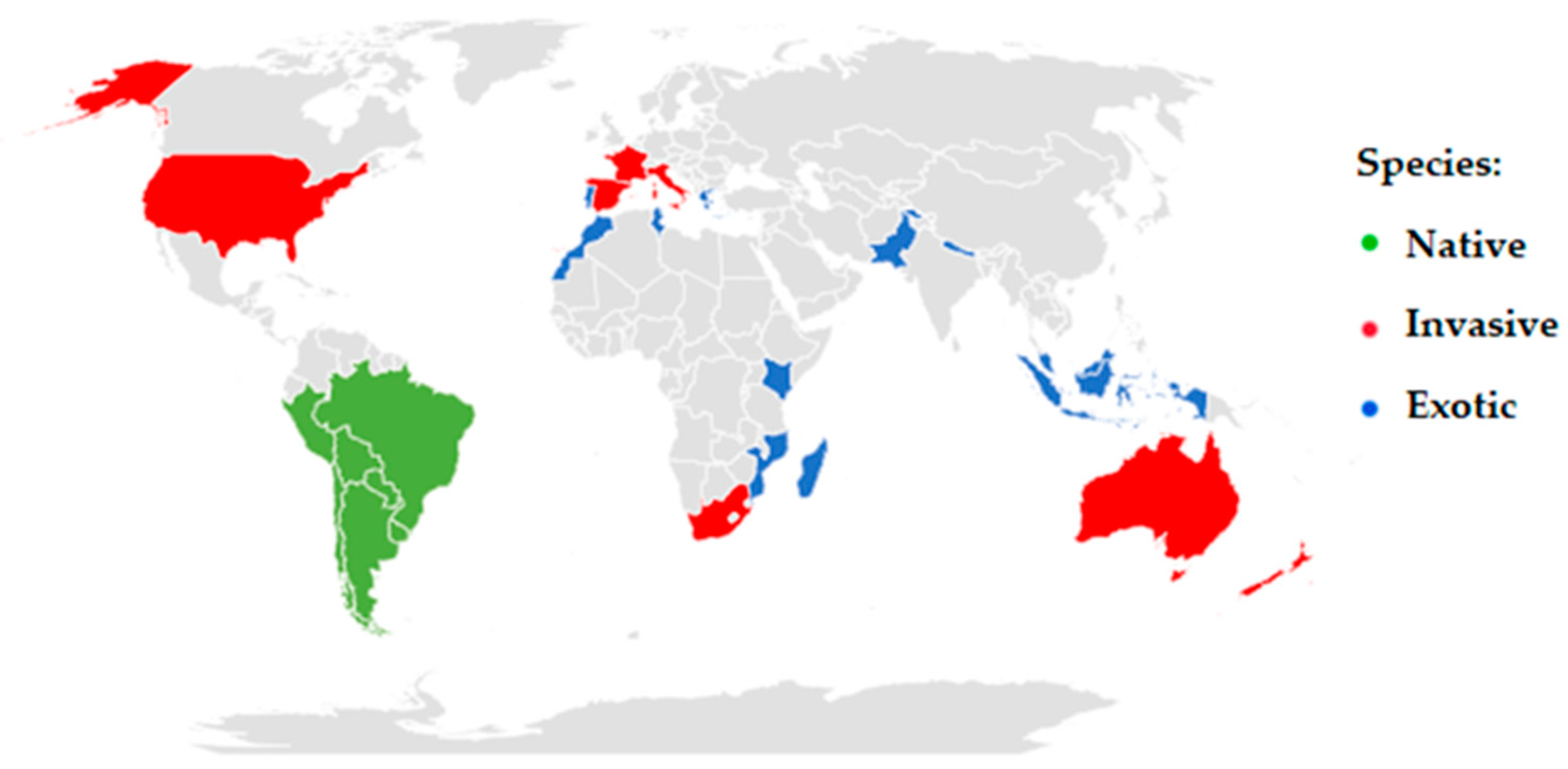
:1. Introduction
2. Traditional Use and Properties
3. Potential Effects of C. parqui
3.1. Insecticidal and Antifeedant Activity
3.2. Molluscicidal Activity
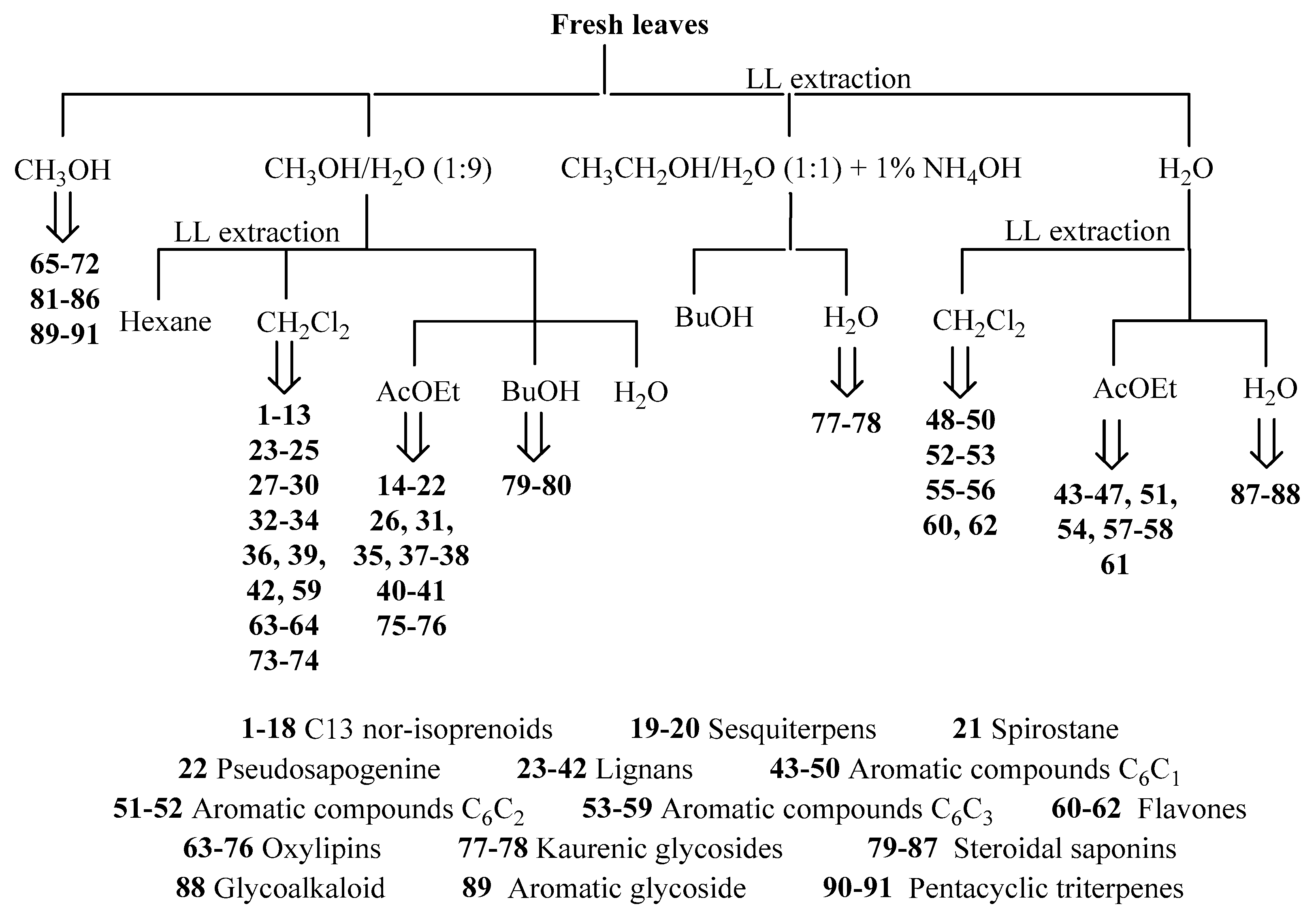
3.3. Phytochemical Composition
3.4. Herbicidal Activity
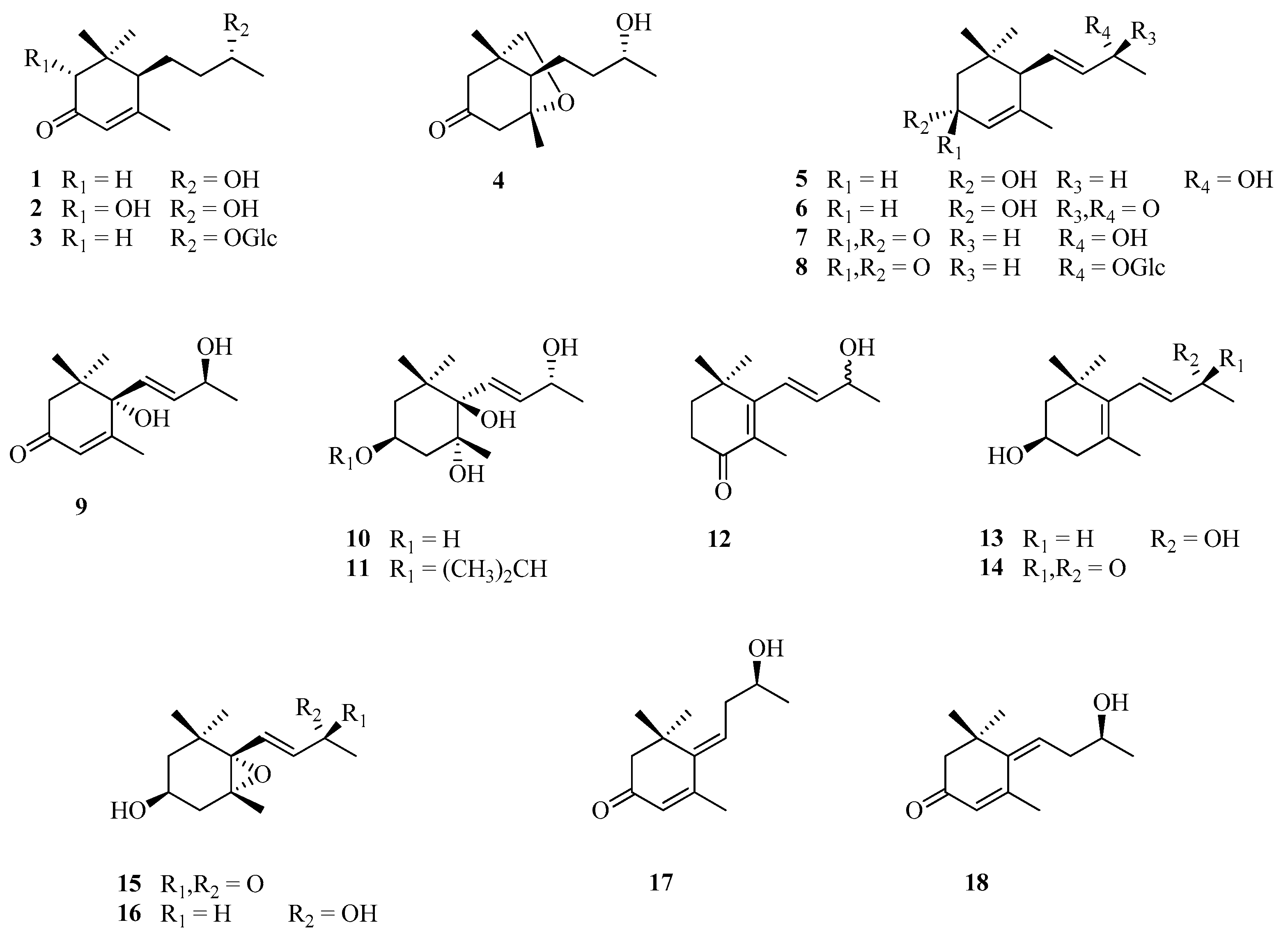
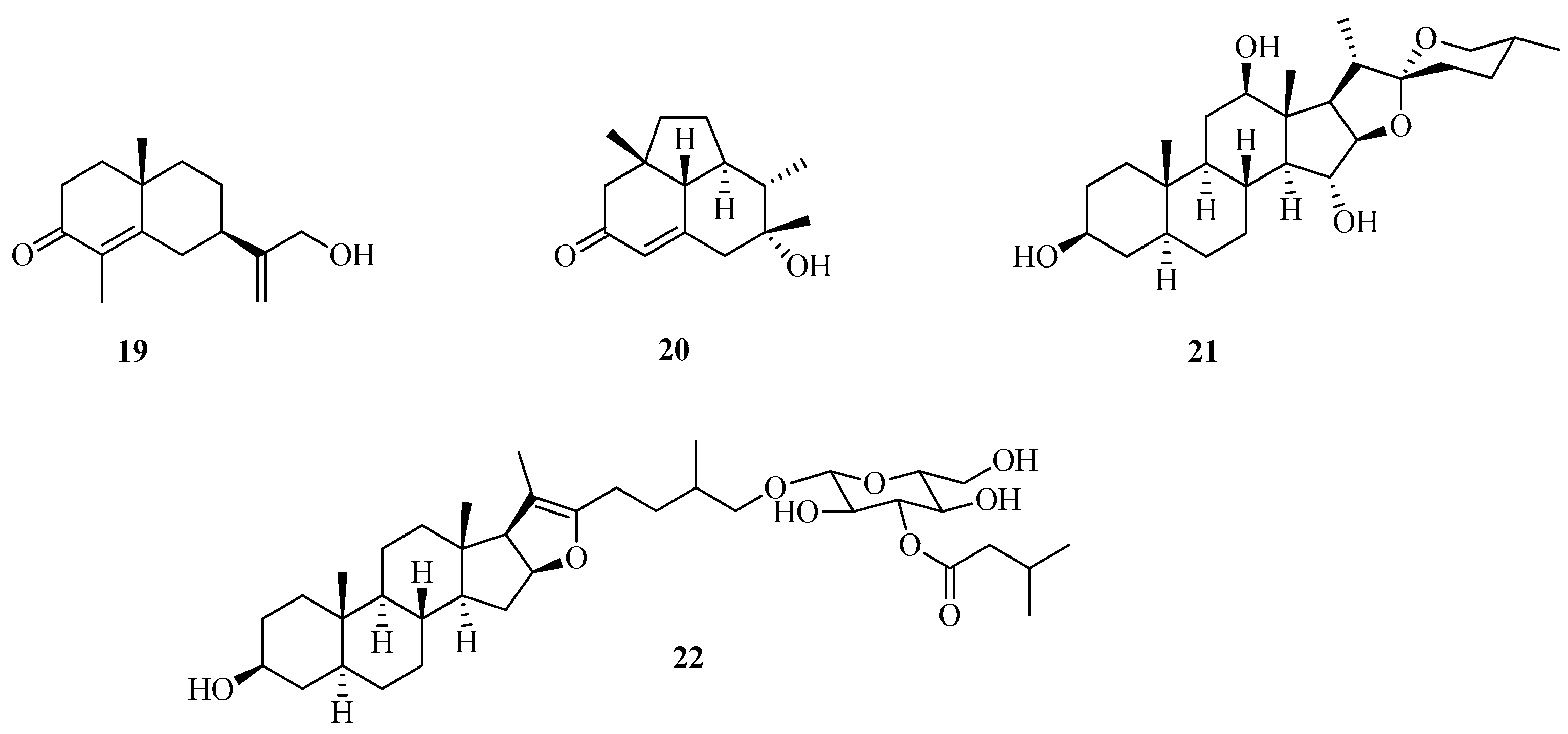
3.4.1. Assay with C13-nor-Isoprenoids (1–18), Sesquiterpenes (19–20), Spirostane (21), and Pseudosapogenin (22)
3.4.2. Assay with Lignans (23–35, 40–42)
3.4.3. Assay with Lignans (36–39)
3.4.4. Assay with Aromatic Compounds (43–59) and Flavones (60–62)
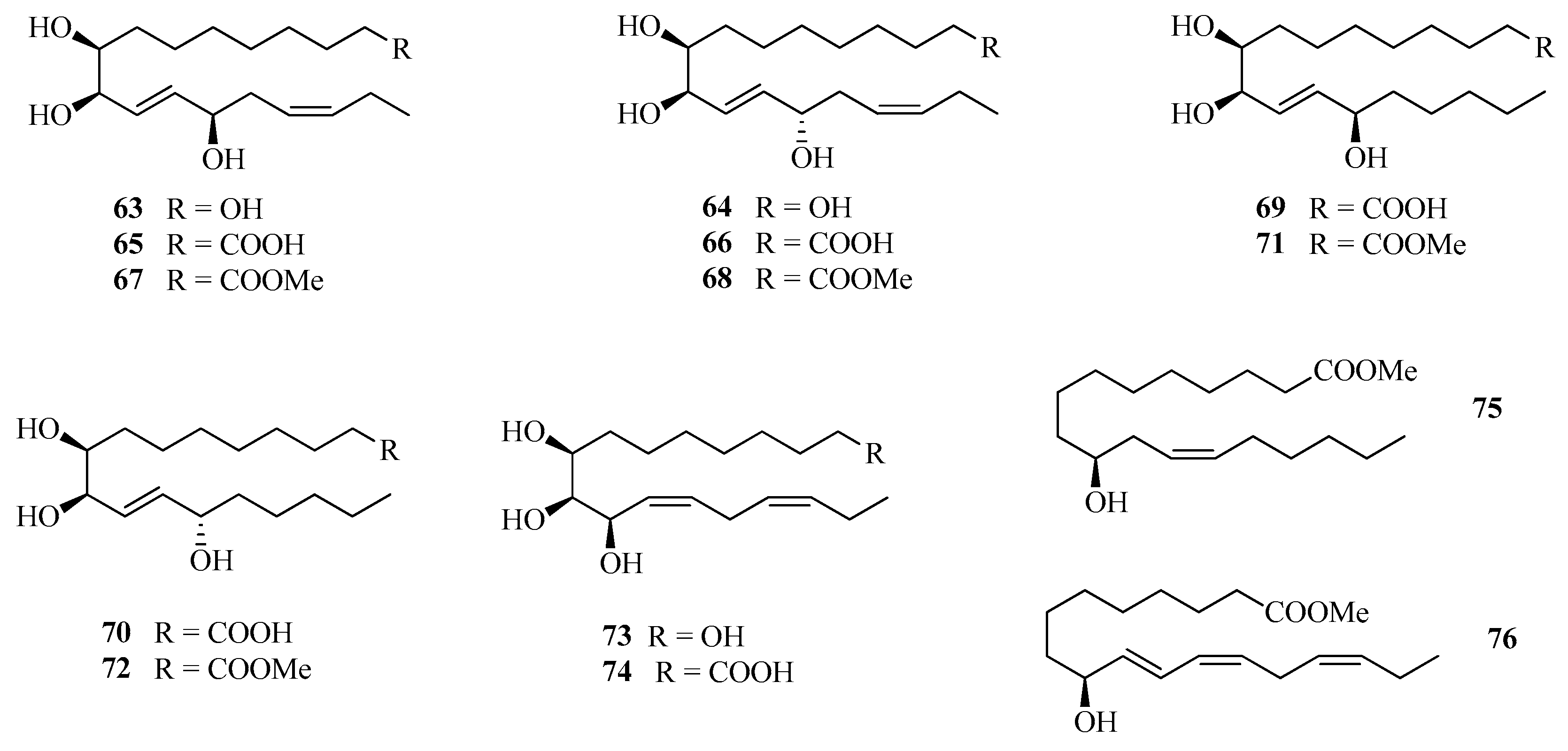
3.4.5. Assay with Oxylipins (63–76)
3.5. Other Isolated Metabolites
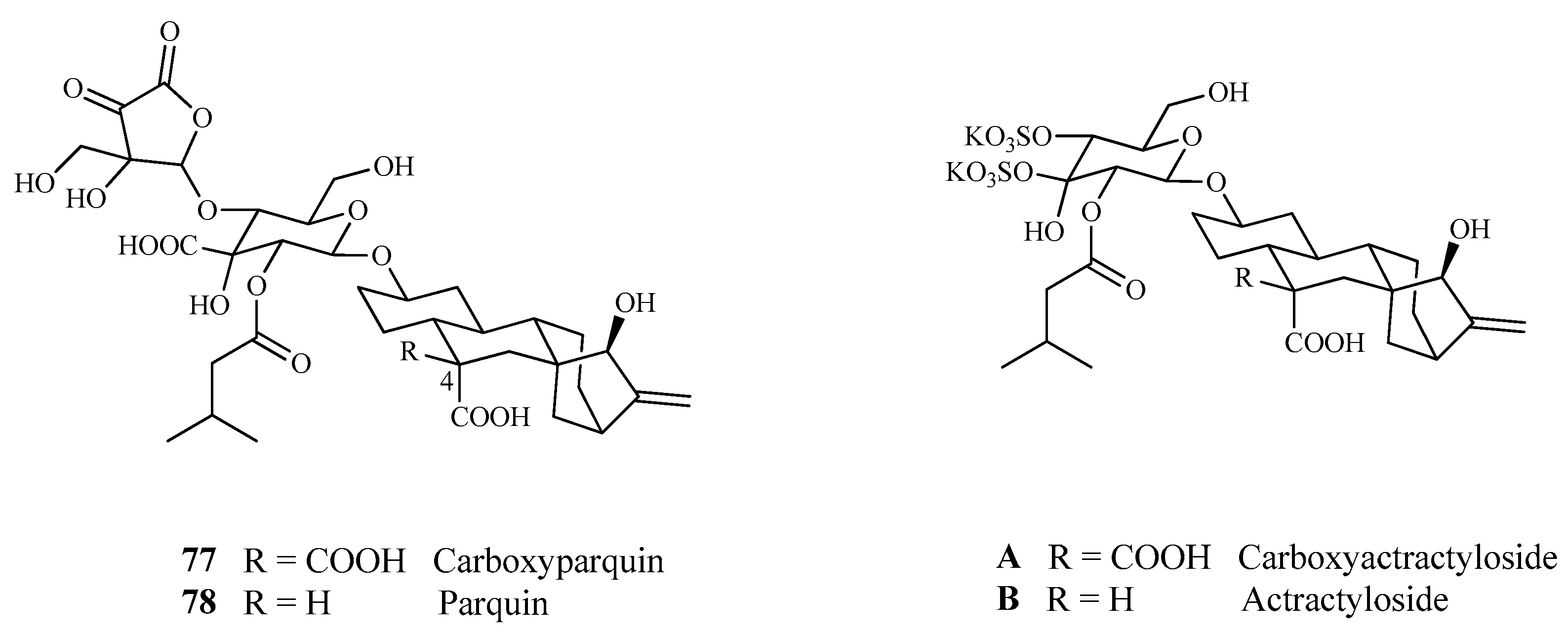
3.5.1. Kaurenic Glycosides (77–78) with Strychnine-Like Action
3.5.2. Cytotoxic Secondary Metabolites
| No. | Common Name/IUPAC Name | Ref. |
|---|---|---|
| 77 | Carboxiparquin | [56] |
| 78 | Parquin | |
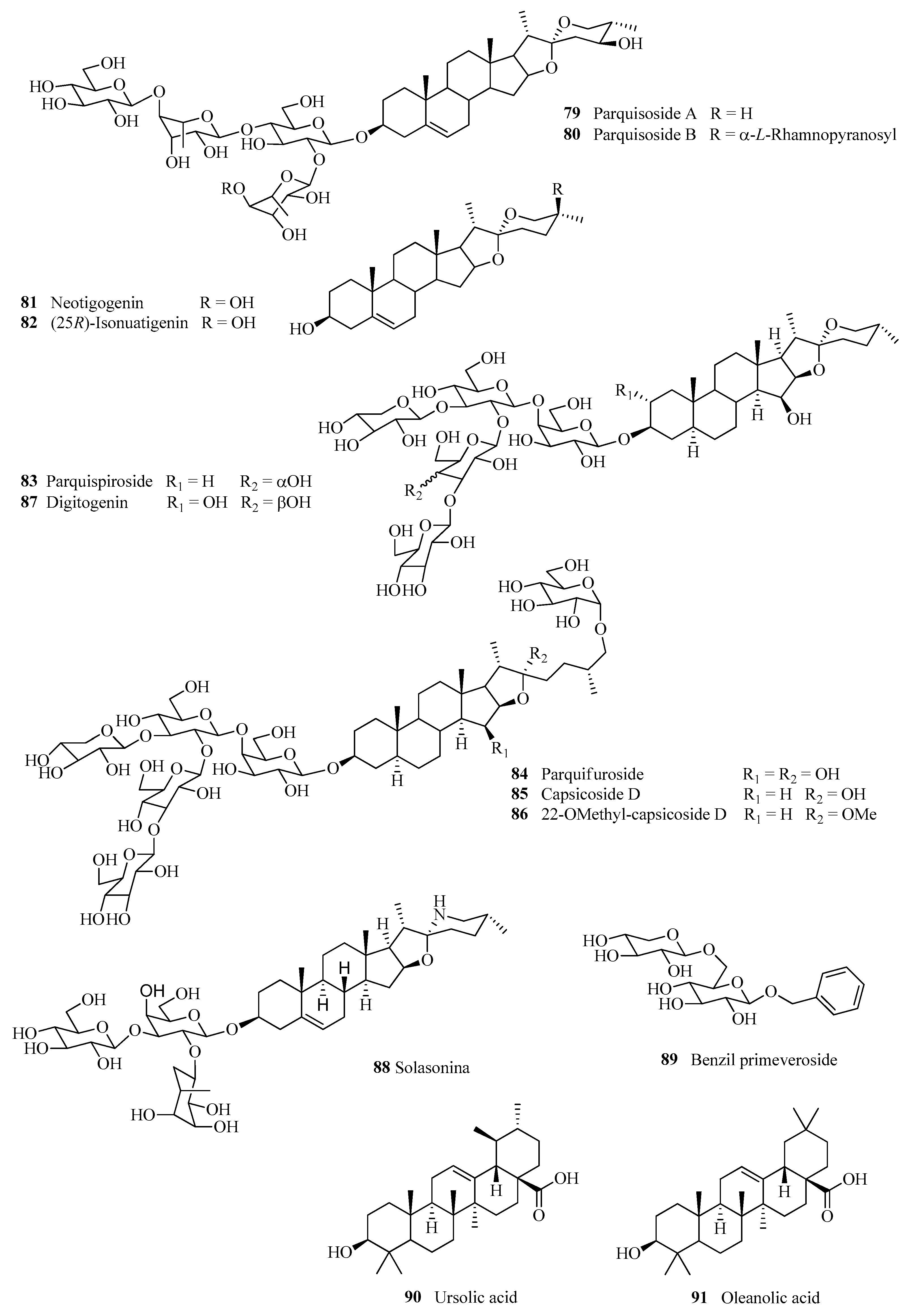
| 79 | Parquisoside A/(3β,24S,25S)-spirost-5-ene-3,24-diol 3-O-{[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | [58] |
| 80 | Parquisoside B/(3β,24S,25S)-spirost-5-ene-3,24-diol 3-O-{[α -L-rhamnopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→4)}-β-D-glucopyranoside | [58] |
| 81 | Neotigogenin | [59] |
| 82 | (25R)-Isonuatigenin | [59] |
| 83 | Parquispiroside/25(R)-3β-[(O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl)oxy]-5α,15β,22R,25R-spirostan-3,15-diol | [58] |
| 84 | Parquifuroside/25(R)-26-[(β-D-Glucopyranosyl)oxy]-(3β[(O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl)oxy],5α,15β,22R,25R)-furostane-3,15,22-triol | [58] |
| 85 | Capsicoside D | [62] |
| 86 | 22-O-Methylcapsicoside D | [58] |
| 87 | Digitogenin | [25,62] |
| 88 | Solasonine | [62] |
| 89 | Benzyl primeveroside | [58] |
| 90 | Ursolic acid | [58] |
| 91 | Oleanolic acid | [19,62] |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Zhan, C.; Shen, S.; Yang, C.; Liu, Z.; Fernie, A.R.; Graham, I.A.; Luo, J. Plant metabolic gene clusters in the multi-omics era. Trends Plant Sci. 2022, 27, 981–1001. [Google Scholar] [CrossRef]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-Derived Pesticides as an Alternative to Pest Management and Sustainable Agricultural Production: Prospects, Applications and Challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef]
- Available online: https://keyserver.lucidcentral.org/weeds/data/media/Html/cestrum_parqui.htm (accessed on 5 May 2024).
- L’Héritier de Brutelle, C.L.; Redouté, P.J.; Fréret, L. Stirpes Novae aut Minus Cognitae, Quas Descriptionibus et Iconibus; Ex Typographia Philippi-Dionysii Pierres; Nabu Press: Miami, NY, USA, 1784. [Google Scholar]
- Ruiz, H. Travels of Ruiz, Pavon, and Dombey in Peru and Chile; Field Museum Press: Chicago, IL, USA, 1940. [Google Scholar]
- Hunziker, A.T. Genera Solanacearum: The Genera of the Solanaceae Illustrated, Arranged According to a New System; Gantner Verlag: Ruggell, Liechtenstein, 2001. [Google Scholar]
- Available online: https://www.actaplantarum.org/flora/flora_info.php?id=2055&nnn=Cestrum%20parqui%20L%27H%C3%A9r (accessed on 17 July 2024).
- Olmstead, R.G.; Bohs, L.; Migid, H.A.; Santiago-Valentin, E.; Garcia, V.F.; Collier, S.M. A molecular phylogeny of the Solanaceae. Taxon 2008, 57, 1159–1181. [Google Scholar] [CrossRef]
- Olmstead, R.G. Phylogeny and Biogeography in Solanaceae, Verbenaceae and Bignoniaceae: A Comparison of Continental and Intercontinental Diversification Patterns. Bot. J. Linn. Soc. 2013, 171, 80–102. [Google Scholar] [CrossRef]
- Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30046363-2 (accessed on 10 May 2024).
- Available online: https://europlusmed.org/cdm_dataportal/taxon/51664b74-b63f-4381-af29-46de5ef8d171 (accessed on 18 July 2024).
- Available online: www.worldplants.de (accessed on 10 May 2024).
- Groves, R.H.; Boden, R.; Lonsdale, W.M. Jumping the Garden Fence: Invasive Garden Plants in Australia and Their Environmental and Agricultural Impacts; WWF: Sydney, Australia, 2005. [Google Scholar]
- Estomba, D.; Ladio, A.; Lozada, M. Plantas medicinales utilizadas por una comunidad Mapuche en las cercanías de Junín de los Andes, Neuquén. BLACPMA 2005, 4, 107–112. [Google Scholar]
- Backhouse, N.; Delporte, C.; Negrete, R.; Salinas, P.; Pinto, A.; Aravena, S.; Cassels, B.K. Antiinflammatory and Antipyretic Activities of Cuscuta chilensis, Cestrum parqui, and Psoralea glandulosa. Int. J. Pharmacogn. Pharm. 1996, 34, 53–57. [Google Scholar] [CrossRef]
- Molgaard, P.; Holler, J.G.; Asar, B.; Liberna, I.; Rosenbæk, L.B.; Jebjerg, C.P.; Jørgensen, L.; Lauritzen, J.; Guzman, A.; Adsersen, A.; et al. Antimicrobial evaluation of Huilliche plant medicine used to treat wounds. J. Ethnopharmacol. 2011, 138, 219–227. [Google Scholar] [CrossRef]
- Chenni, H. Apoptosis induction by Cestrum parqui L’Hér. leaves on HL-60 Cell Line: Identification of active phytomolecules. Int. J. Cancer. Stud. Res. 2015, 1, 1–8. [Google Scholar]
- Chenni, H. Identification of esterified oleanolic acid in Cestrum parqui leaves and its apoptotic induction on HT-29 cell line. J. Med. Pharm. Innov. 2015, 2, 63–68. [Google Scholar]
- Falkenberg, S.S.; Tarnow, I.; Guzman, A.; Mølgaard, P.; Simonsen, H.T. Mapuche herbal medicine inhibits blood platelet aggregation. Evid. Based Complement. Alternat. Med. 2012, 2012, 647620. [Google Scholar] [CrossRef]
- Souad, K.; Ali, S.; Mounir, A.; Mounir, T.M. Spermicidal activity of extract from Cestrum parqui. Contraception 2007, 75, 152–156. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.S.A.; George, N.M.; Abou-Elnour, A.; El-Mekkawy, R.M.; El-Demerdash, M.M. Production and bioprocessing of camptothecin from Aspergillus terreus, an endophyte of Cestrum parqui, restoring their biosynthetic potency by Citrus limonum peel extracts. Microb. Cell Fact. 2023, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Canham, P.A.S.; Warren, F.L. Saponins II. Isolation of gitogenin and digitogenin from Cestrum parqui. S. Afr. J. Chem. 1950, 3, 63–65. [Google Scholar]
- Bianchi, E.; Girardi, F.; Diaz, F.; Sandoval, R.; Gonzales, M. Components of the leaves and fruit of Cestrum parqui: Tigogenin, digallogenin, digitogenin and ursolic acid. Ann. Chim.-Rom. 1963, 53, 1761–1778. [Google Scholar]
- Bezerra, J.J.L.; Pinheiro, A.A.V.; de Lucena, R.B. Poisoning in ruminants caused by species of the genus Cestrum L. (Solanaceae) in Brazil: A review of toxicological and phytochemical evidence. Toxicon 2023, 236, 107348. [Google Scholar] [CrossRef] [PubMed]
- Brevis, C.; Quezada, M.; Sierra, M.A.; Carrasco, L.; Ruiz, A. Lesiones observadas en intoxicaciones accidentales con Cestrum parqui (L’Herit) en bovinos. Arch.Med. Vet. 1999, 31, 109–118. [Google Scholar] [CrossRef]
- Barbouche, N.; Hajem, B.; Lognay, G.; Ammar, M. Contribution for studying the biological activity of the leaves extracts of Cestrum parqui L’Herit. on desert locust Schistocera gregaria. Biotechnol. Agron. Soc. 2001, 5, 85–90. [Google Scholar]
- Ikbal, C.; Ben, H.K.; Ben, H.M. Insect growth regulator activity of Cestrum parqui saponins: An interaction with cholesterol metabolism. Commun. Agric. Appl. Biol. Sci. 2006, 71, 489–496. [Google Scholar]
- Zapata, N.; Budia, F.; Viñuela, E.; Medina, P. Insecticidal effects of various concentrations of selected extractions of Cestrum parqui on adult and immature Ceratitis capitata. J. Econ. Entomol. 2006, 99, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Heppner, J.B.; Woodruff, R.E.; Weems, H.V.; Steck, G.J.; Fasulo, T.R. Mediterranean Fruit Fly, Ceratitis Capitata. (Wiedemann) (Insecta: Diptera, Tephritidae); University of Florida: Gainesville, FL, USA, 2001; IFASA Extension 2001, EENY-214. [Google Scholar]
- Huerta, A.; Chiffelle, I.; Araya, L.; Curkovic, T.; Araya, J.E. Insecticidal capacity of Cestrum parqui (Solanaceae) leaf extracts on Xanthogaleruca luteola (Coleoptera: Chrysomelidae) adults. Rev. Colomb. Entomol. 2021, 47, e10492. [Google Scholar] [CrossRef]
- Ben Hamouda, A.; Ammar, M.; Habib, M.; Hamouda, B. Effect of Olea europea and Cestrum parquii Leaves on the Cuticle and Brain of the Desert Locust, Schistocerca gregaria Forsk (Orthoptera:Acrididae). Pest Technol. 2011, 5, 55–58. [Google Scholar]
- Chaieb, I.; Halima-Kamel, M.B.; Ben, M.H. Antifeedant activity of Cestrum parqui crude saponic extract. Tunis. J. Med. Plants Nat. Prod. 2009, 1, 27–33. [Google Scholar]
- Chaieb, I.; Mounia, B.H.; Habib, B.H. Toxicity experiments of the saponic extract of Cestrum parqui (Solanaceae) on some insect spices. J. Entomol. 2007, 4, 113–120. [Google Scholar]
- Chaieb, I.; Mounia, B.H. The effect of food containing Cestrum paquii (Solanaceae) extract on various damaging Lepidoptera. Meded. Rijksuniv. Gent. Fak. Landbouwkd. Toegepaste Biol. Wet. 2001, 66, 479–490. [Google Scholar]
- Chaieb, I.; Habib, B.; Hichem, B.J.; Monia, B.H.; Habib, B.H.; Zine, M. Purification of a natural insecticidal substance from Cestrum parqui (Solanaceae). PJBS 2007, 10, 3822–3828. [Google Scholar]
- Chaieb, I.; Ben, H.; Trabelsi, M.; Hlawa, W.; Raouani, N.; Ben, A.; Daami, M.; Ben, H. Pesticidal Potentialities of Cestrum parqui saponins. Int. J. Agric. Res. 2003, 2, 275–281. [Google Scholar]
- Huanquilef, C.; Espinoza, J.; Mutis, A.; Bardehle, L.; Hormazábal, E.; Urzúa, A.; Quiroz, A. Antifeedant activities of organic fractions from Cestrum parqui leaves on the red-haired bark beetle Hylurgus ligniperda. J. Soil Sci. Plant Nutrit. 2021, 21, 13–21. [Google Scholar] [CrossRef]
- Chaieb, I.; Halima-Kamel, M.B.; Hammouda, M.B. Experiments for studying the molluscicidal potential of Cestrum parqui (Solanaceae) saponins against Theba pisana (Helicidae) snails. Comm. Agric. Appl. Biol. Sci. 2005, 70, 809–815. [Google Scholar]
- Chaieb, I.; Tayeb, W. Comparison of the molluscicidal activity of Cestrum parqui (Solanaceae) and Quillaja saponaria (Quillajaceae) saponins. Tunis. J. Med. Plants Nat. Prod. 2009, 2, 31–35. [Google Scholar]
- D’Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Monaco, P.; Oriano, P.; Temussi, F. Structure elucidation and phytotoxicity of C13-nor-isoprenoids from Cestrum parqui. Phytochemistry 2004, 65, 497–505. [Google Scholar] [CrossRef]
- D’Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Monaco, P.; Natale, A.; Oriano, P.; Zarrelli, A. Structural characterization of phytotoxic terpenoids from Cestrum parqui. Phytochemistry 2005, 66, 2681–2688. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; DellaGreca, M.; D’Abrosca, B.; Oriano, P.; Golino, A.; Izzo, A.; Monaco, P. Lignans, neolignans and sesquilignans from Cestrum parqui l’Her. Biochem. Syst. Ecol. 2007, 35, 392–396. [Google Scholar] [CrossRef]
- D’Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Golino, A.; Monaco, P.; Zarrelli, A. Isolation and characterization of new lignans from the leaves of Cestrum parqui. Nat. Prod. Res. 2006, 20, 293–298. [Google Scholar] [CrossRef]
- D’Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Monaco, P.; Zarrelli, A. Low molecular weight phenols from the bioactive aqueous fraction of Cestrum parqui. J. Agric. Food Chem. 2004, 52, 4101–4108. [Google Scholar] [CrossRef] [PubMed]
- DellaGreca, M.; Fiorentino, A.; Izzo, A.; Napoli, F.; Purcaro, R.; Zarrelli, A. Phytotoxicity of secondary metabolites from Aptenia cordifolia. Chem. Biodivers. 2007, 4, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; D’Abrosca, B.; DellaGreca, M.; Izzo, A.; Natale, A.; Pascarella, M.T.; Pacifico, S.; Zarrelli, A.; Monaco, P. Chemical characterization of new oxylipins from Cestrum parqui, and their effects on seed germination and early seedling growth. Chem. Biodivers. 2008, 5, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Cangiano, T.; DellaGreca, M.; Fiorentino, A.; Isidori, M.; Monaco, P.; Zarrelli, A. Lactone diterpenes from the aquatic plant Potamogeton natans. Phytochemistry 2001, 56, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Cangiano, T.; DellaGreca, M.; Fiorentino, A.; Isidori, M.; Monaco, P. Effect of ent-labdane diterpenes from Potamogetonaceae on Selenastrum capricornutum and other aquatic organisms. J. Chem. Ecol. 2002, 28, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Pollio, A.; Romanucci, V.; Di Mauro, A.; Barra, F.; Pinto, G.; Crescenzi, E.; Roscetto, E.; Palumbo, G. Polyphenolic profile and targeted bioactivity of methanolic extracts from Mediterranean ethnomedicinal plants on human cancer cell lines. Molecules 2016, 21, 395. [Google Scholar] [CrossRef] [PubMed]
- Cutillo, F.; D’Abrosca, B.; DellaGreca, M.; Zarrelli, A. Chenoalbicin, a novel cinnamic acid amide alkaloid from Chenopodium album. Chem. Biodivers. 2004, 1, 1579–1583. [Google Scholar] [CrossRef] [PubMed]
- DellaGreca, M.; Previtera, L.; Purcaro, R.; Zarrelli, A. Cinnamic ester derivatives from Oxalis pes-caprae (Bermuda buttercup). J. Nat. Prod. 2007, 70, 1664–1667. [Google Scholar] [CrossRef] [PubMed]
- Macıas, F.A.; Castellano, D.; Molinillo, J.M.G. Search for standard phytotoxicity bioassay for allelochemicals. Selection of standard target species. J. Agric. Food Chem. 2000, 48, 2512–2521. [Google Scholar] [CrossRef] [PubMed]
- le Roy, J.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Glycosylation is a major regulator of phenylpropanoid availability and biological activity in plants. Front. Plant Sci. 2016, 7, 735. [Google Scholar] [CrossRef] [PubMed]
- Pearce, C.M.; Skelton, N.J.; Naylor, S.; Kanaan, R.; Kelland, J.; Oelrichs, P.B.; Sanders, J.K.M.; Williams, D.H. Parquin and carboxyparquin, toxic kaurene glycosides from the shrub Cestrum parqui. J. Chem. Soc. 1992, 1, 593–600. [Google Scholar] [CrossRef]
- Daniele, C.; Dahamna, S.; Firuzi, O.; Sekfali, N.; Saso, L.; Mazzanti, G. Atractylis gummifera L. poisoning: An ethnopharmacological review. J. Ethnopharmacol. 2005, 97, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Baqai, F.T.; Ali, A.; Ahmad, V. Two new spirostanol glycosides from Cestrum parqui. Helv. Chim. Acta 2001, 84, 3350–3356. [Google Scholar] [CrossRef]
- Mosad, R.R.; Ali, M.H.; Ibrahim, M.T.; Shaaban, H.M.; Emara, M.; Wahba, A.E. New cytotoxic steroidal saponins from Cestrum parqui. Phytochem. Lett. 2017, 22, 167–173. [Google Scholar] [CrossRef]
- Abdel-Gwad, M.M.; El-Amin, S.M.; El-Sayed, M.M.; Refahy, L.A.; Sabry, W.A. Molluscicidal saponins from Cestrum parqui. AJPS 1997, 20, 80–84. [Google Scholar]
- Torres, R.; Modak, B.; Faini, F. (25R)-Isonuatigenin, an unusual steroidal sapogenin as taxonomicmarker in Cestrum parqui and Vestia lycioides. Biol. Soc. Chil. Quim. 1988, 33, 239–241. [Google Scholar]
- Silva, M.; Mancinelli, P.; Cheul, M. Chemical study of Cestrum parqui. J. Pharm. Sci. 1962, 51, 289. [Google Scholar] [CrossRef] [PubMed]

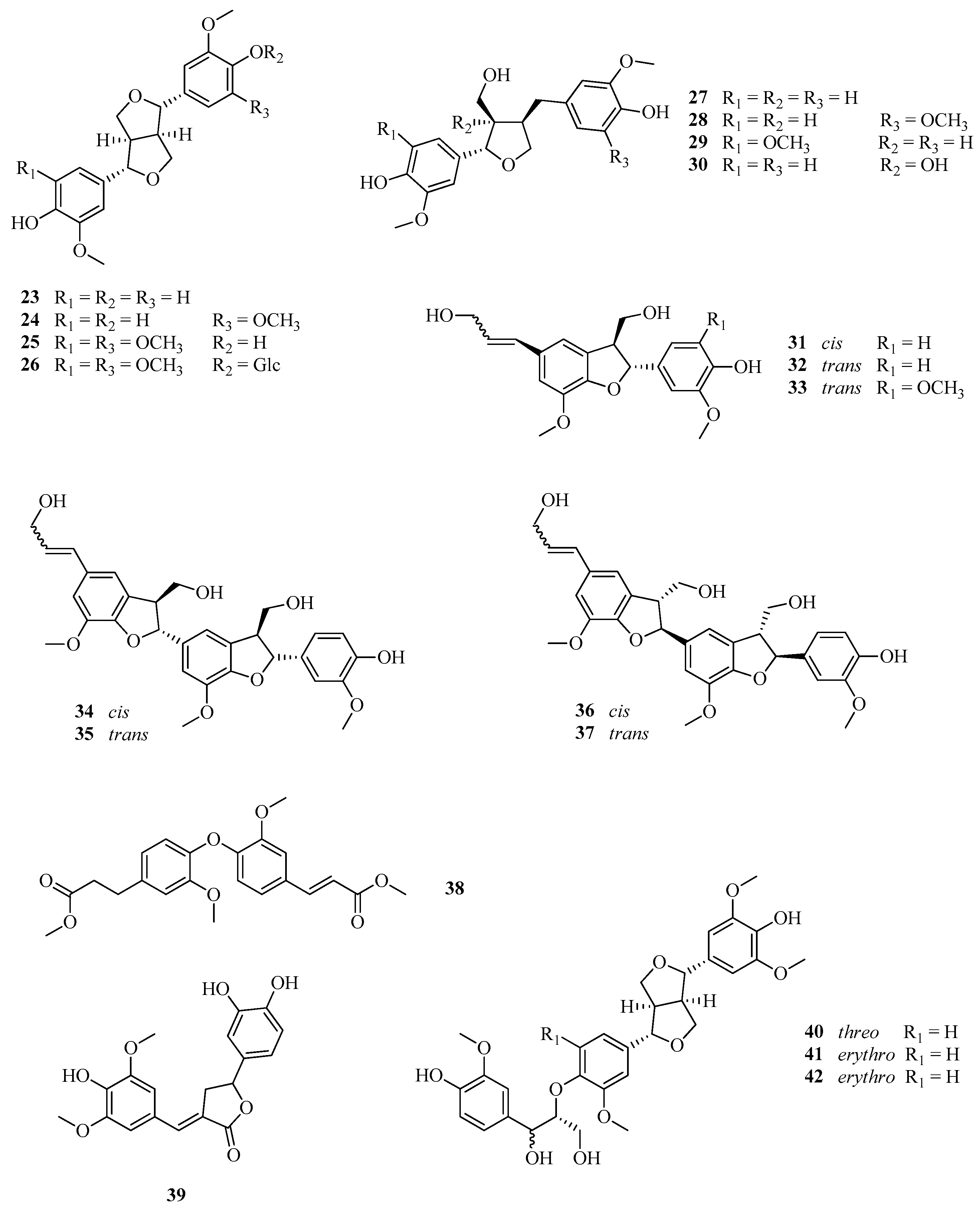
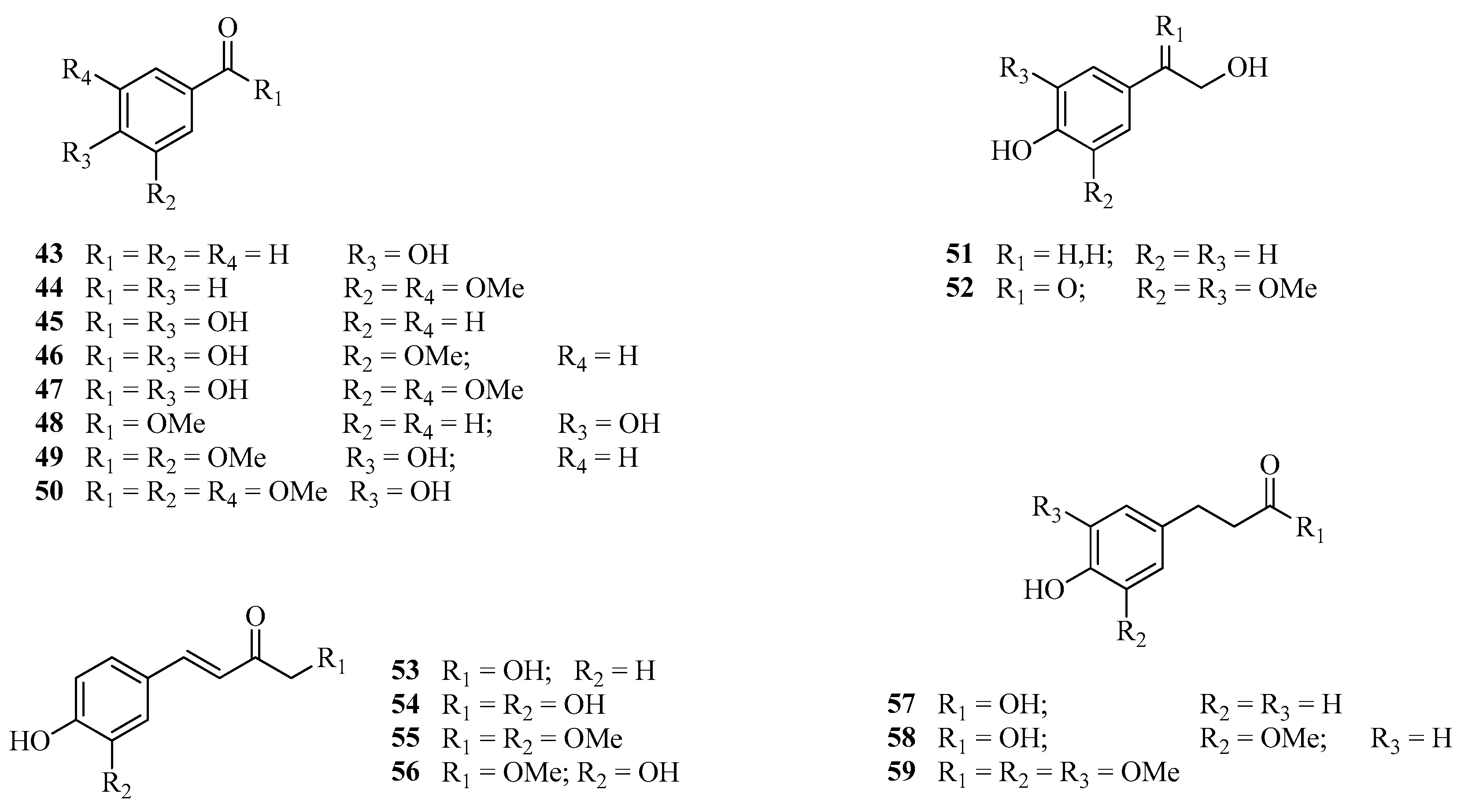
| Kingdom | Plantae |
|---|---|
| Sub-kingdom | Tracheophytes |
| Division | Angiosperms |
| Class | Eudicots |
| Sub-class | Asterids |
| Order | Lamianae Takht |
| Sub-order | Solanales Juss. ex Bercht. and J. Presl |
| Family | Solanaceae’ Juss |
| Tribe | Cestreae |
| Genus | Cestrum L. |
| Species | parqui |
| No. | Name | Ref |
|---|---|---|
| C13-nor-isoprenoids | ||
| 1 | (6R,9R)-9-Hydroxy-4-megastigmen-3-one | |
| 2 | (2R,6R,9R)-2,9-Dihydroxy-4-megastigmen-3-one | |
| 3 | Byzantionoside A | |
| 4 | Annuionone E | |
| 5 | (3R,6R,7E,9R)-3,9-Dihydroxy-4,7-megastigmadiene | |
| 6 | (3R,6R,7E)-3-Hydroxy-4,7-megastigmadien-9-one | |
| 7 | (6R,7E,9R)-9-Hydroxy-4,7-megastigmadien-3-one | |
| 8 | Byzantionoside B | |
| 9 | Corcoionolo C | |
| 10 | (3S,5R,6R,7E,9R)-3,5,6,9-Tetrahydroxy-7-megastigmene | [42,46] |
| 11 | (3S,5R,6R,7E,9R)-5,6,9-Trihydroxy-3-isopropyloxy-7-megastigmene | |
| 12 | 4-Oxo-β-ionol/(7E)-9-Hydroxy-5,7-megastigmadien-4-one | |
| 13 | (3S,7E,9R)-3,9-Dihydroxy-5,7-megastigmadiene | |
| 14 | (3R,7E)-3-Hydroxy-5,7-megastigmadien-9-one | |
| 15 | (3S,5R,6S,7E)-5,6-Epoxy-3-hydroxy-7-megastigmen-9-one | |
| 16 | (3S,5R,6S,7E,9R)-5,6-Epoxy-3,9-dihydroxy-7-megastigmene | |
| 17 | (6E,9S)-9-Hydroxy-4,6-megastigmadien-3-one | |
| 18 | (6Z,9S)-9-Hydroxy-4,6-megastigmadien-3-one | |
| Sesquiterpenes | ||
| 19 | 12-Hydroxy-α-cyperone | [43] |
| 20 | 1,2,2a,3,6,7,8,8a-Octahydro-7-hydroxy-2a,7,8-trimethylacenaphthylen-4(4H)-one | [49,50] |
| Spirostane | ||
| 21 | 5α-Spirostan-3β,12β,15α-triol | [43] |
| Pseudosapogenine | ||
| 22 | 26-O-(30-Isopentanoyl)-β-D-glucopyranosyl-5α-furost-20(22)-ene-3β,26-diol | [43] |
| Lignans Diepoxylignans | ||
| 23 | (+)-Pinoresinol | [44] |
| 24 | (+)-Mediaresinol | |
| 25 | (+)-Syringaresinol | |
| 26 | β-D-Glucopyranoside, 2,6-dimethoxy-4-[(1S,3aR,4S,6aR)-tetrahydro-4-(4-hydroxy-3,5-dimethoxyphenyl)-1H,3H-furo[3,4-c]furan-1-yl]phenyl | |
| Epoxylignans | ||
| 27 | (+)-Lariciresinol | [44] |
| 28 | (+)-Justiciresinol | |
| 29 | 5′-Methoxylariciresinol | |
| 30 | (−)-Berchemol | |
| Neolignans | ||
| 31 | cis-Dehydrodiconiferyl alcohol | [44] |
| 32 | trans-Dehydrodiconiferyl alcohol | |
| 33 | (−)-Simulanol, | |
| Sesquilignans | ||
| 34 | rel-(7Z,7′β,7″β,8′α,8″α)-4″,9,9′,9″-Tetrahydroxy-3,3′,3″-trimethoxy-4,7′:4′,7″-diepoxy-5,8′:5′,8″-sesquilign-7-ene | [44] |
| 35 | Herpetotriol | |
| 36 | rel-(7Z,7′α,7″α,8′β,8″β)-4″,9,9′,9″-Tetrahydroxy-3,3′,3″-trimethoxy-4,7′:4′,7″-diepoxy-5,8′:5′,8″-sesquilign-7-ene | |
| 37 | rel-(7E,7′α,7″α,8′β,8″β)-4″,9,9′,9″-Tetrahydroxy-3,3′,3″-trimethoxy-4,7′:4′,7″-diepoxy-5,8′:5′,8″-sesquilign-7-ene | |
| 40 | threo-4′,4″,7″,9″-Tetrahydroxy-3,3′,3″,5′-tetramethoxy- 4,8″-oxy-7,9′:7′,9-diepoxylignan | |
| 41 | erythro-4′,4″,7″,9″-Tetrahydroxy-3,3′,3″,5′-tetramethoxy- 4,8″-oxy-7,9′:7′,9-diepoxylignan | |
| 42 | erythro-4′,4″,7″,9″-Tetrahydroxy- 3,3′,3″,5,5′-pentamethoxy-4,8″-oxy-7,9′:7′,9-diepoxylignan | |
| Oxyneolignan | ||
| 38 | Dimethyl (7′E)-3,3′-dimethoxy-4,40-oxyneolign-7′-ene-9,9′-dioate | [44,45] |
| Norlignan | ||
| 39 | 9′-Nor-3′,4,4′-trihydroxy-3,5-dimethoxylign-7-eno-9,7′-lactone | [44,45] |
| Aromatic compounds | ||
| 43 | 4-Hydroxybenzaldehyde | [46,47,51,52,53] |
| 44 | 3,5-Dimethoxybenzaldehyde | |
| 45 | 4-Hydroxybenzoic acid | |
| 46 | Vanillic acid | |
| 47 | Syringic acid | |
| 48 | Methyl 4-hydroxybenzoate | |
| 49 | Methyl vanillate | |
| 50 | Methyl syringate | |
| 51 | Tirosol | |
| 52 | 3′,5′-Dimethoxy-4′-hydroxy-2-hydroxy-acetophenone | |
| 53 | p-Coumaric acid | |
| 54 | Caffeic acid | |
| 55 | Methyl ferulate | |
| 56 | Methyl ester of caffeic acid | |
| 57 | p-Dihydrocoumaric acid | |
| 58 | Methyl ester of p-dihydrocoumaric acid | |
| 59 | Dihydrosynapic acid | |
| Flavones | ||
| 60 | 4′-Hydroxy-4-methoxychalcon | [46] |
| 61 | Quercitin | |
| 62 | N-(p-Carboxymethylphenyl)-p-hydroxybenzamide | |
| Oxylipins | ||
| 63 | (8S,9R,10E,12R,14Z)-Heptadeca-10,14-diene-1,8,9,12-tetraol | [48] |
| 64 | (8S,9R,10E,12S,14Z)-Heptadeca-10,14-diene-1,8,9,12-tetraol | |
| 65 | (9S,10R,11E,13R,15Z)-9,10,13-Trihydroxyoctadeca-11,15-dienoic acid | |
| 66 | (9S,10R,11E,13S,15Z)-9,10,13-Trihydroxyoctadeca-11,15-dienoic acid | |
| 67 | Methyl (9S,10R,11E,13R,15Z)-9,10,13-trihydroxyoctadeca-11,15-dienoate | |
| 68 | Methyl (9S,10R,11E,13S,15Z)-9,10,13-trihydroxyoctadeca-11,15-dienoate | |
| 69 | (9S,10R,11E,13R)-9,10,13-Trihydroxyoctadec-11-enoic acid | |
| 70 | (9S,10R,11E,13S)-9,10,13-Trihydroxyoctadec-11-enoic acid | |
| 71 | Methyl (9S,10R,11E,13R)-9,10,13-trihydroxyoctadec-11-enoate | |
| 72 | Methyl (9S,10R,11E,13S)-9,10,13-trihydroxyoctadec-11-enoate | |
| 73 | (8S,9S,10R,11Z,14Z)-Heptadeca-11,14-diene-1,8,9,10-tetraol | |
| 74 | (9S,10S,11R,12Z,15Z)-9,10,11-Trihydoxyoctadeca-12,15-dienoic acid | |
| 75 | Methyl 10-hydroxyoctadec-12-enoate | |
| 76 | Methyl (9S,10E,12Z,15Z)-octadeca-10,12,15-trien-9-ol |
| Compounds | Range of Concentrations | Organism Test | |||||
|---|---|---|---|---|---|---|---|
| L. sativa | S.lycopersicum | A. retroflexus | C. album | P. oleracea | A. cepa | ||
| 1–13 | 10−4–10−7 M | x | |||||
| 14–18 | 10−5–10−7 M | x | |||||
| 19–22 | 10−4–10−7 M | x | |||||
| 23–35 | 10−4–10−8 M | x | x | x | |||
| 36–39 | 10−4–10−7 M | x | x | ||||
| 40–42 | 10−4–10−8 M | x | x | x | |||
| 43–59 | 10−4–10−9 M | x | x | x | |||
| 60–62 | 10−4–10−9 M | x | x | x | |||
| 63–76 | 10−4–10−8 M | x | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Meo, M.C.; Di Marino, C.; Napoletano, P.; De Marco, A.; Bianchi, A.R.; Pedatella, S.; Palatucci, D. Functional, Chemical, and Phytotoxic Characteristics of Cestrum parqui L’Herit: An Overview. Plants 2024, 13, 2044. https://doi.org/10.3390/plants13152044
Di Meo MC, Di Marino C, Napoletano P, De Marco A, Bianchi AR, Pedatella S, Palatucci D. Functional, Chemical, and Phytotoxic Characteristics of Cestrum parqui L’Herit: An Overview. Plants. 2024; 13(15):2044. https://doi.org/10.3390/plants13152044
Chicago/Turabian StyleDi Meo, Maria Chiara, Cinzia Di Marino, Pasquale Napoletano, Anna De Marco, Anna Rita Bianchi, Silvana Pedatella, and Domenico Palatucci. 2024. "Functional, Chemical, and Phytotoxic Characteristics of Cestrum parqui L’Herit: An Overview" Plants 13, no. 15: 2044. https://doi.org/10.3390/plants13152044





