Isolation and Characterization of Plant Growth-Promoting Bacteria from the Rhizosphere of Medicinal and Aromatic Plant Minthostachys verticillata
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physico-Chemical Soil Characteristics
2.2. Strains Isolatated form M. verticillata Rhizosphere
2.3. Phenotypic Characterization
2.4. In Vitro Characterization of PGPR Strains
2.5. Quantitative Values of IAA Production
2.6. Genotypic Identification
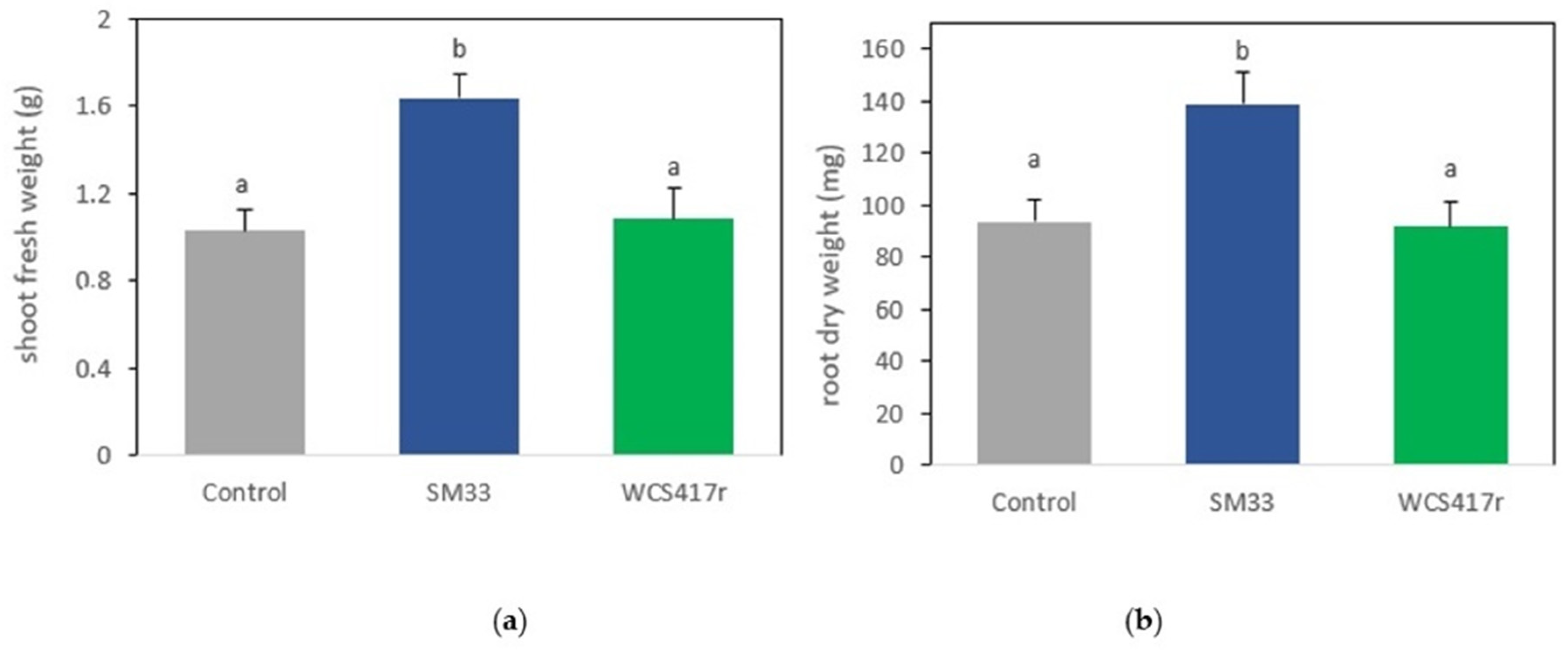
2.7. Growth-Promoting Effects
3. Materials and Methods
3.1. Soil Sample Collection and Strain Isolation
3.2. Determination of Physical and Chemical Properties of the Rhizosphere Soil
3.3. Phenotypic Characterization
3.4. Determination of the Main Direct and Indirect Mechanisms of Plant Promotion
3.4.1. Indole-3-Acetic Acid Production
Qualitative Determination
Quantitative Determination
3.4.2. Siderophore Production
3.4.3. Phosphate Solubilization Ability
3.4.4. Hydrogen Cyanide (HCN) Production
3.4.5. Biocontrol by In Vitro Antibiosis
3.5. Amplification and Bioinformatic Analysis of 16S rRNA Region Sequences
3.5.1. DNA Extraction
3.5.2. 16S rRNA Gene Nucleotide Sequence Analysis
3.5.3. Construction of the Phylogenetic Tree
3.6. Evaluation of Growth-Promoting Effects
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schmidt-Lebuhn, A.N. Ethnobotany, Biochemistry and Pharmacology of Minthostachys (Lamiaceae). J. Ethnopharmacol. 2008, 118, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Juliani, H.R.; Brunetti, P.; Koroch, A.R. Minthostachys verticillata (Griseb.) Epling. In Medicinal and Aromatic Plants of South America Volume 2; Máthé, Á., Bandoni, A., Eds.; Medicinal and Aromatic Plants of the World, Volume 7; Springer: Cham, Switzerland, 2021; pp. 367–378. [Google Scholar] [CrossRef]
- Ojeda, M.; Arroyo, A.; Borgogno, P.; Biderbost, E.; Balzarini, M. Yield of Peperina (Minthostachys mollis (Kunth.) Griseb.) Populations in the Year Following Planting: Response to Cropping Regimen. Span. J. Agric. Res. 2004, 2, 393–399. [Google Scholar] [CrossRef]
- Martínez, G.J. Recolección y Comercialización de Plantas Medicinales en el Departamento Santa María, Provincia de Córdoba. Acta Farm. Bonaer. 2005, 24, 575–584. [Google Scholar]
- Ojeda, M.S.; Martínez, G.J.; Massuh, Y.; Torres, L.E.; Chaves, A.G.; Arizio, O.; Curioni, A.O. Plantas Aromáticas y Medicinales: Modelos para su Domesticación, Producción y usos Sustentables: Carqueja-Orégano-Peperina-Suico-Manzanilla; Editorial Universidad Nacional de Córdoba: Córdoba, Argentina, 2015. [Google Scholar]
- Glinos, E.; Condat, E.; Mulieri, P.; Ashworth, L. Essential Dependence on Wild Pollination Service: A Medicinal Plant under Threat Minthostachys verticillata (Lamiaceae). Arthropod-Plant Interact. 2019, 13, 865–874. [Google Scholar] [CrossRef]
- Roig, F.A. Flora Medicinal Mendocina: Las Plantas Medicinales y Aromáticas, de la Provincia de Mendoza (Argentina): Aborígenes, Exóticas Espontáneas o Naturalizadas y Cultivadas; EDIUNC: Mendoza, Argentina, 2001; Serie Manuales. [Google Scholar]
- Martínez, G.J.; Planchuelo, A.M.; Fuentes, E.; Ojeda, M. A Numeric Index to Establish Conservation Priorities for Medicinal Plants in the Paravachasca Valley, Córdoba, Argentina. Biodivers. Conserv. 2006, 15, 2457–2475. [Google Scholar] [CrossRef]
- Primo, V.; Rovera, M.; Zanón, S.; Oliva, M.; Demo, M.; Daghero, J.; Sabini, L. Determinación de la Actividad Antibacteriana y Antiviral del Aceite Esencial de Minthostachys verticillata (Griseb.) Epling. Rev. Arg. Microbiol. 2001, 33, 113–117. [Google Scholar]
- Cariddi, L.N.; Panero, A.; Demo, M.S.; Sabini, L.I.; Maldonado, A.M.; Grosso, M.; Zygadlo, J. Inhibition of Immediate-Type Allergic Reaction by Minthostachys verticillata (Griseb.) Epling Essential Oil. J. Essent. Oil Res. 2007, 19, 190–196. [Google Scholar] [CrossRef]
- Cariddi, L.; Moser, M.; Andrada, M.C.; Demo, M.S.; Zygadlo, J.A.; Sabini, L.I.; Maldonado, A.M. The Effect of Minthostachys verticillata Essential Oil on the Immune Response of Patients Allergic to Dust Mites. Boletín Latinoam. Y Del Caribe De Plantas Med. Y Aromáticas 2009, 8, 224–233. [Google Scholar]
- Cariddi, L.; Escobar, F.; Moser, M.; Panero, A.; Alaniz, F.; Zygadlo, J.; Sabini, L.; Maldonado, A. Monoterpenes Isolated from Minthostachys verticillata (Griseb.) Epling Essential Oil Modulates Immediate-Type Hypersensitivity Responses In Vitro and In Vivo. Planta Med. 2011, 77, 1687–1694. [Google Scholar] [CrossRef]
- Escobar, F.M.; Magnoli, A.; Sabini, M.C.; Cariddi, L.N.; Bagnis, G.; Soltermann, A.; Cavaglieri, L. Minthostachys verticillata Essential Oils as Potential Phytogenic Additives and Chemoprotective Strategy on Aflatoxin B1 Toxicity. J. Appl. Anim. Res. 2019, 47, 217–222. [Google Scholar] [CrossRef]
- Escobar, F.M.; Sabini, M.C.; Cariddi, L.N.; Sabini, L.I.; Mañas, F.; Cristofolini, A.; Bagnis, G.; Gallucci, M.N.; Cavaglieri, L.R. Safety Assessment of Essential Oil from Minthostachys verticillata (Griseb.) Epling (peperina) 90-days Oral Subchronic Toxicity Study in Rats. Regul. Toxicol. Pharmacol. 2015, 71, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Montironi, I.D.; Cariddi, L.N.; Reinoso, E.B. Evaluation of the Antimicrobial Efficacy of Minthostachys verticillata Essential Oil and Limonene Against Streptococcus uberis Strains Isolated from Bovine Mastitis. Rev. Argent. Microbiol. 2016, 48, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Cerioli, M.F.; Moliva, M.V.; Cariddi, L.N.; Reinoso, E.B. Effect of the Essential Oil of Minthostachys verticillata (Griseb.) Epling and Limonene on Biofilm Production in Pathogens Causing Bovine Mastitis. Front. Vet. Sci. 2018, 5, 146. [Google Scholar] [CrossRef] [PubMed]
- Hiltner, L. Über neuere Erfahrungen und Probleme auf dem Gebiet der Bodenbakteriologie und unterbesonderer Berücksichtigung der Gründüngung und Brachte. Arb. Der Dtsch. Landwirtsch. Ges. 1904, 98, 59–78. [Google Scholar]
- Foster, R.C. Microenvironments of Soil Microorganisms. Biol. Fert. Soils 1998, 6, 189–203. [Google Scholar] [CrossRef]
- McNear, D.H., Jr. The Rhizosphere–Roots, Soil and Everything In Between. Nat. Sci. Educ. 2013, 4, 1. [Google Scholar]
- Tan, S.; Yanga, C.; Meia, X.; Shenb, S.; Razaam, W.; Shena, Q.; Xu, Y. The Effect of Organic Acids from Tomato Root Exudates on Rhizosphere Colonization of Bacillus amyloliquefaciens T-5. Appl. Soil Ecol. 2013, 64, 15–22. [Google Scholar] [CrossRef]
- Walker, S.T.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root Exudation and Rhizosphere Biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Park, S.W.; Weir, T.L.; Callaway, R.M.; Vivanco, J.M. How Plants Communicate Using the Underground Information Superhighway. Trends Plant Sci. 2004, 9, 26–32. [Google Scholar] [CrossRef]
- Ortíz-Castro, R.; Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J. The Role of Microbial Signals in Plant Growth and Development. Plant Signal. Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef]
- Babalola, O.O. Beneficial Bacteria of Agricultural Importance. Biotechnol. Lett. 2010, 32, 1559–1570. [Google Scholar] [CrossRef]
- Kloepper, J.W. Plant-Growth-Promoting Rhizobacteria as Biological Control Agents. In Soil Microbial Ecology: Applications in Agricultural and Environmental Management; Metting, F.B., Jr., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1993; pp. 255–274. [Google Scholar]
- Glick, B.R. Bacteria with ACC Deaminase can Promote Plant Growth and Help to Feed the World. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Boyce, A.N. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability–A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Babalola, O.O.; Fadiji, A.E.; Enagbonma, B.J.; Alori, E.T.; Ayilara, M.S.; Ayangbenro, A.S. The Nexus Between Plant and Plant Microbiome: Revelation of the Networking Strategies. Front. Microbiol. 2020, 11, 548037. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Adl, S.M. Plant Growth-Promoting Rhizobacteria (PGPR) and Their Action Mechanisms in Availability of Nutrients to Plants. In Phyto-Microbiome in Stress Regulation. Environmental and Microbial Biotechnology; Kumar, M., Kumar, V., Prasad, R., Eds.; Springer: Singapore, 2020; pp. 147–203. [Google Scholar] [CrossRef]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant Growth Promoting Rhizobacteria (PGPR): Current and Future Prospects for Development of Sustainable Agriculture. J. Microb. Biochem. Technol. 2015, 7, 96–102. [Google Scholar] [CrossRef]
- Sivasakthi, S.; Usharani, G.A.; Saranraj, P. Biocontrol Potentiality of Plant Growth Promoting Bacteria (PGPR)–Pseudomonas fluorescens and Bacillus subtilis: A review. Afr. J. Agric. Res. 2014, 9, 1265–1277. [Google Scholar]
- David, B.V.; Chandrasehar, G.; Selvam, P.N. Chapter 10: Pseudomonas fluorescens: A Plant-Growth-Promoting Rhizobacterium (PGPR) with Potential Role in Biocontrol of Pests of Crops. In New and Future Developments in Microbial Biotechnology and Bioengineering; Prasad, R., Tuteja, N., Gill, S.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 221–243. [Google Scholar]
- Sah, S.; Krishnani, S.; Singh, R. Pseudomonas Mediated Nutritional and Growth Promotional Activities for Sustainable Food Security. Curr. Res. Microb. Sci. 2021, 2, 100084. [Google Scholar] [CrossRef] [PubMed]
- Lalucat, J.; Gomila, M.; Mulet, M.; Zaruma, A.; García-Valdés, E. Past, Present and Future of the Boundaries of the Pseudomonas Genus: Proposal of Stutzerimonas gen. Nov. Syst. Appl. Microbiol. 2022, 45, 126289. [Google Scholar] [CrossRef]
- Lami, M.J.; Adler, C.; Caram-Di Santo, M.C.; Zenoff, A.M.; de Cristobal, R.E.; Espinosa-Urgel, M.; Vincent, P.A. Pseudomonas stutzeri MJL19, a Rhizosphere-Colonizing Bacterium That Promotes Plant Growth Under Saline Stress. J. Appl. Microbiol. 2020, 129, 1321–1336. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida Indole Acetic Acid in Development of the Host Plant Root System. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef]
- Sergeeva, E.; Hirkala, D.L.; Nelson, L.M. Production of Indole-3-Acetic Acid, Aromatic Amino Acid Aminotransferase Activities and Plant Growth Promotion by Pantoea agglomerans Rhizosphere Isolates. Plant Soil 2007, 297, 1–13. [Google Scholar] [CrossRef]
- El-Ramady, H.R.; Alshaal, T.A.; Amer, M.; Domokos-Szabolcsy, É.; Elhawat, N.; Prokisch, J.; Fári, M. Sustainable Agriculture Reviews; Ozier-Lafontaine, H., Lesueur-Jannoyer, M., Eds.; Springer: Cham, Switzerland, 2014; pp. 345–447. [Google Scholar]
- Calvo Velez, P.; Reymundo Meneses, L.; Zuniga Davila, D. A Study of Potato (Solanum tuberosum) Crop Rhizosphere Microbial Population in Highland Zones. Ecol. Apl. 2008, 7, 141–148. [Google Scholar]
- Santoro, V.M.; Bogino, P.C.; Nocelli, N.; Cappellari, L.; Giordano, W.F.; Banchio, E. Analysis of Plant Growth-Promoting Effects of Fluorescent Pseudomonas Strains Isolated from Mentha piperita Rhizosphere and Effects of Their Volatile Organic Compounds on Essential Oil Composition. Front. Microbiol. 2016, 7, 1085. [Google Scholar] [CrossRef]
- Ahmed, E.A.; Hassan, E.A.; El Tobgy, K.M.K.; Ramadan, E.M. Evaluation of Rhizobacteria of Some Medicinal Plants for Plant Growth Promotion and Biological Control. Ann. Agric. Sci. 2014, 59, 273–280. [Google Scholar] [CrossRef]
- Memenza-Zegarra, M.; Zúñiga-Dávila, D. Bioprospection of Native Antagonistic Rhizobacteria from the Peruvian Coastal Ecosystems Associated with Phaseolus vulgaris. Curr. Microbiol. 2021, 78, 1418–1431. [Google Scholar] [CrossRef]
- Houlden, A.; Timms-Wilson, T.M.; Day, M.J.; Bailey, M.J. Influence of Plant Developmental Stage on Microbial Community Structure and Activity in the Rhizosphere of Three Field Crops. FEMS Microbiol. Ecol. 2008, 65, 193–201. [Google Scholar] [CrossRef]
- Agaras, B.; Wall, L.G.; Valverde, C. Specific Enumeration and Analysis of the Community Structure of Culturable Pseudomonads in Agricultural Soils Under No-Till Management in Argentina. Appl. Soil Ecol. 2012, 61, 305–319. [Google Scholar] [CrossRef]
- Palleroni, N.J. Genus Pseudomonas. In Bergey’s Manual® of Systematic Bacteriology, 2nd ed.; Brenner, D.J., Krieg, N.R., Staley, J.T., Eds.; Springer: New York, NY, USA, 2005; pp. 323–379. [Google Scholar]
- Garrido-Sanz, D.; Meier-Kolthoff, J.P.; Göker, M.; Martín, M.; Rivilla, R.; Redondo-Nieto, M. Genomic and genetic diversity within the Pseudomonas fluorescens complex. PLoS ONE 2016, 11, e0150183. [Google Scholar] [CrossRef]
- Fiodor, A.; Ajijah, N.; Dziewit, L.; Pranaw, K. Biopriming of Seed with Plant Growth-Promoting Bacteria for Improved Germination and Seedling Growth. Front. Microbiol. 2023, 14, 1142966. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.K.; Prasad, G. Integrated management of Sclerotium rolfsii: An overview. Eur. J. Biomed. Pharm. Sci. 2016, 3, 137–146. [Google Scholar]
- Pedraza, R.; Ramirez-Mata, A.; Xiqui, M.; Baca, B. Aromatic Aminoacid Aminotransferase Activity and Indole-3 Acetic Acid Production by Associative Nitrogen-Fixing Bacteria. FEMS Microbiol. Lett. 2004, 233, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The Rhizosphere: A Playground and Battlefield for Soilborne Pathogens and Beneficial Microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Choudhary, D.K. Plant Growth-Promotion (PGP) Activities and Molecular Characterization of Rhizobacterial Strains Isolated from Soybean (Glycine max L. Merril) Plants Against Charcoal Rot Pathogen, Macrophomina phaseolina. Biotechnol. Lett. 2011, 33, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Lyu, D.; Backer, R.; Smith, D.L. Three Plant Growth-Promoting Rhizobacteria Alter Morphological Development, Physiology, and Flower Yield of Cannabis sativa L. Ind. Crops Prod. 2022, 178, 114583. [Google Scholar] [CrossRef]
- Chepsergon, J.; Moleleki, L.N. Rhizosphere Bacterial Interactions and Impact on Plant Health. Curr. Opin. Microbiol. 2023, 73, 102297. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, L.R.; Santoro, M.V.; Reinoso, H.; Travaglia, C.; Giordano, W.; Banchio, E. Anatomical, Morphological, and Phytochemical Effects of Inoculation with Plant Growth-Promoting Rhizobacteria on Peppermint (Mentha piperita). J. Chem. Ecol. 2015, 41, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Ordookhani, K.; Sharafzadeh, S.; Zare, M. Influence of PGPR on Growth, Essential Oil and Nutrients Uptake of Sweet Basil. Adv. Environ. Biol. 2011, 5, 672–677. [Google Scholar]
- Mohammadi, H.; Dashi, R.; Farzaneh, M. Effects of Beneficial Root Pseudomonas on Morphological, Physiological, and Phytochemical Characteristics of Satureja hortensis (Lamiaceae) Under Water Stress. Braz. J. Bot. 2017, 40, 41–48. [Google Scholar] [CrossRef]
- Dehghani Bidgoli, R.; Azarnezhad, N.; Akhbari, M.; Ghorbani, M. Salinity Stress and PGPR Effects on Essential Oil Changes in Rosmarinus officinalis L. Agric. Food Secur. 2019, 8, 2. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant Growth Promoting Rhizobacteria as Biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- van Loon, L.C. Plant Responses to Plant Growth-Promoting Rhizobacteria. In New Perspectives and Approaches in Plant Growth-Promoting Rhizobacteria Research; Bakker, P.A.H.M., Raaijmakers, J.M., Bloemberg, G., Höfte, M., Lemanceau, P., Cooke, B.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 243–254. [Google Scholar] [CrossRef]
- Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R.A.; del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López Baena, F.J.; Ollero, F.J.; Cubo, T. Plant Growth Promotion in Cereal and Leguminous Agricultural Important Plants: From Microorganism Capacities to Crop Production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.V.; Zygadlo, J.; Giordano, W.; Banchio, E. Volatile Organic Compounds from Rhizobacteria Increase Biosynthesis of Essential Oils and Growth Parameters in Peppermint (Mentha piperita). Plant Physiol. Biochem. 2011, 49, 1177–1182. [Google Scholar] [CrossRef]
- Santoro, M.; Cappellari, L.; Giordano, W.; Banchio, E. Plant Growth Promoting Effects of Native Pseudomonas Strains on Mentha piperita (peppermint): An in Vitro Study. Plant Biol. 2015, 17, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, L.; Santoro, M.V.; Schmidt, A.; Gershenzon, J.; Banchio, E. Induction of Essential Oil Production in Mentha x piperita by Plant Growth Promoting Bacteria Was Correlated with an Increase in Jasmonate and Salicylate Levels and a Higher Density of Glandular Trichomes. Plant Physiol. Biochem. 2019, 141, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Mekureyaw, M.F.; Pandey, C.; Hennessy, R.C.; Nicolaisen, M.H.; Liu, F.; Nybroe, O.; Roitsch, T. The Cytokinin-Producing Plant Beneficial Bacterium Pseudomonas fluorescens G20-18 Primes Tomato (Solanum lycopersicum) for Enhanced Drought Stress Responses. J. Plant Physiol. 2022, 270, 153629. [Google Scholar] [CrossRef] [PubMed]
- Kalozoumis, P.; Savvas, D.; Aliferis, K.; Ntatsi, G.; Marakis, G.; Simou, E.; Tampakaki, A.; Karapanos, I. Impact of Plant Growth-Promoting Rhizobacteria Inoculation and Grafting on Tolerance of Tomato to Combined Water and Nutrient Stress Assessed via Metabolomics Analysis. Front. Plant Sci. 2021, 12, 670236. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.; Poulev, A.; Chrisler, W.; Acosta, K.; Orr, G.; Lebeis, S.; Lam, E. Auxin-Producing Bacteria from Duckweeds Have Different Colonization Patterns and Effects on Plant Morphology. Plants 2022, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Askari, K.; Kumari, M. Optimization of Indole Acetic Acid Production by Isolated Bacteria from Stevia rebaudiana Rhizosphere and its Effects on Plant Growth. J. Genet. Eng. Biotechnol. 2018, 16, 581–586. [Google Scholar] [CrossRef]
- Yousef, N.M. Capability of Plant Growth-Promoting Rhizobacteria (PGPR) for Producing Indole Acetic Acid (IAA) Under Extreme Conditions. Eur. J. Biol. Res. 2018, 8, 174–182. [Google Scholar] [CrossRef]
- Wahyudi, A.T.; Astuti, R.P.; Widyawati, A.; Meryandini, A.; Nawangsih, A.A. Characterization of Bacillus sp. Strains Isolated from Rhizosphere of Soybean Plants for Their Use as Potential Plant Growth for Promoting Rhizobacteria. J. Microbiol. Antimicrob. 2011, 3, 34–40. [Google Scholar]
- Zhang, H.; Zheng, D.; Hu, C.; Cheng, W.; Lei, P.; Xu, H.; Gao, N. Certain Tomato Root Exudates Induced by Pseudomonas stutzeri NRCB010 Enhance Its Rhizosphere Colonization Capability. Metabolites 2023, 13, 664. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Rediers, H.; Ghequire, M.G.; Nguyen, H.H.; De Mot, R.; Vanderleyden, J.; Spaepen, S. The Plant Growth-Promoting Effect of the Nitrogen-Fixing Endophyte Pseudomonas stutzeri. Arch. Microbiol. 2017, 199, 513–517. [Google Scholar] [CrossRef] [PubMed]
- King, E.O.; Ward, M.K.; Randey, D.E. Two Simple Media for the Demonstration of Pyocyanin and Fluorescein. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar] [PubMed]
- Mehnaz, S.; Weselowski, B.; Aftab, F.; Zahid, S.; Lazarovits, G.; Iqbal, J. Isolation, Characterization and Effect of Fluorescent Pseudomonads on Micropropagated Sugarcane. Can. J. Microbiol. 2009, 55, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Whittles, C.L.; Little, R.C. A Colorimetric Method for the Determination of Potassium and its Application to the Analysis of Soil Extracts. J. Sci. Food Agric. 1950, 1, 323–326. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer Method Improved for Making Particle Size Analysis of Soil. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Lysenko, O. Pseudomonas–an attempt at a general classification. J. Gen. Microbiol. 1961, 25, 379–408. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Palleroni, N.J.; Doudoroff, M. The Aerobic Pseudomonads: A Taxonomic Study. J. Gen. Microbiol. 1966, 43, 159–271. [Google Scholar] [CrossRef]
- Moaledj, K. Comparison of Gram-Staining and Alternate Methods, KOH Test and Aminopeptidase Activity in Aquatic Bacteria: Their Application to Numerical Taxonomy. J. Microbiol. Methods 1986, 5, 303–310. [Google Scholar] [CrossRef]
- Urzí, C.; Brusetti, L.; Salamone, P.; Sorlini, C.; Stackebrandt, E.; Daffonchio, D. Biodiversity of Geodermatophilaceae Isolated from Altered Stones and Monuments in the Mediterranean Basin. Environ. Microbiol. 2001, 3, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Sierra, G. A Simple Method for the Detection of Lipolytic Activity of Microorganisms and Some Observations on the Influence of the Contact Between Cells and Fatty Substrates. Antonie Van Leeuwenhoek 1957, 23, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.E.; Mihm, J.M. A comparative study of some strains received as Nocardia. J. Bacteriol. 1956, 73, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, W.A.; Lykos, J.J. Hydrolysis of Casein: A Differential Aid for the Identification of Serratia marcescens. J. Clin. Path. 1972, 25, 1083–1085. [Google Scholar] [CrossRef] [PubMed]
- Sneath, P.H.A. Cultural and Biochemical Characteristics of the Genus Chromobacterium. J. Gen. Microbiol. 1956, 15, 70–98. [Google Scholar] [CrossRef]
- Sachdev, D.P.; Chaudhari, H.G.; Kasture, V.M.; Dhavale, D.D.; Chopade, B.A. Isolation and Characterization of Indole Acetic Acid (IAA) Producing Klebsiella pneumoniae Strains from Rhizosphere of Wheat (Triticum aestivum) and Their Effect on Plant Growth. Indian J. Exp. Biol. 2009, 47, 993–1000. [Google Scholar] [PubMed]
- Masciarelli, O.; Llanes, A.; Luna, V. A New PGPR Co-Inoculated with Bradyrhizobium japonicum Enhances Soybean Nodulation. Microbiol. Res. 2014, 169, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Milagres, A.M.; Machuca, A.; Napoleao, D. Detection of siderophore production from several fungi and bacteria by a modification of chrome azurol S (CAS) agar plate assay. J. Microbiol. Methods 1999, 37, 1–6. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal Chemical Assay for the Detection and Determination of Siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Pikovskaya, R. Mobilization of Phosphorus in Soil in Connection with Vital Activity of Some Microbial Species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Kang, B.R.; Anderson, A.J.; Kim, Y.C. Hydrogen Cyanide Produced by Pseudomonas chlororaphis O6 is a Key Aphicidal Metabolite. Can. J. Microbiol. 2019, 65, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.; Lone, R.; Mohamed, H.I. Production of Antibiotics from PGPR and Their Role in Biocontrol of Plant Diseases. In Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management; Mohamed, H.I., El-Beltagi, H.E.-D.S., Abd-Elsalam, K.A., Eds.; Springer: Cham, Switzerland, 2021; pp. 441–461. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.; Madden, T.; Schäffer, A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]



| Chemical Characteristics | |
|---|---|
| pH | 7.50 |
| OM (%) | 5.48 |
| H° (%) | 32.10 |
| P (ppm) | 57.00 |
| N-NO3 (ppm) | 23.30 |
| NO3 (ppm) | 103.22 |
| Ca+2 (cmol/Kg) | 16.00 |
| Mg+2 (cmol/Kg) | 6.50 |
| Na+ (cmol/Kg) | 0.90 |
| K+ (cmol/Kg) | 2.31 |
| CEC (cmol/Kg) | 27.83 |
| Strain | Gram | Catalase | Oxidase | Pigmentation | Growth | Hydrolysis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| King B | 4 °C | 42 °C | NaCl 5% | NaCl 6.5% | Lipids | Starch | Casein | Lecithinase | ||||
| SM 2 | − | + | + | green | − | − | +/− | − | − | − | − | |
| SM 6 | − | + | + | orange | − | − | − | − | + | − | + | − |
| SM 8 | − | + | + | green | − | − | +/− | − | − | − | − | − |
| SM 12 | − | + | + | orange | − | − | − | − | − | − | − | |
| SM 17 | − | + | − | − | − | − | − | + | − | + | − | |
| SM 18 | − | + | − | orange | − | − | +/− | − | − | − | − | − |
| SM 19 | − | + | − | orange | − | − | +/− | − | − | − | − | − |
| SM 20 | − | + | + | green | + | − | − | − | − | − | + | − |
| SM 21 | − | + | + | green | + | − | − | − | − | − | + | − |
| SM 24 | − | − | + | − | − | − | − | − | − | − | − | |
| SM 25 | − | + | − | − | + | − | − | + | − | − | + | |
| SM 27 | − | + | + | green | − | − | − | − | − | − | − | − |
| SM 29 | − | + | + | + | − | − | − | − | − | − | − | |
| SM 33 | − | + | + | + | − | +/− | − | − | − | − | − | |
| SM 35 | − | + | + | green | − | + | − | − | − | − | − | − |
| SM 40 | − | + | + | + | + | + | − | + | + | + | + | |
| SM 41 | − | + | + | + | − | + | − | − | − | + | − | |
| SM 42 | − | + | + | + | − | − | − | − | − | + | − | |
| SM 46 | − | + | + | green | − | − | +/− | − | − | − | − | − |
| SM 47 | − | + | + | green | + | − | − | − | − | − | − | − |
| SM 49 | − | + | + | + | − | − | − | − | − | + | − | |
| SM 51 | − | + | + | + | + | − | − | + | + | − | − | |
| Strain | Phosphate Solubilization | Siderophores Production | IAA Production | CHN Production | Antibiosis % |
|---|---|---|---|---|---|
| SM2 | − | − | + | − | 19 |
| SM6 | − | − | − | + | 5 |
| SM8 | − | − | + | − | 21 |
| SM12 | − | − | + | − | 5 |
| SM17 | − | − | − | − | 0 |
| SM18 | + | − | + | − | 12 |
| SM19 | + | − | + | − | 9 |
| SM20 | + | − | + | − | 31 |
| SM21 | − | − | + | + | 14 |
| SM24 | + | − | + | − | 14 |
| SM25 | − | − | − | − | 50 |
| SM27 | + | − | − | − | 29 |
| SM29 | + | − | − | − | 19 |
| SM33 | + | − | + | − | 34 |
| SM35 | − | − | − | − | 38 |
| SM40 | + | − | − | − | 33 |
| SM41 | − | − | + | − | 14 |
| SM42 | + | − | + | − | 12 |
| SM46 | + | − | − | − | 14 |
| SM47 | + | − | + | − | 19 |
| SM49 | + | − | + | − | 19 |
| SM51 | − | − | − | − | 19 |
| Sample | IAA (ng/mL) |
|---|---|
| Control LB | 30.9 |
| SM 8 | 556.7 |
| SM 20 | 578.1 |
| SM 21 | 717.8 |
| SM 33 | 936.3 |
| Native Strain | Aligned Species (Access Number) | Homology | Coverage | Annotation (Access Number) |
|---|---|---|---|---|
| SM 8 | Pseudomonas sp. | 99% | ~61–63% | Pseudomonas sp. |
| SM 21 | Pseudomona putida IR7-6 (MW686888.1) | 99% | 99% | Pseudomonas putida SM21 (OR343518) |
| SM 33 | Stutzerimona stutzeri KGS-2 (CP018050.1) | 99% | 95% | Stutzerimona stutzeri SM33 (OR343508) |
| SM 20 | Pseudomonas sp. | 99% | ~62–63% | Pseudomonas sp. |
| Leaf Number | Ramification Number | Node Number | Shoot Lenght (cm) | |
|---|---|---|---|---|
| control | 17.50 ± 1.20 a | 0.5 0± 0.13 a | 7.17 ± 0.22 a | 15.67 ± 0.99 a |
| SM 33 | 24.07 ± 1.12 b | 1.70 ± 0.23 b | 8.30 ± 0.22 b | 20.07 ± 0.83 b |
| WCS417r | 17.27 ± 1.33 a | 0.85 ± 0.21 a | 7.00 ± 0.18 a | 15.75 ± 1.15 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meneguzzi, R.d.V.; Fernandez, M.; Cappellari, L.d.R.; Giordano, W.; Banchio, E. Isolation and Characterization of Plant Growth-Promoting Bacteria from the Rhizosphere of Medicinal and Aromatic Plant Minthostachys verticillata. Plants 2024, 13, 2062. https://doi.org/10.3390/plants13152062
Meneguzzi RdV, Fernandez M, Cappellari LdR, Giordano W, Banchio E. Isolation and Characterization of Plant Growth-Promoting Bacteria from the Rhizosphere of Medicinal and Aromatic Plant Minthostachys verticillata. Plants. 2024; 13(15):2062. https://doi.org/10.3390/plants13152062
Chicago/Turabian StyleMeneguzzi, Romina del Valle, Marilina Fernandez, Lorena del Rosario Cappellari, Walter Giordano, and Erika Banchio. 2024. "Isolation and Characterization of Plant Growth-Promoting Bacteria from the Rhizosphere of Medicinal and Aromatic Plant Minthostachys verticillata" Plants 13, no. 15: 2062. https://doi.org/10.3390/plants13152062





