The Biotechnological Potential of Plant Growth-Promoting Rhizobacteria Isolated from Maize (Zea mays L.) Cultivations in the San Martin Region, Peru
Abstract
:1. Introduction
2. Results
2.1. Isolation of Rhizobacteria from Maize Plants
2.2. Promoters of Plant Growth Parameters Produced by Rhizobacteria
2.2.1. Production of Indole Acetic Acid (IAA)
2.2.2. Solubilization of Aluminum Phosphate (AlPO4)
2.2.3. Production of Siderophores
2.3. Seed Germination Assay
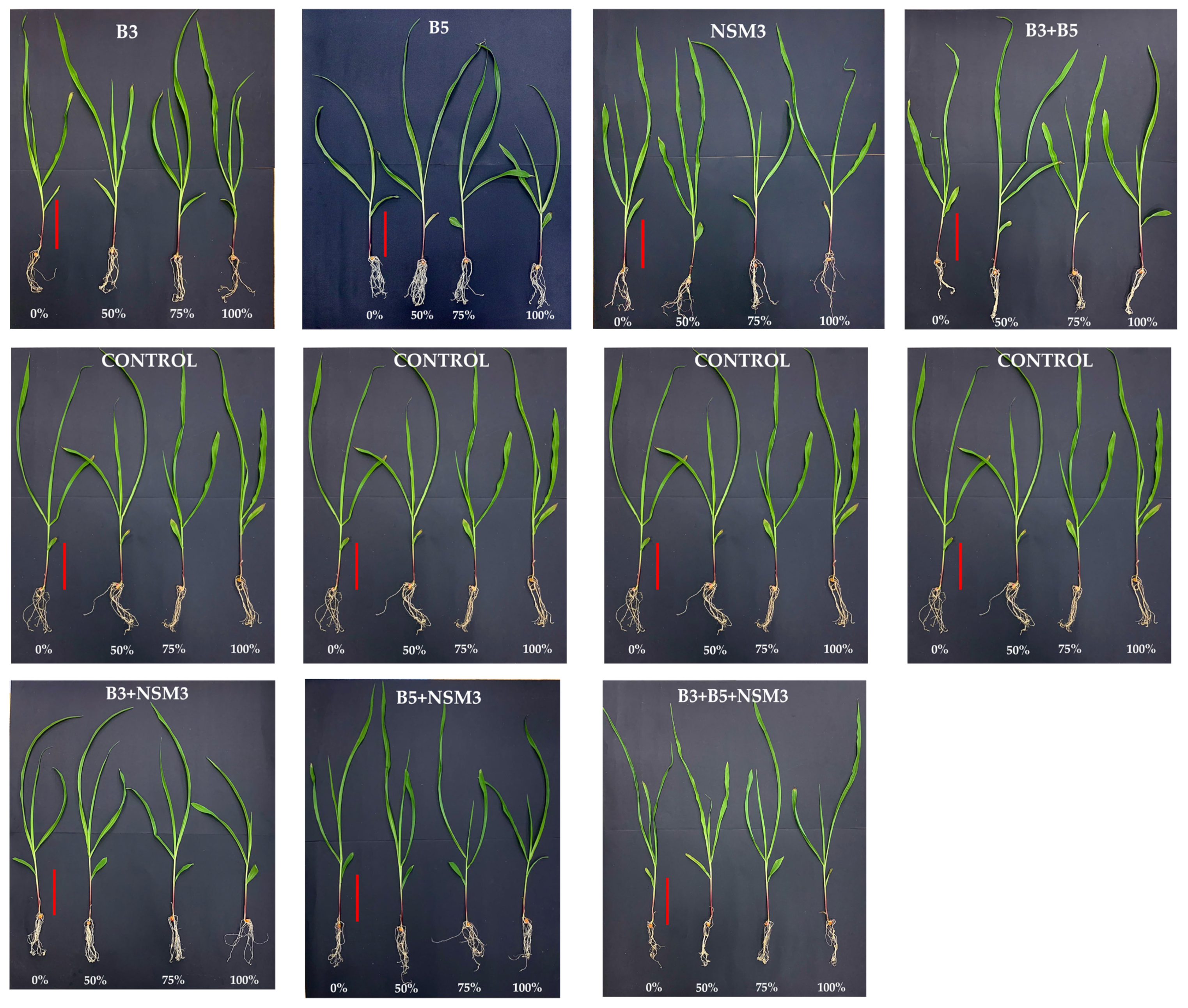
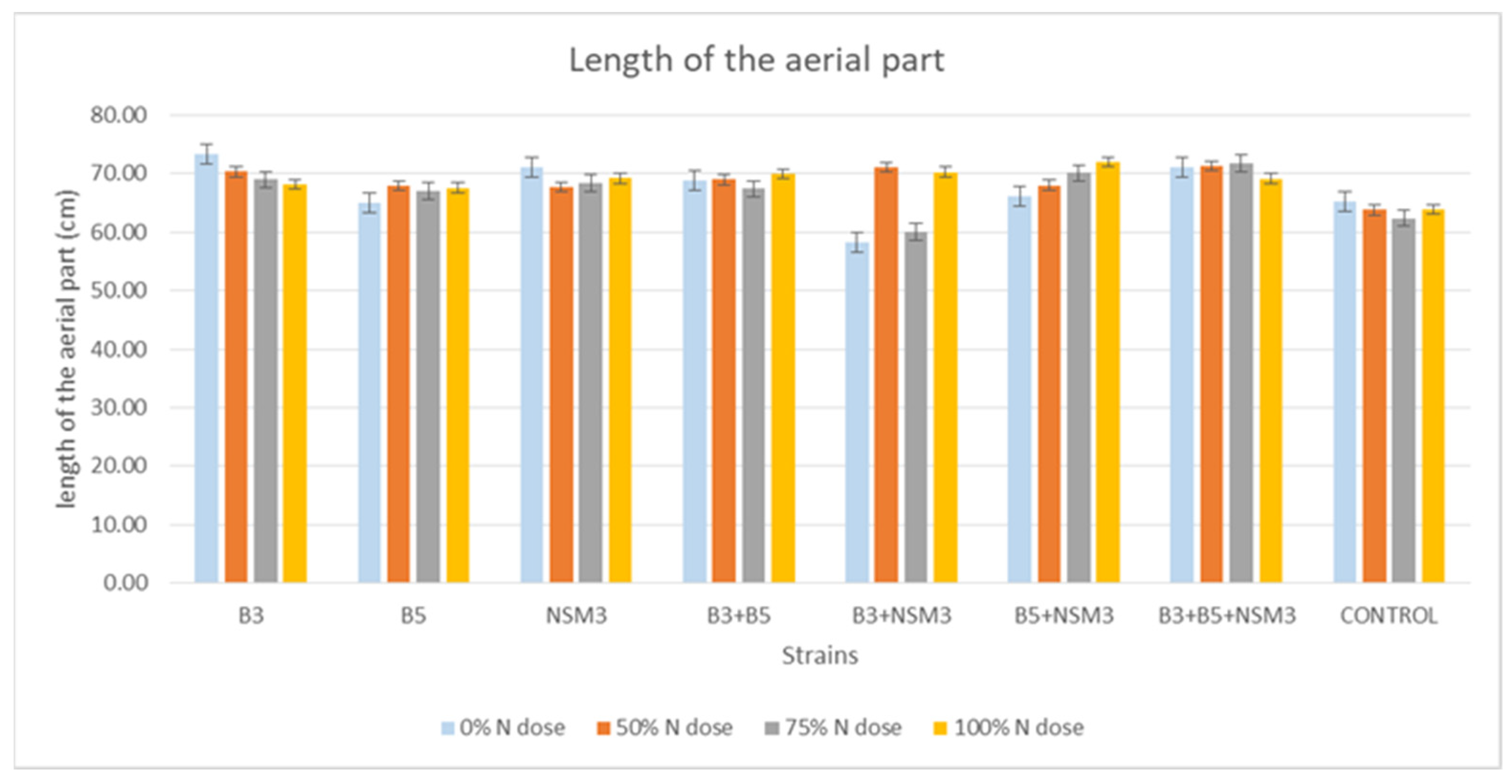
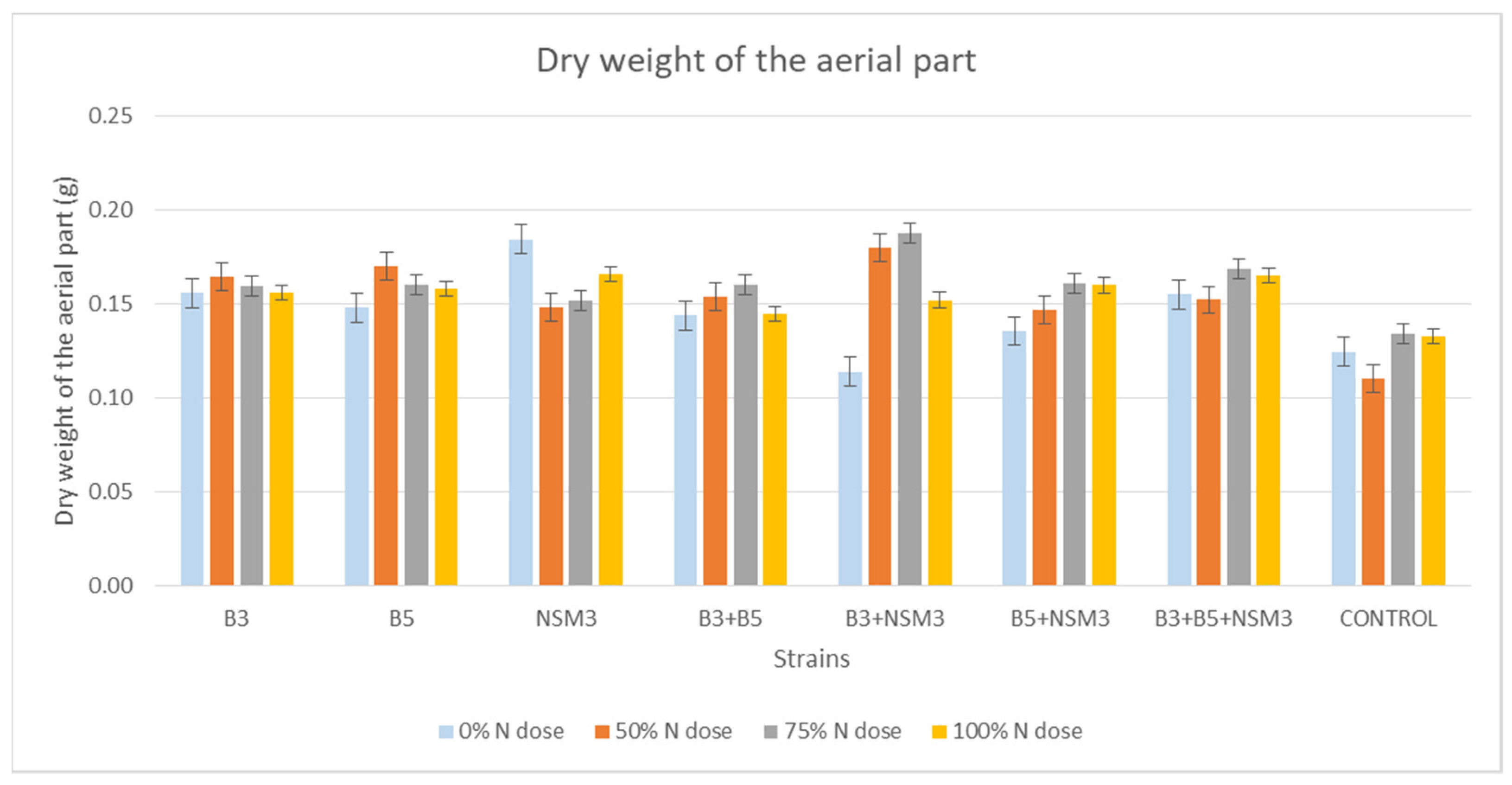
2.4. Gnotobiotic Experiment
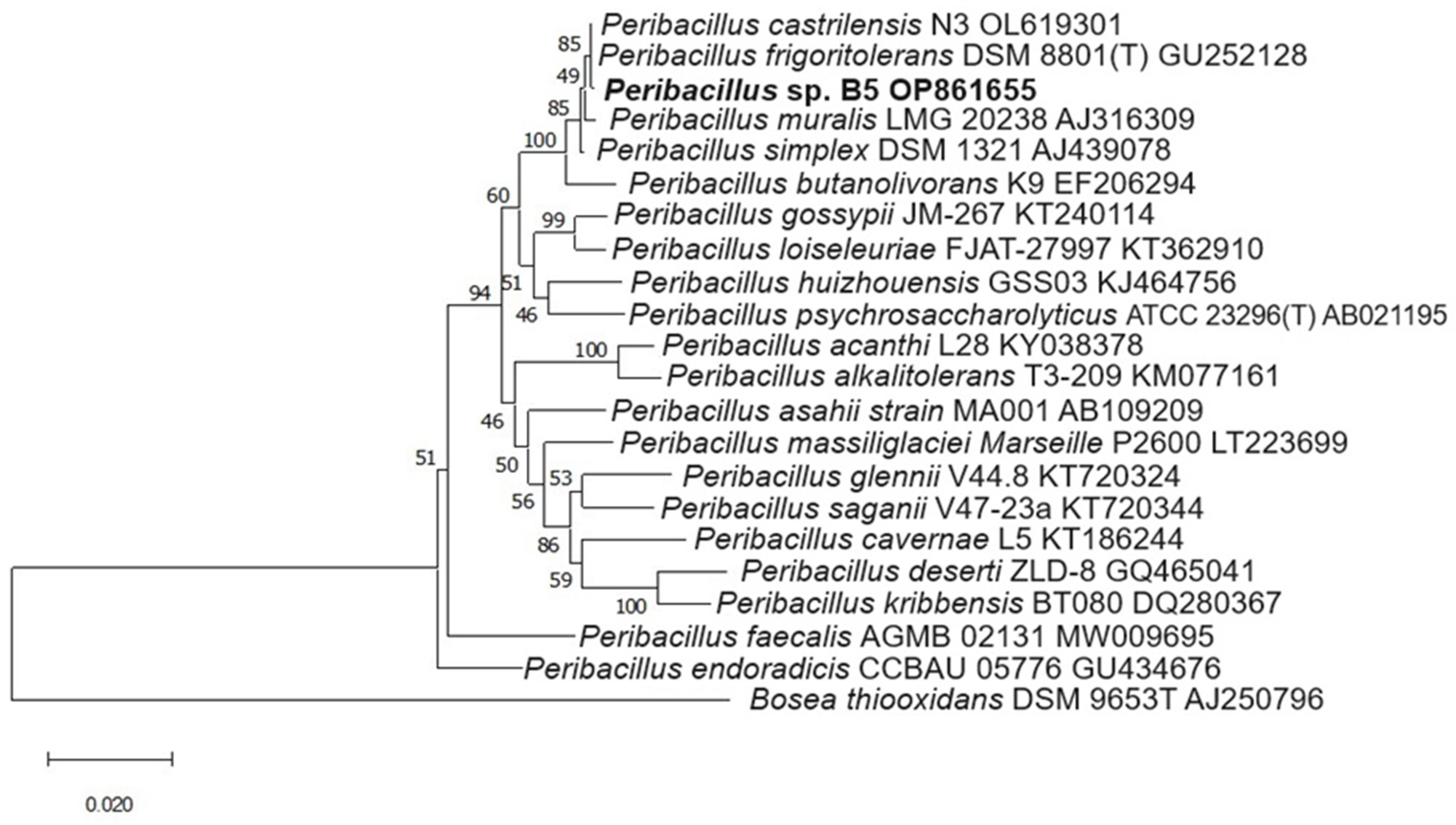
2.5. Molecular Identification of Isolates
3. Discussion
3.1. Isolation of Rhizobacteria from Maize Plants
3.2. Plant Growth-Promoting Parameters Produced by Rhizobacteria
3.2.1. Production of IAA
3.2.2. Solubilization of AlPO4
3.2.3. Siderophore Production
3.3. Germination Assay
3.4. Gnotobiotic Experiment
3.5. Molecular Identification of Isolates
4. Materials and Methods
4.1. Isolation of Rhizobacteria from Maize Plants
4.2. Evaluation of Plant Growth-Promoting Parameters Produced by Rhizobacteria
4.2.1. Determination of IAA Production
4.2.2. Determination of AlPO4 Solubilization
4.2.3. Measurement of Siderophore Production
4.3. Germination Assay
4.4. Gnotobiotic Experiment
4.5. Molecular Identification of the Isolates
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chávez, A.; Narro León, L.A.; Jara Calvo, T.W.; Narro León, T.P.; Medina Hoyos, A.E.; Cieza Ruiz, I.; Díaz Chuquizuta, P.; Alvarado Rodríguez, R.; Escobal Valencia, F. Tecnologías disponibles para incrementar la producción de maíz en Perú. ACI Av. Cienc. Ing. 2022, 14, 2507. [Google Scholar] [CrossRef]
- Ministerio de Desarrollo Agrario y Riego (MIDAGRI). Perfil Productivo Regional. 2024. Available online: https://siea.midagri.gob.pe/portal/siea_bi/index.html (accessed on 16 June 2024).
- Abdelkhalek, S.T.; Moussa, M.A.; Gomaa, S.I.; Qiu, C.L.; Wang, M.Q. Agrochemicals: Safety Evaluation and Characterization for Humans and Biodiversity. In One Health Implications of Agrochemicals and Their Sustainable Alternatives. Sustainable Development and Biodiversity; Ogwu, M.C., Chibueze Izah, S., Eds.; Springer: Singapore, 2023; Volume 34, pp. 3–51. [Google Scholar] [CrossRef]
- Van Deynze, A.; Zamora, P.; Delaux, P.M.; Heitmann, C.; Jayaraman, D.; Rajasekar, S.; Graham, D.; Maeda, J.; Gibson, D.; Schwartz, K.D.; et al. Nitrogen fixation in a landrace of maize is supported by a mucilage-associated diazotrophic microbiota. PLoS Biol. 2018, 16, e2006352. [Google Scholar] [CrossRef]
- Ríos-Ruiz, W.F.; Torres-Chávez, E.E.; Torres-Delgado, J.; Rojas-García, J.C.; Bedmar, E.J.; Valdez-Nuñez, R.A. Inoculation of bacterial consortium increases rice yield (Oryza sativa L.) reducing applications of nitrogen fertilizer in San Martin region, Peru. Rhizosphere 2020, 14, 100200. [Google Scholar] [CrossRef]
- Ríos-Ruiz, W.F.; Tuanama-Reátegui, C.; Huamán-Córdova, G.; Valdez-Nuñez, R.A. Co-Inoculation of Endophytes Bacillus siamensis TUR07-02b and Priestia megaterium SMBH14-02 Promotes Growth in Rice with Low Doses of Nitrogen Fertilizer. Plants 2023, 12, 524. [Google Scholar] [CrossRef] [PubMed]
- Galindo, F.S.; Pagliari, P.H.; da Silva, E.C.; de Lima, B.H.; Fernández, G.C.; Thiengo, C.C.; Bernardes, J.V.S.; Jalal, A.; Oliveira, C.E.S.; de Sousa Vilela, L.; et al. Impact of nitrogen fertilizer sustainability on corn crop yield: The role of beneficial microbial inoculation interactions. BMC Plant Biol. 2024, 24, 268. [Google Scholar] [CrossRef] [PubMed]
- Gil, T.; Romão, I.R.; do Carmo Gomes, J.; Vergara-Diaz, O.; de Carvalho, L.A.L.; Sousa, A.; Kasa, F.; Teixiera, R.; Mateus, S.; Katamadze, A.; et al. Comparing native and non-native seed-isolated strains for drought resilience in maize (Zea mays L.). Plant Stress 2024, 12, 100462. [Google Scholar] [CrossRef]
- Magotra, S.; Negi, N.P.; Kumar, H. Co-shaping and Co-evolution of Microbial Biodiversity: Study for Identification of Potential Plant Growth Promoting Microbes. In Metabolomics, Proteomics and Gene Editing Approaches in Biofertilizer Industry; Kaur, S., Dwibedi, V., Sahu, P.K., Eds.; Springer: Singapore, 2024; pp. 261–281. [Google Scholar] [CrossRef]
- Sharma, P.; Bano, A.; Singh, S.P. Diversity of Microbes Inside Plants and Their Reaction to Biotic and Abiotic Stress. In Planet Earth: Scientific Proposals to Solve Urgent Issues; Núñez-Delgado, A., Ed.; Springer: Cham, Switzerland, 2024; pp. 207–239. [Google Scholar] [CrossRef]
- Vaghela, N.; Gohel, S. Medicinal plant-associated rhizobacteria enhance the production of pharmaceutically important bioactive compounds under abiotic stress conditions. J. Basic Microbiol. 2023, 63, 308–325. [Google Scholar] [CrossRef]
- Rojas-Sánchez, B.; Castelán-Sánchez, H.; Garfias-Zamora, E.Y.; Santoyo, G. Diversity of the Maize Root Endosphere and Rhizosphere Microbiomes Modulated by the Inoculation with Pseudomonas fluorescens UM270 in a Milpa System. Plants 2024, 13, 954. [Google Scholar] [CrossRef]
- Pedrinho, A.; Mendes, L.W.; do Rêgo Barros, F.M.; Bossolani, J.W.; Kühn, T.N.; Quecine, M.C.; Andreote, F.D. The interplay between Azospirillum brasilense and the native bacterial communities in the soil and rhizosphere of maize (Zea mays L.). Soil Biol. Biochem. 2024, 189, 109292. [Google Scholar] [CrossRef]
- Ramírez-Sánchez, D.; Gibelin-Viala, C.; Roux, F.; Vailleau, F. Genetic architecture of the response of Arabidopsis thaliana to a native plant-growth-promoting bacterial strain. Front. Plant Sci. 2023, 14, 1266032. [Google Scholar] [CrossRef]
- Agbodjato, N.A.; Babalola, O.O. Promoting sustainable agriculture by exploiting plant growth-promoting rhizobacteria (PGPR) to improve maize and cowpea crops. PeerJ 2024, 12, e16836. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Pandove, G.; Kaur, A. Role of PGPR as Microbial Inoculants in Improving Fodder Crops Productivity and Quality: A Review. Forage Res. 2023, 48, 420–429. Available online: https://forageresearch.in/wp-content/uploads/2023/06/420-429.pdf (accessed on 14 June 2024).
- Prisa, D.; Fresco, R.; Spagnuolo, D. Microbial Biofertilisers in Plant Production and Resistance: A Review. Agriculture 2023, 13, 1666. [Google Scholar] [CrossRef]
- Ercole, T.G.; Savi, D.C.; Adamoski, D.; Kava, V.M.; Hungria, M.; Galli-Terasawa, L.V. Diversity of maize (Zea mays L.) rhizobacteria with potential to promote plant growth. Braz. J. Microbiol. 2021, 52, 1807–1823. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; Singh, R.K.; Meena, V.S.; Meena, R.K. ¿Can we use maize (Zea mays) rhizobacteria as plant growth promoter? Vegetos 2015, 28, 86–99. [Google Scholar] [CrossRef]
- Oliveira, I.J.; Fontes, J.R.; Pereira, B.F.; Muniz, A. Inoculation with Azospirillum brasiliense increases maize yield. Chem. Biol. Technol. Agric. 2018, 5, 6. [Google Scholar] [CrossRef]
- Moreira, R.C.; Valadão, F.C.A.; Valadão-Júnior, D.D. Desempenho agronômico do milho em função da inoculação com Azospirillum brasilense e adubação nitrogenada. RCA-Amaz. J. Agric. Environ. Sci. 2019, 62, 1–10. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Abu Alhmad, M.F.; Kordrostami, M.; Abo Baker, A.E.; Zakir, A. Inoculation with Azospirillum lipoferum or Azotobacter chroococcum Reinforces Maize Growth by Improving Physiological Activities Under Saline Conditions. J. Plant Growth Regul. 2020, 39, 1293–1306. [Google Scholar] [CrossRef]
- Javoreková, S.; Cinkocki, R.; Maková, J.; Hricáková, N. Isolation and identification of rhizobacteria from maize (Zea mays L.) In luvisols and documentation their plant growth promoting traits. J. Microb. Biotechnol. Food Sci. 2020, 10, 505–510. [Google Scholar] [CrossRef]
- Sukweenadhi, J.; Theda, J.A.; Artadana, I.B.M.; Kang, S.C. Isolation and in vitro Screening of Plant Growth Promoting Rhizospheric Bacteria from Corn (Zea mays var. indentata). Appl. Biochem. Microbiol. 2022, 58, 806–812. [Google Scholar] [CrossRef]
- Roy, B.; Maitra, D.; Biswas, A.; Chowdhury, N.; Ganguly, S.; Bera, M.; Dutta, S.; Golder, S.; Roy, S.; Ghosh, J.; et al. Efficacy of High-Altitude Biofilm-Forming Novel Bacillus subtilis Species as Plant Growth-Promoting Rhizobacteria on Zea mays L. Appl. Biochem. Biotechnol. 2023, 196, 643–666. [Google Scholar] [CrossRef]
- Ercole, T.G.; Kava, V.M.; Aluizio, R.; Pauletti, V.; Hungria, M.; Galli-Terasawa, L.V. Co-inoculation of Bacillus velezensis and Stenotrophomonas maltophilia strains improves growth and salinity tolerance in maize (Zea mays L.). Rhizosphere 2023, 27, 100752. [Google Scholar] [CrossRef]
- Reed, L.; Glick, B.R. The recent use of plant-growth-promoting bacteria to promote the growth of agricultural food crops. Agriculture 2023, 13, 1089. [Google Scholar] [CrossRef]
- John, C.J. PGPR Biotechnology for Management of Biotic and Abiotic Stresses in Agricultural Plants: Recent Developments. In Bioresources and Bioprocess in Biotechnology for a Sustainable Future; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 247–269. [Google Scholar]
- Martínez, S.A.C.; Quispe, R.N.G. Bacterias endofíticas de Zea mays “maíz” productoras de auxinas. Ayacucho 2019. Investigación 2020, 28, 200–209. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, M.G.; Gallegos-Robles, M.A.; Rodríguez-Sifuentes, L.; Fortis-Hernández, M.; Luna-Ortega, L.G.; González-Salas, U. Cepas nativas de Bacillus spp. como una alternativa sostenible en el rendimiento de forraje de maíz. Terra Latinoam. 2020, 38, 313–321. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhou, J.; Sun, M.; Li, H.; Han, Y.; Lv, J.; Li, Y.; Zhan, X.; George, T.S.; Lui, W.; et al. A newly isolated Bacillus megaterium OQ560352 promotes maize growth in saline soils by altering rhizosphere microbial communities and organic phosphorus utilization. Rhizosphere 2023, 27, 100746. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Bacterial indole-3-acetic acid: A key regulator for plant growth, plant-microbe interactions, and agricultural adaptive resilience. Microbiol. Res. 2024, 281, 127602. [Google Scholar] [CrossRef]
- Parra-Cota, F.I.; Los Santos-Villalobos, S.; Lugo-Valdez, M.A.; Cruz-Ibarra, R.A.; Fuentes-Dávila, G.; Peinado-Fuentes, L.A. Potencial agrobiotecnológico de bacterias aisladas de suelos agrícolas asociados al cultivo de maíz en el Valle del Fuerte, Sinaloa. Rev. Latinoam. Rec. Nat. 2017, 13, 51–57. [Google Scholar]
- Bolivar-Anillo, H.J.; González-Rodríguez, V.E.; Cantoral, J.M.; García-Sánchez, D.; Collado, I.G.; Garrido, C. Endophytic bacteria Bacillus subtilis, isolated from Zea mays, as potential biocontrol agent against Botrytis cinerea. Biology 2021, 10, 492. [Google Scholar] [CrossRef]
- Navid, S.; Tanveer, S.; Ali, B. Auxin Production by Bacillus simplex Enhanced the Growth of Zea mays (L.) under In-Vitro and In-Vivo Conditions. LGU–J. Life Sci. 2023, 7, 459–473. [Google Scholar] [CrossRef]
- Agunbiade, V.F.; Fadiji, A.E.; Agbodjato, N.A.; Babalola, O.O. Isolation and Characterization of Plant-Growth-Promoting, Drought-Tolerant Rhizobacteria for Improved Maize Productivity. Plants 2024, 13, 1298. [Google Scholar] [CrossRef]
- Pang, F.; Li, Q.; Solanki, M.K.; Wang, Z.; Xing, Y.X.; Dong, D.F. Soil phosphorus transformation and plant uptake driven by phosphate-solubilizing microorganisms. Front. Microbiol. 2024, 15, 1383813. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Ruiz, W.F.; Casique-Huamanguli, R.D.; Valdez-Nuñez, R.A.; Rojas-García, J.C.; Calixto-García, A.R.; Ríos-Reátegui, F.; Pompa-Vásquez, D.F.; Padilla-Santa-Cruz, E. Rhizospheric Bacteria of Cover Legumes from Acidic Soils Are Capable of Solubilizing Different Inorganic Phosphates. Microorganisms 2024, 12, 1101. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, R.; Oudot, M.; Hernández, I.; Nápoles, M.C.; Pérez-Martínez, S.; Castillo, S.D. Aislamiento y caracterización de Stenotrophomonas asociada a rizosfera de maíz (Zea mays L.). Cul. Trop. 2020, 41, 1–17. [Google Scholar]
- Azizah, H.; Rahajeng, S.M.; Jatmiko, Y.D. Isolation and screening of phosphate and potassium solubilizing endophytic bacteria in Maize (Zea mays L.). J. Exp. Life Sci. 2020, 10, 165–170. [Google Scholar] [CrossRef]
- Sangoquiza-Caiza, C.A.; Pincay-Verdezoto, A.K.; Park, C.H.; Zambrano-Mendoza, J.L. Diversity of nitrogen-fixing and phosphorus-solubilizing bacteria associated with the rhizosphere of Andean maize in Ecuador. Braz. J. Biol. 2023, 83, e273632. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Culbert, C.T.; Barraclough, T.G. Community composition drives siderophore dynamics in multispecies bacterial communities. BMC–Ecol. Evo. 2023, 23, 45. [Google Scholar] [CrossRef]
- Parra-Cota, F.I.; Coronel-Acosta, C.B.; Amézquita-Avilés, C.F.; Santos-Villalobos, S.D.L.; Escalante-Martínez, D.I. Diversidad metabólica de microorganismos edáficos asociados al cultivo de maíz en el Valle del Yaqui, Sonora. Rev. Mex. Cienc. Agríc. 2018, 9, 431–442. [Google Scholar] [CrossRef]
- Yılmaz, S.; Ünlü, E.; Mamoori, K.; Çetin, A. Indole acetic acid (IAA) production potential of PGPR bacterial isolates and their effect on seed germination in Zea mays L. Curr. Trends Nat. Sci. 2022, 11, 90–99. [Google Scholar] [CrossRef]
- Ribeiro, V.P.; Gomes, E.A.; de Sousa, S.M.; de Paula-Lana, U.G.; Coelho, A.M.; Marriel, I.E.; de Oliveira-Paiva, C.A. Co-inoculation with tropical strains of Azospirillum and Bacillus is more efficient than single inoculation for improving plant growth and nutrient uptake in maize. Arch. Microbiol. 2022, 204, 143. [Google Scholar] [CrossRef]
- Cardarelli, M.; Woo, S.L.; Rouphael, Y.; Colla, G. Seed Treatments with Microorganisms Can Have a Biostimulant Effect by Influencing Germination and Seedling Growth of Crops. Plants 2022, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.C.; Alves, G.C.; Silva, T.F.R.D.; Reis, V.M. Inoculation effects of growthpromoting bacteria on corn root architecture: Influence of nitrogen levels, bacterial populations, and plant genotypes. Rev. Bras. Ciênc. Solo 2023, 47, e0230059. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Plant growth-promoting soil bacteria: Nitrogen fixation, phosphate solubilization, siderophore production, and other biological activities. Plants 2023, 12, 4074. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Gupta, R.S. A phylogenomic and comparative genomic framework for resolving the polyphyly of the genus Bacillus: Proposal for six new genera of Bacillus species, Peribacillus gen. nov., Cytobacillus gen. nov., Mesobacillus gen. nov., Neobacillus gen. nov., Metabacillus gen. nov. and Alkalihalobacillus gen. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 406–438. [Google Scholar] [CrossRef] [PubMed]
- Manetsberger, J.; Caballero Gómez, N.; Soria-Rodríguez, C.; Benomar, N.; Abriouel, H. Simply Versatile: The Use of Peribacillus simplex in Sustainable Agriculture. Microorganisms 2023, 11, 2540. [Google Scholar] [CrossRef] [PubMed]
- Kämpfer, P.; Falsen, E.; Lodders, N.; Schumann, P. Sporosarcina contaminans sp. nov. and Sporosarcina thermotolerans sp. nov., two endospore-forming species. Int. J. Syst. Evol. Microbiol. 2010, 60, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Xu, C.; Liu, J.; Sun, F.; Yu, H.; Wang, S.; Pei, Z. Static composting of cow manure and corn stalk covered with a membrane in cold regions. Front. Bioeng. Biotechnol. 2022, 10, 969137. [Google Scholar] [CrossRef]
- Burt, R. Soil Survey Staff: Soil Survey Field and Laboratory Methods Manual Report No. 51; US Department of Agriculture: Washington, DC, USA, 2014; Volume 2, pp. 227–234. Available online: https://www.nrcs.usda.gov/sites/default/files/2023-01/SSIR51.pdf (accessed on 12 June 2024).
- Gravel, V.; Antoun, H.; Tweddell, R.J. Effect of indole-acetic acid (IAA) on the development of symptoms caused by Pythium ultimum on tomato plants. Eur. J. Plant Pathol. 2007, 119, 457–462. [Google Scholar] [CrossRef]
- Marra, L.M.; Oliveira, S.M.D.; Soares, C.R.F.S.; Moreira, F.M.D.S. Solubilization of inorganic phosphates by inoculant strains from tropical legumes. Sci. Agric. 2011, 68, 603–609. [Google Scholar] [CrossRef]
- Sayyed, R.Z.; Badjugar, M.D.; Sonawane, H.M.; Mhaske, M.M.; Chincholkar, S.B. Production of microbial iron chelators (siderophores) by fluorescent Pseudomonads. Indian J. Biotechnol. 2005, 4, 484–490. [Google Scholar]
- Swift, R. Plant Growth-Promoting Bacteria from Western Australian Soils. Ph.D. Thesis, Murdoch University, Perth, Australia, 2016. [Google Scholar]
- Etesami, H.; Alikhani, H.A. Co-inoculation with endophytic and rhizosphere bacteria allows reduced application rates of N-fertilizer for rice plant. Rhizosphere 2016, 2, 5–12. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA and whole genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]






| Strains of Rhizobacteria | Microscopic Characteristics | Colony Characteristics | Origin | ||||
|---|---|---|---|---|---|---|---|
| Gram | Shape | Shape | Edge | Elevation | Consistency | ||
| L2 | - | Bacillus | Irregular | Undulated | Raised | Creamy | Lamas |
| L3-1 | - | Bacillus | Circular | Entire | Raised | Creamy | Lamas |
| L4 | - | Bacillus | Circular | Entire | Raised | Creamy | Lamas |
| L6 | - | Bacillus | Circular | Entire | Raised | Creamy | Lamas |
| NSM3 | + | Bacillus | Circular | Entire | Raised | Creamy | El Dorado |
| B3 | + | Bacillus | Irregular | Entire | Raised | Creamy | Picota |
| B5 | + | Bacillus | Circular | Entire | Raised | Creamy | Picota |
| P1 | + | Cocci | Circular | Entire | Flat | Creamy | Picota |
| P3 | + | Bacillus | Circular | Entire | Raised | Creamy | Picota |
| P4 | - | Bacillus | Circular | Entire | Raised | Creamy | Picota |
| SJ1 | - | Bacillus | Circular | Entire | Flat | Yellow | Bellavista |
| SJ2 | - | Cocci | Circular | Entire | Flat | Creamy | Bellavista |
| Strains of Rhizobacteria | BNF | Production of IAA (μg mL−1) | Solubilization of AlPO4 (μg mL−1) | Production of Siderophores (%) | |
|---|---|---|---|---|---|
| JMV | Burk | ||||
| L2 | + | + | 6.04 ± 1.03 fg | 150.57 ± 1.48 g | 5.95 ± 1.08 d |
| L3-1 | + | + | 1.15 ± 2.03 g | 146.30 ± 2.96 gh | 2.70 ± 1.87 d |
| L4 | + | + | 8.52 ± 1.98 fg | 153.56 ± 0.00 g | 8.47 ± 0.62 d |
| L6 | + | + | 4.57 ± 4.42 g | 181.77 ± 7.80 e | 3.06 ± 0.62 d |
| NSM3 | + | + | 26.94 ± 0.33 d | 193.31 ± 3.39 d | 89.19 ± 0.00 a |
| B3 | + | + | 44.54 ± 0.45 a | 180.91 ± 4.12 e | 32.61 ± 7.97 c |
| B5 | + | + | 35.65 ± 1.14 bc | 233.91 ± 1.96 a | 34.05 ± 2.86 c |
| P1 | + | + | 31.79 ± 4.51 cd | 208.26 ± 1.96 c | 10.99 ± 4.87 d |
| P3 | + | + | 36.21 ± 3.50 bc | 165.96 ± 1.48 f | 9.91 ± 1.25 d |
| P4 | + | + | 41.59 ± 3.39 ab | 165.96 ± 0.74 f | 57.12 ± 1.25 b |
| SJ1 | + | + | 19.10 ± 0.89 e | 224.08 ± 2.22 b | 10.63 ± 2.50 d |
| SJ2 | + | + | 12.60 ± 3.12 ef | 192.88 ± 0.74 d | 7.03 ± 1.08 d |
| Positive control | + | + | NE | 141.60 ± 1.48 h | NE |
| CV (%) | 11.88 | 1.68 | 13.45 | ||
| Strains of Rhizobacteria | Place of Origin | Host | Most Related Species | Similarity (%) | Identified Strain/ Accession Number in NCBI GenBank |
|---|---|---|---|---|---|
| B5 | Picota, Barranquita | Zea mays | Peribacillus frigoritolerans DSM 8801T | 99.93 | Peribacillus sp. B5/OP861655 |
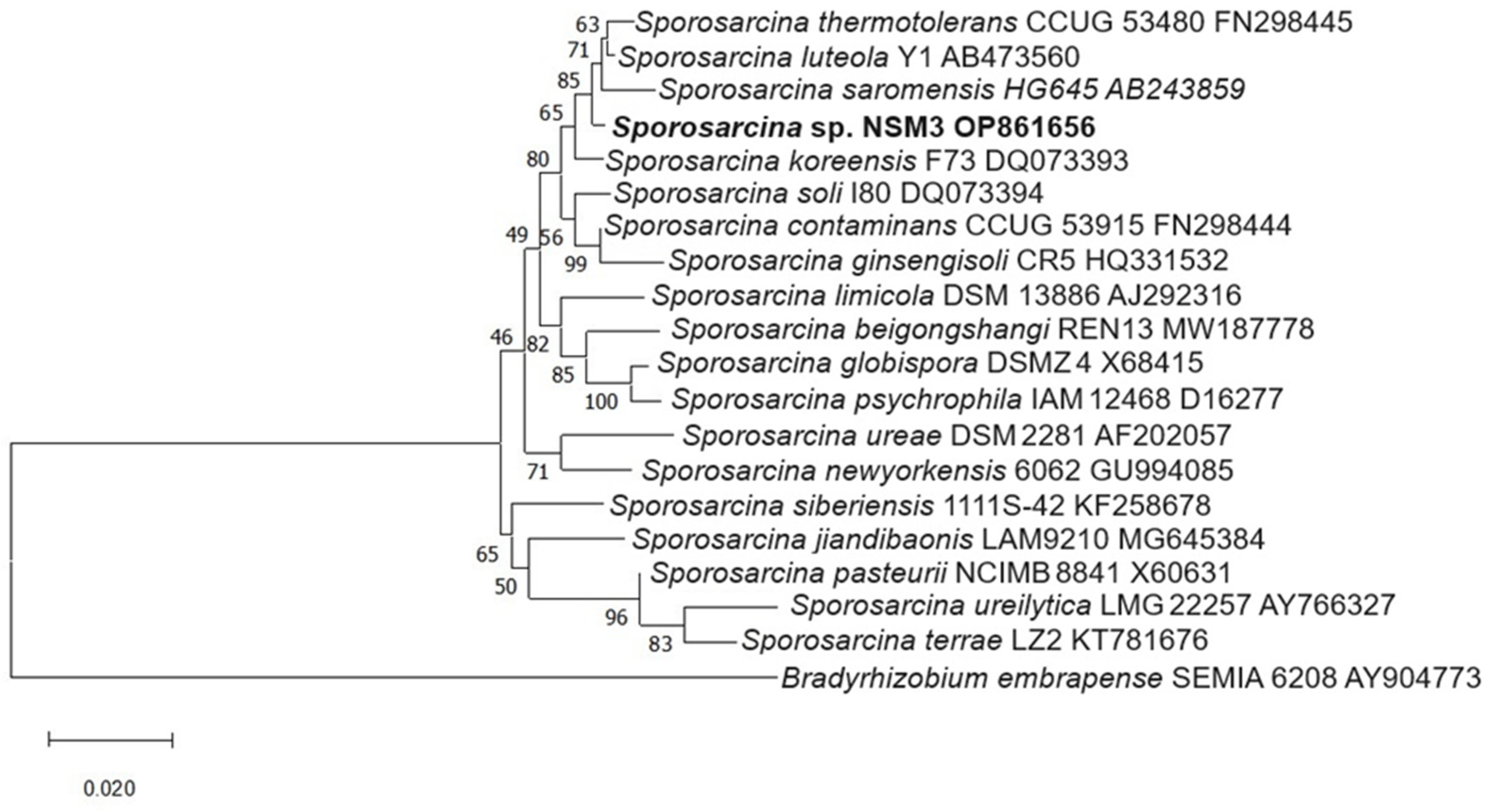
| NSM3 | El Dorado, Nuevo San Martin | Zea mays | Sporosarcina luteola Y1 AB473560 | 99.44 | Sporosarcina sp. NSM3/OP861656 |
| Province | Sector | Coordinates | Altitude (masl) | |
|---|---|---|---|---|
| South Latitude | West Longitude | |||
| Lamas | Huapo | 06°22.869′ S | 076°32.883′ W | 536 m |
| Alto Pucalpillo | 06°25.204′ S | 076°33.536′ W | 680 m | |
| Cochapata | 06°22.869′ S | 076°32.052′ W | 584 m | |
| Picota | Barranquita | 06°54.346′ S | 076°18.226′ W | 216 m |
| Ponaza | 06°54.126′ S | 076°15.660′ W | 229 m | |
| Ponaza 1 | 06°54.337′ S | 076°15.407′ W | 246 m | |
| El Dorado | Nuevo San Martin | 06°41.672′ S | 076°37.250′ W | 312 m |
| Bellavista | San Jose | 07°02.077′ S | 076°29.306′ W | 233 m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ríos-Ruiz, W.F.; Tarrillo-Chujutalli, R.E.; Rojas-García, J.C.; Tuanama-Reátegui, C.; Pompa-Vásquez, D.F.; Zumaeta-Arévalo, C.A. The Biotechnological Potential of Plant Growth-Promoting Rhizobacteria Isolated from Maize (Zea mays L.) Cultivations in the San Martin Region, Peru. Plants 2024, 13, 2075. https://doi.org/10.3390/plants13152075
Ríos-Ruiz WF, Tarrillo-Chujutalli RE, Rojas-García JC, Tuanama-Reátegui C, Pompa-Vásquez DF, Zumaeta-Arévalo CA. The Biotechnological Potential of Plant Growth-Promoting Rhizobacteria Isolated from Maize (Zea mays L.) Cultivations in the San Martin Region, Peru. Plants. 2024; 13(15):2075. https://doi.org/10.3390/plants13152075
Chicago/Turabian StyleRíos-Ruiz, Winston Franz, Rosslinn Esmith Tarrillo-Chujutalli, Jose Carlos Rojas-García, Cicerón Tuanama-Reátegui, Danny Fran Pompa-Vásquez, and Carlos Alberto Zumaeta-Arévalo. 2024. "The Biotechnological Potential of Plant Growth-Promoting Rhizobacteria Isolated from Maize (Zea mays L.) Cultivations in the San Martin Region, Peru" Plants 13, no. 15: 2075. https://doi.org/10.3390/plants13152075
APA StyleRíos-Ruiz, W. F., Tarrillo-Chujutalli, R. E., Rojas-García, J. C., Tuanama-Reátegui, C., Pompa-Vásquez, D. F., & Zumaeta-Arévalo, C. A. (2024). The Biotechnological Potential of Plant Growth-Promoting Rhizobacteria Isolated from Maize (Zea mays L.) Cultivations in the San Martin Region, Peru. Plants, 13(15), 2075. https://doi.org/10.3390/plants13152075






